Abstract
Mycobacteria were isolated and characterised from 49 cats with extensive infections of the subcutis and skin. Cats were generally between 3 and 10 years of age, and female cats were markedly over-represented. All isolates were rapid-growers and identified as either Mycobacteria smegmatis (40 strains) or M fortuitum (nine strains). On the basis of Etest for minimum inhibitory concentration and/or disc diffusion susceptibility testing, all strains of M smegmatis were susceptible to trimethoprim while all strains of M fortuitum were resistant. M smegmatis strains were typically susceptible to doxycycline, gentamicin and fluoroquinolones but not clarithromycin. All M fortuitum strains were susceptible to fluoroquinolones, and often also susceptible to gentamicin, doxycycline and clarithromycin. Generally, M smegmatis strains were more susceptible to antimicrobial agents than M fortuitum strains. Treatment of mycobacterial panniculitis involves long courses of antimicrobial agents, typically of 3–6 months, chosen on the basis of in vitro susceptibility testing and often combined with extensive surgical debridement and wound reconstruction. These therapies will result in effective cure of the disease. One or a combination of doxycycline, ciprofloxacin/enrofloxacin or clarithromycin are the drugs of choice for long-term oral therapy.
Mycobacterial panniculitis refers to a clinical syndrome characterised by chronic infection of the subcutis and skin of cats with ‘rapidly growing’ mycobacteria (RGM). This term refers to a heterogeneous group of saprophytic organisms that produce visible colonies on synthetic media within 5 days when cultured at room temperature. They are distributed ubiquitously in nature, and can be isolated commonly from soil and bodies of water. Bacteria in this group of organisms include Mycobacterium fortuitum, M chelonae, M smegmatis, M abscessus and M thermoresistibile (Dewevre et al 1977, Wilkinson et al 1978, 1982, Kunkle et al 1983, White et al 1983, Wallace et al 1985, Willemse et al 1985, Pedersen 1988, Wilkinson & Mason 1991, Wayne & Shramek 1992, Lotti & Hautmann 1993).
If introduced through some breach in the integument, saprophytic mycobacteria are capable of replication in mammalian tissues (Pedersen 1988, Street et al 1991, Wilkinson & Mason 1991). Like other mycobacteria, they are Gram-positive rods with a cell wall rich in complex fatty acids and waxes. The major cell wall lipids are the mycolic acids which enable organisms to survive and replicate within phagocytes, and to retain variable amounts of carbol fuchsin after heating and exposure to acid and/or alcohol (Wolinsky 1973, Youmans 1980).
The tropism of RGM for fat results in their propensity to produce disease in obese individuals and in tissues rich in lipid, such as the inguinal fat pad (Wilkinson et al 1982, Kunkle et al 1983, White et al 1983, White & Kowalski 1991, Studdert & Hughes 1992). The same phenomenon accounts for scenarios where these organisms give rise to human infections, for example, in athletes that inject themselves with anabolic steroids suspended in oily vehicles from contaminated multi-use vials, as a complication of lipoid pneumonia and following augmentation mammoplasty and median sternotomy (Wallace et al 1985, 1988). Experimental and clinical observations suggest that adipose tissue offers a favourable environment for the survival and proliferation of RGM, either by providing triglycerides for growth of organisms, or protecting them from the phagocytic or immune response of the host (Hagan & Levine 1932, Richardson 1971, Wilkinson et al 1982). Initial reports suggested that mycobacterial panniculitis in cats was more common in warm humid tropical and subtropical climates (Wilkinson & Mason 1991). This would appear to be the case in human beings, where, for example, most reported cases in the USA occur in Texas, Florida and Louisiana (Wallace et al 1985). However, cats from a variety of temperate climates have subsequently been reported to develop these infections (White & Kowalski 1991, Studdert & Hughes 1992, Malik et al 1994), and the causal organisms have been readily cultivated in temperate soil samples collected from Japan (Tsukamura 1976).
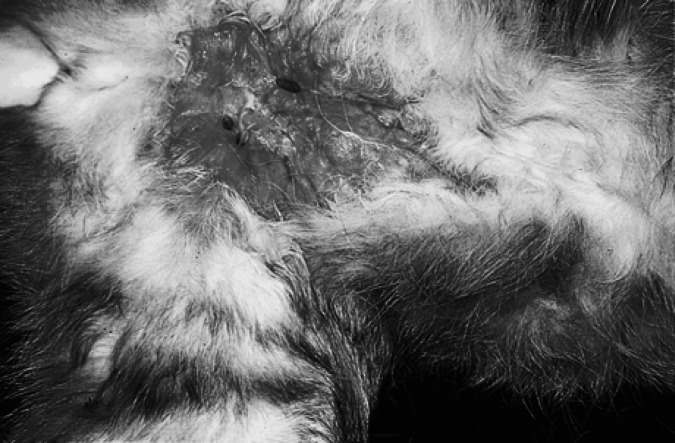
In cats, infections tend to start in the inguinal region, usually following environmental contamination of catfight injuries, typically bites or raking wounds inflicted with the hind claws. The infection may spread subsequently to contiguous subcutaneous tissues of the ventral and lateral abdominal wall and perineum (Wilkinson & Mason 1991). Penetrating injury by sticks, metallic objects and vehicular trauma may also give rise to these infections (Wilkinson et al 1982, Newton et al 1993), as can dog bite injuries contaminated with soil or dirt. Sometimes infections start in the axillae and spread into adjacent tissues. Early in their clinical course, infections can resemble conventional catfight abscesses, but without the characteristic pungent odour and turbid pus. Instead, a circumscribed plaque or nodule is apparent at the site of injury. Later there is progressive thickening of the nearby subcutis to which the overlying skin becomes adherent. Affected areas become denuded of hair and numerous punctate fistulae appear, discharging a watery exudate (Fig 1). Fistulae are intermingled with focal purple depressions, which correspond to thinning of the epidermis over accumulations of pus (Fig 2). The ‘lesion’ gradually increases in area and depth, and may eventually involve the entire ventral abdomen, adjacent flanks or limbs.
Fig 1.
Inguinal panniculitis in a cat caused by M smegmatis (strain 15). Note the two small draining sinus tracts.
Fig 2.
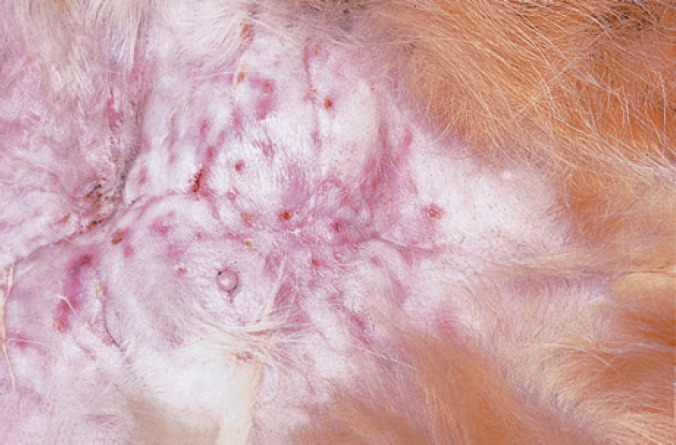
Inguinal panniculitis in a cat caused by M smegmatis (strain 24). Note the focal depressions that correspond to thinned epidermis overlying focal accumulations of watery exudate. Aspirates from such lesions, preceded by treatment of the overlying skin with 70% ethanol, provide ideal specimens for cytology and culture.
If cats are presented promptly for veterinary attention and the lesion confused with an anaerobic cat bite abscess, standard treatment consisting of surgical drainage and administration of a synthetic penicillin is typically followed by wound breakdown and development of a large non-healing sinus tract surrounded by indurated suppurating tissue (Monroe et al 1988, Kunkle 1993; Fig 3). Some severely affected cats become depressed, pyrexic, inappetent, lose weight and become reluctant to move. Surprisingly, other cats remain otherwise well despite extensive pyogranulomatous disease. Usually the problem remains localised to the cutaneous and subcutaneous tissues, as might be expected from an opportunistic infection in an immunocompetent host. Although adjacent structures such as the abdominal or thoracic wall can eventually be affected, widespread dissemination of the infection to the internal organs and lymph nodes is unusual (Kunkle et al 1983). Histologically, there is a pyogranulomatous inflammation of subcutaneous adipose tissue, overlying dermis and underlying abdominal fascia and musculature. Organisms are generally difficult to find in Ziehl– Neelsen stained sections, although acid-fast bacilli can usually be detected in macrophages or extracellular lipid vacuoles after a thorough search (Wilkinson & Mason 1991, Malik et al 1994).
Fig 3.
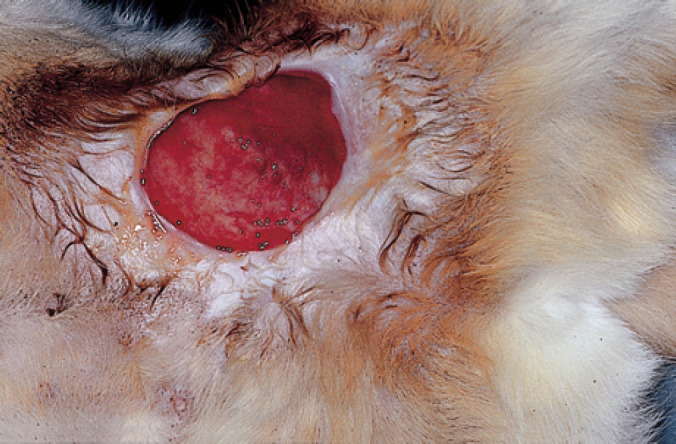
Inguinal panniculitis in a cat caused by M fortuitum (strain 2). Attempted surgical removal of affected tissues resulted in wound breakdown and the development of a large non-healing sinus tract surrounded by indurated suppurating granulation tissue.
Although the clinical syndrome of feline mycobacterial panniculitis has been well described and several case reports and small case series have concentrated on aspects of diagnosis and treatment, the microbiological features of a substantial number of cases have not been reported. The present study provides microbiological data concerning 49 cases of mycobacterial panniculitis in cats from which our laboratory has received samples. In addition, our evolving experience with the clinical management of these cases is reviewed.
Materials and methods
Of the 49 cases reviewed, 20 cases were submitted from the University of Sydney Veterinary Centre (UVCS) and the remainder were submitted by private practitioners or private clinical pathology laboratories.
Sampling and preparation of material from clinical cases
A tentative diagnosis of mycobacteriosis was confirmed by collection, usually under general anaesthesia, of aspirated pus or deep tissue samples. Smears prepared from homogenised biopsy material or swabs or aspirates of the purulent exudate were stained using DiffQuik, Burke's modification of the Gram stain and a modified acid-fast procedure (decolourising with 5% sulphuric acid for 3–5 min). Samples of pus obtained from needle aspirates of affected tissues produced the best laboratory specimens (Fig 2).
Bacteriology
Tissue homogenates and pus were streaked onto duplicate 5% sheep blood agar plates and 1% Ogawa egg yolk medium (Ogawa & Motomura 1970) and incubated aerobically at 37 and 25°C. Contaminated tissue homogenates were treated with 4% sodium hydroxide followed by neutralisation with dilute hydrochloric acid. In several instances, RGM were selectively differentiated from contaminant skin flora by primary isolation around antibiotic discs (first generation cephalosporins, isoxazolyl penicillins) on blood agar, and in time this became the preferred method of dealing with contaminated skin samples.
Strain identification
A total of 49 strains of RGM were isolated during the study period. Of these, a representative 27 strains were identified at a Mycobacteria Reference Laboratory. Subsequently, three further strains were identified to the species level. Speciation was carried out using a modification of the identification scheme of Dawson (1971), taking into account the following phenotypic features: organism morphology and degree of acid-fastness in Ziehl-Neelsen stained smears of growth taken from Lowenstein-Jensen medium, colonial morphology (rough or smooth), pigmentation in the dark and light, rate of growth at room temperature and 37°C, ability to grow at 42 and 52°C, arylsulphatase activity, iron uptake, p-amino salicylic acid (PAS) degradation, nitrate reduction, β-galactosidase activity (Tsukamura 1984), acid production from carbohydrates (glucose, inositol, mannitol) (Gordon et al 1974), utilisation of compounds (glucose, fructose, inositol, mannitol, citrate) as the sole carbon source (Silcox et al 1981), tolerance to 5% sodium chloride in Lowenstein-Jensen medium and susceptibility to polymyxin B. The mycolic acid profile of 10 strains was determined by reverse phase HPLC, using previously reported methods (Butler et al 1988, Chew et al 1996).
Antimicrobial susceptibility testing
For 27 strains identified at the reference laboratory, minimum inhibitory concentrations (MICs) were determined using the Etest (AB Biodisk, Solna, Sweden) method according to the manufacturer's recommendations and using one Etest strip per 90 mm plate containing 20 ml Brucella agar (Difco Laboratories, Detroit, USA) (Hoffner et al 1994, Koontz et al 1994). The antimicrobials tested were azithromycin, ciprofloxacin, gentamicin, trimethoprim, clarithromycin and doxycycline and values were determined after 72 h incubation.
Disc diffusion susceptibility tests were performed on 27 of the strains identified in the reference laboratory and an additional 22 strains tentatively identified as M fortuitum (one strain) or M smegmatis (21 strains) on the basis of growth characteristics, colonial morphology and their susceptibility to trimethoprim. Disc diffusion methodology was based on that of Wallace et al (1979, 1985) and strains were tested against discs containing doxycycline (30 μg), gentamicin (10 μg), tobramycin (10 μg), ciprofloxacin (5 μg), norfloxacin (10 μg), trimethoprim (5 μg), polymyxin B (300 μg), enrofloxacin (5 μg) and clarithromycin (30 μg). Some antibiotics were included to determine a suitable agent for long-term oral therapy, while others (trimethoprim, polymyxin B, tobramycin) were included to provide additional phenotypic information.
Results
Patient information and case management
The 27 strains for which a species identification and MICs were obtained (Table 1) were isolated between 1983 and 1998. Twenty-two strains were obtained from cats residing in the greater Sydney area, two were from the Central Coast of New South Wales, and three were from rural locations within New South Wales (Mittagong, Blayney, Bargo). Of the 27 cats, 18 were spayed females, seven were castrated males, while information was unavailable for the remaining two cases. The cats ranged in age from 2 to 12 years.
Table 1.
Minimum inhibitory concentrations (μg/ml) determined by Etest method for 27 strains of RGM speciated in the reference laboratory and isolated from cats with mycobacterial panniculitis
| Strain | Identity | Trimethoprim | Doxycycline | Gentamicin | Ciprofloxacin | Clarithromycin | Azithromycin |
|---|---|---|---|---|---|---|---|
| 1 | M fortuitum(3) | >32 | 12 | 0.75 | 0.125 | 0.125 | 1.5 |
| 2 | M fortuitum(3) | >32 | 4 | 3 | 0.047 | 0.75 | 16 |
| 3 | M fortuitum(f) | >32 | 0.064 | 4 | 0.016 | 1 | 3 |
| 4 | M fortuitum(f) | >32 | 0.094 | 3 | 0.016 | 1.5 | 6 |
| 5 | M fortuitum(f) | >32 | 0.125 | 3 | 0.012 | 1.5 | 6 |
| 6 | M fortuitum(f) | >32 | >256 | 3 | 0.023 | 21 | 6 |
| 7 | M fortuitum(f) | >32 | 0.094 | 3 | 0.016 | 2 | >256 |
| 8 | M fortuitum(f) | >32 | 96 | 4 | 0.023 | 3 | >256 |
| 9 | M smegmatis | 0.75 | 0.125 | 0.125 | 0.094 | 0.047 | 0.19 |
| 10 | M smegmatis | 0.19 | 0.047 | 0.064 | 1 | 4 | 6 |
| 11 | M smegmatis | 0.75 | 0.094 | 0.19 | 0.125 | 1.5 | 8 |
| 12 | M smegmatis | 0.38 | 0.064 | 0.125 | 0.094 | 4 | 8 |
| 13 | M smegmatis | 0.25 | 0.032 | 0.38 | 0.032 | 0.5 | 32 |
| 14 | M smegmatis | 0.38 | 0.064 | 0.094 | 0.064 | 1.5 | >256 |
| 15 | M smegmatis | 1.5 | 0.064 | 0.125 | 0.125 | 3 | >256 |
| 16 | M smegmatis | 0.25 | 0.047 | 0.75 | 0.064 | 3 | >256 |
| 17 | M smegmatis | 1 | 0.094 | 0.094 | 0.094 | 3 | >256 |
| 18 | M smegmatis | 0.75 | 0.094 | 0.094 | 0.094 | 3 | >256 |
| 19 | M smegmatis | 1 | 0.064 | 0.094 | 0.094 | 3 | >256 |
| 20 | M smegmatis | 0.5 | 0.094 | 0.125 | 0.064 | 6 | >256 |
| 21 | M smegmatis | 0.5 | 0.094 | 0.125 | 0.125 | 6 | >256 |
| 22 | M smegmatis | 0.75 | 0.125 | 0.125 | 0.125 | >256 | >256 |
| 23 | M smegmatis | 0.38 | 0.094 | 0.094 | 0.094 | >256 | >256 |
| 24 | M smegmatis | 0.19 | 0.064 | 0.094 | 0.125 | >256 | >256 |
| 25 | M smegmatis | 1 | 0.064 | 0.125 | 0.094 | >256 | >256 |
| 26 | M smegmatis | 0.75 | 0.094 | 0.125 | 0.125 | >256 | >256 |
| 27 | M smegmatis | 0.5 | 0.094 | 0.125 | 0.094 | >256 | >256 |
| MIC breakpoints* | 16 | 8 (16) | 8 | 2 (4) | 8 (8) | 2 |
M fortuitum(f)=M fortuitum biovar fortuitum; M fortuitum(3)=unnamed third biovar of M fortuitum.
*Values for interpretation of susceptibility as recommended by NCCLS (1999) and Woods et al (1999) (in parentheses).
The 22 strains for which only disc diffusion antimicrobial susceptibility data were available (strains 28–49 inclusive; Table 2) had been obtained from patients examined between 1992 and 1999. Three of the patients were castrated males, 12 were spayed females, while the sex of seven was not provided. These cats ranged in age from 3 to 11 years. Ten cats normally resided in Sydney and its environs, six were from Victoria (strains provided by the Central Veterinary Diagnostic Laboratory) and one each were from the Blue Mountains, Hunter Valley, south coast and central coast of New South Wales. Only five of these cases were treated as in-patients at the UVCS.
Table 2.
Antimicrobial susceptibility test data for 49 strains of RGM from cats with mycobacterial panniculitis determined using a disc diffusion method
| Strain | Identity | TMP5 | DO30 | GM10 | CIP5 | CLR15 | NOR10 | ENR5 | PB300 |
|---|---|---|---|---|---|---|---|---|---|
| (a) Species identification confirmed at a Mycobacteria Reference Laboratory | |||||||||
| 1 | M fortuitum(3) | R | R | S | NT | NT | S | NT | S |
| 2 | M fortuitum(3) | NT | NT | NT | NT | NT | NT | NT | NT |
| 3 | M fortuitum(f) | R | S | R | NT | NT | S | NT | S |
| 4 | M fortuitum(f) | R | S | R | NT | NT | S | NT | S |
| 5 | M fortuitum(f) | R | S | S | NT | NT | S | NT | S |
| 6 | M fortuitum(f) | R | R | S | NT | NT | S | NT | S |
| 7 | M fortuitum(f) | R | S | S | S | NT | S | NT | S |
| 8 | M fortuitum(f) | NT | NT | NT | NT | NT | NT | NT | NT |
| 9 | M smegmatis | S | S | S | NT | NT | S | NT | S |
| 10 | M smegmatis ‡ | S | S | S | R | NT | R | NT | S |
| 11 | M smegmatis | S | S | S | S | NT | S | NT | S |
| 12 | M smegmatis | S | S | S | NT | NT | S | NT | S |
| 13 | M smegmatis | S | S | S | S | NT | S | NT | NT |
| 14 | M smegmatis | S | S | S | NT | NT | S | NT | S |
| 15 | M smegmatis | S | S | S | NT | NT | S | NT | S |
| 16 | M smegmatis | S | S | S | NT | NT | S | NT | S |
| 17 | M smegmatis | S | S | S | NT | NT | S | NT | S |
| 18 | M smegmatis | S | S | S | NT | NT | S | NT | S |
| 19 | M smegmatis | S | S | S | NT | NT | S | NT | S |
| 20 | M smegmatis | S | S | S | NT | NT | S | NT | S |
| 21 | M smegmatis | S | S | S | NT | NT | S | NT | S |
| 22 | M smegmatis | S | S | S | NT | NT | S | NT | S |
| 23 | M smegmatis | S | S | S | NT | NT | S | NT | S |
| 24 | M smegmatis | S | NT | S | NT | NT | S | NT | S |
| 25 | M smegmatis | S | S | S | S | NT | S | NT | S |
| 26 | M smegmatis | S | S | S | S | NT | S | NT | S |
| 27 | M smegmatis | S | S | S | S | NT | S | S | S |
| (b) Species identification based on susceptibility to trimethoprim | |||||||||
| 28 | M fortuitum | R | R | S | S | I | NT | S | NT |
| 29 | M smegmatis | S | S | S | NT | NT | S | NT | S |
| 30 | M smegmatis † | S | NT | S | NT | NT | S | NT | S |
| 31 | M smegmatis † | S | S | S | NT | NT | S | NT | S |
| 32 | M smegmatis | S | S | S | S | NT | S | NT | NT |
| 33 | M smegmatis | S | S | S | S | NT | NT | NT | S |
| 34 | M smegmatis | S | S | S | S | NT | NT | NT | S |
| 35 | M smegmatis | S | S | S | S | NT | NT | NT | S |
| 36 | M smegmatis | S | S | S | S | NT | NT | NT | S |
| 37 | M smegmatis | S | S | S | S | NT | NT | NT | S |
| 38 | M smegmatis | S | S | S | S | NT | S | NT | S |
| 39 | M smegmatis | S | S | S | S | NT | S | NT | S |
| 40 | M smegmatis | S | S | S | S | NT | S | NT | S |
| 41 | M smegmatis | S | S | S | S | NT | S | NT | S |
| 42 | M smegmatis | S | S | S | S | NT | S | NT | S |
| 43 | M smegmatis | S | S | S | S | NT | S | NT | S |
| 44 | M smegmatis | S | S | S | S | NT | S | NT | S |
| 45 | M smegmatis | S | S | S | S | NT | S | NT | S |
| 46 | M smegmatis | S | S | S | S | NT | NT | S | NT |
| 47 | M smegmatis | S | S | S | S | NT | S | S | NT |
| 48 | M smegmatis | S | S | S | S | NT | S | S | S |
| 49 | M smegmatis ‡ | S | S | S | R | S | R | R | NT |
| Inhibition zone (mm)* | 20 | 30 | 16 | 30 | 30 | 17 | 30 | 10 | |
Organisms conformed to growth and microscopic characteristics of rapidly growing mycobacterial species and were confirmed as M smegmatis by testing at the Mycobacteria Reference Laboratory.
Isolated following prolonged monotherapy with enrofloxacin or ciprofloxacin.
DO30, doxycycline 30 μg; TMP5, trimethoprim 5 μg; GM10, gentamicin 10 μg; NOR10, norfloxacin 10 μg; CIP5, ciprofloxacin 5 μg; ENR5, enrofloxacin 5 μg; CLR15, clarithromycin 15 μg; PB300, polymyxin B 300 μg. S, susceptible; R, resistant; I, intermediate; NT, not tested.
M fortuitum(3), unnamed third biovar of M fortuitum; M fortuitum(f), M fortuitum biovar fortuitum.
Zone diameter to be exceeded to be classed as susceptible.
Mycobacteriology
In all cases it was possible to visualise Gram-positive and/or acid-fast rods in smears, although an exhaustive search of several smears was sometimes required. Many organisms showed beading. Cytology invariably demonstrated pyogranulomatous inflammation. Moderate to heavy growth of pinpoint, smooth, non-haemolytic colonies was usually visible after 36–48 h on sheep blood agar at 37°C.
Of the 27 strains sent to the reference laboratory, all were considered to be ‘rapid growers’ and 19 were identified as M smegmatis. Isolates were identified as M smegmatis if they grew in less than 7 days, grew well at 43°C but not at 52°C, were positive for iron uptake, usually tolerant of 5% sodium chloride, and had a negative 3-day arylsulphatase reaction. Further, colonies of M smegmatis grown from clinical material were invariably smooth and not immediately pigmented, although a late-developing yellow-to-orange brown pigmentation was apparent upon aging, but in only some strains. Pigment developed in the dark but was enhanced by light, and seemed to be dependent on oxygen availability. The strains identified as M smegmatis all gave negative nitrogen reduction tests initially. However, as the cultures aged, some tubes developed a brownish pigment and became more mucoid. These cultures then gave a positive nitrate reduction test. The M smegmatis strains were not uniform in their β-galactosidase activity and sodium chloride tolerance; these tests are of little value for discrimination of this species, but were included to differentiate M fortuitum strains from M chelonae strains. On culture, M smegmatis strains were generally non-acid-fast by the decolourisation method used (5% sulphuric acid for 5 min), although some strains retained the carbol fuchsin. One strain classified as M smegmatis produced acid from melibiose and was niacin positive, both unusual properties for this species. All M smegmatis strains failed to degrade PAS.
Strains were identified as M fortuitum if they were acid-fast, grew in less than 7 days at 28°C and 37°C, produced non-pigmented colonies, produced a positive three-day arylsulphatase reaction, were positive for iron uptake, reduced nitrate and were susceptible to polymyxin B (Silcox et al 1981, Wallace et al 1982). The M fortuitum strains were divided into biovarieties according to their utilisation of mannitol, inositol and citrate as sole sources of carbon for growth: M fortuitum biovar fortuitum strains failed to utilise mannitol, inositol or citrate whereas strains were included as members of the ‘third biovariant complex’ if they used inositol and mannitol as a carbon source (Silcox et al 1981, Wallace et al 1991). Eight strains belonged to the M fortuitum complex by virtue of their arylsulphatase activity and iron uptake. Six of these conformed with the fortuitum biovariant, although the sodium chloride susceptibility of one strain was unusual, and the growth observed at 43°C observed for several strains was rare in the reference laboratory's experience. Two strains corresponded with the ‘third biovariant complex’ of M fortuitum, although the susceptibility of one of these strains to sodium chloride was atypical. The cellular morphology of M fortuitum strains was generally more pleomorphic than the M smegmatis strains and M fortuitum strains were consistently acid-fast using 5% sulphuric acid as the decolouriser.
The 10 strains (nine M smegmatis, one M fortuitum biovar fortuitum) subjected to mycolic acid profile analysis by reverse phase HPLC were considered to be typical of M smegmatis, i.e. the mycolic acid profile incorrectly classified one M fortuitum strain as M smegmatis. This discordance was not surprising given that, despite their genetic differences, the mycolic acid profile of M smegmatis on two dimensional thin layer chromatography is very similar to that of M fortuitum (Minnikin et al 1984). Trimethoprim resistance correctly identified this ‘aberrant’ strain as M fortuitum (see below).
Antimicrobial susceptibility data
The MIC data for the 27 strains tested are presented in Table 1. All M smegmatis strains were susceptible to trimethoprim (MIC ranging from 0.19 to 1.5 μg/ml; MIC90 1.0 μg/ml), doxycycline (MIC ranging from 0.032 to 0.125 μg/ml; MIC90 0.094 μg/ml), gentamicin (MIC ranging from 0.094 to 0.75 μg/ml; MIC90 0.38 μg/ml) and ciprofloxacin (MIC ranging from 0.064 to 1.0 μg/ml; MIC90 0.125 μg/ml). Strains were generally resistant to azithromycin (MIC ranging from 0.064 to >256 μg/ml; MIC50 and MIC90 >256 μg/ml) and variable in their susceptibility to clarithromycin (MICs ranging from 0.047 to >256 μg/ml; MIC50 3 μg/ml, MIC90 >256 μg/ml).
All M fortuitum strains were susceptible to ciprofloxacin (MIC ranging from 0.016 to 0.125 μg/ml; MIC90 0.047 μg/ml). All strains of M fortuitum were resistant to trimethoprim (MIC >32 μg/ml) as has been reported previously (Wallace et al 1981). The susceptibility of M fortuitum to the other agents tested varied but, in general, the MICs for clarithromycin were lower than those for M smegmatis, while the MICs for gentamicin and doxycycline were higher.
As can be seen from Table 2, of the 22 strains tested using only disc diffusion susceptibility testing methodology (strains 28–49 inclusive), all 17 tested against polymyxin B were susceptible. Twenty-one of these strains were also susceptible to trimethoprim. By extrapolation from the MIC data set results for the species identified, it would be reasonable to assume that these 21 strains were M smegmatis. Subsequently, two of these strains were confirmed as M smegmatis at the reference laboratory.
There was almost complete agreement in results for the 27 stains tested by both MIC and disc diffusion methods. Susceptibility data obtained using the disc diffusion method for the 49 strains (Table 2) taken together were generally similar to those for the Etest data on 27 strains identified definitively. Mycobacterium smegmatis strains were generally susceptible to ciprofloxacin or enrofloxacin, doxycycline, gentamicin and tobramycin. Two strains (both confirmed as M smegmatis at the reference laboratory) were resistant to enrofloxacin and/or ciprofloxacin using the disc diffusion method but susceptible to doxycycline and gentamicin. Interestingly, both were obtained from cats that had been receiving monotherapy with a fluoroquinolone for a period of time prior to referral. Etest data were available for one of these strains; although the strain was considered to be susceptible to ciprofloxacin, the MIC value (1 μg/ml) was higher than for any other strains for which MIC values for ciprofloxacin were determined using Etests (Table 1).
Response to treatment
Treatment regimens and outcomes for 19 cases of mycobacterial panniculitis referred to the UVCS are summarised in Table 3. Case management details of five of these cats (cases 5, 6, 7, 8 and 9) have been published in detail previously (Malik et al 1994). The handling of these cases continued to evolve through the study period according to accumulating clinical experience and the availability of new antimicrobial agents and surgical procedures. There was great variation in the severity and extent of lesions in individual cats. Typically, cats had been seen by other veterinarians on several occasions and had been subjected to at least one attempt at surgical excision of lesions, usually followed by treatment using an antibiotic such as amoxycillin/clavulanic acid. The marked variation in disease severity makes it difficult to compare different treatment strategies. In addition, three cases were euthanased prior to therapy because owners were not prepared to participate in a treatment utilising an expensive operative procedure followed by long-term antibiotic therapy; these cases, however, were not amongst the most severe encountered in this series. The most common strategy utilised an approach similar to that of Malik et al (1994). Treatment commenced with either doxycycline or a fluoroquinolone, followed some weeks later by radical surgical excision of all or nearly all infected tissues, with intra- and peri-operative intravenous gentamicin followed subsequently by a long period (typically months) of follow-up antimicrobial therapy using either doxycycline or a fluoroquinolone. These cases benefited from the use of the radical excision technique of Hunt (1995) where infected tissue is resected en bloc followed by rearrangement of nearby skin to fill the often substantial tissue deficits created (Fig 4 a, b; Fig 5 a–c). This strategy was successful and all treated cases were cured on the basis of absence of disease recurrence for years after treatment. Gentamicin was administered intraoperatively and in the early post-operative period because it was bactericidal, available in a parenteral form, inexpensive and displayed good in vitro activity against all mycobacterial strains. Ciprofloxacin (Ciproxin; Bayer) was used in many of the cases because enrofloxacin (Baytril; Bayer) was not available commercially in Australia when this treatment was first instituted. Subsequent use of enrofloxacin has given comparable efficacy to ciprofloxacin at a considerable cost advantage and with the convenience of once daily dosing.
Table 3.
Response to therapy of cats admitted to the University Veterinary Centre (UVC) with mycobacterial panniculitis and dermatitis associated with RGM infections*
| Case No. | Strain No. | Species | Year | Surgery at UVC | Antimicrobial therapy | Cured |
|---|---|---|---|---|---|---|
| 1 | 21 | M smegmatis | 1985 | No | Trimethoprim | Yes |
| 2 | 16 | M smegmatis | 1987 | Yes | Tetracycline | Yes |
| 3 | 19 | M smegmatis | 1988 | Multiple | Tetracycline | Yes |
| 41 | M fortuitum (3)† | 1988 | Multiple | Tetracycline | Yes | |
| 5 | 23 | M smegmatis | 1992 | Yes | Doxycycline/gentamicin/ciprofloxacin | Yes |
| 6 | 24 | M smegmatis | 1992 | Yes | Doxycycline/gentamicin/ciprofloxacin | Yes |
| 7 | 25 | M smegmatis | 1992 | Yes | Doxycycline/gentamicin/ciprofloxacin | Yes |
| 8 | 15 | M smegmatis | 1992 | Yes | Doxycycline/gentamicin/ciprofloxacin | Yes |
| 9 | 27 | M smegmatis | 1993 | Yes | Doxycycline/gentamicin/ciprofloxacin | Yes |
| 10 | 10 | M smegmatis | 1994 | Yes | * Gentamicin/doxycycline | Yes |
| 11 | 29 | M smegmatis | 1996 | No | Ciprofloxacin | Yes |
| 12 | 12 | M smegmatis | 1997 | No | Doxycycline | Yes |
| 13 | 47 | M smegmatis | 1997 | Yes | Enrofloxacin | Yes |
| 14 | 48 | M smegmatis | 1998 | No | Enrofloxacin | Yes |
| 15 | 14 | M smegmatis | 1998 | Yes | # Enrofloxacin/gentamicin/doxycycline | Yes |
| 16 | 49 | M smegmatis | 1998 | Yes | ** Doxycycline | Yes |
| 17 | 28 | M fortuitum | 1998 | No | ‡ Clarithromycin | Yes |
Two additional cases likely to have mycobacterial panniculitis based on characteristic histopathology but where suitable material was not obtained for culture, were cured using long courses of enrofloxacin.
M fortuitum(3)=unnamed third biovar of M fortuitum.
Resistant to fluoroquinolones in vitro. Changed to doxycycline and cured.
Cat salivated excessively following enrofloxacin dosing, so doxycycline was substituted.
Resistant to doxycycline in vitro. Changed to clarithromycin and cured.
Fig 4.
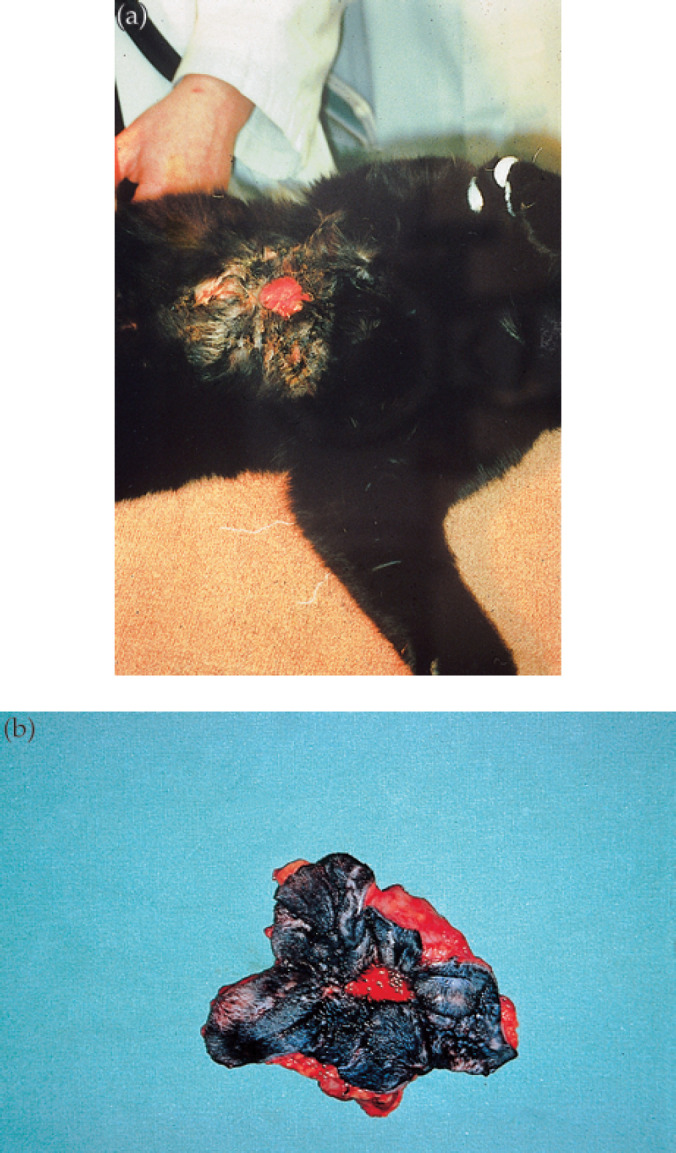
Axillary panniculitis in a cat caused by M smegmatis (strain 25). The infection has spread from the primary focus in the axilla (a) to involve a very large portion of the cat's ventral thorax and abdomen. Following preliminary treatment using doxycycline, affected tissues were removed by en bloc resection (b), and the resulting skin deficit corrected using advancement flaps utilising the method of Hunt (1995).
Fig 5.
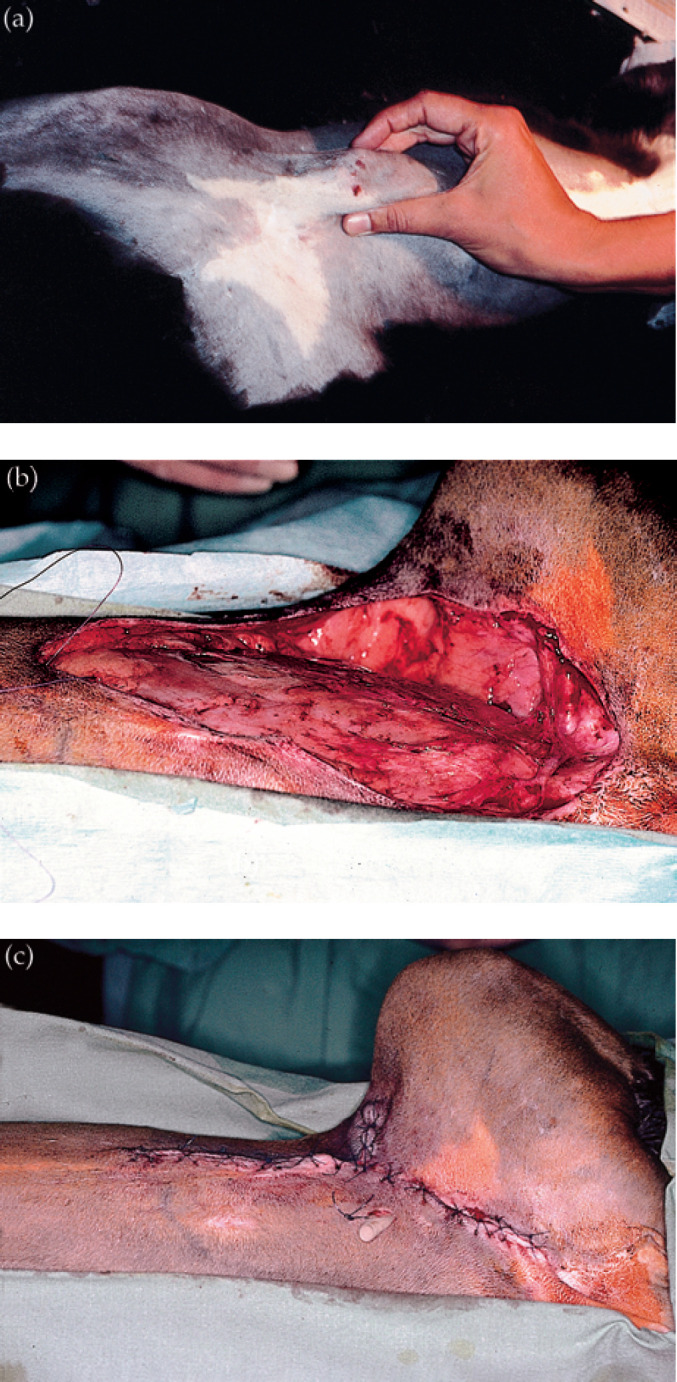
Inguinal panniculitis in a cat caused by M smegmatis (strain 10). The infection had recurred as a focal pyogranuloma following attempted removal by another veterinarian (a). Following preliminary treatment with doxycycline, the mass was resected en bloc (b), and the resulting wound closed (c).
Interestingly, some cases treated in a preliminary fashion using doxycycline (25–50 mg per cat every 12 h) or a fluoroquinolone continued to improve to such an extent that surgery was delayed indefinitely. These cases were subsequently cured using medical therapy alone, although this involved treating patients with oral antimicrobials for long periods of up to 6 months. As a generalisation, cases that resolved without the need for (further) surgical intervention involved a lesser depth of subcutaneous tissue than those cases that required surgery. There was very good agreement between the in vitro susceptibility test results and the response to antimicrobial agents in vivo. In particular, disease in two cats infected with strains resistant to fluoroquinolones in vitro did not respond to dosing with these drugs but were cured subsequently using doxycycline. A cat infected with one strain resistant to doxycycline in vitro did not respond to treatment with doxycycline but was cured using clarithromycin to which the organism involved had recorded intermediate in vitro susceptibility.
Discussion
This study is by far the largest case series concerning panniculitis and dermatitis in cats infected with RGM. Other studies have described the clinical manifestations of these infections and attempted treatment strategies in a small number of cats. Our investigation, although also concerned with treatment outcomes, was principally concerned with the microbiological characterisation of organisms and their in vitro antimicrobial susceptibility patterns, to provide information concerning agents likely to be most suitable for long-term oral therapy.
The clinical features of our cases largely conform to what has been reported previously. There was, however, a striking preponderance of females, which accounted for 31 of the 41 cases for which the sex of the patient was recorded. As neutered female cats generally are less likely to fight, roam and suffer injuries compared with their male counterparts, we suspect this over-representation reflects the tendency of neutered females to become obese, as the presence of a prominent inguinal fat pad no doubt contributes to the likely development of infection following penetrating injury of this region. A wide age range of cats was affected, although most of the cases were in mature adults between 3 and 10 years of age. The feline leukaemia virus (FeLV) and feline immunodeficiency virus (FIV) status of cats was not investigated systematically as it was considered that these infections generally reflected a localised opportunistic infection of an immunocompetent host. In all cases where retro-virus status had been tested by the referring veterinarian using commercial ELISA kits, cats tested FIV and FeLV negative.
Cases were recorded from a wide geographical range throughout New South Wales and Victoria, including suburban, rural and coastal regions of temperate to subtropical climates. Mycobacterium smegmatis comprised the great majority of cases, accounting for 40 of 49 cases. This finding conforms with previous studies in Australia from temperate New South Wales (Malik et al 1994, Vere 1996) and Victoria (Studdert & Hughes 1992), and subtropical southeast Queensland (Mason et al 1989, Wilkinson & Mason 1991). On the other hand, there is the suggestion in the literature that M fortuitum may be more common than M smegmatis in North American cats (Kunkle et al 1983, White et al 1983). The over-representation of M smegmatis in Australia presumably reflects something about the way in which feline inguinal and axillary wounds become contaminated that favours inoculation from environmental sources heavily colonised by this organism. The preponderance of M smegmatis in Australia does not extend to human patients who can develop similar infections following penetrating injuries (classically stepping on a nail), vehicular trauma, and following contamination of surgical wounds and multi-use vials containing depot preparations or vaccines. For example, in Queensland where some 100 such cases are seen each year in humans, M smegmatis accounts for only approximately 5% of infections (D Dawson unpublished observations). During the study period, comparable infections of the subcutis and skin of dogs were not reported and the only isolation of RGM from dog was from a patient with M fortuitum pneumonia. This further suggests a unique factor in the aetiopathogenesis of these infections, such as wound inoculation by large number of these mycobacterial organisms at the expense of others, possibly on the claws of the feline perpetrator. No M chelonae strain was encountered in this study, inferring this species is less common in environmental niches frequented by cats in the study areas. We believe this organism accounts for some feline infections in far northern Queensland, based on susceptibility patterns reported by private clinical pathology laboratories located there.
The in vitro susceptibility data conformed to those written previously concerning these organisms in human patients. The Etest method was easily applicable to this group of organisms and provided quantitative information pertinent to selection of optimal drug therapy. Susceptibility testing using a disc diffusion method provided very similar information however, and would be cheaper to use in a general diagnostic laboratory. Furthermore, the in vitro data correlated very closely with responses in vivo for all cases studied. As a group, M smegmatis strains were susceptible to a range of different antimicrobial agents well suited to treating chronic infections in cats. Similar observations have been made for strains from human patients, which are generally resistant to rifampicin and isoniazid, but susceptible to ethambutol, doxycycline, sulphamethoxazole, amikacin and ciprofloxacin (Wallace et al 1988). Importantly, the tendency for many M smegmatis strains to be resistant to clarithromycin in vitro has been reported recently (Wallace 1994). Mycobacterium fortuitum strains generally demonstrated resistance to one or several agents and often had higher MICs than M smegmatis for agents to which strains were susceptible. All M fortuitum strains were susceptible to fluoroquinolones, however, and most were susceptible to doxycycline. The propensity for M fortuitum strains to be more resistant to antimicrobial agents than M smegmatis strains has been reported previously for isolates from human patients (Wallace et al 1985).
Of the agents tested, we consider the fluoroquinolones (ciprofloxacin and enrofloxacin) and doxycycline to be the agents of choice for treating these infections in our cohort of cats. All strains but two were susceptible to ciprofloxacin (and therefore probably also to enrofloxacin, as inferred by identical sensitivities for all strains in which both quinolones were tested). Peak concentrations of ciprofloxacin, enrofloxacin and doxycycline during therapy are likely to be in the vicinity of 2–5 μg/ml, substantially higher than the MIC of the vast majority of strains (Leysen et al 1989, Walker et al 1990, Kordick et al 1999). Fluoroquinolones have the advantage that they are bactericidal, penetrate well into tissues including fat and are concentrated in polymorphs and macrophages (Leysen et al 1989, Hooper & Wolfson 1991). Thus one of the fluoro-quinolones is recommended for empirical therapy where microbiological data are lacking, or while susceptibility data are pending. Doxycycline is the tetracycline of choice for use in the cat, being well tolerated orally, present in a readily available form (Vibravet tablets; Pfizer Australia) and having good lipid solubility. Furthermore, it has a cost advantage, very similar efficacy in vivo to enrofloxacin or ciprofloxacin in our experience, and is equally well suited to long-term oral therapy. In general, it is necessary to use as high doses as possible of tetracyclines and fluoroquinolones for treating these infections because affected subcutaneous tissues are not well perfused, and considerable diffusion barriers can prevent blood levels of antimicrobial agents reaching the organisms in tissues.
Doxycycline (25–50 mg orally every 8–12 h), enrofloxacin (25–75 mg orally once a day) and ciprofloxacin (62.5 mg orally every 8 h to 125 mg orally every 12 h) (Watson 1991) all proved effective for monotherapy in cats in this series. In general, treatment was commenced using the low end of the dose rates given above, and the dosage slowly increased (over several weeks) until adverse side effects (inappetence, vomiting) indicated the need for slight dose reduction. Two strains of M smegmatis isolated in this series were resistant to enrofloxacin and/or ciprofloxacin. Interestingly both cats had been treated with fluoroquinolones prior to referral and culture, but cultures of the strains prior to antimicrobial therapy were not available. We suspect that resistance developed during a course of therapy as has been reported previously for human patients (Wallace et al 1990). The propensity of mycobacteria species to develop resistance during the course of therapy is well known, especially for species such as M tuberculosis, M avium and M leprae, although this phenomenon has been far less problematic for potentially pathogenic mycobacteria (Iredell et al 1992). It should be borne in mind that the development of resistance is common for the quinolone agents, probably as a result of selection of pre-existing mutants (Leysen et al 1989). There is probably insufficient information to recommend routine combination therapy in these cases, especially in the cat, where administration of multiple agents is often problematic. However, the possibility of development of resistance during monotherapy, especially using fluoroquinolones, should be considered in cases where the favourable response to therapy does not continue during a course of treatment. The same dilemma is debated in the human literature, where it is sometimes recommended that fluoroquinolones be given in combination with another agent (Wallace et al 1991), while others consider treatment with a single agent to be appropriate (Iredell et al 1992). There was no evidence of acquired resistance to doxycycline in the present investigation and experience in human patients indicates the likelihood of mutational resistance to doxycycline is low. Thus, single-agent therapy with this agent may be employed with a lesser risk of the development of resistance than for the fluoroquinolones. The total duration of therapy should be in the order of 3–6 months and typically agents should be administered for at least 1–2 months after the affected tissues look and feel completely normal. Rosychuk (1992) has recently reported a similar approach to the management of these cases.
Clarithromycin and azithromycin are azalides, macrolide derivatives with an extended spectrum of activity and prolonged pharmacokinetics. Clarithromycin (Klacid; Abbott) in particular has proved an extremely useful drug in treating mycobacterial infections in human patients, especially M avium complex infections, and we have also found it useful for certain mycobacterial infections in cats at a dose of 62.5 mg every 12–24 h. Unfortunately, our susceptibility data suggest this agent will prove useful in only a proportion of RGM infections, and this unpredictability makes it unsuitable for empiric therapy. Azithromycin would appear to have little to recommend it for treating RGM infections in cats, except for the very occasional susceptible strain for which its very long inter-dosing interval (Hunter et al 1995) may prove useful.
Trimethoprim, or sulphamethoxazole/trimethoprim combinations, were not evaluated clinically in this study, even though in vitro susceptibility data showed many strains were susceptible to both components of the combination (data not shown). One cat early in the series was successfully treated using a short course of trimethoprim. Previous reports, however, imply that this combination was inferior to doxycycline or a fluoroquinolone for long-term therapy of these deep-seated infections in cats.
An interesting finding of the present study was that some cats could be treated successfully using long courses of doxycycline or enrofloxacin and without a requirement for surgery. Successful management of mycobacterial panniculitis using enrofloxacin without surgery has been reported previously (Studdert & Hughes 1992). On the other hand, some of our cases were so severe that only limited improvement could be achieved with antimicrobial therapy alone, and surgical intervention was essential. Because it is not possible to predict with certainty which cases will require operative debridement, our current recommendation is to start treatment using a fluoroquinolone or doxycycline, determine the in vitro susceptibility of the strain to ensure the cat is on appropriate therapy, then re-access the case every 3–4 weeks to decide if continued improvement is occurring, or whether surgery is indicated to effect a cure.
Given the extent and severity of the pathology in many of these cats (Fig 4), it is understandable that adequate levels of antimicrobial agent may not be achieved throughout all involved tissues and that in these cases the best chance for a successful long-term outcome is to remove as much infected tissue as possible following preliminary antimicrobial therapy (Malik et al 1994). Residual foci of infection can then be targeted by the high concentrations of antibiotics achieved during and after surgery. Peri- and postoperative antimicrobial therapy is vital to ensure primary intention healing of the surgical wound. Similar recommendations have been made for humans with deep-seated RGM infections, where antibiotic therapy is considered to be adjunctive to aggressive surgical debridement (Wallace et al 1985, 1988, Plaus & Hermann 1991, Newton et al 1993). In people, the current recommendation is to pack the infected wound open following surgical debridement (Wallace et al 1985) although our preference is a one-stage procedure (including the judicious use of drains) because of practical difficulties of maintaining good wound toilet for a prolonged period in a fractious patient.
To sum up, mycobacterial panniculitis is an eminently treatable disease in the cat. Diagnosis is straightforward, especially for a practitioner familiar with the syndrome. The prognosis is excellent even in cases with severe, extensive and longstanding disease. Treatment involves long courses, typically 3–6 months, of antimicrobial agents chosen on the basis of in vitro susceptibility testing, often combined with extensive surgical debridement and wound reconstruction. A variety of drugs are now available which are generally devoid of renal or hepatic toxicity and therefore are well tolerated even when administered for very prolonged periods. Although many cases require surgical intervention to effect a cure, even when this is not possible because of financial considerations, long-term once-daily therapy with doxycycline or enrofloxacin will usually confine the infection sufficiently to enable the cat to lead a normal life.
An unexpected but potentially useful finding of the present study was that all M smegmatis strains were susceptible to trimethoprim in vitro. In contrast, all M fortuitum strains were resistant to this agent, as has been reported previously (Wallace et al 1981). This finding should prove useful for veterinary microbiology laboratories in which detailed identification of mycobacteria to the species level using biochemical tests is impractical. In the same way that it is possible to differentiate M fortuitum strains from M chelonae strains based on in vitro susceptibility to polymyxin B (M fortuitum susceptible, M chelonae resistant), it should be possible to differentiate M smegmatis (susceptible) from M fortuitum/chelonae (resistant) using a trimethoprim susceptibility disc. This finding should be tested using a larger number of strains from different geographical regions to establish the generality of the observation.
Acknowledgements
We wish to thank the numerous veterinarians and veterinary clinical pathologists that provided samples for this investigation. RM is supported by the Valentine Charlton Bequest of the Post Graduate Foundation of Veterinary Science of The University of Sydney. HPLC determinations of mycolic acid profiles were performed by Mr William Chew at Westmead Hospital. Sarah Goldsmid, Craig Macpherson, David Simpson, Penny Tisdall, Vanessa Barrs and Sue Foster all contributed to the cases managed at the University Veterinary Centre Sydney. Clarithromycin used to treat one cat in this series was generously provided at no charge by Abbott Australasia.
Note added in proof
Some recent work has suggested that, with respect to mycobacteria, the breakpoint for clarithromycin may be 2–4 μg/ml rather than 8 μg/ml.
References
- Butler WR, Kilburn JO. (1988) Identification of major slow growing pathogenic mycobacteria and Mycobacteria gordonae by high performance liquid chromatography of their mycolic acids. Journal of Clinical Microbiology 26, 50–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chew W, Sorrel T, Gilbert L. (1996) Identification of mycobacteria by high-performance liquid chromatography. Microbiology Australia 70, S13.2, A23. [Google Scholar]
- Dawson DJ. (1971) A simple identification scheme for mycobacteria. Australian Journal of Medical Technology 2, 7–15. [Google Scholar]
- Dewevre PJ, McAllister HA, Schirmer RG, Weinacker A. (1977) Mycobacterium fortuitum infection in a cat. Journal of the American Animal Hospital Association 13, 68–70. [Google Scholar]
- Gordon RE, Barnett DA, Handerhan JE, Pang CH. (1974) Nocardia coeliaca, Nocardia autotrophica, and the Nocardin strain. International Journal of Systematic Bacteriology 24, 54–63. [Google Scholar]
- Hagan WA, Levine P. (1932) The pathogenicity of the saprophytic acid-fast bacilli. Journal of the American Veterinary Medical Association 81, 723–733. [Google Scholar]
- Hoffner S, Klintz L, Olsson-Liljequist B, Bolstrom A. (1994) Evaluation of Etest for rapid susceptibility testing of Mycobacterium chelonae and M fortuitum. Journal of Clinical Microbiology 32, 1846–1849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hooper DC, Wolfson JS. (1991) Fluoroquinolone antimicrobial agents. New England Journal of Medicine 324, 384–394. [DOI] [PubMed] [Google Scholar]
- Hunt GB. (1995) Skin-fold advancement flaps for closing large sternal and inguinal wounds in cats and dogs. Veterinary Surgery 24, 172–175. [DOI] [PubMed] [Google Scholar]
- Hunter RP, Lynch MJ, Ericson JF, Millas WJ, Fletcher AM, Ryan NI, Olson JA. (1995) Pharmacokinetics, oral bioavail-ability and tissue distribution of azithromycin in cats. Journal of Veterinary Pharmacology and Therapeutics 18, 38–46. [DOI] [PubMed] [Google Scholar]
- Iredell J, Whitby M, Blacklock Z. (1992) Mycobacterium marinum infection: Epidemiology and presentation in Queensland 1971–1990. Medical Journal of Australia 157, 596–598. [DOI] [PubMed] [Google Scholar]
- Koontz FP, Erwin ME, Barrett MS, Jones RN. (1994) Etest for routine clinical antimicrobial susceptibility testing of rapid-growing mycobacteria isolates. Diagnostic Microbiology and Infectious Diseases 19, 183–186. [DOI] [PubMed] [Google Scholar]
- Kordick DL, Papich MG, Breitschwerdt EB. (1999) Efficacy of enrofloxacin or doxycycline for treatment of Bartonella henselae or Bartonella clarridgeiae infection in cats. Antimicrobial Agents and Chemotherapy 41, 2448–2455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kunkle GA. (1993) Differential diagnosis of nodules and draining tracts in the cat. Veterinary International 1, 3–9. [Google Scholar]
- Kunkle GA, Gulbas NK, Fakok V, Halliwell REW, Connelly M. (1983) Rapidly-growing mycobacteria as a cause of cutaneous granulomas: Report of five cases. Journal of the American Animal Hospital Association 19, 513–521. [Google Scholar]
- Leysen DC, Haemers A, Pattyn SR. (1989) Mycobacteria and the new quinolones. Antimicrobial Agents and Chemotherapy 33, 1–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lotti T, Hautmann G. (1993) Atypical mycobacterial infections: A difficult and emerging group of infectious dermatoses. International Journal of Dermatology 7, 499–501. [DOI] [PubMed] [Google Scholar]
- Malik R, Hunt GB, Goldsmid SE, Martin P, Wigney DI, Love DN. (1994) Diagnosis and treatment of pyo-granulomatous panniculitis due to Mycobacterium smegmatis in cats. Journal of Small Animal Practice 35, 524–530. [Google Scholar]
- Mason KV, Wilkinson GT, Blacklock Z. (1989) Results of treatment of atypical mycobacteriosis. In: Advances in Veterinary Dermatology, von Tscharner C, Halliwell REW. (eds). Bailliere Tindall, London, 1, pp. 452–453. [Google Scholar]
- Minnikin DE, Minnikin SM, Hutchinson IG, Goodfellow M, Grange JM. (1984) Mycolic acid patterns of representative strains of Mycobacterium fortuitum, ‘Mycobacterium peregrinum’ and Mycobacterium smegmatis. Journal of General Microbiology 130, 363–367. [DOI] [PubMed] [Google Scholar]
- Monroe WE, August JR, Chickering WR, Sriranganathan N. (1988) Atypical mycobacterial infections in cats. Compendium of Continuing Education for the Practising Veterinarian 10, 1044–1048. [Google Scholar]
- National Committee for Clinical Laboratory Standards (1999) Performance Standards for Antimicrobial Susceptibility Testing; 9th Informational Supplement. M-100-S9. Vol. 19, No. 1. [Google Scholar]
- Newton JA, Weiss PJ, Bowler WA, Oldfield EC. (1993) Soft-tissue infection due to Mycobacterium smegmatis: Report of two cases. Clinical Infectious Diseases 16, 531–533. [DOI] [PubMed] [Google Scholar]
- Ogawa T, Motomura K. (1970) Studies on murine leprosy bacillus. 1. Attempt to cultivate in vitro the Hawaiian strain of Mycobacterium lepraemurium. Kitasato Archives of Experimental Medicine 43, 21–36. [PubMed] [Google Scholar]
- Pedersen NC. (1988) Atypical mycobacteriosis. In: Feline Infectious Diseases. American Veterinary Publications, Golete, pp. 197–200. [Google Scholar]
- Plaus WJ, Hermann G. (1991) The surgical management of superficial infections caused by atypical mycobacteria. Surgery 110, 99–103. [PubMed] [Google Scholar]
- Richardson A. (1971) The experimental production of mastitis in sheep by Mycobacterium smegmatis and Mycobacterium fortuitum. Cornell Veterinarian 61, 640–646. [PubMed] [Google Scholar]
- Rosychuk RAW. (1992) New therapies in veterinary dermatology. In: Proceedings of the 10th American College of Veterinary Internal Medicine Forum, San Diego, California, pp. 119–121.
- Silcox VA, Good RC, Floyd MM. (1981) Identification of clinically significant Mycobacterium fortuitum complex isolates. Journal of Clinical Microbiology 14, 686–691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Street ML, Umbert-Millet IJ, Roberts GD, Su WP. (1991) Nontuberculous mycobacterial infections of the skin. Report of fourteen cases and review of the literature. Journal of the American Academy of Dermatology 24, 208–215. [DOI] [PubMed] [Google Scholar]
- Studdert VP, Hughes KL. (1992) Treatment of opportunistic mycobacterial infections with enrofloxacin in cats. Journal of the American Veterinary Medical Association 201, 1388–1390. [PubMed] [Google Scholar]
- Tsukamura M. (1976) Properties of Mycobacterium smegmatis freshly isolated from soil. Japanese Journal of Microbiology 20, 355–356. [DOI] [PubMed] [Google Scholar]
- Tsukamura M. (1984) Identification of Mycobacteria. National Chuba Hospital, Mycobacteriosis Research Laboratory, Japan. [Google Scholar]
- Vere H. (1996) Mycobacterial dermatitis in a cat. University of Sydney Post Graduate Foundation in Veterinary Science. Control and Therapy 191, 872. [Google Scholar]
- Walker RD, Stein GE, Hauptman JG, MacDonald KH, Budsberg SC, Rosser EJ. (1990) Serum and tissue cage fluid concentrations of ciprofloxacin after oral administration of the drug to healthy dogs. American Journal of Veterinary Research 51, 896–900. [PubMed] [Google Scholar]
- Wallace RJ., Jr (1994) Recent changes in taxonomy and disease manifestations of the rapidly growing mycobacteria. European Journal of Clinical Microbiology and Infectious Diseases 13, 953–960. [DOI] [PubMed] [Google Scholar]
- Wallace RJ, Jr, Dalovisio JR, Pankey GA. (1979) Disk diffusion testing of susceptibility of Mycobacterium fortuitum and Mycobacterium chelonei to antibacterial agents. Antimicrobial Agents and Chemotherapy 16, 611–614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wallace RJ, Jr, Jones DB, Wiss K. (1981) Sulfonamide activity against Mycobacterium fortuitum and Mycobacterium chelonei. Reviews of Infectious Diseases 3, 898–904. [DOI] [PubMed] [Google Scholar]
- Wallace RJ, Jr, Swenson JM, Silcox VA, Good RC. (1982) Disk diffusion testing with polymyxin and amikacin for differentiation of Mycobacterium fortuitum and Mycobacterium chelonei. Journal of Clinical Microbiology 16, 1003–1006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wallace RJ, Jr, Swenson JM, Silcox VA, Bullen MG. (1985) Treatment of nonpulmonary infections due to Mycobacterium fortuitum and Mycobacterium chelonei on the basis of in vitro susceptibilities. Journal of Infectious Diseases 152, 500–514. [DOI] [PubMed] [Google Scholar]
- Wallace RJ, Jr, Nash DR, Tsukamura M, Blacklock ZM, Silcox VA. (1988) Human disease due to Mycobacterium smegmatis. Journal of Infectious Diseases 158, 52–59. [DOI] [PubMed] [Google Scholar]
- Wallace RJ, Jr, Bedsole G, Sumpter G, Sanders CV, Steele LC, Brown BA, Smith J, Graham DR. (1990) Activities of ciprofloxacin and ofloxacin against rapidly-growing mycobacteria with demonstration of acquired resistance following single drug therapy. Antimicrobial Agents and Chemotherapy 34, 65–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wallace RJ, Jr, Brown BA, Silcox VA, Tsukamura M, Nash DR, Steele LC, Steingrube VA, Smith J, Sumter G, Zhang YS. (1991) Clinical disease, drug susceptibility and biochemical patterns of the unnamed third biovariant complex of Mycobacterium fortuitum. Journal of Infectious Diseases 163, 598–603. [DOI] [PubMed] [Google Scholar]
- Watson ADJ. (1991) Systemic anti-microbial drug therapy in cats. In Practice 13, 73–79. [Google Scholar]
- Wayne LG, Sramek HA. (1992) Agents of newly recognized or infrequently encountered mycobacterial diseases. Clinical Microbiology Reviews 5, 1–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- White PD, Kowalski JJ. (1991) Enrofloxacin-responsive cutaneous atypical mycobacterial infection in two cats. In: Proceedings of 7th Meeting of the American College of Veterinary Dermatology, Scottsdale, p. 95.
- White SD, Ihrke PJ, Stannard AA, Cadmus C, Griffin C, Kruth SA, Rosser EJ, Jr, Reinke SI, Jang S. (1983) Cutaneous atypical mycobacteriosis in cats. Journal of the American Veterinary Medical Association 182, 1218–1222. [PubMed] [Google Scholar]
- Wilkinson GT, Mason KV. (1991) Clinical aspects of mycobacterial infections of the skin. In: Consultations in Feline Internal Medicine,August JR. (ed.). WB Saunders, Philadelphia, pp. 129–136. [Google Scholar]
- Wilkinson GT, Kelly WR, O'Boyle D. (1978) Cutaneous granulomas associated with Mycobacterium fortuitum infection in a cat. Journal of Small Animal Practice 19, 357–362. [DOI] [PubMed] [Google Scholar]
- Wilkinson GT, Kelly WR, O'Boyle D. (1982) Pyogranulomatous panniculitis in cats due to Mycobacterium smegmatis. Australian Veterinary Journal 58, 77–78. [DOI] [PubMed] [Google Scholar]
- Willemse T, Groothuis DG, Koeman JP, Beyer EG. (1985) Mycobacterium thermoresistibile: Extrapulmonary infection in a cat. Journal of Clinical Microbiology 21, 854–856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolinsky E. (1973) Mycobacteria. In: Microbiology, Davis BD, Dulbecco R, Eisen HN, Ginsberg HS, Wood WB. (eds). Harper and Row, Hagerstown, p. 884. [Google Scholar]
- Woods GL, Bergmann JS, Witebsky FG, Fahle GA, Wanger A, Boulet B, Plaunt M, Brown BA, Wallace RJ., Jr (1999) Multisite reproducibility of results obtained by the broth microdilution method for susceptibility testing of Mycobacterium abscessus, Mycobacterium chelonae and Mycobacterium fortuitum. Journal of Clinical Microbiology 37, 1676–1682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Youmans GP. (1980) General characteristics of Mycobacteria. In: The Biologic and Clinical Basis of Infectious Diseases (2nd edn), Youmans GP, Paterson PY, Sommers HM. (eds). WB Saunders, Philadelphia, p. 367. [Google Scholar]