Abstract
Peripheral vestibular disease referable to otitis media/interna was the main reason for presentation in three cats with cryptococcosis. In two cats, Cryptococcus neoformans var neoformans was isolated from the tympanic bulla. In the remaining cat, otitis media/interna was considered to be secondary to occlusion of the auditory tube by a nasopharyngeal granuloma associated with a C neoformans var gattii infection. This report emphasises the importance of maintaining an index of suspicion for a fungal aetiology in cats with signs of otitis media/interna, particularly in countries with a high prevalence of cryptococcosis. The presence of C neoformans may be overlooked with potentially fatal consequences where only standard methods for bacterial isolation are used to examine samples obtained from the middle ear.
Cryptococcus neoformans is a basidiomycetous, saprophytic fungus and a potentially fatal pathogen of many species worldwide, including cats and humans (Mitchell & Perfect 1995, Jacobs & Medleau 1998). Two varieties have been described; C neoformans var neoformans and C neoformans var gattii, which differ in environmental niche, geographical distribution, serologically defined antigens, biochemical characteristics and pathogenicity (Levitz 1991).
Cryptococcosis is the most common systemic mycosis of cats. Feline cryptococcosis has been extensively reviewed (Wilkinson 1979, Legendre 1986, 1994, Holzworth 1987, Malik et al 1992, Jacobs & Medleau 1998). The source of infection is considered to be environmental and cases of disease are sporadic. Cats can develop cryptococcosis at any age (Legendre 1986, Holzworth 1987, Malik et al 1992) and there is no significant sex or breed predisposition, although Siamese cats are over-represented in some studies and a slightly higher number of cases have been reported in male cats (Holzworth 1987, Malik et al 1992). Most cats (greater than 80%) present with signs of nasal cavity disease including sneezing, nasal discharge, respiratory stridor and subcutaneous masses at the nostrils or nasal bridge. There is sometimes concurrent involvement of other organs including the skin, the central nervous system (CNS) and the eyes. Cutaneous lesions, other than those involving the nose, are usually multiple, non-pruritic and range from small papules to large ulcerative lesions. Clinical signs of CNS involvement depend on the structures which are affected and include seizures, head pressing, circling and ataxia. Ocular signs, particularly dilated, unresponsive pupils, usually associated with optic neuritis and retinitis, are seen in some cases (Jacobs & Medleau 1998).
Inhalation of basidiospores or dessicated blastoconidia is believed to be the principle route of infection, consistent with the clinical observation of primary disease in the respiratory tract (Levitz 1991, Malik et al 1997b). Disease in other sites usually results from local or haematogenous spread of organisms from the nasal cavity (Jacobs & Medleau 1998). Direct inoculation of cryptococcal organisms may occur in a minority of cases presenting with isolated or superficial lesions (Martin et al 1996). In humans, dogs and ferrets, the gastrointestinal tract has also been incriminated as a rare portal of entry (Washington et al 1991, Malik et al 1999a, M France, personal communication).
Three cases of feline cryptococcosis where peripheral vestibular signs were a major feature are presented. The aetiopathogenesis of the disease process in these cases is discussed.
Case reports
Case 1
A 15.5-year-old female neutered Cornish Rex (3.2 kg) was presented with a head tilt and inappetance. Relevant historical findings included an upper respiratory tract infection 4 months previously which had apparently responded to treatment with amoxycillin/clavulanic acid (Clavulox; Pfizer) and chronic bilateral otitis externa of 2 years' duration. Three months prior to admission the right ear had become intensely pruritic. Cytological examination of swabs taken from the ear canal at that time had demonstrated many broad-based, budding yeasts with morphology typical of a Malassezia species. A pure, heavy growth of a non-haemolytic, coagulasenegative Staphylococcus species was obtained on culture on 5% sheep blood agar. Clinical signs improved following treatment with amoxycillin/clavulanic acid.
On physical examination the cat was ataxic and had a head tilt to the right. Rectal temperature (37.6°C) was within the normal range. Although neurological examination was impeded by the cat's aggressive temperament, postural reaction deficits were not detected, mentation was judged to be normal and nystagmus was not a feature. Otoscopy under general anaesthesia demonstrated stenosis of the external ear canal. The tympanic membrane was intact but discoloured. Cytology of material obtained from the middle ear following myringotomy demonstrated squamous epithelial cells and Malassezia-like yeasts. No organisms were grown following aerobic or anaerobic culture of this sample on 5% sheep blood agar after 48 h. With the cat in dorsal recumbency and the soft palate retracted rostrally with a spey hook, the nasopharynx was examined using a dental mirror and appeared normal. Skull radiographs showed decreased aeration of the right tympanic bulla and thickening of its bony wall. Peripheral vestibular disease due to otitis media/interna was considered to be the most likely diagnosis and the cat was treated with amoxycillin/clavulanic acid (62.5 mg every 12 h) and ketoconazole (Nizoral; Jansen Cilag) (25 mg every 24 h). When no improvement was seen after 2 weeks of therapy, a lateral bulla osteotomy and total ear canal ablation were performed. Cytology of bone and soft tissue removed from the bulla showed mononuclear cells, squamous epithelial cells, polymorphs and occasional Gram-positive cocci. On aerobic and anaerobic culture on blood agar a yeast was grown which was subsequently identified at a reference laboratory as C neoformans var neoformans and found to have in vitro susceptibility to amphotericin B, 5 flucytosine, ketoconazole, fluconazole and itraconazole using a disc diffusion method. The serum latex cryptococcal antigen agglutination test (LCAT) titre (Malik et al 1996b) was four. Treatment was changed to itraconazole (50 mg PO every 24 h). When the cat was presented 3 months later the head tilt had resolved. Although serum was not available for further LCAT determination in this case, clinical signs did not recur and the cat was euthanased 4 years later for an unrelated problem.
Case 2
An 8-year-old neutered female domestic longhair (3.7 kg) was presented at an emergency clinic. Immediately prior to presentation the cat had suffered two brief episodes of incoordination 15 min apart, which the owner described as ‘seizures’. There were no premonitory signs and the cat was housed indoors. Intermittent falling to the right side was the only abnormality on admission, but over the next 12 h the ataxia progressed and the cat developed a head tilt to the right and resting nystagmus with the fast phase to the left. Seizures were not observed. Treatment with clindamycin, B-group vitamins and phenobarbitone was initiated and blood was collected for a LCAT. After 48 h, a serosanguinous discharge was noted in the right ear canal. The cat was referred to the University of Sydney Veterinary Centre 1 week later when the LCAT was reported as positive.
Signs consistent with right-sided peripheral vestibular disease were present on physical examination (head tilt, ataxia and intermittent resting nystagmus with normal postural reactions) and the cat was pyrexic (40.2°C). Indirect opthalmoscopic findings were normal. Screening serology for feline immunodeficiency virus (FIV) antibody and feline leukaemia virus (FeLV) antigen was negative. Numerous, capsulate, narrow-necked, budding yeasts were seen on cytological examination of swabs taken from the right external ear canal and a moderate growth of C neoformans was evident on bird seed agar after incubation at 28°C for 72 h. The organism was identified at a reference laboratory as C neoformans var neoformans, with in vitro susceptibility to amphotericin B (E-test minimal inhibitory concentration [MIC] 0.064 mg/l), flucytosine (E-test MIC 0.004 mg/l), itraconazole (E-test MIC 0.064 mg/l) and fluconazole (E-test MIC 3.0 mg/l). Cryptococcus neoformans was not evident on cytological examination or culture of superficial nasal swabs. The LCAT titre was 32. Treatment was initiated with amphotericin B (Fungizone; Bristol-Myers Squibb) (by subcutaneous infusion twice weekly for 4 weeks; initial dose 0.4 mg/kg, increasing to 0.8 mg/kg), 5-flucytosine (Ancotil; Roche) (250 mg every 8 h for 7 weeks, then every 12 h for 5 weeks) and fluconazole (Diflucan; Pfizer) (32 mg every 8 h for 7 weeks, then every 12 h for 17 weeks). Resting nystagmus resolved after 2 weeks. Amphotericin B was stopped after 4 weeks as sterile abscesses developed at the injection sites. This is a potential complication of subcutaneous administration of amphotericin B due to the local irritant effect of this drug (Malik et al 1996a). The cat had a fluctuating pyrexia (39.8–40.1°C) which persisted for 3 weeks, despite antibiotic treatment.
The cat's condition deteriorated during the 3rd week of hospitalisation when it developed a severe upper respiratory tract infection and became anorectic. To provide nutritional support, a gastrostomy tube was placed percutaneously under general anaesthesia. Skull radiographs taken at this time demonstrated loss of aeration of the right tympanic bulla cavity and subtle thickening and irregularity of the osseous bulla, consistent with otitis media. When the cat's respiratory signs improved, a ventral bulla osteotomy was performed. Material removed from the bulla cavity was submitted for microbiological evaluation. No organisms were grown using routine bacterial or fungal culture of the middle ear contents, though poorly encapsulated, weakly staining cryptococcal organisms were seen in cytological preparations. The cat began to eat after 8 weeks in hospital, though the gastrostomy tube was left in place for a further 5 weeks to facilitate medication and supplementary feeding. The serum LCAT titre declined to 16 after 6 weeks of therapy and was negative after 12 and 24 weeks. Treatment was stopped at 24 weeks. Despite a residual head tilt (Fig 1) the cat compensated well and regained its previous body weight.
Fig 1.
Residual head tilt in case 2 after cessation of treatment.
Case 3
A 10-year-old male neutered, domestic shorthair (5.6 kg) was presented for investigation of ataxia and an intermittent head tilt. The owners had also noticed noisy breathing and gradual weight loss for 6 months prior to admission. On physical examination the cat had an inspiratory stridor, a small lesion on the right nasal plane, left-sided Horner's syndrome and mild ataxia. Postural reactions were normal and no head tilt was evident at this time. No abnormalities were detected using indirect opthalmoscopy.
A purulent discharge was detected in the left external ear canal. Cytological examination of the discharge demonstrated moderate numbers of neutrophils and many Gram-negative rods. A moderate growth of a Pseudomonas species (with in vitro susceptibility to gentamicin, ciprofloxacin, polymyxin B and ticarcillin) was isolated following culture. Skull radiographs showed decreased aeration and bony changes in the left tympanic bulla. Using a retroflexed paediatric gastroscope to examine the nasopharynx, a fleshy mass was detected protruding from the choanae (Fig 2a). Diff Quik-stained smears of a sample obtained from the mass by fine needle aspiration through the soft palate demonstrated numerous clusters of heavily capsulate, spherical, budding yeasts and an inflammatory component consisting mainly of vacuolated macrophages. The mass was identified as a cryptococcal granuloma (Malik et al 1997a). It was considered most likely that occlusion of the auditory tube by this mass had resulted in secondary otitis media, evidenced by Horner's syndrome and radio-graphic changes in the bulla. Extension of this process to the inner ear could account for the ataxia and the intermittent head tilt reported in the history. The absence of paresis on presentation and the non-progressive nature of the complaint were considered to indicate that a central lesion was unlikely. Cryptococcus neoformans var gattii with in vitro susceptibility to amphotericin B (E-test MIC 0.094 mg/l), flucytosine (E-test MIC 0.002 mg/l), itraconazole (E-test MIC 0.016 mg/l) and fluconazole (E-test MIC 0.5 mg/l) was cultured from a sample obtained by retrograde nasal flushing. On serology, the cat had a LCAT titre of 256 and was negative for FIV antibody and FeLV antigen. Treatment with itraconazole (Sporanox; Jansen Cilag) (50 mg PO every 24 h) for the cryptococcal infection and topical gentamicin for otitis externa was initiated. There was minimal clinical improvement after 5 weeks, so the granuloma was removed by blunt dissection via an incision through the soft palate (Fig 2b). Histological examination demonstrated sheets of foamy macrophages containing scattered cryptococcal organisms, the latter in association with a local suppurative response. Treatment with itraconazole was continued. At re-examination 1 month later, all clinical signs had resolved. After 4 months of treatment, the serum LCAT titre had declined to 32 and the cat had gained 2.4 kg in weight since its initial presentation. The dose of itraconazole was consequently increased (100 mg PO every 24 h) and treatment is on-going at the time of writing.
Fig 2.
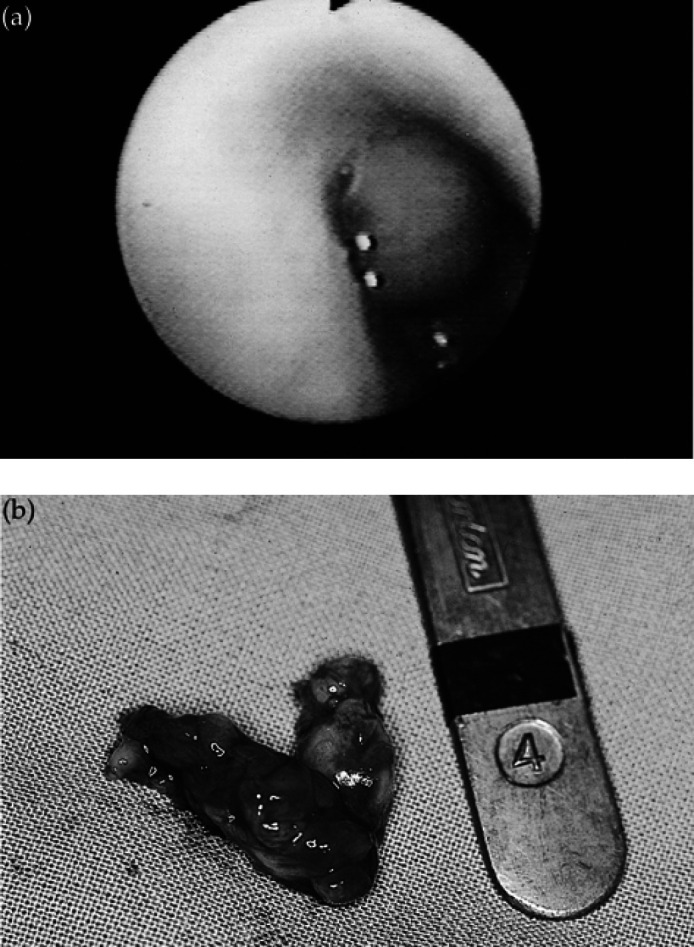
(a) Endoscopic view of cryptococcal granuloma occupying the caudal nasal cavity/nasopharynx in case 3. (b) Cryptococcal granuloma following surgical removal. The mass measured approximately 3 × 1 cm.
Discussion
The majority of cases of feline cryptococcosis present with signs referable to mycotic rhinitis. The predominance of signs of peripheral vestibular disease in these three cats with cryptococcosis is atypical. Signs of unilateral peripheral vestibular disease (head tilt, circling, ataxia and spontaneous nystagmus in the absence of postural reaction deficits) indicate dysfunction of the membranous labyrinth or of the peripheral portion of the eighth cranial nerve. Components of Horner's syndrome (ptosis, miosis, enophthalmos, prolapse of the nictitating membrane) or facial nerve palsy are often seen concurrently, indicating involvement of sympathetic nerve fibres or of the facial nerve which pass through and adjacent to the middle ear respectively.
The differential diagnoses for peripheral vestibular disorders in the cat include otitis interna, usually in association with otitis media (Smith 1996), and feline idiopathic vestibular syndrome (Burke et al 1985). Less common aetiologies include neoplasia (Indrieri & Taylor 1984), trauma, congenital disorders in Burmese, Siamese and Tonkinese breeds (Chrisman 1991), and ototoxic agents, particularly aminoglycosides (Pickrell et al 1993). Congenital and toxic aetiologies typically result in bilateral signs. Otitis media/interna usually results from extension of an infection in the upper respiratory tract or the external ear canal and commonly has a bacterial aetiology. Organisms commonly isolated from affected bullae include Staphylococcus spp, Streptococcus spp, Pasteurella spp, Proteus spp, Escherichia coli, Enterococcus spp, Pseudomonas spp and obligate anaerobes; yeasts are an uncommon cause of otitis media (Braund 1995). Non-septic otitis media/interna may occur secondary to occlusion of the auditory tube, for example by a neoplasm or a polyp (Little 1997). A comprehensive review of feline vestibular disorders has recently been published (LeCouteur & Vernau 1999, Vernau & LeCouteur 1999).
In the three cases presented here signs of peripheral vestibular disease were attributable to otitis media/interna. In Cases 1 and 2, C neoformans var neoformans was considered to be the aetiological agent. This is based on the demonstration of organisms within the affected tympanic bulla with serological confirmation of infection. Asymptomatic carriage of C neoformans has been demonstrated in the nasal cavity of 7% of cats (Malik et al 1997b). However, these cats have a negative LCAT. A positive serum LCAT titre is considered to indicate active or previous infection (Malik et al 1999b). Thus, positive culture of C neoformans from the middle ear in association with clinical signs of otitis media/interna and concurrent demonstration of serum antigen is considered to indicate that C neoformans had a pathogenic role in these cases. In case 3, middle ear contents were not examined. However, given the rapid resolution of clinical signs following surgical debulking of the cryptococcal granuloma, otitis media/interna in this case was considered most likely to have been secondary to intermittent occlusion of the auditory tube in the nasopharynx by the granuloma. As emphasised elsewhere (Malik et al in press), the physical removal of accessible fungus impregnated tissues facilitates subsequent antifungal chemotherapy.
The portal of entry for cryptococcal organisms in cats is presumed to be the nasal cavity in most cases (Malik et al 1997a, Jacobs & Medleau 1998). In case 1, the previous episode of upper respiratory tract signs implicates the nasal cavity as the primary site of infection. Because of the unusual history and physical findings, cryptococcosis was not suspected in this case, so nasal swabs were not obtained. The significance of the Malassezia species seen on cytology of the middle ear sample is not clear and it may have been a contaminant from the external ear canal. The presence of C neoformans may have been detected earlier if the sample from the middle ear had been cultured on mycological media. For example, colonies of C neoformans are highlighted by the characteristic brown colour effect on bird seed agar (Staib et al 1989). In case 2, primary subclinical infection of the nasal cavity with ascending spread to the middle, inner and external ear is considered the most likely route of infection. Failure to isolate yeasts from nasal swabs in this case may have reflected preferential involvement of the caudal nasal cavity or nasopharynx. CNS involvement was suspected initially in this case, prompting early testing for cryptococcosis. The signs described by the owner may have been consistent with local extension of infection from the labyrinth to the CNS or with peracute, peripheral vestibular disease. Advanced imaging using computed tomography or magnetic resonance imaging may have provided additional information to differentiate between these possibilities.
Case 3 had nasopharyngeal cryptococcosis, a condition with more subtle clinical features than classical mycotic rhinitis, as sneezing and nasal discharge are often absent from the clinical picture (Malik et al 1997a). Nasal cavity involvement was suggested by the stertorous breathing and a lesion on the nasal plane. However, it was the signs of middle/inner ear disease (Horner's syndrome, ataxia, head tilt) which prompted veterinary attention in this case.
These cases emphasise the importance of a systematic approach to the investigation of peripheral vestibular disease in cats. In the initial investigation, signalment and history (including respiratory or otic infections, drug therapies, traumatic incidents) should be evaluated in conjunction with physical findings, including those on neurological, otoscopic, opthalmoscopic and nasopharyngeal examination. Where findings are supportive of otitis media/interna, broad spectrum antibiotic therapy, for example with amoxycillin/clavulanic acid, doxycycline or clindamycin should be initiated as many cases have a bacterial aetiology. However, since C neoformans should be considered among the differential diagnoses of otitis media in cats, particularly in areas with a high prevalence of cryptococcosis, collection of serum for LCAT is indicated. Failure to respond to antibiotic therapy should prompt further investigations including imaging of the tympanic bullae using radiography or advanced imaging, and sampling of middle ear contents by myringotomy or bulla osteotomy. In addition to routine bacteriology, media appropriate for the isolation of cryptococcal organisms, such as Sabouraud's dextrose agar or bird seed agar (Jacobs & Medleau 1998), should be used. Finally, the improved prognosis for cryptococcosis is clear from these three cases which were all successfully treated.
Acknowledgements
JB was supported by a Veterinary Research Career Development Fellowship from the Wellcome Trust, UK. RM is supported by The Valentine Charlton Bequest of the Postgraduate Foundation in Veterinary Science, University of Sydney, Australia. The fluconazole used in these cases was kindly provided free of charge by Pfizer, Australia.
References
- Braund KG. (1995) Peripheral nerve disorders. In: Textbook of Veterinary Internal Medicine (4th edn), Ettinger SJ, Feldman EC. (eds). WB Saunders, Philadelphia, pp. 701–726. [Google Scholar]
- Burke EE, Moise NS, de Lahunta A, Erb HN. (1985) Review of idiopathic feline vestibular syndrome in 75 cats. Journal of the American Veterinary Medical Association 187, 941–943. [PubMed] [Google Scholar]
- Chrisman CL. (1991) Head tilt, circling, nystagmus and other vestibular deficits. In: Problems in Small Animal Neurology, Chrisman CL. Lea & Febiger, Philadelphia, pp. 269–294. [Google Scholar]
- Holzworth J. (1987) Mycotic diseases. In: Diseases of the Cat, Holzworth J. (ed.). WB Saunders, Philadelphia, pp. 320–358. [Google Scholar]
- Indrieri RJ, Taylor RF. (1984) Vestibular dysfunction caused by squamous cell carcinoma involving the middle ear and inner ear in two cats. Journal of the American Veterinary Medical Association 184, 471–473. [PubMed] [Google Scholar]
- Jacobs GJ, Medleau L. (1998) Cryptococcosis. In: Infectious Diseases of the Dog and Cat (2nd edn), Green CE. (ed.). WB Saunders, Philadelphia, pp. 383–390. [Google Scholar]
- LeCouteur RA, Vernau KM. (1999) Feline vestibular disorders. Part I: Anatomy and clinical signs. Journal of Feline Medicine and Surgery 1, 71–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Legendre AM. (1986) Systemic mycotic infections of dogs and cats. In: Infectious Diseases, Scott FW. (ed.). Churchill Livingstone, New York, pp. 29–53. [Google Scholar]
- Legendre AM. (1994) Systemic mycotic infections. In: The Cat: Diseases and Clinical Management, Sherding RG. (ed.). WB Saunders, Philadelphia, pp. 553–564. [Google Scholar]
- Levitz SM. (1991) The ecology of Cryptococcus neoformans and the epidemiology of cryptococcosis. Review of Infectious Diseases 13, 1163–1169. [DOI] [PubMed] [Google Scholar]
- Little CJ. (1997) Nasopharyngeal polyps. In: Consultations in Feline Medicine (3rd edn). WB Saunders, Philadelphia, pp. 310–316. [Google Scholar]
- Malik R, Wigney DI, Muir DB, Gregory DJ, Love DN. (1992) Cryptococcosis in cats: Clinical and mycological assessment of 29 cases and evaluation of treatment using orally administered fluconazole. Journal of Medical and Veterinary Mycology 30, 133–144. [DOI] [PubMed] [Google Scholar]
- Malik R, Craig AJ, Wigney DI, Martin P, Love DN. (1996a) Combination chemotherapy of crypotococcosis using subcutaneously administered amphotericin B. Australian Veterinary Journal 73, 124–128. [DOI] [PubMed] [Google Scholar]
- Malik R, McPetrie R, Wigney DI, Craig AJ, Love DN. (1996b) A latex cryptococcal antigen agglutination test for diagnosis and monitoring of therapy for cryptococcosis. Australian Veterinary Journal 74, 358–364. [DOI] [PubMed] [Google Scholar]
- Malik R, Martin P, Wigney DI, Church DB, Bradley W, Bellenger CR, Lamb WA, Barrs VR, Foster S, Hemsley S, Canfield PJ, Love DN. (1997a) Nasopharyngeal cryptococcosis. Australian Veterinary Journal 75, 483–488. [DOI] [PubMed] [Google Scholar]
- Malik R, Wigney DI, Muir DB, Love DN. (1997b) Asymptomatic carriage of Cryptococcus neoformans in the nasal cavity of dogs and cats. Journal of Medical and Veterinary Mycology 35, 27–31. [PubMed] [Google Scholar]
- Malik R, Hunt GB, Bellenger CR, Cairns B, Pegorer M, Wigney DI, Love DN. (1999a) Intra-abdominal cryptococcosis in two dogs. Journal of Small Animal Practice 40, 387–391. [DOI] [PubMed] [Google Scholar]
- Malik R, Speed BR, Kaldor J, Allan GS, Martin P, Canfield PJ, Love DN. (1999b) Serum antibody response to Cryptococcus neoformans in cats, dogs and koalas with and without active infection. Medical Mycology 37, 43–51. [PubMed] [Google Scholar]
- Malik R, Jacobs G, Love DN. (in press) Feline cryptococcosis: New perspectives on aetiology, pathogenesis, diagnosis and clinical management In: Consultations in Feline Medicine (4th edn), August JR. (ed.). WB Saunders, Philadelphia. [Google Scholar]
- Martin CL, Stiles J, Willis M. (1996) Ocular adnexal cryptococcosis in a cat. Veterinary and Comparative Opthalmology 6, 225–229. [Google Scholar]
- Mitchell TG, Perfect JR. (1995) Cryptococcosis in the era of AIDS—100 years after the discovery of Cryptococcus neoformans. Clinical Microbiology Reviews 8, 515–548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pickrell JA, Oehme FW, Cash WC. (1993) Ototoxicity in dogs and cats. Seminars in Veterinary Medicine and Surgery 8, 42–49. [PubMed] [Google Scholar]
- Smith MO. (1996) Vestibular disease in small animals. Veterinary Annual 36, 162–172. [Google Scholar]
- Staib F, Seibold M, Antweiler E, Frolich B. (1989) Staib agar supplemented with a triple antibiotic combination for the detection of Cryptococcus neoformans in clinical specimens. Mycoses 32, 448–454. [DOI] [PubMed] [Google Scholar]
- Vernau KM, LeCouteur RA. (1999) Feline vestibular disorders. Part II: Diagnostic approach and differential diagnosis. Journal of Feline Medicine and Surgery 1, 81–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Washington K, Gottfried MR, Wilson ML. (1991) Gastrointestinal cryptococcosis. Modern Pathology 2, 4707–4711. [PubMed] [Google Scholar]
- Wilkinson GT. (1979) Feline cryptococcosis: A review and seven case reports. Journal of Small Animal Practice 20, 749–768. [DOI] [PubMed] [Google Scholar]



