Abstract
Background
Dry eye is one of the most common ophthalmic conditions and can significantly impact quality of life. Meibomian gland dysfunction (MGD) is a major cause of evaporative dry eye.
We sought to conduct a systematic review and meta-analysis to estimate the prevalence and incidence of dry eye and MGD in Central and South America and to identify factors associated with disease burden.
Methods
Data sources Ovid MEDLINE and Embase.
Study selection
A search conducted on August 16, 2021, identified studies published between January 1, 2010, and August 16, 2021, with no restrictions regarding participant age or language of publication. Case reports, case series, case–control studies, and interventional studies were excluded.
Data extraction and synthesis
The review was based on a protocol registered on PROSPERO (CRD42021256934). Risk of bias was assessed in duplicate using a risk of bias tool designed for the purposes of descriptive epidemiological studies. Data were extracted by one investigator and verified by another for accuracy. Prevalence of dry eye and MGD were grouped based on study participant characteristics.
Main outcomes and measures
Prevalence and incidence of dry eye and MGD in Central and South America. Summary estimates from meta-analysis with 95% confidence intervals (CI).
Results
Fourteen studies (11,594 total participants) were included. The population prevalence of dry eye was 13% (95% CI, 12%-14%) in Brazil and 41% (95% CI, 39%-44%) in Mexico based on one study each. Meta-analyses suggested that dry eye prevalence was 70% among indoor workers (95% CI, 56%-80%; I2, 82%; 3 studies), 71% among students (95% CI, 65%-77%; I2, 92%; 3 studies), and 83% in general ophthalmology clinics (95% CI, 77%-88%; I2, 88%; 2 studies). MGD prevalence ranged from 23% among indoor workers (95% CI, 16%-31%; 1 study) to 68% in general ophthalmology clinics (95% CI, 62%-72%; 1 study). No studies reported incidence of dry eye or MGD.
Conclusions
This systematic review and meta-analysis demonstrated considerable variation in the published prevalence of dry eye and MGD among the general population and subpopulations in Central and South America. Local and subpopulation estimates of dry eye disease burden may be valuable to assist needs assessments and implementation of measures to mitigate the condition.
Supplementary Information
The online version contains supplementary material available at 10.1186/s12886-023-03249-w.
Keywords: Dry eye syndrome, Meibomian gland disease, Meta-analysis, Prevalence, Central America, South America
Background
Dry eye disease (DED) is defined as “a multifactorial disease of the ocular surface characterized by a loss of homeostasis of the tear film, and accompanied by ocular symptoms, in which tear film instability and hyperosmolarity, ocular surface inflammation and damage, and neurosensory abnormalities play etiological roles.” [1] Etiologies of DED are classified as aqueous deficient, evaporative, or mixed. Meibomian gland dysfunction (MGD) is characterized by an alteration of the tear film lipid layer and is a major cause of DED [2]. DED has been shown to have significant social and economic burden and adverse effects on quality of life around the globe [3–8]. We will use the term dry eye to encompass the wide range of both symptomatic and clinical presentations of DED.
In 2017, the Tear Film and Ocular Surface Society (TFOS) Dry Eye Workshop (DEWS) II Epidemiology Report estimated the prevalence of dry eye to be 5% to 50% worldwide, depending on the study population and definition and diagnostic methods used [9]. Many of the studies used to determine this estimate studied populations in North America, Europe, and Asia. Recently, our group conducted a systematic review and meta-analysis, estimating dry eye prevalence in the United States to be 8.1% (95% confidence interval [CI] 4.9%-13.1%) [10]. At the time of publication of the TFOS DEWS II Epidemiology Report, there were no population-based studies south of the equator, leaving significant gaps in knowledge about the global epidemiology of dry eye.
Central and South America had a combined estimated population of about 616 million people in 2022 [11]. These regions have different socioeconomic and geo-environmental factors from those of previously studied regions, which may affect the magnitude and impact of the dry eye burden. Characterizing the prevalence and incidence of dry eye in these regions will provide greater insight into the population burden of the condition.
The objectives of the current systematic review and meta-analysis are to estimate the prevalence and incidence of dry eye and MGD in Central and South America and to identify factors associated with disease burden.
Methods
We adapted the methods from a previously registered protocol (CRD42021256934) [12] and followed the Preferred Reporting Items for Systematic Reviews and Meta-analyses (PRISMA) and Meta-analysis of Observational Studies in Epidemiology (MOOSE) guidelines for reporting [13, 14].
Eligibility criteria
We considered eligible population-based, clinic-based, and secondary healthcare database studies that reported prevalence or incidence of dry eye or MGD in Central and South American countries. We did not exclude studies based on diagnostic criteria used to define dry eye in the studies. We followed guidance from the TFOS DEWS-II report to categorize case definitions, including: (1) Women’s Health Study criteria (i.e., self-reported physician diagnosis and/or self-reported constant or often symptoms) [15], (2) symptoms when signs were not measured (e.g., measured by the 5-item Dry Eye Questionnaire), (3) clinical signs when symptoms were not measured (e.g., tear breakup time), (4) combination of signs and symptoms (distinct from Women’s Health Study criteria), and (5) MGD (e.g., meibomian gland assessment) [9]. We also considered dry eye and MGD definitions based on relevant Current Procedural Terminology (CPT) and International Classification of Disease (ICD) codes.
We excluded case reports, case series, case–control studies, interventional studies, as well as studies reported only as abstracts.
Search strategies
In collaboration with an information specialist from the University of Colorado Strauss Health Sciences Library, we searched Ovid MEDLINE and Embase for studies published between January 1, 2010 and August 16, 2021, to provide current estimates of dry eye and MGD frequency. We included relevant controlled-vocabulary terms (i.e., medical subject headings in MEDLINE, Emtree terms in Embase) and text words (eTable 1). We hand-searched the reference lists of included studies.
We searched the Cochrane Eyes and Vision US Satellite (CEV@US) database of systematic reviews on March 15, 2023, for systematic reviews tagged with condition: dry eye and review type: epidemiology – prevalence/incidence,and reviewed available reference lists of relevant systematic reviews [16]. We also searched the World Health Organization site on March 15, 2023, using the keywords dry eye and meibomian gland dysfunction. We retrieved one systematic review and the World Health Organization World Report on Vision, and reviewed the reference lists [17, 18]. No additional studies were retrieved from any of the reference lists.
Study selection
At both the title/abstract and full-text stages, each record was independently screened by two investigators using Covidence [19]. Discrepancies were resolved via discussion or with a third investigator as needed.
Data extraction and risk-of-bias assessment
One investigator extracted all relevant study characteristics, methods, and results from each included study using a data extraction form developed on the web-based platform—Systematic Review Data Repository Plus [20]. An independent investigator verified all extracted data with discrepancies resolved via discussion or with a third investigator as needed. For included studies, two investigators independently assessed risk-of-bias using a published risk of bias tool for the purposes of descriptive epidemiological studies [21]. Discrepancies were resolved via discussion or a third investigator as needed.
Evaluation of heterogeneity
We summarized study characteristics using evidence tables. We investigated clinical heterogeneity by assessing demographic characteristics (e.g., age, sex, region, type of study population). We assessed methodological heterogeneity by evaluating study designs [21]. We assessed statistical heterogeneity by estimating the amount of between-study variance (τ2) and the contribution of between-study variance to the total variability across studies (I2) [22, 23]. We also generated 95% prediction intervals (PIs) – intervals within which the prevalence of a new study would fall if this study were selected at random from the same population of the studies already included in the meta-analysis.
Meta-analysis
We used mixed-effects models for meta-analyses of dry eye prevalence. We combined dry eye prevalence from each study using symptomatic disease definitions. To model prevalence, we applied generalized linear mixed-effects models with a logit link using the maximum likelihood approach [24]. We reported the summary prevalence and its 95% CIs and 95% PIs. Our primary analysis focused on indoor workers, students or general ophthalmology clinic-based subpopulations for meta-analysis. We also extracted factors associated with dry eye and MGD from included studies and summarized their associations according to their reported odds ratios across studies. We did not conduct meta-analyses for these associations due to methodological heterogeneity. All statistical analyses were conducted using the metafor package version 3.8.1 in R version 4.2.2 [25].
Results
Search results
Our original search yielded 11,133 records. We excluded 11,117 records due to ineligible populations. After screening 16 full-text reports, we included 14 studies (eFigure 1 in the Supplement). Two full-text reports were excluded due to the study population consisting of pre-existing dry eye disease and neither reported prevalence of dry eye disease [26, 27].
Characteristics of included studies
The 14 included studies covered three outcomes: dry eye prevalence (n = 13), MGD prevalence (n = 2) and computer vision syndrome prevalence (n = 2) (Table 1) [28–41]. No studies reported incidence of dry eye or MGD. Characteristics of the study populations varied across the 14 studies: general population-based (n= 2) [33, 41], indoor and outdoor worker populations (n= 3) [28–30], student populations (n= 3) [37, 39, 40], and hospital- and clinic-based populations (n= 6) [31, 32, 34–36, 38]. Two of the hospital- and clinic-based studies were from general ophthalmology clinics [35, 36]. Definitions of dry eye varied across studies: symptom questionnaires without signs and/or self-reported diagnosis (n= 7) [33, 35, 35, 37, 39–41], symptoms and signs (n= 4) [28, 32, 34, 36], and signs alone (n = 2) (Table 2) [31, 38]. The two MGD prevalence studies used different diagnostic criteria to define MGD: meibum gland quality [36] and MGD stage based on meibum gland quality and expressibility [28]. The two studies that reported prevalence of computer vision syndrome used different symptom questionnaires to define the dry eye component: the Ocular Surface Disease Index (OSDI) [30] and the 5-item Dry Eye Questionnaire (DEQ-5) [37], respectively.
Table 1.
Study characteristics
| Design details | Characteristics of study population | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Population category |
Author, publication year |
Etiology | Country | Source population | Geographical locations |
Data source (national, regional, local) |
Sampling scheme; (response rate) |
Female (%) | Age (years) | Race / ethnicity (%) |
| General population-based | Castro, 2018 [33] | DED | Brazil | Brazilian adults aged ≥ 18 years selected from general urban population, from any labor activities, workplace environment, and socioeconomic status | Brazil—North, Northeast, Central-west, Southeast, South geopolitcal regions | Population-based epidemiological study (national) |
Sampling scheme—cluster sampling by geopolitical region, consecutive enrollment; (77.7%) |
65.5 |
Mean: 40.5 SD: 17.1 |
n.r |
| Graue-Hernandez, 2018 [41] | DED | Mexico | People aged ≥ 50 years in 39 municipalities | Tlaxcala, Mexico | Population-based epidemiological study (regional) |
Sampling scheme—cluster sampling; (58.5%) |
59.6 |
Mean: 64.7 SD: 10.6 |
n.r | |
| Working populations | Castellanos-Gonzalez, 2016 [28] | DED / MGD | Mexico | Surgical residents (physicians) of the surgical branches of the Specialty Hospital, Western National Medical Center | Guadalajara, Jalisco, Mexico | Single institution clinic or hospital-based study (local) |
Sampling scheme—unspecified; (n.r.) |
27 |
Mean: 27.8 SD: 2.1 |
n.r |
| Sanchez-Valerio, 2020 [30] | CVS | Mexico | Office workers at the Autonomous University of Puebla | Puebla, Mexico | Population-based epidemiological study (local) |
Sampling scheme—unspecified; (n.r.) |
54.6 |
Mean: 32.1 SD: 7.8 Range: 18 – 45 |
n.r | |
| Hernandez-Llamas, 2020 [29] | DED | Mexico | Office workers at Universidad de Monterrey (UDEM) rectory office and construction workers at a new UDEM campus module | Monterrey, Mexico | Single institution clinic or hospital-based study (local) |
Sampling scheme-random sampling; (n.r.) |
Construction workers 1.3 Office workers 70.3 |
Construction workers Mean: 35.12 SD: 10.63 Office workers Mean: 33.03 SD: 9.77 |
n.r | |
| Student populations | Garza-Leon, 2016 [39] | DED | Mexico | All undergraduate and graduate students officially registered at University of Monterrey for the academic year 2014–2015 | Monterrey, Mexico | Single institution clinic or hospital-based study (local) |
Other—voluntary response sampling; (95.7%) |
59.8 |
Mean: 21.38 SD: 1.79 |
n.r |
| Cartes, 2021 [37] | CVS, DED | Chile | University students from different universities all over Chile who moved their classes online due to the COVID-19 pandemic | Nationwide, Chile | Population-based epidemiological study (national) |
Sampling scheme—unspecified; (27.1%) |
65 |
Mean: 21.1 SD: 2.7 |
n.r | |
| Garza-Leon, 2021 [40] | DED | Mexico | High school students from Medical Technical High School at Nuevo León Autonomous University | Monterrey, Mexico | Single institution clinic or hospital-based study (local) |
Sampling scheme—non-probabalistic random sampling; (n.r.) |
55.7 |
Mean: 16 SD: 0.96 |
n.r | |
| Hospital- and clinic-based populations | Skare, 2012 [32] | DED | Brazil | Pregnant patients in prenatal care at the Obstetrics Service and non-pregnant patients who had a gynecological appointment at the same hospital | Curitiba, Brazil | Single institution clinic or hospital-based study (local) |
Sampling scheme—unspecified; (n.r.) |
100 |
Pregnant Mean: 28.3 SD: 8.3; Non-pregnant Mean: 27.5 SD: 8.5 |
n.r |
| Martinez JD, 2016 [36] | DED/MGD | Mexico | All new patients age 16 to 85 who presented to the tertiary care outpatient clinic of a referral ophthalmology center (Asociación para Evitar la Ceguera) | Mexico City, Mexico | Single institution clinic or hospital-based study (local) | Sampling scheme -consecutive enrollment; (96.6%) | 55 |
Mean: 45 SD: 16 |
n.r | |
| Garza-Leon, 2017 [35] | DED | Mexico | Patients who attended for the first time a public or private ophthalmology high specialty center with doctors who are members of the Mexican Group of Research in Visual Sciences | 19 states across Mexico | Multi-institution clinic or hospital-based study (national) |
Sampling scheme -consecutive enrollment; (n.r.) |
59.4 |
Mean: 50.0 SD: 17.68 |
n.r | |
| da Cruz, 2018 [34] | DED | Brazil | Dermatology and ophthalmology clinics of Universidade do Estado do Pará | Belém, Pará, Brazil | Single institution clinic or hospital-based study (local) |
Sampling scheme—unspecified; (n.r.) |
48.8 |
Mean: 47.9 SD: 14.6 |
n.r | |
| Surmacz, 2021 [31] | DED | Brazil | Adults who visited dermatology clinics for cosmetic reasons and treatment of superficial mycosis during the period of one year in a single Dermatological tertiary center | Curitiba, Brazil | Single institution clinic or hospital-based study (local) |
Sampling scheme—convenience sampling; (n.r.) |
67.5 |
Median: 42 IQR: 28 – 55 |
Eurodescendant: 74.1; Afrodescendant: 25.9; Asiatic: 1.5 |
|
| De Freitas, 2021 [38] | DED | Brazil | Brazilian patients seen at a single university hospital with diabetes mellitus and controls without diabetes. DM patients were recruited from a single Endocrinology outpatient clinic of a university Faculdade Evangélica Mackenzie de Curitiba, PR, Brazil hospital and controls from Dermatology Clinic seeking consultation for cosmetic reasons | Curitiba, Brazil | Single institution clinic or hospital-based study (local) |
Sampling scheme—convenience sampling; (n.r.) |
Diabetes patients 57.5 Controls 55 |
Diabetes patients Median 59 IQR: 47.2 – 67.0 Controls Median 57 IQR 46.2 – 67.0 |
n.r | |
CVS Computer vision syndrome, DED Dry eye disease, IQR Interquartile range, n/a Not applicable, n.r. Not reported, SD Standard deviation
Table 2.
Prevalence of dry eye and Meibomian gland dysfunction
| Population category | Author, publication year | Etiology | Measurement method: component(s) contributing to diagnostic standard(s) | Diagnostic standard(s) | Prevalence denominator | Prevalence numerator | Prevalence (%), 95% confidence interval | Point or period of data collection | Characteristics used to stratify prevalence or report an association | Overall risk of bias |
|---|---|---|---|---|---|---|---|---|---|---|
| General population-based | Castro, 2018 [33] | DED |
Self-reported diagnosis; Symptoms |
Self-reported diagnosisa | 3107 | 317 | 10.2, 95%CI* 9.2, 11.3 | Point: n.r |
¶ Sex, country / region; † Age, sex, medical comorbidities, medication use, other—computer exposure, smoking; ‡ Age, sex, medical comorbidities, medication use, other—computer exposure, smoking |
M |
| Symptoms (questionnaire)b | 3107 | 151 | 4.9, 95%CI* 4.1, 5.7 | |||||||
| Self reported diagnosis or symptoms (questionnaire) | 3107 | 398 | 12.9, 95%CI* 11.7, 14.0 | |||||||
| Graue-Hernandez, 2018 [41] | DED | Symptoms | Symptoms (questionnaire)c | 1508 | 621 | 41.1, 95%CI 38.6, 43.6 | Period: July to September 2013 |
¶ Dry eye symptom severity; † Sex, dry eye symptom severity, medical comorbidities (hypertension, diabetes mellitus), medication use (hypoglycemic, anti-hypertensive), country or region (rural/urban), other-smoking status, alcohol consumption, wearing glasses, cataract surgery, occupation, level of education; ‡ Sex, dry eye symptom severity, other–smoking index, ever alcohol consumption, medication use (anti-hypertensive) |
M | |
| Working populations | Castellanos-Gonzalez, 2016 [28] | DED / MGD | Symptoms; signs | Symptoms (questionnaire)d | 123 | 69 | 56.1, 95%CI* 46.9, 65.0 | Point: 2014 |
¶ Medical comorbidities, medication use; other—year of residency, makeup use, microscope use; † n.r.; ‡ n.r. |
M |
| TBUTe | 123 | 70 | 56.9, 95%CI* 47.7, 65.8 | |||||||
| Corneal stainingf | 123 | 30 | 24.4, 95%CI* 17.1, 32.9 | |||||||
| Schirmer I testg | 123 | 0 | 0, 95%CI* 0, 2.9§ | |||||||
| Meibomian gland dysfunctionh | 123 | 28 | 22.7, 95%CI* 15.7, 31.2 | |||||||
| Sanchez-Valerio, 2020 [30] | CVS | Symptoms; signs | Symptoms (questionnaire)d | 108 | 86* | 79.7, 95%CI* 70.8, 86.7 | Point: n.r |
¶ Other—computer exposure degree; † Other—computer exposure times; ‡ n.r. |
M | |
| TBUTe | 108 | 105* | 97.2, 95%CI* 92.1, 99.4 | |||||||
| Ocular surface stainingi | 108 | 48* | 44.4, 95%CI* 34.8, 54.3 | |||||||
| Schirmer I testj | 108 | 29* | 26.9, 95%CI* 18.8, 36.2 | |||||||
| Hernandez-Llamas, 2020 [29] | DED | Symptoms | Symptoms (questionnaire)d |
Construction workers 149 |
Construction workers 53 |
Construction workers 35.6, 95% CI* 27.9, 43.8 |
Period: October and December 2017 |
¶ Age, sex, medical comorbidities (systemic disease), other–smoking status, contact lens use, computer hours/day, working hours/day, ocular disease, gender, smoking status, medical comorbidities, other– contact lens use, smoking status, office vs. construction worker, ocular disease; † Gender, smoking status, medical comorbidities, other– contact lens use, smoking status, office vs. construction worker, ocular disease; ‡ Gender, smoking status, medical comorbidities, other– contact lens use, smoking status, office vs. construction worker, ocular disease |
H | |
|
Office workers 155 |
Office workers 112 |
Office workers 72.3, 95% CI* 64.5, 79.1 |
||||||||
| Student populations | Garza-Leon, 2016 [39] | DED | Symptoms | Symptoms (questionnaire)d | 823 | 579 | 70.4, 95% CI* 67.1, 73.5 | Point: 2014 |
¶ Sex, dry eye symptom severity; † n.r.; ‡ Sex, other-smoking status, hours in front of computer, refractive surgery, eye drops user, contact lens user |
M |
| Cartes, 2021 [37] | CVS, DED |
Self-reported diagnosis; Symptoms |
Self-reported diagnosis (questionnaire)k | 1450 | 85 | 5.9, 95%CI* 4.7, 7.2 | Point: May 2020 |
¶ Sex, dry eye symptoms; † Sex, medical comorbidities, medication use, other-smoking, contact lens use, screen exposure time; ‡ Sex, screen exposure time, medical comorbidities (previous dry eye disease diagnosis), medication use (allergy medication) |
H | |
| Symptoms (questionnaire)c | 1450 | 1123 | 77.4, 95%CI* 75.2, 79.6 | |||||||
| Garza-Leon, 2021 [40] | DED | Symptoms | Symptoms (questionnaire)d | 759 | 496 | 65.3, 95% CI* 61.8, 68.7 | Point: n.r |
¶ Sex, dry eye symptom severity; † Sex, other-contact lens use, contact lens type, contact lens wear overnight; ‡ Sex, other-contact lens use, contact lens type, contact lens wear overnight |
M | |
| Hospital- and clinic-based populations | Skare, 2012 [32] | DED | Symptoms; signs | Dry eye sensation |
Pregnant 150 |
Pregnant 24 |
Pregnant 16.0, 95%CI* 10.5, 22.9 |
Point: n.r |
¶ n.r.; † Pregnant vs non-pregnant and lacrimal dysfunction vs no lacrimal dysfunction, medication use, others—length of pregnancy, number of pregnancies, number of full-term pregnancies, number of abortions; ‡ n.r. |
H |
|
Non-pregnant 150 |
Non-pregnant 28 |
Non-pregnant 18.7, 95%CI* 12.8, 25.8 |
||||||||
| Schirmer's testl |
Pregnant 150 |
Pregnant 26 |
Pregnant 17.3, 95%CI* 11.7, 24.4 |
|||||||
|
Non-pregnant 150 |
Non-pregnant 10 |
Non-pregnant 6.7, 95%CI* 3.2, 11.9 |
||||||||
| Martinez JD, 2016 [36] | DED, MGD | Symptoms, Signs | Symptoms (questionnaire)d | 338 | 264 | 78.1, 95% CI* 73.3, 82.4 | Period: November 2012 to February 2013 |
¶ Sex, age, dry eye symptom severity (OSDI, DEQ), medical comorbidities (diabetes mellitus, arthritis, thyroid problems, dry mouth, acne, depression), medication use (antihypertensive, antihistamine, diuretic, GI ulcer medication, multivitamins, lubricant eye drops), other–smoking, indoors occupation, exposure to air conditioning, contact lens use, ocular sugery; † Symptom severity (OSDI, DEQ-5), aqueous tear deficiency (Schirmer's), evaporative deficiency (TBUT), meibomian gland disease, corneal staining (Oxford) vs. Sex, age, medical comorbidities (diabetes mellitus, arthritis, thyroid problems, dry mouth, acne, depression), medication use (antihypertensive, antihistamine, diuretic, GI ulcer medication, multivitamins, lubricant eye drops), other–smoking, indoors occupation, exposure to air conditioning, contact lens use, ocular sugery; ‡ n.r. |
M | |
| Symptoms (questionnaire)c | 338 | 248 | 73.4, 95% CI* 68.3, 78.0 | |||||||
| TBUTm | 338 | 319 | 94.4, 95% CI* 91.4, 96.6 | |||||||
| Corneal stainingn | 338 | 37 | 11.0, 95% CI* 7.8, 14.8 | |||||||
| Schirmer I testo | 338 | 74 | 21.9, 95% CI* 17.6, 26.7 | |||||||
| Meibomian gland dysfunctionp | 338 | 228 | 67.5, 95% CI* 62.2, 72.4 | |||||||
|
Garza-Leon, 2017 [35] |
DED | Symptoms | Symptoms (questionnaire)d | 2270 | 1967 |
86.4, 95% CI* 85.2, 88.0 |
Period: September to December 2014 |
¶ Sex, dry eye symptoms severity; † Age, dry eye symptoms severity; ‡ Sex, age, other- referring physician specialty |
M | |
| da Cruz, 2018 [34] | DED | Symptoms; signs | Japanese criteriaq |
Psoriasis patients 43 Controls 86 |
Psoriasis patients 7 Controls 3 |
Psoriasis patients 16.3, 95%CI* 6.8, 30.7 Controls 3.5, 95%CI* 0.7, 9.9 |
Period: October 2013 to August 2014 |
¶ n.r; † n.r.; ‡ n.r. |
H | |
| Symptoms (questionnaire)d |
Psoriasis patients 43 Controls 86 |
Psoriasis patients 17 Controls 16 |
Psoriasis patients 39.5, 95%CI* 24.9, 55.6 Controls 18.6, 95%CI* 11.0, 28.5 |
|||||||
| TBUTe |
Psoriasis patients 43 Controls 86 |
Psoriasis patients 23 Controls 35 |
Psoriasis patients 53.5, 95%CI* 37.7, 68.8 Controls 40.7, 95%CI* 30.2, 51.8 |
|||||||
| Schirmer I testr |
Psoriasis patients 43 Controls 86 |
Psoriasis patients 6 Controls 5 |
Psoriasis patients 13.9, 95%CI* 5.3, 27.9 Controls 5.8, 95%CI* 1.9, 13.1 |
|||||||
| Rose Bengal stainings |
Psoriasis patients 43 Controls 86 |
Psoriasis patients 23 Controls 25 |
Psoriasis patients 53.5, 95%CI* 37.7, 68.8 Controls 29.1, 95%CI* 19.8, 39.9 |
|||||||
| Surmacz, 2021 [31] | DED | Signs | Schirmer I testt | 135 | 60 | 44.4, 95% CI* 35.9, 53.2 | Point: n.r |
¶ n.r.; † Altered Schirmer's test vs normal and altered TBUT vs normal, age, sex, race/ethnicity, medical comorbidities, medication use, others—smoking, BMI, body composition, OSDI; ‡ n.r. |
H | |
| TBUTe | 135 | 108 | 80, 95%CI* 72.3, 86.4 | |||||||
| De Freitas, 2021 [38] | DED | Signs | Schirmer I testl |
Diabetes patients 120 |
Diabetes patients 46 |
Diabetes patients 38.3, 95%CI* 29.6, 47.7 |
Period: April 2018 to March 2019 |
¶ Severity of dry eye symptoms † Sex, age, medical comorbidities, medication use (metformin, insulin), Other– women at menopause, smoking status ‡ Age medical comorbidities, medication use (metformin, insulin) |
H | |
|
Controls 120 |
Controls 30 |
Controls 25, 95%CI* 17.6, 33.7 |
CI Confidence interval; H High, MGD Meibomian gland dysfunction, M Moderate, n.r. Not reported, SD standard deviation, ¶ Stratified by characteristic, † Characteristic included in a univariate analysis, ‡ Characteristic included in a multivariate analysis, * derived by binomial "exact" calculation (https://sample-size.net/confidence-interval-proportion/); § one-sided 97.5% confidence interval, a – 'Yes' answer to the question “Have you ever been diagnosed (by a clinician) as having dry eye disease?”, b – Womens Health Study questionnaire: Evaluated using the following questions: “How often do your eyes feel dry? and “How often do your eyes feel irritated?”. Response options included “never”, “sometimes”, "often”, and “constantly”. Dry eye defined as the presence of severe symptoms, indicated by "constantly" or "often" response to BOTH questions, c – DEQ-5 score: 7–12 indicates mild to moderate symptoms of DE; > 12 indicates severe symptoms of DE, d – OSDI scale from 0 to 100: 0–12 are considered normal, 13–22 light dry eye symptoms, 23–32 moderate, and > 32 severe symptoms, e – TBUT ≤ 10 s, f – Oxford Schema fluorescein grade ≥ 1, g – Schirmer's I test with anaesthesia < 10 mm, h – Meibomian gland dysfunction stage 2 (Meibomian gland secretion, or “meibum”, quality was assessed in each of the 8 glands of the central third of the lower lid, on a 0–3 scale for each gland: 0 = clear meibum; 1 = cloudy meibum; 2 = cloudy with debris (granular); 3 = thick, like toothpaste. The expressibility of the meibum was assessed from 5 glands: 0 = all glands were expressible; 1 = 3–4 glands were expressible; 2 = 1–2 glands were expressible; 3 = no glands were expressible. Gland dysfunction was classified according to the score obtained: Stage 1 = minimally altered secretions: grade > 2 – < 4; expressiveness: 1. Stage 2 = moderately altered secretions: grade > 4 – < 8; expressiveness: 1. Stage 3 = moderately altered secretions: grade > 8 – < 13; expressiveness: 2. Stage 4 = severely altered secretions: grade > 13; expressiveness: 3), i – Ocular surface damage (fluorescein and Lissamine green) grade ≥ 1, j – Schirmer I test with anesthesia ≤ 5 mm, k – VDT-related symptoms included: soreness, itchiness, pain, dryness, foreign body sensation, redness, visual fatigue, and blurry vision. The frequencies of these symptoms were categorized using a frequency scale in five categories: never, rarely, sometimes, often, and always, and they were graded from 1 (never) to 5 (always) in relation to each answer, l – Schirmer I test without anesthesia ≤ 10 mm, m – TBUT ≤ 5 s, n – Oxford schema corneal fluorescein grade ≥ 2, o – Schirmer I test without anesthesia ≤ 5 mm, p – Meibum gland quality ≥ 2, q – Dry eyes were diagnosed according to Japanese criteria, which required that patients had clinical symptoms and at least two positive results from among the Schirmer I test, the TBUT test, the rose bengal test, and the presence of keratitis. Patients who met only two criteria were classified as having probable dry eyes, r – Schirmer I test without anesthesia ≤ 10 mm / 5 min, s – Rose bengal test, scored from 0–9, considered abnormal if value > 3, t – Schirmer I test without anesthesia ≤ 5 mm in ≥ 1 eye
Risk-of-bias assessment
Summaries of risk-of-bias assessments for the studies are presented in eTable 2. In total eight studies were deemed moderate risk of bias [28, 30, 33, 35, 36, 39–41] and six studies deemed high risk of bias [29, 31, 32, 34, 37, 38]. None of the studies were determined to be representative of the national population. Further, studies at moderate to high risk of bias were not representative of the target population as defined by the primary studies. Studies with bias introduced by sampling strategies and response rates were also judged to be at moderate to high risk of bias.
Prevalence of dry eye
Although included in the systematic review, we excluded from meta-analysis cohorts defined by medical conditions (i.e., diabetes, pregnancy, psoriasis) and their controls to focus on the burden of dry eye in general ophthalmology clinics. Of the worker populations in the included studies, all were indoor occupations except for one study of outdoor construction workers. Due to the qualitative difference in working environments, we excluded the outdoor working cohort from meta-analyses. We conducted an exploratory meta-analysis of subpopulations exposed to sustained computer use either for work or for school.
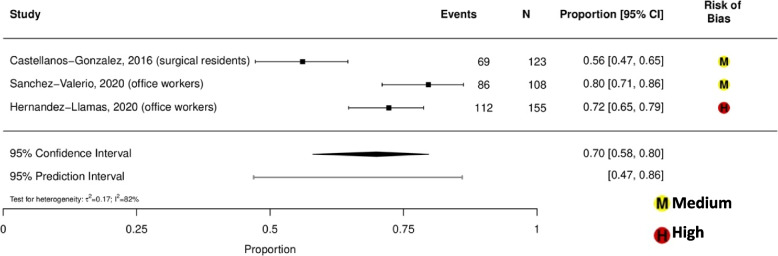
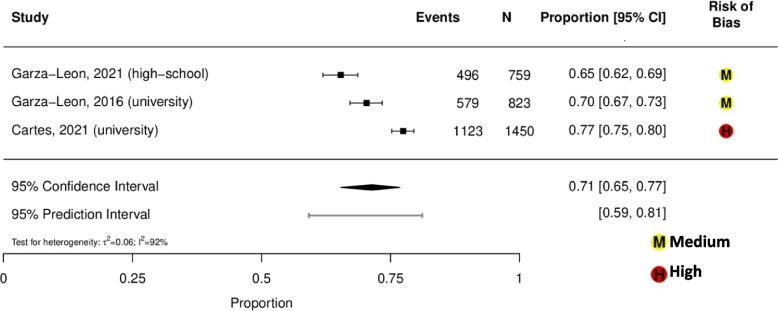
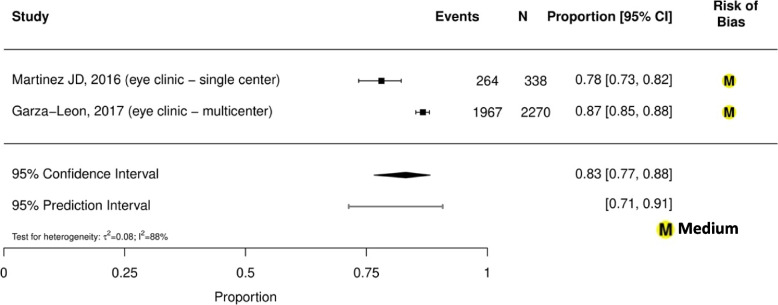
Prevalence estimates of dry eye among general population-based studies were highly variable, ranging from 13% (95% CI, 12%-14%) in Brazil (aged ≥ 18 years old) to 41% (95% CI, 39%-44%) in Mexico (aged ≥ 50 years old) (eFigure 2) [33, 41]. Prevalence of dry eye among indoor working populations ranged from 56 to 80%, with a summary estimate of 70% (95% CI, 58%-80%; τ2 = 0.17; I2 = 82%; 95% PI, 47%-86%; 3 studies, 386 participants; Fig. 1) [28–30]. Prevalence of dry eye among student populations ranged from 65 to 77%, with a summary estimate of 71% (95% CI, 65%-77%; τ2 = 0.06; I2 = 92%; 95% PI, 59%-81%; 3 studies, 3,032 participants; Fig. 2) [37, 39, 40]. Prevalence of dry eye among general ophthalmology clinic-based populations ranged from 78 to 87%, with a summary estimate of 83% (95% CI, 77%-88%; τ2 = 0.08; I2 = 88%; 95% PI, 71%-91%; 2 studies, 2,608 participants; Fig. 3) [35, 36]. Prevalence of dry eye among student and indoor working populations exposed to sustained computer use ranged from 72 to 80%, with a summary estimate of 77% (95% CI, 75%-79%; τ2 = 0.00; I2 = 0%, 95% PI, 75%-79%; 3 studies, 386 participants; eFigure 3).
Fig.1.
Meta-analysis of the prevalence of dry eye among indoor working populations
Fig. 2.
Meta-analysis of the prevalence of dry eye among student populations
Fig. 3.
Meta-analysis of the prevalence of dry eye among general ophthalmology clinic-based populations
Summaries of the associations with sex, age, ocular and medical comorbidities, and medication use are outlined in eTables 3–5 in the Supplement. Prevalence of dry eye appears to be associated with increased age (odds ratio [OR] range 1.04–2.02) [31, 33, 35], female sex (OR range 1.49–3.82) [29, 31, 33, 35, 37, 39, 40], contact lens use (OR range 1.12–4.67) [29, 33, 37, 39, 40], and smoking (OR range 1.24–1.44) [33, 37, 39]. While generally associated with increased dry eye prevalence, there is some inconsistent evidence regarding the direction of association with computer use (OR range 0.82–1.49) [30, 33, 37, 39]. In terms of medical comorbidities, one study reported dry eye association with menopause (OR 1.92 [CI 1.37–2.68]), connective tissue disorder (OR 1.93 [CI 1.23–3.02]) and cancer treatment (OR 3.59 [CI 1.71–7.55]) [33].
Prevalence of MGD and associations
Two studies reported MGD prevalence, which was 68% among general tertiary eye clinic patients and 23% among surgical residents [28, 36]. We observed in both studies that dry eye prevalence assessed by the OSDI was higher than MGD prevalence. One study (338 participants) reported risk factors associated with MGD including older age (per year, OR 1.07, 95% CI 1.05–1.09), male sex (OR 1.7, 95% CI 1.04–2.6), arthritis (OR 7.7, 95% CI 1.001–59), and anti-hypertensive use (OR 2.7, 95% CI 1.3–5.7) [36].
Discussion
This systematic review and meta-analysis of Central and South American studies published since 2010 estimates the prevalence of dry eye to range from 13% (95% CI, 12%-14%) in Brazil to 41% (95% CI, 39%-44%) in Mexico. Considerable statistical heterogeneity prevented meaningful pooling of their results in meta-analysis. Sources of clinical and methodological heterogeneity between these studies may be attributed to different diagnostic criteria, different eligible age ranges, and different geographic regions. However, a recent systematic review and meta-analysis decided to combine these results using a Bayesian approach and provided a pooled prevalence estimate for South America of 14.7% [18]. In addition, a study published in 2022 reported that the overall prevalence of dry eye was 24.4% in adults from Sao Paulo, Brazil, using the Women’s Health Study criteria [42]. Our meta-analyses of Central and South American populations showed pooled subpopulation estimates of dry eye prevalence ranging from 70% (95% CI, 58%-80%) among indoor working populations [28–30], to 71% (95% CI, 65%-77%) in student populations [37, 39, 40], and 83% (95% CI, 77%-88%) in general ophthalmology clinic-based populations [35, 36]. MGD prevalence ranged from 23% among indoor workers and 68% among general tertiary eye clinic patients [28, 36]. We found no studies of the incidence of dry eye or MGD in these regions.
Interestingly, dry eye was highly prevalent among younger populations, such as high-school and university students, which is consistent with recent dry eye prevalence estimates in Caribbean (84%) and Spanish (51%) university students [43, 44]. Furthermore, a study published in 2021 reported that the overall prevalence of dry eye was 59.6% in undergraduate and medical students from Sao Paulo, Brazil, using OSDI [45]. These additional studies taken together with our meta-analysis, which included studies from three countries in Central and South America, comprise only a small body of evidence. It is worth noting that none our included studies for this subpopulation evaluated local environmental factors, while only one study [37] included medical history in the participant questionnaire. Therefore, we recommend cautious interpretation of these high prevalence estimates and suggest that they warrant further investigation in these and other regions. Nevertheless, we surmise that such high prevalence estimates of dry eye among student populations may stem from prolonged computer or other digital device use and contact lens wear. Given increased screen time since the COVID-19 pandemic, it is possible dry eye prevalence among this population may continue to increase [46–48]. Indeed, our meta-analysis of student and working populations in Central and South America exposed to sustained computer use showed that these subgroups have relatively high prevalence of dry eye [28–30, 37, 39, 40]. Furthermore, most studies that reported an association between computer use and dry eye showed higher dry eye prevalence with more computer use, although one study reported an inverse relationship between computer use and dry eye [39]. However, these associations are limited by cross-sectional designs of the primary studies and causality, reverse or otherwise, cannot be determined.
MGD was reported as less prevalent than dry eye in one eye clinic-based population and one working population [28, 36]. However, without individual participant data, we were unable to determine if MGD represented a subset of dry eye in these populations or if some cases of MGD were asymptomatic.
Risk-of-bias, generalizability, and heterogeneity require consideration. The clinical and methodological heterogeneity across studies corresponds to the wide range of reported prevalence estimates for dry eye, even within well-characterized subpopulations. Several studies used multiple diagnostic criteria to estimate dry eye prevalence [36, 37]. Within each of these studies the reported estimates varied by the diagnostic criteria. We noted poor correlation between prevalence as estimated by patient reported symptom questionnaire cutoff values compared to self-reported diagnosis, suggesting considerable clinical under-ascertainment of disease among university students [37]. For the purposes of meta-analysis, we selected the result provided by patient reported symptom questionnaire cutoff values rather than self-reported diagnosis in order to minimize methodological heterogeneity. Given these findings, and the established poor correlation between subjective and objective measures of DED [49, 50], a set of working diagnostic criteria for DED is necessary for standardization across dry eye epidemiological studies. Our attempts to address between-study heterogeneity in subgroup analysis by population characteristics did not successfully reduce statistical heterogeneity. The remaining residual heterogeneity may be associated with differential exposure to dry eye risk factors within each subpopulation, such as duration of computer use [37, 39, 40], and the variety of occupational exposures [28–30].
We noted that Mexican populations working in indoor environments may have higher prevalence of dry eye compared with Mexican construction workers [29]. However, these results were taken from a single study. Elsewhere, dry eye prevalence was reported to be 51% among Chinese coal workers, and duration and levels of dust exposure were associated with dry eye [51]. We were unable to determine the levels of occupational dust exposure in Mexican construction workers in which dry eye prevalence was 36%. Local climate-related factors (e.g., humidity, temperature, ultraviolet light), pollution, altitude, duration of working shifts, and occupational protection practices may all influence differences in dry eye prevalence among these working populations [48]. Also, the highly localized source populations and small sample sizes included in our systematic review may limit the generalizability and comparability with other populations. Overall, there is some evidence that dry eye is highly prevalent in younger and older South American adults and occupational exposures may have an impact.
The prevalence of dry eye was estimated at 11.59% worldwide and 8.1% in the United States [10, 18]. Variations in the prevalence have been noted depending on criterion of symptoms only, TFOS DEWS II criteria, or signs only [18]. In our meta-analysis we focused on the prevalence of symptomatic dry eye to concentrate on outcomes shown to be most important to patients [52]. Dry eye prevalence has also been noted to vary dependent on country income classification level by the World Bank. Studies included in our systematic review reported dry eye prevalence for Mexico, Brazil and Chile; According to 2023 World Bank Classification, Mexico and Brazil are upper-middle income countries, while Chile is a high-income country [53, 54]. Economic status may influence health literacy, access to care, and burden of chronic conditions and it is possible that these factors impact the burden of dry eye among Central and South American populations [55].
Study limitations
Our initial search for studies was limited to Ovid MEDLINE and Embase which aligned with recommendations by Cochrane [56]. There could be studies beyond these databases, like Scopus and the Latin America and the Caribbean literature on health sciences (LILACS) database, that were not searched in our systematic review. We recognize that our search strategy was conducted two years ago; and we mitigated this by integrating recent literature into the discussion.
Conclusions
Overall, there is some evidence that dry eye is highly prevalent in young and older South American adults and occupational exposures may have an impact. Low-cost interventions such as awareness campaigns and environmental modifications in university and workplace settings to improve local ergonomics could mitigate development, progression and complications of DED and reduce the evident healthcare burden among eye clinics [57, 58]. We recommend cautious interpretation of these high prevalence estimates due to the enriched populations with respect to risk factor exposures and the risk of bias in the primary studies.
Supplementary Information
Supplementary file 1: eFigure S1. PRISMA Search Flow Diagram. eFigure S2. eFigure S3. Meta-analysis of dry eye prevalence among student and indoor working populations exposed to sustained computer use. eTable S1. MEDLINE and Embase search strategies. eTable S2. Risk of bias assessments for prevalence studies. eTable S3. Stratified associations with dry eye and meibomian gland dysfunction. eTable S4. Univariable model associations with dry eye and meibomian gland dysfunction. eTable S5. Multivariable model associations with dry eye and meibomian gland dysfunction.
Acknowledgements
We would like to acknowledge the contribution of Kristen Desanto, MSLS, MS, RD, AHIP, information specialist at University of Colorado, who assisted with developing the search strategy for electronic databases, a library service for which a fee was paid.
Abbreviations
- CI
Confidence interval
- DED
Dry eye disease
- DEWS
Dry Eye Workshop
- MGD
Meibomian gland dysfunction
- PRISMA
Preferred reporting items for systematic reviews and meta-analyses
- TFOS
Tear film and ocular surface society
Authors’ contributions
HC, PM and T Li had full access to all of the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis. Concept and design: HC, PM, T Li Acquisition, analysis, or interpretation of data: All authors Drafting of the manuscript: HC, PM, T Lien, T Li Critical revision of the manuscript for important intellectual content: All authors. Statistical analysis: XM, PM, AA, RQ, SL, IS, T Li Obtained funding: T Li Administrative, technical, or material support: T Li Supervision: T Li.
Funding
This work was supported by a grant from the National Institutes of Health National Eye Institute (UG1EY020522). The funder had no role in the design, conduct, analysis, interpretation, and reporting of the study.
Availability of data and materials
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
HC has no conflicts of interest to report PM has no conflicts of interest to report TL has no conflicts of interest to report MX has no conflicts of interest to report AGA has no conflicts of interest to report DG has the following disclosures: Claris Biotherapeutics (I), Oyster Point Pharma (I), Sylentis (I)SH has the following disclosures: Allergan/Abbvie (C), BioTissue, Inc (C), Claris Biotherapeutics (I), TearScience, Inc (C), Johnson & Johnson Vision (C), Novartis (C), Sun Pharmaceuticals (C), TearRestore (C/I), Sight Sciences (C), Kala Pharmaceuticals (C), Dompe USA (C/I), Horizon Therapeutics (C), Science Based Health (C), Ocular Therapeutix (C/I), Nusight Medical (C), Oyster Point Pharma (C/I), Thea Pharma (C)RQ has no conflicts of interest to report SL has no conflicts of interest to report IJS has no conflicts of interest to report TL has no conflicts of interest to report C = paid consultant I = Investigator.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Hongan Chen and Paul McCann are co-first authors.
References
- 1.Craig JP, Nichols KK, Akpek EK, Caffery B, Dua HS, Joo CK, et al. TFOS DEWS II definition and classification report. Ocul Surf. 2017;15(3):276–283. doi: 10.1016/j.jtos.2017.05.008. [DOI] [PubMed] [Google Scholar]
- 2.Nichols KK, Foulks GN, Bron AJ, Glasgow BJ, Dogru M, Tsubota K, et al. The international workshop on meibomian gland dysfunction: executive summary. Invest Ophthalmol Vis Sci. 2011;52(4):1922–1929. doi: 10.1167/iovs.10-6997a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Yu J, Asche CV, Fairchild CJ. The economic burden of dry eye disease in the United States: a decision tree analysis. Cornea. 2011;30(4):379–387. doi: 10.1097/ICO.0b013e3181f7f363. [DOI] [PubMed] [Google Scholar]
- 4.Uchino M, Uchino Y, Dogru M, Kawashima M, Yokoi N, Komuro A, et al. Dry eye disease and work productivity loss in visual display users: the Osaka study. Am J Ophthalmol. 2014;157(2):294–300. doi: 10.1016/j.ajo.2013.10.014. [DOI] [PubMed] [Google Scholar]
- 5.Clegg JP, Guest JF, Lehman A, Smith AF. The annual cost of dry eye syndrome in France, Germany, Italy, Spain, Sweden and the United Kingdom among patients managed by ophthalmologists. Ophthalmic Epidemiol. 2006;13(4):263–274. doi: 10.1080/09286580600801044. [DOI] [PubMed] [Google Scholar]
- 6.Ahn JM, Lee SH, Rim THT, Park RJ, Yang HS, Kim TI, et al. Prevalence of and risk factors associated with dry eye: the Korea national health and nutrition examination survey 2010–2011. Am J Ophthalmol. 2014;158(6):1205–1214.e7. doi: 10.1016/j.ajo.2014.08.021. [DOI] [PubMed] [Google Scholar]
- 7.Buchholz P, Steeds CS, Stern LS, Wiederkehr DP, Doyle JJ, Katz LM, et al. Utility assessment to measure the impact of dry eye disease. Ocul Surf. 2006;4(3):155–161. doi: 10.1016/S1542-0124(12)70043-5. [DOI] [PubMed] [Google Scholar]
- 8.Paulsen AJ, Cruickshanks KJ, Fischer ME, Huang GH, Klein BEK, Klein R, et al. Dry Eye in the beaver dam offspring study: prevalence, risk factors, and health-related quality of life. Am J Ophthalmol. 2014;157(4):799–806. doi: 10.1016/j.ajo.2013.12.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Stapleton F, Alves M, Bunya VY, Jalbert I, Lekhanont K, Malet F, et al. TFOS DEWS II Epidemiology Report. Ocul Surf. 2017;15(3):334–365. doi: 10.1016/j.jtos.2017.05.003. [DOI] [PubMed] [Google Scholar]
- 10.McCann P, Abraham AG, Mukhopadhyay A, Panagiotopoulou K, Chen H, Rittiphairoj T, et al. Prevalence and Incidence of Dry Eye and Meibomian Gland Dysfunction in the United States: A Systematic Review and Meta-analysis. JAMA Ophthalmol. 2022;140(12):1181–1192. doi: 10.1001/jamaophthalmol.2022.4394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.World Population Prospects - Population Division - United Nations [Internet]. [cited 2023 Feb 20]. Available from: https://population.un.org/wpp/.
- 12.McCann P, Abraham A, Hauswirth S, Ifantides C, Gregory DG, Saldanha IJ, et al. Prevalence and incidence of dry eye in the United States: a systematic review [Internet]. PROSPERO; 2021. Available from: www.crd.york.ac.uk/prospero/display_record.php?ID=CRD42021256934. [DOI] [PMC free article] [PubMed]
- 13.Page MJ, McKenzie JE, Bossuyt PM, Boutron I, Hoffmann TC, Mulrow CD, et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ. 2021;29(372):n71. doi: 10.1136/bmj.n71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Stroup DF, Berlin JA, Morton SC, Olkin I, Williamson GD, Rennie D, et al. Meta-analysis of observational studies in epidemiology: a proposal for reporting, meta-analysis Of observational studies in epidemiology (MOOSE) group. JAMA. 2000;283(15):2008–12. doi: 10.1001/jama.283.15.2008. [DOI] [PubMed] [Google Scholar]
- 15.Schaumberg DA, Sullivan DA, Buring JE, Dana MR. Prevalence of dry eye syndrome among US women. Am J Ophthalmol. 2003;136(2):318–326. doi: 10.1016/S0002-9394(03)00218-6. [DOI] [PubMed] [Google Scholar]
- 16.Lê JT, Qureshi R, Rouse B, Twose C, Rosman L, Lindsley K, et al. Development and content of a database of systematic reviews for eyes and vision. Eye Lond Engl. 2022;36(4):883–885. doi: 10.1038/s41433-021-01514-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.World Health Organization [Internet]. 2019. World Report on Vision. Available from: https://www.who.int/publications-detail-redirect/9789241516570.
- 18.Papas EB. The global prevalence of dry eye disease: a Bayesian view. Ophthalmic Physiol Opt. 2021;41(6):1254–1266. doi: 10.1111/opo.12888. [DOI] [PubMed] [Google Scholar]
- 19.Covidence Systematic Review Software [Internet]. Melbourne, Australia: Veritas Health Innovation; [cited 2023 Feb 19]. Available from: https://www.covidence.org/.
- 20.Systematic Review Data Repository [Internet]. Agency for Healthcare Research and Quality; Available from: https://srdrplus.ahrq.gov/.
- 21.Hoy D, Brooks P, Woolf A, Blyth F, March L, Bain C, et al. Assessing risk of bias in prevalence studies: modification of an existing tool and evidence of interrater agreement. J Clin Epidemiol. 2012;65(9):934–939. doi: 10.1016/j.jclinepi.2011.11.014. [DOI] [PubMed] [Google Scholar]
- 22.Higgins JPT, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta-analyses. BMJ. 2003;327(7414):557–560. doi: 10.1136/bmj.327.7414.557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.DerSimonian R, Laird N. Meta-analysis in clinical trials. Control Clin Trials. 1986;7(3):177–188. doi: 10.1016/0197-2456(86)90046-2. [DOI] [PubMed] [Google Scholar]
- 24.Lin L, Chu H. Meta-analysis of Proportions Using Generalized Linear Mixed Models. Epidemiol Camb Mass. 2020;31(5):713–717. doi: 10.1097/EDE.0000000000001232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Viechtbauer W. Conducting meta-Analyses in R with the metafor Package | journal of statistical software. J Stat Softw. 2010;36(3):1–48. doi: 10.18637/jss.v036.i03. [DOI] [Google Scholar]
- 26.Rodriguez-Garcia A, Loya-Garcia D, Hernandez-Quintela E, Navas A. Risk factors for ocular surface damage in Mexican patients with dry eye disease: a population-based study. Clin Ophthalmol. 2019;13:53–62. doi: 10.2147/OPTH.S190803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Damasceno RW, Osaki MH, Dantas PEC, Belfort R. Involutional entropion and ectropion of the lower eyelid: prevalence and associated risk factors in the elderly population. Ophthal Plast Reconstr Surg. 2011;27(5):317–320. doi: 10.1097/IOP.0b013e3182115229. [DOI] [PubMed] [Google Scholar]
- 28.Castellanos-González JA, Torres-Martínez V, Martínez-Ruiz A, Fuentes-Orozco C, Rendón-Félix J, Irusteta-Jiménez L, et al. Prevalence of dry eye syndrome in residents of surgical specialties. BMC Ophthalmol. 2016;16(16):108. doi: 10.1186/s12886-016-0292-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hernandez-Llamas S, Paz-Ramos AK, Marcos-Gonzalez P, Amparo F, Garza-Leon M. Symptoms of ocular surface disease in construction workers: comparative study with office workers. BMC Ophthalmol. 2020;20(1):272. doi: 10.1186/s12886-020-01548-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sánchez-Valerio MDR, Mohamed-Noriega K, Zamora-Ginez I, Baez Duarte BG, Vallejo-Ruiz V. Dry eye disease association with computer exposure time among subjects with computer vision syndrome. Clin Ophthalmol Auckl NZ. 2020;14:4311–4317. doi: 10.2147/OPTH.S252889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Surmacz HU, Cotlinski AL, Gehlen ML, Nisihara R, Skare TL. Dry eye and percentage of body fat: a cross-sectional prospective study. Int Ophthalmol. 2021;41(5):1855–1861. doi: 10.1007/s10792-021-01747-8. [DOI] [PubMed] [Google Scholar]
- 32.Skare TL, Gehlen ML, Silveira DMG, Uema de MMS. Lacrimal dysfunction and pregnancy. Rev Bras Ginecol E Obstet Rev Fed Bras Soc Ginecol E Obstet. 2012;34(4):170–4. doi: 10.1590/S0100-72032012000400006. [DOI] [PubMed] [Google Scholar]
- 33.de Castro JS, Selegatto IB, de Castro RS, Miranda ECM, de Vasconcelos JPC, de Carvalho KM, et al. Prevalence and Risk Factors of self-reported dry eye in Brazil using a short symptom questionnaire. Sci Rep. 2018;8(1):2076. doi: 10.1038/s41598-018-20273-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.da Cruz NFS, da, Brandão LS, Cruz SFS da, Cruz SAS da, Pires CAA, Carneiro FRO Ocular manifestations of psoriasis. Arq Bras Oftalmol. 2018;81(3):219–225. doi: 10.5935/0004-2749.20180044. [DOI] [PubMed] [Google Scholar]
- 35.Garza-Leon M, Hernandez-Quintela E, Cámara-Castillo H, De La Parra-Colín P, Covarrubias-Espinosa P, Sanchez-Huerta V, et al. Prevalence of symptoms of ocular surface disease in patients of ophthalmic practices. Gac Med Mex. 2017;153:696–700. [DOI] [PubMed]
- 36.Martinez JD, Galor A, Ramos-Betancourt N, Lisker-Cervantes A, Beltrán F, Ozorno-Zárate J, et al. Frequency and risk factors associated with dry eye in patients attending a tertiary care ophthalmology center in Mexico City. Clin Ophthalmol Auckl NZ. 2016;10:1335–1342. doi: 10.2147/OPTH.S106451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Cartes C, Segovia C, Salinas-Toro D, Goya C, Alonso MJ, Lopez-Solis R, et al. dry eye and visual display terminal-related symptoms among university students during the coronavirus disease pandemic. Ophthalmic Epidemiol. 2021;12:1–7. doi: 10.1080/09286586.2021.1943457. [DOI] [PubMed] [Google Scholar]
- 38.De Freitas GRD, Ferraz GAM, Gehlen M, Skare TL. Dry eyes in patients with diabetes mellitus. Prim Care Diabetes. 2021;15(1):184–186. doi: 10.1016/j.pcd.2020.01.011. [DOI] [PubMed] [Google Scholar]
- 39.Garza-León M, Valencia-Garza M, Martínez-Leal B, Villarreal-Peña P, Marcos-Abdala HG, Cortéz-Guajardo AL, et al. Prevalence of ocular surface disease symptoms and risk factors in group of university students in Monterrey, Mexico. J Ophthalmic Inflamm Infect. 2016;18(6):44. doi: 10.1186/s12348-016-0114-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Garza-León M, López-Chavez E, De La Parra-Colín P. Prevalence of Ocular Surface Disease Symptoms in High School Students in Monterrey. Mexico J Pediatr Ophthalmol Strabismus. 2021;58(5):287–291. doi: 10.3928/01913913-20210308-01. [DOI] [PubMed] [Google Scholar]
- 41.Graue-Hernández EO, Serna-Ojeda JC, Estrada-Reyes C, Navas A, Arrieta-Camacho J, Jiménez-Corona A. Dry eye symptoms and associated risk factors among adults aged 50 or more years in Central Mexico. Salud Publica Mex. 2018;60(5):520–527. doi: 10.21149/9024. [DOI] [PubMed] [Google Scholar]
- 42.Marculino LGC, Hazarbassanov RM, Hazarbassanov NGT de Q, Hirai F, Milhomens Filho JAP, Wakamatsu TH, et al. Prevalence and risk factors for dry eye disease: the Sao Paulo dry eye study. Arq Bras Oftalmol. 2022;85(6):549–57. [DOI] [PubMed]
- 43.Ezinne N, Alemu HW, Cheklie T, Ekemiri K, Mohammed R, James S. High prevalence of symptomatic dry eye disease among university students during the covid-19 pandemic in university of west indies. Trinidad Tobago Clin Optom. 2023;3(15):37–43. doi: 10.2147/OPTO.S396135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.García-Ayuso D, Di Pierdomenico J, Moya-Rodríguez E, Valiente-Soriano FJ, Galindo-Romero C, Sobrado-Calvo P. Assessment of dry eye symptoms among university students during the COVID-19 pandemic. Clin Exp Optom. 2022;105(5):507–513. doi: 10.1080/08164622.2021.1945411. [DOI] [PubMed] [Google Scholar]
- 45.Yang I, Wakamatsu T, Sacho IBI, Fazzi JH, de Aquino AC, Ayub G, et al. Prevalence and associated risk factors for dry eye disease among Brazilian undergraduate students. PLoS ONE. 2021;16(11):e0259399. doi: 10.1371/journal.pone.0259399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Ehrlich JR, Ramke J, Macleod D, Burn H, Lee CN, Zhang JH, et al. Association between vision impairment and mortality: a systematic review and meta-analysis. Lancet Glob Health. 2021;9(4):e418–e430. doi: 10.1016/S2214-109X(20)30549-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Saldanha IJ, Petris R, Makara M, Channa P, Akpek EK. Impact of the COVID-19 pandemic on eye strain and dry eye symptoms. Ocul Surf. 2021;22:38–46. doi: 10.1016/j.jtos.2021.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Wolffsohn JS, Lingham G, Downie LE, Huntjens B, Inomata T, Jivraj S, et al. TFOS Lifestyle: Impact of the digital environment on the ocular surface. Ocul Surf. 2023;14(28):213–252. doi: 10.1016/j.jtos.2023.04.004. [DOI] [PubMed] [Google Scholar]
- 49.Schmidl D, Witkowska KJ, Kaya S, Baar C, Faatz H, Nepp J, et al. The Association Between Subjective and Objective Parameters for the Assessment of Dry-Eye Syndrome. Invest Ophthalmol Vis Sci. 2015;56(3):1467–1472. doi: 10.1167/iovs.14-15814. [DOI] [PubMed] [Google Scholar]
- 50.Kyei S, Dzasimatu SK, Asiedu K, Ayerakwah PA. Association between dry eye symptoms and signs. J Curr Ophthalmol. 2018;30(4):321–325. doi: 10.1016/j.joco.2018.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Qiu C, Fang Y. The Prevalence of Symptomatic Dry Eye Disease Among Coal Workers in Huainan Region of China. Int J Gen Med. 2023;19(16):203–209. doi: 10.2147/IJGM.S396670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Saldanha IJ, Petris R, Han G, Dickersin K, Akpek EK. Research questions and outcomes prioritized by patients with dry eye. JAMA Ophthalmol. 2018;136(10):1170–1179. doi: 10.1001/jamaophthalmol.2018.3352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.The global prevalence of dry eye disease and its association with economy: a systematic review [Internet]. 2019 [cited 2023 Feb 20]. Available from: https://www.researchsquare.com.
- 54.New World Bank country classifications by income level: 2022–2023 [Internet]. 2022 [cited 2023 Feb 20]. Available from: https://blogs.worldbank.org/opendata/new-world-bank-country-classifications-income-level-2022-2023.
- 55.Abegunde DO, Mathers CD, Adam T, Ortegon M, Strong K. The burden and costs of chronic diseases in low-income and middle-income countries. The Lancet. 2007;370(9603):1929–1938. doi: 10.1016/S0140-6736(07)61696-1. [DOI] [PubMed] [Google Scholar]
- 56.Lefebvre C, Glanville J, Briscoe S, Littlewood A, Marshall C, Metzendorf M-I, et al. et al. Chapter 4: Searching for and selecting studies. In: Higgins JPT, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, et al.et al., editors. Cochrane Handbook for Systematic Reviews of Interventions. 2. Chichester, UK: John Wiley & Sons; 2019. pp. 67–108. [Google Scholar]
- 57.Byber K, Radtke T, Norbäck D, Hitzke C, Imo D, Schwenkglenks M, et al. Humidification of indoor air for preventing or reducing dryness symptoms or upper respiratory infections in educational settings and at the workplace. Cochrane Database Syst Rev. 2021;12:CD012219. [DOI] [PMC free article] [PubMed]
- 58.Fan Z, Du Y, Tang C, Tian R, Lu X, Zheng L, et al. Awareness, prevalence, and knowledge of dry eye among internet professionals: a cross-sectional study in China. Eye Contact Lens. 2023;49(3):92. doi: 10.1097/ICL.0000000000000968. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary file 1: eFigure S1. PRISMA Search Flow Diagram. eFigure S2. eFigure S3. Meta-analysis of dry eye prevalence among student and indoor working populations exposed to sustained computer use. eTable S1. MEDLINE and Embase search strategies. eTable S2. Risk of bias assessments for prevalence studies. eTable S3. Stratified associations with dry eye and meibomian gland dysfunction. eTable S4. Univariable model associations with dry eye and meibomian gland dysfunction. eTable S5. Multivariable model associations with dry eye and meibomian gland dysfunction.
Data Availability Statement
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.