Abstract
Background
Guidelines recommend cardiovascular risk assessment and counseling for cancer survivors. For effective implementation, it is critical to understand survivor cardiovascular health (CVH) profiles and perspectives in community settings. We aimed to (1) Assess survivor CVH profiles, (2) compare self-reported and EHR-based categorization of CVH factors, and (3) describe perceptions regarding addressing CVH during oncology encounters.
Methods
This cross-sectional analysis utilized data from an ongoing NCI Community Oncology Research Program trial of an EHR heart health tool for cancer survivors (WF-1804CD). Survivors presenting for routine care after potentially curative treatment recruited from 8 oncology practices completed a pre-visit survey, including American Heart Association Simple 7 CVH factors (classified as ideal, intermediate, or poor). Medical record abstraction ascertained CVD risk factors and cancer characteristics. Likert-type questions assessed desired discussion during oncology care.
Results
Of 502 enrolled survivors (95.6% female; mean time since diagnosis = 4.2 years), most had breast cancer (79.7%). Many survivors had common cardiovascular comorbidities, including high cholesterol (48.3%), hypertension or high BP (47.8%) obesity (33.1%), and diabetes (20.5%); 30.5% of survivors received high cardiotoxicity potential cancer treatment. Less than half had ideal/non-missing levels for physical activity (48.0%), BMI (18.9%), cholesterol (17.9%), blood pressure (14.1%), healthy diet (11.0%), and glucose/ HbA1c (6.0%). While > 50% of survivors had concordant EHR-self-report categorization for smoking, BMI, and blood pressure; cholesterol, glucose, and A1C were unknown by survivors and/or missing in the EHR for most. Most survivors agreed oncology providers should talk about heart health (78.9%).
Conclusions
Tools to promote CVH discussion can fill gaps in CVH knowledge and are likely to be well-received by survivors in community settings.
Trial registration
NCT03935282, Registered 10/01/2020
Supplementary Information
The online version contains supplementary material available at 10.1186/s12885-024-11912-8.
Keywords: Cancer survivorship, Electronic Health Records, Lifestyle risk factors, Care delivery model
Background
Cancer survivors have almost twice the risk of fatal heart disease compared to the general population, and deaths related to heart disease exceed deaths from the primary cancer for many common cancer types [1–9]. Over 85% of survivors have one or more cardiovascular (CV) risk factors [10–12], increasing their risk of poor CV and cancer outcomes [13–22]. Contributors to heightened CV risk among cancer survivors include: (1) shared mechanisms of cancer and CV disease, including inflammation, tobacco, and obesity; [13, 23] (2) adverse changes in lifestyle factors during cancer treatment (e.g., weight gain) [24, 25]; and (3) cardiotoxic effects of certain cancer treatments [1, 26]. Consequently, the NCCN Clinical Practice Guidelines in Oncology (NCCN Guidelines) for Survivorship recommend cardiovascular risk assessment and counseling for all cancer survivors throughout the survivorship continuum [27]. ASCO guidelines also recommend CVH assessment and counseling, specifically:
Clinicians should regularly evaluate and manage cardiovascular risk factors such as smoking, hypertension, diabetes, dyslipidemia, and obesity in patients previously treated with cardiotoxic cancer therapies. A heart-healthy lifestyle, including the role of diet and exercise, should be discussed as part of long-term follow-up care [28].
To effectively implement these and similar guidelines to prevent CV morbidity in cancer survivors, it is critical to understand survivors’ current understanding of their heart health, as well as their perspectives on addressing CV risk during routine oncology care. In our pilot work with breast cancer survivors treated at an academic medical center, we uncovered gaps in survivors’ knowledge of heart health, as well as their attainment of ideal cardiovascular health (CVH) [29]. More than half of these survivors reported not knowing their level for one or more CVH factors, and less than 50% had ideal blood pressure (BP), body mass index (BMI), cholesterol, diet, and physical activity per American Heart Association guidelines [30]. Considering that the majority of survivors receive their care in community settings [31], it is important to understand whether these results generalize to a more diverse group of cancer patients seen in community oncology practices for routine follow-up.
We present baseline data from an ongoing hybrid effectiveness-implementation study (WF-1804CD) of a novel electronic health record (EHR)-embedded heart health assessment tool, Automated Heart-Health Assessment (AH-HA) [13, 29, 32, 33]. The AH-HA trial collects data on CVH factors and care coordination among cancer survivors receiving routine survivorship care in NCI Community Oncology Research Program (NCORP) outpatient oncology practices [34]. The AH-HA tool assesses the American Heart Association’s Life’s Simple 7 CVH factors [35] (body mass index, smoking status, blood pressure (BP), total cholesterol, hemoglobin A1c (HbA1c) or blood glucose, physical activity, and diet) using a combination of self-report and EHR data. The objectives of these baseline analyses were to: (1) assess the CVH profiles of post-treatment cancer survivors receiving routine follow up care in community oncology settings, (2) compare self-reported and EHR-based categorization of CVH factors, and (3) describe cancer survivors’ perceived health risks, motivation to improve CVH, as well as the perceived appropriateness of addressing CVH during outpatient oncology encounters.
Methods
Study sample
This cross-sectional analysis utilized baseline data from participants in an ongoing study to examine the effects of the AH- HA tool among survivors receiving routine follow-up care in community oncology settings, using a group-randomized trial design [34]. This study was conducted in partnership with 9 outpatient oncology practices affiliated with the Wake Forest NCI Community Oncology Research Program (WF NCORP) Research Base (NCT03935282, registered 10/01/2020). Eligible survivors included those presenting for routine cancer-related follow-up care ≥ 6 months post-potentially curative treatment for breast, prostate, colorectal, or endometrial cancers or Hodgkin and non-Hodgkin lymphomas; ongoing hormonal therapies such as tamoxifen, aromatase inhibitors (with or without adjuvant CDK 4/6 inhibitors such as abemaciclib), or androgen deprivation were allowed. To be eligible, survivors had to be free of disease at their last medical visit for all cancers. The trial was available in English and Spanish. Local NCORP site staff contacted potentially eligible survivors prior to their appointment via mail, telephone, e-mail, patient portals, or in-person to provide study information, screen, and ascertain interest in participating.
Data collection
The study was approved by the NCI Central Institutional Review Board (CIRB), and all participants provided informed consent prior to participation. Patient surveys were generally administered using a web-based platform, with paper and phone as back-up options. Enrolled survivors completed a baseline assessment (~ 15 min) within two weeks prior to their regularly scheduled follow-up clinic visit and then a second survey immediately following their visit. A trained research staff member completed the EHR chart abstraction form following the designated clinic visit. Survivors received a $10 gift card after completing the post-visit survey.
Measures
Survivor CVH factors were collected via both self-reported numerical values [weight, height, smoking status, blood pressure (BP), total cholesterol, hemoglobin A1c (HbA1c), physical activity, and diet] and the most recent value abstracted from the EHR. Physical activity and diet were not available from the EHR. In the case where survivors were unsure of the numerical value for a heart health factor, they could select “I don’t know” as an option. Self-report items (Supplementary Material 1) were based on prior work in both primary care and oncology [13, 29]. Each factor for both methods (EHR and self-report) was scored as ideal, intermediate, poor, or missing/unknown according to the American Heart Association Simple 7 framework [30]. Self-report and EHR were considered concordant if they resulted in the same categorization (e.g., ideal, intermediate, poor). Survivor knowledge of CVH and perceived importance and appropriateness of heart health discussions during oncology care were evaluated with 6 questions used in prior work [29] assessing: 1) confidence in understanding risk of heart disease, 2 & 3) understand (or plan to take) steps needed to maintain or improve heart health, 4 & 5) perception that cancer (or heart disease) pose a risk to health, 6) importance of talking with oncology provider about heart health, and 7) oncology providers should talk to patients about heart health. These questions were rated on a 5-point Likert scale from strongly agree to strongly disagree. Survivors also reported age, gender, race/ethnicity, years of education, marital status, and visits to primary care and cardiology within the past six months. Key medical information from the EHR, including cancer type, stage, date of diagnosis, and cancer treatment was abstracted by research staff at each site. Cancer treatments with cardiotoxic potential were classified according to Herrmann 2020 [36]; specifically, those cancer therapies classified as having very common (> 10%) frequency of either cardiac or arrhythmia toxicity were defined as high cardiotoxicity potential therapies. We also collected information on treatments with less frequent occurrence of cardiotoxicity. Non-cancer cardiovascular comorbidities included: high cholesterol, hypertension, obesity, diabetes with and without complications, heart failure, and atherosclerotic vascular disease (ASCVD, inclusive of heart disease, myocardial infarction, peripheral vascular disease, and cerebrovascular disease/stroke). Rural residence is defined as residing in a zip-code that qualifies as rural according to the Federal Office of Rural Health Policy definition [37].
Statistical considerations
Descriptive statistics of count (frequency) and mean (standard deviation) were used to characterize baseline patient demographics, healthcare utilization, and cardiovascular comorbidities categorical and continuous variables respectively. Counts and frequencies were also used to characterize cardiovascular health perceptions and receipt of cardiotoxic cancer treatments. We classified the AHA Simple 7 cardiovascular health factors from both self-report and the EHR as ideal, intermediate, or poor according to the American Heart Association Simple 7 rubric [30].
Results
Sample characteristics
Data for the present analyses were available from 502 enrolled survivors (10/1/2020-10/21/2022) with complete baseline self-report and medical record data from 8 enrolling practices in 7 states. At the time of analysis, we had screened 590 participants; 2 were ineligible, 77 declined participation, and 9 did not respond. Breast cancer survivors comprised most of the sample (79.7%, n = 400), with smaller proportions of endometrial (10.6%, n = 53), colorectal (5.8%, n = 29), lymphoma (2.4%, n = 12), and prostate (0.2%, n = 1) cancers (Table 1). The mean time since cancer diagnosis was 4.21 years (SD = 3.32). Diagnosis dates for survivors’ most recent cancer ranged from 10/1995 to 12/2021. Females comprised 95.6% (n = 480) of the sample; participants identified primarily as non-Hispanic/Latino, white (86.3%, n = 433). A little more than half of survivors were between 40 and 65 years of age (51.2%, n = 257), with 4.0% (n = 20) under 40 and 44.8% (n = 225) 65 years and older.
Table 1.
Demographic and cancer characteristics of enrolled post-treatment cancer survivors (N = 502)
| N | % | |
|---|---|---|
| Age | ||
| 18–39 | 20 | 4.0 |
| 40–64 | 257 | 51.2 |
| 65–74 | 175 | 34.8 |
| 75+ | 50 | 10.0 |
| Female Gender | 480 | 95.6 |
| Race/ Ethnicity | ||
| American Indian or Alaska Native | 3 | 0.6 |
| Asian | 4 | 0.8 |
| Black/African American | 35 | 7.0 |
| More than one race | 7 | 1.4 |
| White, non-Hispanic or Latino | 433 | 86.3 |
| White/Other/Unknown, Hispanic or Latino | 17 | 3.4 |
| Other/Unknown, Not Hispanic or Latino | 3 | 0.6 |
| Rural Residence | ||
| Non-metro# | 89 | 17.7 |
| Education | ||
| High School or Less | 107 | 21.3 |
| Some College (including vocational/ technical) | 170 | 33.9 |
| College degree or more | 223 | 44.4 |
| Prefer not to answer | 2 | 0.4 |
| Marital Status | ||
| Married/Living as Married | 340 | 67.7 |
| Single, Divorced, Separated, or Widowed | 161 | 32.1 |
| Prefer not to answer | 1 | 0.2 |
| Cancer Type | ||
| Breast | 400 | 79.7 |
| Colorectal | 29 | 5.8 |
| Endometrial | 53 | 10.6 |
| Prostate | 1 | 0.2 |
| Lymphoma | 12 | 2.4 |
| Multiple Cancer Types | 7 | 1.4 |
| Time Since Diagnosis (years) | ||
| Mean (SD), Range | 455 | 4.21 (3.32), 0.52–26.72 |
| AJCC Cancer Stage for Most Recent Cancer | ||
| 0 | 25 | 5.0 |
| 1 | 210 | 41.8 |
| 2 | 117 | 23.3 |
| 3 | 64 | 12.8 |
| 4 | 3 | 0.6 |
| Unknown/NA | 83 | 16.5 |
| Health Care Utilization* (self-reported) | ||
| PCP (last 6 months) | 397 | 80.0 |
| Cardiologist (last 6 months) | 71 | 14.2 |
| How confident are you filling out medical forms by yourself? | ||
| Extremely | 352 | 70.1 |
| Quite a bit | 103 | 20.5 |
| Somewhat/A little bit/ Not at all | 47 | 9.4 |
| During the past 4 weeks, did you have enough money to meet the daily needs of your family? | ||
| No | 17 | 3.4 |
#Defined as residing in a zip-code that qualifies as rural according to the Federal Office of Rural Health Policy definition
*Six participants reported not knowing if they had seen a primary care provider in the last six months; Two participants reported not knowing if they had seen a cardiologist in the last six months
Objective 1: cardiovascular health profiles
Comorbidities
The most common cardiovascular comorbidities (Table 2) were high cholesterol (48.3% n = 241), hypertension or high BP (47.8%, n = 240), obesity (33.1%, n = 166), and diabetes (20.5%, n = 103).
Table 2.
Cardiovascular comorbidities and cancer therapy with cardiotoxicity potential among post-treatment cancer survivors (N = 502)
| Cardiovascular Comorbidities | N | % |
|---|---|---|
| High Cholesterol1 | 241 | 48.3 |
| Hypertension or high BP | 240 | 47.8 |
| Obesity2 | 166 | 33.1 |
| Diabetes | 103 | 20.5 |
| Atherosclerotic Vascular Disease (ASCVD)2** | 67 | 13.4 |
| Heart Failure | 15 | 3.0 |
| Receipt of Cancer Treatment with High Cardiotoxicity Potential*** | 153 | 30.5 |
| 1n=499; 2n=501 | ||
**ASCVD includes Heart Disease, Peripheral Vascular Disease, Myocardial Infarction, and Cerebrovascular Disease
***classified according to Herrmann 2020 [36]
Receipt of cancer treatment with cardiotoxic potential
Almost a third of survivors (n = 153, 30.5%) received at least one cancer treatment with high cardiotoxicity potential (Table 2). Anthracycines were the most common (n = 109, 21.7%), followed by monoclonal antibodies (n = 53, 10.6%, Supplementary Material 2). Receipt of cancer treatment with lower risk of cardiotoxicity was common in the sample; 93.8% of survivors received treatment with at least some cardiotoxicity potential (Supplementary Material 2).
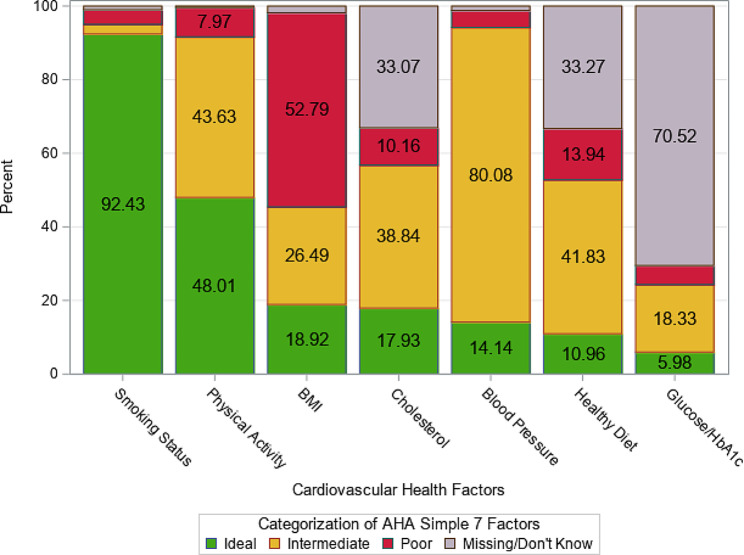
Cardiovascular factors
Using the AHA Simple 7 ratings (ideal, intermediate, poor), most survivors (92.4%, n = 464) had an ideal smoking status; less than half of survivors (48%, n = 241) reported ideal levels of physical activity (Fig. 1). Smaller proportions of survivors had ideal levels for BMI (18.9%, n = 95), cholesterol (17.9%, n = 90), BP (14.1%, n = 71), and glucose/ HbA1c (6.0%, n = 207). More than half (52.8%, n = 265) of survivors had poor BMI. Among survivors with available data (i.e., survivor known and/or available from EHR), intermediate was the most common level for cholesterol (38.8%, n = 195), BP (80.1%, n = 402), a healthy diet (41.8%, n = 210), and glucose/ HbA1c (18.3%, n = 92).
Fig. 1.
Simple 7 cardiovascular health factors among post-treatment cancer survivors (N = 502). American heart association simple 7 physical activity and diet components are self-reported prior to a routine oncology visit; all other components are from the electronic health record. Missing/Don’t know means EHR missing and/or survivor unknown
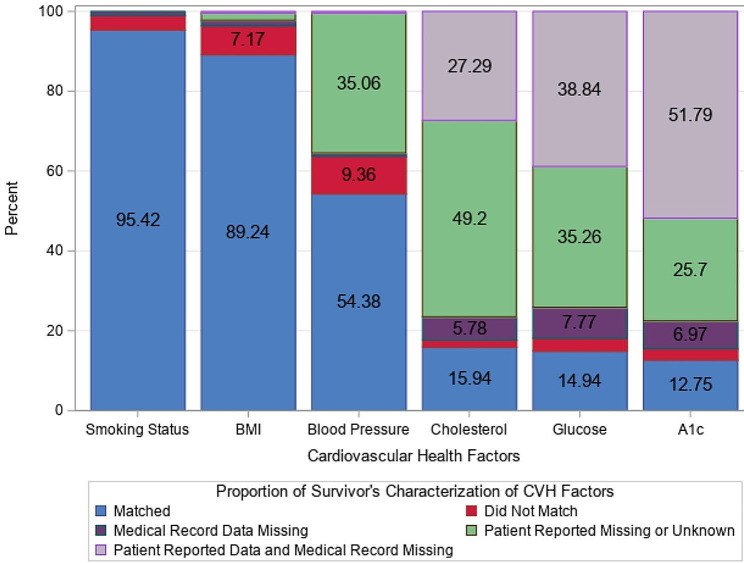
Objective 2: CVH concordance between survivor self-report and EHR
Most participants (> 80%) had blood pressure, smoking status, and BMI values reported from the same day as their designated clinic visit (median number of days between appointment day and reported day = 0 for each measure); the median number of days between the designated clinic visit and the test reported day was longer for cholesterol (median = 205 days), glucose (median = 121 days), and HbA1c (median = 173 days), which are less frequently measured. The proportion of survivors with self-report and EHR concordance for categorization varied across CVH factors assessed (Fig. 2). There was concordance for most survivors with smoking status (95.4%, n = 479) and BMI (89.2%, n = 448); 54.4% (n = 273) had concordance for BP. Concordance rates were low for cholesterol (15.9%, n = 80), glucose (14.9%, n = 75), and HbA1c (12.8%, n = 64) primarily because self-reported categorization was unknown and/or values were missing in the EHR. Many survivors reported that they were unaware of their BP (35.1%, n = 176), cholesterol (49.2%, n = 247), glucose (35.3%, n = 177), and HbA1c (25.7%, n = 129). Risk category was unknown (survivor reported “don’t know” and missing from EHR) for a substantial number of survivors for cholesterol (27.3%, n = 137), glucose (38.8%, n = 195), and HbA1c (51.8%, n = 260).
Fig. 2.
Concordance of survivor cardiovascular factors from self-report and EHR (N = 502). American heart association simple 7 cardiovascular health factors were classified according to the 2011 framework [30]. These five components were self-reported prior to a routine post-treatment oncology visit and abstracted from the electronic health record at the same visit. BMI = body mass index, AIC = hemoglobin A1c
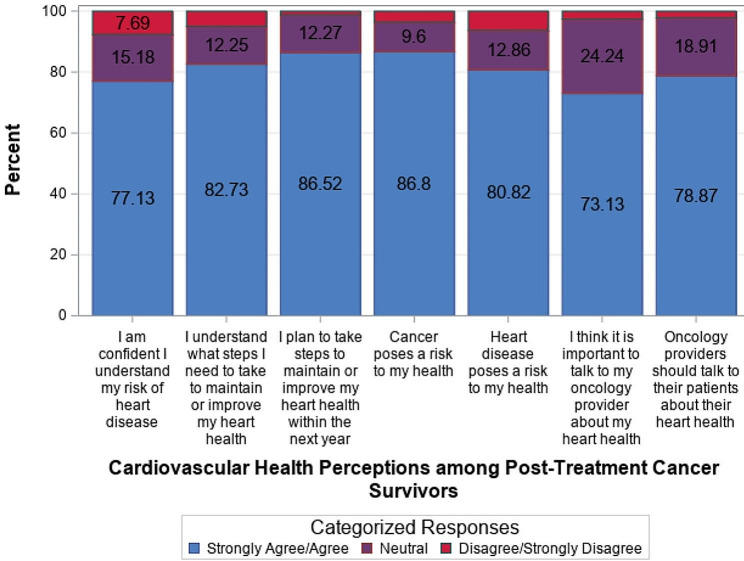
Objective 3: cardiovascular health perceptions among post-treatment cancer survivors
The majority of survivors strongly agreed/ agreed with each of the cardiovascular health perception items ranging from 73.1% (n = 362) (important to talk to my oncology provider about heart health) to 86.8% (n = 434) (cancer poses a risk to my health) (Fig. 3). Few survivors (1.2 − 7.7%, n = 6–38) strongly disagreed/disagreed with the cardiovascular health perception items.
Fig. 3.
Cardiovascular health perceptions among post-treatment cancer survivors (N = 502)
Discussion
There was considerable burden from cardiovascular comorbidities and risk factors among the post-treatment cancer survivors treated in community settings enrolled in this study. Nearly one third received at least one cancer treatment with high cardiotoxicity potential, and a large majority received a treatment with at least some cardiotoxicity potential. Although most survivors had an ideal smoking status, many had intermediate or poor levels for the other AHA Simple 7 cardiovascular factors. Many survivors correctly self-reported smoking andBMI status; however, most survivors had BMI values in the intermediate or poor level. Several of the CVH factors (e.g., cholesterol, glucose, HbA1c) were not known among survivors and not reported in the EHR. For the relatively small proportion of survivors (< 10% for each CVH factor) whose EHR values and self-reported characterization did not match, self-reported values were generally better than those documented in the EHR (ranging from 50% for HbA1c to 88.9% for cholesterol). Survivors and clinicians cannot take appropriate action if they are unaware of or underestimate cardiovascular health risk factors. These results mirror other studies which show similar rates of cardiovascular comorbidities [6, 33], and highlight the importance of clinical practice guidelines recommending cardiovascular health assessment as part of routine follow-up care for cancer survivors [27, 28]. We also found that most survivors were motivated to improve their CVH, perceived that there are risks posed by cancer and heart disease, and agreed that oncology providers should talk to patients about their CVH. This suggests survivors will be receptive to efforts to implement CVH assessment and management into their oncology care.
Very few cancer survivors in this sample and others [38] report ideal CVH, suggesting that most survivors routinely seen for follow-up care could benefit from CVH assessment and education, with potential long-term improvements in survival and risk of CV disease [39–42]. An emerging consensus suggests optimal management of a cancer survivor should be done collaboratively using a team approach between oncologists, primary care providers, and cardiologists, among others [43–45]. Survivors want oncologists to address their heart health. While oncologists are aware of the cardiotoxicity profile of cancer treatments, especially those with the highest associated risk, they may not routinely incorporate management of CV risk factors into follow-up care once cancer treatment is completed, assuming this issue will be managed by others such as primary care providers. Nearly 80% of the survivors in our study had a recent primary care visit, suggesting the availability of primary care for primary management of cardiovascular risk. By contrast, only 14% of those in our study received care from a cardiologist in the prior 6 months.
Regardless of whether they were seen by primary care or cardiology, many survivors were unaware of key CVH factors, suggesting an opportunity for education and brief intervention. Innovative tools, such as the one being tested in the current study, can improve the implementation of clinical practice guidelines calling for a CVH assessment in the oncology setting. Work-flow compatible strategies for collecting information from patients about CVH factors not routinely available from the EHR (i.e., physical activity & diet) are needed. Other CVH factors were often missing in the EHR (i.e., cholesterol and glucose/HbA1c), and survivors frequently reported not knowing their values for these same variables. Patient’s CVH cannot be effectively assessed, and their risk factors appropriately managed, without these important data elements. Public health campaigns such as the AHA’s “Know Your Numbers” [46] may be valuable for cancer survivors, as would targeted efforts to fill in missing test data by primary care and oncology providers.
There were numerous strengths to this study, including a large sample of survivors seen in outpatient community oncology practice. We included many cancer types to reflect the variety of providers seen for long-term follow-up care and recruited a sample diverse with respect to age. We also had staff on site at community practices to abstract medical information from the EHR to improve data quality and completeness. Specific limitations of the study are discussed below. During our funding period, the AHA also updated its framework for CVH by adding sleep as the eighth modifiable risk factor to Life’s Simple 7, creating Life’s Essential 8 [47]. Although this addition does not affect the results of the present analysis [48], we plan to prospectively collect sleep data (average hours per night) at later time points, providing information which can inform routine assessment and management of sleep health, an understudied issue in survivorship care [49].
Our baseline data demonstrate a high prevalence of multiple cardiovascular comorbidities among cancer survivors, as well as the desire to discuss CVH with their oncologist. The AH-HA platform delivered through the EHR is one promising strategy to facilitate the delivery of guideline concordant cardiovascular health assessment and discussion in outpatient oncology care. Such efforts to combine health information technology tools and cancer care delivery implementation approaches are needed to improve cancer survivors‘ morbidity and mortality from both CV disease and cancer.
Limitations
Although many cancer types were eligible for our study, breast cancer cases predominated, which may reflect the specialty of enrolling providers and the community medical oncology context, as well as the established role of breast cancer survivorship programs in community practices. Nevertheless, future research should seek to extend these findings to survivors of other prevalent cancer types (e.g., colorectal, lung, prostate). Due to the pragmatic nature of our trial, we also did not have data available about specific doses of chemotherapy agents and/or precise targets for radiation therapy, additional factors which may impact CVH.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Acknowledgements
We would like to acknowledge the following NCI Community Oncology Research Program (NCORP) Sites for their participation: Cancer Research for the Ozarks NCORP, Baptist Memorial Health Care/Mid-South Minority Underserved NCORP, Geisinger Cancer Institute NCORP, Iowa-Wide Oncology Research Coalition NCORP, Wisconsin NCORP, and VCU Massey Cancer Center Minority Underserved NCORP. We would also like to thank Wake Forest NCORP Research Base staff members Karen Craver, Jessica Sheedy, William Stanfield, and Cheyenne Wagi.
Abbreviations
- AH-HA
Automated Heart-Health Assessment
- BP
Blood pressure
- cIRB
NCI Central Institutional Review Board
- CV
Cardiovascular
- CVH
Cardiovascular health
- EHR
Electronic health record
- HbA1c
Hemoglobin A1c
- NCCN Guidelines
NCCN Clinical Practice Guidelines in Oncology
- NCORP
NCI Community Oncology Research Program
- WF NCORP
Wake Forest NCI Community Oncology Research Program
Author contributions
Conception and/or design of the work: KW, ED, HK, SL BW, WH, RF. Acquisition, analysis, and/or interpretation of data: KW, ED, SS, CN, HK, SL BW, WH, RF. Drafted the work and/or substantively revised it: KW, ED, SS, CN, HK, SL BW, WH, JD, SP, RF. All authors have approved the submitted version of the manuscript and have agreed to be both personally accountable for the author’s own contributions and to ensure that questions related to the accuracy or integrity of any part of the work.
Funding
Support for this study was provided by National Cancer Institute grants: R01CA226078 and 5UG1CA189824. The NCI reviewed this manuscript prior to submission for adherence to NCORP guidelines.
Data availability
The datasets used and/or analysed during the current study are available from the corresponding author on reasonable request.
Declarations
Ethics approval and consent to participate
The study was approved by the NCI Central Institutional Review Board (cIRB), the Wake IRB Coordinating Center number for AH-HA is IRB00056774. All participants provided informed consent prior to participation.
Consent for publication
N/A.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Shelburne N, Adhikari B, Brell J, Davis M, Desvigne-Nickens P, Freedman A et al. Cancer treatment–related cardiotoxicity: current state of knowledge and future research priorities. J Natl Cancer Inst. 2014;106(9). [DOI] [PMC free article] [PubMed]
- 2.Lajous M, Mozaffarian D, Mozaffarian R, Schrag D, Adami HO. Lifestyle prescriptions for cancer survivors and their communities. J Intern Med. 2011;269(1):88–93. doi: 10.1111/j.1365-2796.2010.02273.x. [DOI] [PubMed] [Google Scholar]
- 3.Patnaik JL, Byers T, DiGuiseppi C, Dabelea D, Denberg TD. Cardiovascular disease competes with breast cancer as the leading cause of death for older females diagnosed with breast cancer: a retrospective cohort study. Breast Cancer Res. 2011;13(3):R64. doi: 10.1186/bcr2901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.van Erning FN, van Steenbergen LN, Lemmens VEPP, Rutten HJT, Martijn H, van Spronsen DJ, et al. Conditional survival for long-term colorectal cancer survivors in the Netherlands: who do best? Eur J Cancer. 2014;50(10):1731–9. doi: 10.1016/j.ejca.2014.04.009. [DOI] [PubMed] [Google Scholar]
- 5.Ward KK, Shah NR, Saenz CC, McHale MT, Alvarez EA, Plaxe SC. Cardiovascular disease is the leading cause of death among endometrial cancer patients. Gynecol Oncol. 2012;126(2):176–9. doi: 10.1016/j.ygyno.2012.04.013. [DOI] [PubMed] [Google Scholar]
- 6.Stoltzfus KC, Zhang Y, Sturgeon K, Sinoway LI, Trifiletti DM, Chinchilli VM, et al. Fatal heart disease among cancer patients. Nat Commun. 2020;11(1):2011. doi: 10.1038/s41467-020-15639-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sturgeon KM, Deng L, Bluethmann SM, Zhou S, Trifiletti DM, Jiang C, et al. A population-based study of cardiovascular disease mortality risk in US cancer patients. Eur Heart J. 2019;40(48):3889–97. doi: 10.1093/eurheartj/ehz766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Florido R, Daya NR, Ndumele CE, Koton S, Russell SD, Prizment A, et al. Cardiovascular Disease Risk among Cancer survivors: the Atherosclerosis Risk in communities (ARIC) Study. J Am Coll Cardiol. 2022;80(1):22–32. doi: 10.1016/j.jacc.2022.04.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Coughlin SS, Datta B, Guha A, Wang X, Weintraub NL. Cardiovascular conditions and obesity among gynecologic cancer survivors: results from the 2020 behavioral risk factor surveillance system survey. Gynecol Oncol. 2022;165(3):405–9. doi: 10.1016/j.ygyno.2022.03.025. [DOI] [PubMed] [Google Scholar]
- 10.Underwood J, Townsend J, Stewart S, Buchannan N, Ekwueme D, Hawkins N, et al. Surveillance of Demographic Characteristics and Health behaviors among Adult Cancer survivors — behavioral risk factor Surveillance System, United States, 2009. MMWR Surveill Summ. 2012;61(1):1–23. [PubMed] [Google Scholar]
- 11.Hershman DL, Till C, Shen S, Wright JD, Ramsey SD, Barlow WE, et al. Association of Cardiovascular Risk factors with cardiac events and survival outcomes among patients with breast Cancer enrolled in SWOG clinical trials. J Clin Oncol. 2018;36(26):2710–7. doi: 10.1200/JCO.2017.77.4414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Rubens M, Appunni S, Ramamoorthy V, Saxena A, Das S, Bhatt C, et al. Prevalence of Cardiovascular Risk factors among Cancer patients in the United States. Metab Syndr Relat Disord. 2019;17(8):397–405. doi: 10.1089/met.2018.0137. [DOI] [PubMed] [Google Scholar]
- 13.Foraker RE, Shoben AB, Kelley MM, Lai AM, Lopetegui MA, Jackson RD, et al. Electronic health record-based assessment of cardiovascular health: the stroke prevention in healthcare delivery environments (SPHERE) study. Prev Med Rep. 2016;4:303–8. doi: 10.1016/j.pmedr.2016.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Folsom AR, Yatsuya H, Nettleton JA, Lutsey PL, Cushman M, Rosamond WD. Community Prevalence of Ideal Cardiovascular Health, by the American Heart Association Definition, and Relationship with Cardiovascular Disease incidence. J Am Coll Cardiol. 2011;57(16):1690–6. doi: 10.1016/j.jacc.2010.11.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Rasmussen-Torvik LJ, Shay CM, Abramson JG, Friedrich CA, Nettleton JA, Prizment AE, et al. Ideal Cardiovascular Health is inversely Associated With Incident Cancer: the atherosclerosis risk in communities Study. Circulation. 2013;127(12):1270–5. doi: 10.1161/CIRCULATIONAHA.112.001183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kulshreshtha A, Vaccarino V, Judd SE, Howard VJ, McClellan WM, Muntner P, et al. Life’s simple 7 and risk of Incident Stroke: the reasons for Geographic and racial differences in Stroke Study. Stroke. 2013;44(7):1909–14. doi: 10.1161/STROKEAHA.111.000352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hurt RD, Ebbert JO, Hays JT, McFadden DD. Preventing Lung Cancer by treating Tobacco Dependence. Clin Chest Med. 2011;32(4):645–57. doi: 10.1016/j.ccm.2011.08.004. [DOI] [PubMed] [Google Scholar]
- 18.Byers T, Sedjo RL. Does intentional weight loss reduce cancer risk? Diabetes. Obes Metabolism. 2011;13(12):1063–72. doi: 10.1111/j.1463-1326.2011.01464.x. [DOI] [PubMed] [Google Scholar]
- 19.Thomson C, Rock C, Thompson P, Caan B, Cussler E, Flatt S, et al. Vegetable intake is associated with reduced breast cancer recurrence in tamoxifen users: a secondary analysis from the women’s healthy eating and living study. Breast Cancer Res Treat. 2011;125(2):519–27. doi: 10.1007/s10549-010-1014-9. [DOI] [PubMed] [Google Scholar]
- 20.Oeffinger KC, Tonorezos ES. The cancer is over. now what? Cancer. 2011;117(S10):2250–7. doi: 10.1002/cncr.26051. [DOI] [PubMed] [Google Scholar]
- 21.Davies NJ, Batehup L, Thomas R. The role of diet and physical activity in breast, colorectal, and prostate cancer survivorship: a review of the literature. Br J Cancer. 2011;105(S1):52–S73. doi: 10.1038/bjc.2011.423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lau ES, Paniagua SM, Liu E, Jovani M, Li SX, Takvorian K, et al. Cardiovascular Risk factors are Associated with Future Cancer. JACC CardioOncol. 2021;3(1):48–58. doi: 10.1016/j.jaccao.2020.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Duprez DA, Otvos J, Sanchez OA, Mackey RH, Tracy R, Jacobs DR. Comparison of the predictive value of GlycA and other biomarkers of inflammation for total death, Incident Cardiovascular events, Noncardiovascular and Noncancer Inflammatory-related events, and total Cancer events. Clin Chem. 2016;62(7):1020–31. doi: 10.1373/clinchem.2016.255828. [DOI] [PubMed] [Google Scholar]
- 24.Kroenke CH, Chen WY, Rosner B, Holmes MD, Weight Weight gain, and Survival after breast Cancer diagnosis. J Clin Oncol. 2005;23(7):1370–8. doi: 10.1200/JCO.2005.01.079. [DOI] [PubMed] [Google Scholar]
- 25.Caan BJ, Kwan ML, Shu XO, Pierce JP, Patterson RE, Nechuta SJ, et al. Weight change and survival after breast Cancer in the after breast Cancer Pooling Project. Cancer Epidemiol Biomarkers Prevention: Publication Am Association Cancer Res Cosponsored Am Soc Prev Oncol. 2012;21(8):1260–71. doi: 10.1158/1055-9965.EPI-12-0306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Jones LW, Haykowsky MJ, Swartz JJ, Douglas PS, Mackey JR. Early breast Cancer Therapy and Cardiovascular Injury. J Am Coll Cardiol. 2007;50(15):1435–41. doi: 10.1016/j.jacc.2007.06.037. [DOI] [PubMed] [Google Scholar]
- 27.Denlinger CS, Sanft T, Moslehi JJ, Overholser L, Armenian S, Baker KS, et al. NCCN guidelines insights: Survivorship, Version 2.2020. J Natl Compr Canc Netw. 2020;18(8):1016–23. doi: 10.6004/jnccn.2020.0037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Armenian SH, Lacchetti C, Barac A, Carver J, Constine LS, Denduluri N, et al. Prevention and Monitoring of Cardiac Dysfunction in survivors of adult cancers: American Society of Clinical Oncology Clinical Practice Guideline. J Clin Oncol. 2017;35(8):893–911. doi: 10.1200/JCO.2016.70.5400. [DOI] [PubMed] [Google Scholar]
- 29.Weaver KE, Klepin HD, Wells BJ, Dressler EV, Winkfield KM, Lamar ZS, et al. Cardiovascular Assessment Tool for breast Cancer survivors and Oncology providers: Usability Study. JMIR Cancer. 2021;7(1):e18396. doi: 10.2196/18396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sacco RL. The new American Heart Association 2020 goal: achieving ideal cardiovascular health. J Cardiovasc Med (Hagerstown) 2011;12(4):255–7. doi: 10.2459/JCM.0b013e328343e986. [DOI] [PubMed] [Google Scholar]
- 31.About NCORP. 2023 [Available from: https://ncorp.cancer.gov/about/
- 32.Foraker R, Shoben AB, Lopetegui MA, Lai AM, Payne PR, Kelley M, Roth C, Tindle H, Schreiner A, Jackson RD. Assessment of Life’s simple 7 in the primary care setting: the Stroke Prevention in Healthcare Delivery EnviRonmEnts (SPHERE) study. Contemp Clin Trials. 2014;38(2):182–9. doi: 10.1016/j.cct.2014.03.007. [DOI] [PubMed] [Google Scholar]
- 33.Foraker RE, Kite B, Kelley MM, Lai AM, Roth C, Lopetegui MA, et al. EHR-based visualization Tool: Adoption Rates, satisfaction, and patient outcomes. eGEMs. 2015;3(2):1159. doi: 10.13063/2327-9214.1159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Foraker RE, Davidson EC, Dressler EV, Wells BJ, Lee SC, Klepin HD, et al. Addressing cancer survivors’ cardiovascular health using the automated heart health assessment (AH-HA) EHR tool: initial protocol and modifications to address COVID-19 challenges. Contemp Clin Trials Commun. 2021;22:100808. doi: 10.1016/j.conctc.2021.100808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lloyd-Jones DM, Hong Y, Labarthe D, Mozaffarian D, Appel LJ, Van Horn L, et al. Defining and setting national goals for cardiovascular health promotion and disease reduction: the American Heart Association’s strategic impact goal through 2020 and beyond. Circulation. 2010;121(4):586–613. doi: 10.1161/CIRCULATIONAHA.109.192703. [DOI] [PubMed] [Google Scholar]
- 36.Herrmann J. Adverse cardiac effects of cancer therapies: cardiotoxicity and arrhythmia. Nat Rev Cardiol. 2020;17(8):474–502. doi: 10.1038/s41569-020-0348-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Administration HRS. Federal Office of Rural Health Policy (FORHP) Data Files 2022 [Available from: https://www.hrsa.gov/rural-health/about-us/what-is-rural/data-files
- 38.Byers T, Patnaik JL. Missed opportunities for chronic disease prevention after breast cancer. Women’s Health. 2011;7(6):619–21. doi: 10.2217/whe.11.66. [DOI] [PubMed] [Google Scholar]
- 39.de Moor JS, Mariotto AB, Parry C, Alfano CM, Padgett L, Kent EE, et al. Cancer survivors in the United States: prevalence across the survivorship trajectory and implications for care. Cancer Epidemiol Biomarkers Prev. 2013;22(4):561–70. doi: 10.1158/1055-9965.EPI-12-1356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Daher I, Daigle T, Bhatia N, Durand J. The prevention of cardiovascular disease in cancer survivors. Tex Heart Inst J. 2012;39(2):190–8. [PMC free article] [PubMed] [Google Scholar]
- 41.Singla A, Kumar G, Bardia A. Personalizing cardiovascular disease prevention among breast cancer survivors. Curr Opin Cardiol. 2012;27(5):515–24. doi: 10.1097/HCO.0b013e3283570040. [DOI] [PubMed] [Google Scholar]
- 42.Haque R, Prout M, Geider A, Kamineni A, Thwin S, Avila C, et al. Comorbidities and Cardiovascular Disease Risk in older breast Cancer survivors. Am J Manag Care. 2014;20(1):86–92. [PMC free article] [PubMed] [Google Scholar]
- 43.Nekhlyudov L, Mollica MA, Jacobsen PB, Mayer DK, Shulman LN, Geiger AM. Developing a quality of Cancer Survivorship Care Framework: implications for Clinical Care, Research, and policy. J Natl Cancer Inst. 2019;111(11):1120–30. doi: 10.1093/jnci/djz089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Halpern MT, Viswanathan M, Evans TS, Birken SA, Basch E, Mayer DK. Models of Cancer Survivorship Care: overview and Summary of current evidence. J Oncol Pract. 2015;11(1):e19–27. doi: 10.1200/JOP.2014.001403. [DOI] [PubMed] [Google Scholar]
- 45.Jefford M, Howell D, Li Q, Lisy K, Maher J, Alfano CM, et al. Improved models of care for cancer survivors. Lancet. 2022;399(10334):1551–60. doi: 10.1016/S0140-6736(22)00306-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.American Heart Association. Know Your Numbers 2020 Available from: https://www.goredforwomen.org/en/know-your-risk/know-your-numbers
- 47.Lloyd-Jones DM, Allen NB, Anderson CAM, Black T, Brewer LC, Foraker RE, et al. Life’s essential 8: updating and enhancing the American Heart Association’s construct of Cardiovascular Health: a Presidential Advisory from the American Heart Association. Circulation. 2022;146(5):e18–e43. doi: 10.1161/CIR.0000000000001078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Lloyd-Jones DM, Ning H, Labarthe D, Brewer L, Sharma G, Rosamond W, et al. Status of Cardiovascular Health in US adults and Children Using the American Heart Association’s New Life’s essential 8 Metrics: Prevalence Estimates from the National Health and Nutrition Examination Survey (NHANES), 2013 through 2018. Circulation. 2022;146(11):822–35. doi: 10.1161/CIRCULATIONAHA.122.060911. [DOI] [PubMed] [Google Scholar]
- 49.Zhou ES, Partridge AH, Syrjala KL, Michaud AL, Recklitis CJ. Evaluation and treatment of insomnia in adult cancer survivorship programs. J Cancer Surviv. 2017;11(1):74–9. doi: 10.1007/s11764-016-0564-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The datasets used and/or analysed during the current study are available from the corresponding author on reasonable request.