ABSTRACT
Background:
The prevalence of oral diseases has been increasing alarmingly in the state of Kerala. Screening for periodontal disease (PD) is crucial due to its negative impact on oral and overall health. Since the occurrence and severity of PD depend on its risk factors, a structured survey in randomly selected districts in the state can be a valuable tool for policymakers to envisage strategies to enhance oral health care and control shared systemic illnesses. Data on the prevalence and risk factors of PD among the residents of the Thiruvananthapuram district of Kerala is not currently available in the public domain. This data could also be representative of the other 13 districts with more or less similar topographical, cultural, and lifestyle characteristics.
Aim:
To study the prevalence of PD and its risk factors among the residents of the Thiruvananthapuram district of Kerala and to compare the urban–rural differences.
Materials and Methods:
In this community-based cross-sectional study, a multistage cluster random sampling method was used to select the participants. Among the 1285 participants, 560 were from urban areas, and 725 were from rural areas. A modification of the Ramfjord PD index was used to assess periodontal health. The epidemiological risk factors were evaluated using sociodemographic data, personal histories, and physical and biochemical parameters. Multivariate logistic regression was used to determine the relationship of PD with independent variables. Mediation analysis was performed to examine the mediating effects of independent factors.
Results:
The rural population (61.4%) had a higher frequency of PD than the urban (35.5%) and an overall prevalence of 50%. Aging, poor oral hygiene, and low educational level (EL) were significant risk factors for PD in urban and rural settings, with hypertension only being significant in the latter. A higher odds ratio (9.07–29.68) with a confidence interval of (5.45–48.94) for poor oral hygiene was noted. Poor oral hygiene and tobacco use had mediating effects between low EL and PD.
Conclusions:
In this study, the overall prevalence of PD was 50%, with the rural population being more afflicted. Poor oral hygiene has been identified as a modifiable risk factor for PD in urban and rural populations. Poor oral hygiene and tobacco use have been demonstrated to be mediators of the strong link between low EL and PD. Therefore, this study reiterates the need for better oral health awareness and treatment facilities to minimize the impact of the above risk factors on the periodontium. A shared risk relationship between PD and hypertension in the rural population emphasizes the need for an integrated approach to public health by including oral health as part of noncommunicable disease prevention and intervention programs.
Keywords: Mediators, periodontal disease, prevalence, risk factors, rural, urban
INTRODUCTION
Oral diseases are a group of noncommunicable diseases (NCDs) affecting approximately 50% of mankind and creating a considerable disease burden. The majority of these patients reside in middle-income countries such as India.[1] Regardless of income level, oral diseases, including periodontal disease (PD), dental caries, malocclusion, dental fluorosis, and oral cancer, are among the most prevalent diseases.[1,2]
PD is a chronic inflammatory condition affecting the periodontal membrane of the teeth. One billion individuals worldwide suffer from severe periodontitis, according to the 2019 Global Burden of Disease report.[1] A policy statement adopted by the Federation Dentaire Internationale (FDI) general assembly in 2018 approved “Global Periodontal Health” as one of the policy statements and declared PD as a major global disease burden because of its deleterious effects on oral health, close link with general health, and the enormous healthcare costs associated with it.[3] PD is aggravated by various risk factors such as low socioeconomic status (SES), low educational level (EL), lack of physical activity, old age, smoking, stress, alcoholism, obesity, and poor oral hygiene.[4,5,6] Screening for PD and its risk factors is crucial for prevention, as understanding these factors may help prevent occurrence and severity.[7,8]
A few authors[9,10,11] have documented the prevalence and risk factors of PD in the Indian community. The evidence from published studies suggests that PD shares a common risk factor with diseases such as chronic respiratory illness, cardiovascular disease, cancer, diabetes mellitus, and unfavorable pregnancy outcomes, among others,[2,12,13,14] many of which are modifiable.[7,8] By utilizing a common risk factor approach, it is possible to manage both PD and NCDs effectively.[2,12,13,14,15] Therefore, adopting an integrated approach to public health is essential by including oral health as part of NCD prevention and intervention programs. This is true for a state like Kerala, where the prevalence of oral diseases and NCDs has been increasing alarmingly.[2,16] Under these circumstances, updating existing data can be a valuable tool for policymakers to envisage strategies to enhance oral health care in the country. Since the occurrence and severity of PD depend on its risk factors, a structured survey in randomly selected districts in the state could be helpful in understanding its epidemiology. Currently, there is no publicly available data on the various risk factors of PD among the residents of the Thiruvananthapuram district of Kerala. This data could also be representative of the other 13 districts with more or less similar topographical, cultural, and lifestyle characteristics. So, the current study aimed to assess the environmental risk factors for PD that may be modifiable and endeavored to compare the urban–rural differences. The confounding and mediating roles of various risk factors and their interactions were also explored.
MATERIALS AND METHODS
SETTING AND DESIGN
This study was done as a part of a cross-sectional, community-based research conducted by the Cardiological Society of India, Kerala Chapter; Coronary Artery Disease and Its Risk Factors Prevalence Study (CSI Kerala CRP Study). In the parent study, subjects in the 20–79 age range were chosen using a multistage cluster sampling technique from urban and rural regions in Kerala’s southern, central, and northern districts. In the southern region, Thiruvananthapuram district was randomly selected. The KISH technique (WHO STEPS Manual)[17] was used to choose one subject from each household between the ages of 20 and 59, and all individuals aged 60–79 were admitted. Edentulous patients, as well as individuals who were apprehensive about taking part in the study, were excluded. The CSI Kerala CRP study’s design, sample, and methodologies have all been described earlier.[18,19] The present study has been reported based on STROBE guidelines.[20]
ORAL EXAMINATION
The methods of the oral examination and scoring criteria were discussed in detail in an article published previously.[21] Using standardized techniques, the periodontal health condition of participants from the Thiruvananthapuram district of Kerala’s rural and urban areas was assessed. The periodontal health of the teeth and oral hygiene were evaluated using a modification of the Ramfjord PD index. A qualified dental surgeon, aided by a resident, conducted a clinical examination of the oral cavity, including the hard and soft tissues, using a mouth mirror and periodontal probe under sufficient lighting. The six index teeth that were evaluated include the maxillary right first molar, left central incisor, left first premolar, mandibular left first molar, right central incisor, and right first premolar. The mesial, distal, buccal, and lingual aspects of each indexed tooth were examined, and the highest possible score was noted.[21] The periodontal condition of the teeth was assessed using a standard periodontal probe graded in millimeters, which gauged the clinical attachment loss by measuring between the tooth’s cementoenamel junction and the bottom of the gingival sulcus. The gingival and periodontal components of the PD index were assessed. Diagnoses of PD were made in patients with established periodontitis that showed a periodontal attachment loss ranging from ≥3 to >6 mm. PD index scores of 4, 5, and 6 were assigned based on the grading of periodontal attachment loss between ≥3 and >6 mm.[21] Oral hygiene status was assessed based on a modification of the calculus component of the Ramfjords PD index. The calculus score was graded as 1, 2, and 3 based on the amount of supragingival and subgingival calculus on the buccal and lingual surfaces of the six indexed teeth.
ASSESSMENT OF EPIDEMIOLOGICAL RISK FACTORS
The methodologies and descriptions of the epidemiological risk variables were presented in detail in an earlier published work.[18] In the CSI Kerala CRP Study, the urban and rural populations in the southern region were selected from the Thiruvananthapuram district. One ward randomly selected from the 100 wards in the Thiruvananthapuram municipal corporation constituted the urban area. However, randomization was not done in the selection of rural areas due to logistic reasons. One adjoining panchayat of the Thiruvananthapuram city along the coastal line was randomly selected, and the hilly areas were avoided. The participants who were recruited for the CSI Kerala CRP Study in Thiruvananthapuram district also undertook an oral examination and periodontal health assessment. A final sample size difference was noted between the parent study participants (N = 1643) and periodontal health study participants (n = 1285). This is because 358 participants were excluded for edentulousness or declining the intraoral examination. Age (below < or above > 45 years), sex, SES (below the poverty line [BPL]/above poverty line [APL]), EL (< or >10 years of education), tobacco use, sedentary lifestyle, body mass index (BMI) (<25 kg/m2 and ≥25 kg/m2), diabetes mellitus, hypertension, and dyslipidemia were the risk variables studied. Demographic details, personal histories, and physical and biochemical profiles were collected using standard protocols. BMI was computed as weight in kilograms/(height in meters squared); weight was measured using a portable electronic weighing scale in kilograms to the closest 0.5 kg, and height was determined using a wall-mounted stadiometer to the next centimeter.[18] BMI was measured as a continuous variable and divided into two categories: BMI < 25 kg/m2 and BMI ≥ 25 kg/m2. According to the consensus statement, a BMI of 18.0–22.9 kg/m2 was regarded as normal, 23.0–24.9 kg/m2 to be overweight, and ≥25 kg/m2 to be obese.[22] In a seated posture, with the left arm lying on a table at heart level, blood pressure was measured using an electronic device. Blood pressure above 140/90 mmHg and/or existing antihypertensive medication usage were suggestive of hypertension.[18] Fasting glucose levels ≥126 mg/dL and/or ongoing antiglycemic drug usage were indicative of diabetes mellitus. Dyslipidemia was characterized as having any of the following: serum total cholesterol ≥200 mg/dL, serum low-density lipoprotein cholesterol ≥130 mg/dL, serum high-density lipoprotein cholesterol <40 mg/dL in men and <50 mg/dL in women, and serum triglycerides ≥150 mg/dL.[18]
STATISTICAL ANALYSIS
Data were analyzed using IBM SPSS Statistics for Windows, Version 11.0, analytic software (Armonk, NY, USA: IBM Corporation). Categorical data are presented as numbers and percentages. Binary logistic regression was performed to ascertain the link between the dependent variable and other independent factors. The odds ratio (OR) with a 95% confidence interval (CI) was calculated to assess the influence of independent factors on the dependent variable. To identify the differences among the groups, univariate and multivariate logistic regressions were performed. A P-value of <0.05 was considered significant. A mediation analysis was carried out to look into the mediating effects of independent factors on the predictor and outcome variables using the whole set of data. The R package (R Studio version 4.1.1.) mediation was used to conduct a causal mediation analysis. The confounding and mediating effects of independent variables have been determined through logistic regression models and mediation analysis.
RESULTS
BASELINE CHARACTERISTICS OF THE STUDY POPULATION
The study had a total of 1643 participants. Periodontal status was evaluated in 1285 participants who met the selection criteria. Out of all the participants, 560 were from urban areas, and 725 were from rural areas. In the urban region, there were 292 males and 268 women, while in the rural region, there were 227 men and 498 women. Missing data for diabetes (urban—9, rural—8), dyslipidemia (urban—13, rural—7), and poor oral hygiene (urban—4, rural—24) were excluded from the analysis and were managed using the available data for each variable.
The prevalence of PD was 50% (644 out of 1285) among the participants, with higher rates observed in rural areas (61.4%) compared to urban areas (35.5%). Table 1 displays the baseline characteristics and the prevalence of risk factors in rural and urban settings of the population. The analyzed risk factors include age, male sex, poor oral hygiene, low SES, tobacco use, sedentary lifestyle, low EL, hypertension, diabetes mellitus, dyslipidemia, and obesity (BMI ≥ 25). Most of the urban participants were males >45 years old. Poor oral hygiene was more prevalent among the rural population (44%). There was a striking difference in the SES and EL between the two groups. The tobacco use in all its forms was comparable in the two groups with an overall 23%. A higher prevalence of NCD risk factors, such as hypertension, diabetes, obesity, and dyslipidemia, was observed in urban populations. A chi-square test has been performed to compare any significant difference in the prevalence of risk factors between urban and rural areas. There was a significant difference in the prevalence of risk factors among the urban and rural populations. However, tobacco use and sedentary lifestyle failed to demonstrate any significant difference between the two communities.
Table 1.
Baseline characteristics of the study population
| Variables | Urban N = 560 (N and %) | Rural N = 725 (N and %) | Overall N = 1285 (N and %) | P value |
|---|---|---|---|---|
| Age > 45 years | 408 (72.9) | 429 (59.2) | 837 (65.1) | <0.001* |
| Male sex | 292 (52.1) | 227 (31.3) | 519 (40.4) | <0.001* |
| Poor oral hygiene* | 126 (22.7) | 318 (45.4) | 444 (35.3) | <0.001* |
| Low socioeconomic status | 20 (3.6) | 285 (39.3) | 305 (23.7) | <0.001* |
| Tobacco use | 119 (21.3) | 171 (23.6) | 290 (22.6) | 0.321 |
| Sedentary lifestyle | 202 (36.1) | 298 (41.1) | 500 (38.9) | 0.067 |
| Low education level | 86 (15.4) | 442 (61) | 528 (41.1) | <0.001* |
| Hypertension | 258 (46.1) | 234 (32.3) | 492 (38.3) | <0.001* |
| Diabetes* | 192 (34.8) | 181 (25.2) | 373 (29.4) | <0.001* |
| Dyslipidemia* | 182 (33.3) | 157 (21.9) | 339 (26.8) | <0.001* |
| Obesity (BMI ≥25) | 294 (52.5) | 309 (42.6) | 603 (46.9) | <0.001* |
*Missing data for poor oral hygiene (urban—4, rural—24), diabetes (urban—9, rural—8), and dyslipidemia (urban—13, rural—7) were addressed using the available data for each variable for calculating percentages. A chi-square test has been performed to compare any significant difference in the prevalence of risk factors between urban and rural areas
REGRESSION ANALYSIS FOR THE VARIOUS RISK FACTORS CAUSING PD
Table 2 presents ORs from univariate and multivariate logistic regression for PD risk factors in urban populations. The ORs were, for age >45 years, 2.06 (95% CI [1.16–3.67]) and significant (P = 0.014), poor oral hygiene, 9.07 (95% CI [5.45–15.07]) and significant (P < 0.001), and low EL, 2.18 (95% CI [1.19–3.99]) and significant (P = 0.012), respectively, in multiple regression analysis. Although tobacco use, obesity, hypertension, and diabetes were significant in univariate regression analysis, they were no longer significant after correcting for age, poor oral hygiene, and low EL.
Table 2.
Odds ratios by univariate and multivariate logistic regression for various risk factors causing PD in urban population
| Variables | Odds ratio Univariate logistic regression (CI) | P value | Odds ratio Multivariate logistic regression (CI) | P value |
|---|---|---|---|---|
| Age > 45 years | 4.26 (2.62-6.91) | <0.001* | 2.06 (1.16-3.67) | 0.014* |
| Male sex | 1.38 (0.97–1.95) | 0.071 | 1.36 (0.83–2.23) | 0.226 |
| Poor oral hygiene | 10.42 (6.54–16.62) | <0.001* | 9.07 (5.45–15.07) | <0.001* |
| Low socioeconomic status | 0.77 (0.29–2.04) | 0.599 | 0.57 (0.16–2.02) | 0.386 |
| Tobacco use | 1.77 (1.17–2.68) | 0.006* | 1.59 (0.91–2.78) | 0.105 |
| Sedentary lifestyle | 1.32(0.92–1.88) | 0.131 | 1.13 (0.72–1.76) | 0.604 |
| Low education level | 2.86 (1.79-4.57) | <0.001* | 2.18 (1.19-3.99) | 0.012* |
| Hypertension | 1.90 (1.34–2.69) | <0.001* | 1.45 (0.93–2.26) | 0.104 |
| Diabetes | 1.57 (1.09–2.25) | 0.015* | 1.34 (0.86–2.09) | 0.196 |
| Dyslipidemia | 1.32 (0.91–1.91) | 0.140 | 1.31 (0.84–2.04) | 0.236 |
| Obesity (BMI ≥ 25) | 0.66 (0.46–0.93) | 0.017* | 0.74 (0.48–1.13) | 0.166 |
Table 3 displays the ORs resulting from univariate and multivariate logistic regression for different risk factors causing PD in rural populations. The ORs for age >45 years, 5.25 (95% CI [3.27–8.44]) and significant (P < 0.001), poor oral hygiene, 20.48 (95% CI [11.83–35.44]) and significant (P < 0.001), low EL, 1.84 (95% CI [1.13–2.99]) and significant (P = 0.004), and hypertension, 1.93 (95% CI [1.12–3.34]) and significant (P = 0.019) in multivariate regression analysis. Although male sex, tobacco use, sedentary lifestyle, and diabetes were significant in univariate regression analysis, they were not significant after adjusting for age, low EL, poor oral hygiene, and hypertension.
Table 3.
Odds ratios by univariate and multivariate logistic regression for various risk factors causing PD in rural population
| Variables | Odds ratio Univariate logistic regression (CI) | P value | Odds ratio Multivariate logistic regression (CI) | P value |
|---|---|---|---|---|
| Age > 45 years | 11.72 (8.22–16.72) | <0.001* | 5.25 (3.27–8.44) | <0.001* |
| Male sex | 1.46 (1.05–2.03) | 0.025* | 1.63 (0.86–3.10) | 0.135 |
| Poor oral hygiene | 29.68 (18–48.94) | <0.001* | 20.48 (11.83–35.44) | <0.001* |
| Low SES | 1.08 (0.79–1.47) | 0.632 | 0.85 (0.52–1.39) | 0.526 |
| Tobacco use | 1.99 (1.36–2.90) | <0.001* | 1.01 (0.55–1.86) | 0.979 |
| Sedentary lifestyle | 1.85 (1.36–2.53) | <0.001* | 0.72 (0.41–1.28) | 0.264 |
| Low EL | 2.70 (1.98–3.69) | <0.001* | 1.84 (1.13–2.99) | 0.004* |
| Hypertension | 5.22 (3.52–7.73) | <0.001* | 1.93 (1.12–3.34) | 0.019* |
| Diabetes | 2.82 (1.91–4.16) | <0.001* | 1.28 (0.74–2.21) | 0.372 |
| Dyslipidemia | 1.41 (0.97–2.05) | 0.070 | 0.96 (0.56–1.64) | 0.869 |
| Obesity (BMI ≥ 25) | 0.81 (0.60–1.10) | 0.182 | 0.56 (0.45–1.37) | 0.563 |
CAUSAL MEDIATION ANALYSIS
In mediation analysis, [Figure 1] the mediating effect of risk factors on the predictor and outcome variables was examined using the whole data set for both populations together. Low EL was selected as the predictor variable, poor oral hygiene, tobacco use, low SES as mediating risk factors, and PD as the outcome variable. The series of regression coefficients between low EL, poor oral hygiene, and PD were significant: low EL and PD (P < 0.001), low EL and poor oral hygiene (P < 0.001), and poor oral hygiene and PD (P < 0.001). The series of regression coefficients between low EL, tobacco use, and PD were significant: low EL and tobacco use (P < 0.001) and tobacco use and PD (P = 0.002). The series of regression coefficients between low EL, low SES, and PD demonstrated no significance between low SES and PD (P = 0.531), although the regression coefficient between low EL and low SES was significant (P < 0.001).
Figure 1.
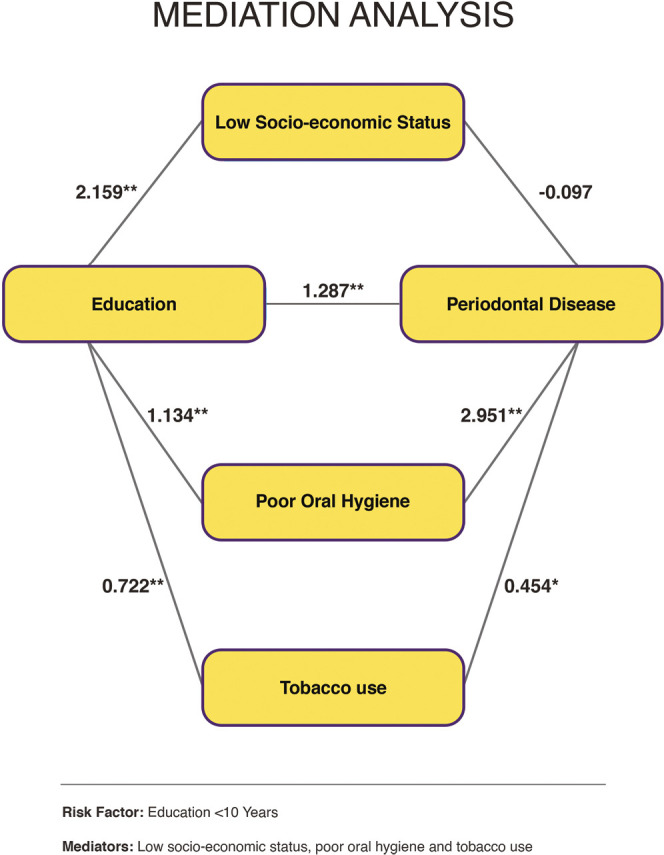
Mediation analysis demonstrating the mediating effect of risk factors
DISCUSSION
The present study compared the various risk factors of PD in the urban and rural populations and found that most of the risk factors were commonly influencing PD outcomes in both populations. Aging, poor oral hygiene, and low EL were significant in both populations as per both multivariate analysis and univariate analysis. Tobacco use, hypertension, and diabetes emerged as additional significant risk factors in both populations as per univariate analysis, whereas its role could not be substantiated in the multivariate analysis except hypertension in rural populations. Poor oral hygiene and tobacco use, according to mediation analysis, mediated the link between low EL and PD.
PD is a chronic inflammatory condition of the periodontium that arises due to the complex interactions between pathogens in the dental plaque and the host immune system, and various risk factors aggravate the disease progression. The estimated global prevalence of PD is approximately 45%, with 11% of patients suffering from severe forms of the disease.[23,24] In the present study, an overall prevalence of 50% was observed, which is in accordance with the global data. Many previous studies have established poor oral hygiene as the most common risk factor for PD.[25,26,27] It can lead to plaque and calculus deposition, leading to gingivitis and the destruction of periodontium. Poor oral hygiene was found to enhance the risk of periodontitis (PD) by five times, according to a published meta-analysis on the topic.[25] The current study’s authors found a much higher OR (9.07–29.68), consistent with an Indian study, where the reported OR was 21.8.[28] Although a high OR for poor oral hygiene was noticed for both populations in univariate and multivariate analysis, it was more discernible in rural populations, emphasizing the need for better oral care services and awareness in rural areas of the state.
Periodontal destruction is enhanced with aging due to the incremental effects of long-term exposure to periodontal pathogens, sustained host immune response, genetic predisposition, inadequate dietary intake and absorption, and diminutive dexterity to remove dental plaque or systemic illnesses that are detrimental to periodontal health, among many other causes.[23,29,30,31,32,33] A recent systematic review and meta-analysis also reported age as a relevant confounding variable for periodontitis, and our results are according to the literature that has been reported.[34] In the present study, age >45 years was a significant risk factor in rural and urban populations, even after controlling the confounding factors. According to a recent study, periodontal health conditions deteriorated with increasing age up to 60 years, after which it remained mostly unchanged.[35] In this study, the prevalence of periodontitis above the age of 60 years was not evaluated, which could be done in future studies.
Various studies have reported low EL as a risk factor for PD, similar to the observation in this study.[6,36] A meta-analysis by Boillot et al.[6] examined education as a covariate of chronic periodontitis and found that people with low education had an increased risk of PD. Plausible mechanisms include a lack of self-prestige, low SES, low income resulting in higher stress, lack of motivation and drive to maintain the full complement of teeth for a longer span, poor oral hygiene due to ineffective and unreliable methods of plaque removal, incompetency to perceive the pivotal role of dentition and periodontium toward general well-being, inability to understand the importance of regular dental visits, increased tobacco use and the practice of other deleterious habits that may hasten periodontal breakdown, to name a few. The mediating effect of factors like low SES, poor oral hygiene, and tobacco use on low EL and PD was examined further using a mediation analysis. Low SES did not show any mediation between low EL and PD, whereas poor oral hygiene and tobacco use had mediating effects. Thus, the link between low EL and PD was found to be mediated through poor oral hygiene and tobacco use [Figure 1]. This also implied that low EL was not predominantly confounded by poor access to regular professional dental care. Since low EL per se is difficult to modify, interventions could be targeted toward its mediating risk factors. This study is the first of its type to explain how mediating variables contribute to a strong link between low EL and PD.
Tobacco use and diabetes have also been recognized as well-established periodontitis risk factors.[7,8,37,38,39,40,41,42] However, in the present study, multivariate analysis failed to find any connection between tobacco use or diabetes mellitus and periodontitis in any of the groups examined. This could be attributed to various extrinsic or intrinsic factors such as lifestyle, culture, social, ethnic, demographic, and genetic factors, which might have mitigated the harmful effects of the above-mentioned risk factors on periodontal health in the selected cohorts. Alternatively, it could be a result of failure to identify unknown confounders that may influence the link between these risk factors and periodontitis. In this study, failure to categorize tobacco users as smokers, chewers, or dual users may be another reason, as the quantity and mode of consumption may impact periodontal breakdown.[40,41,42] Interestingly, a few studies have found no significant link between different forms of smokeless tobacco and PD.[41,42] Likewise, diabetes cases were not categorized as newly diagnosed or established, poorly controlled or optimally controlled, which could have acted as confounding factors that weakened the significant relationship.
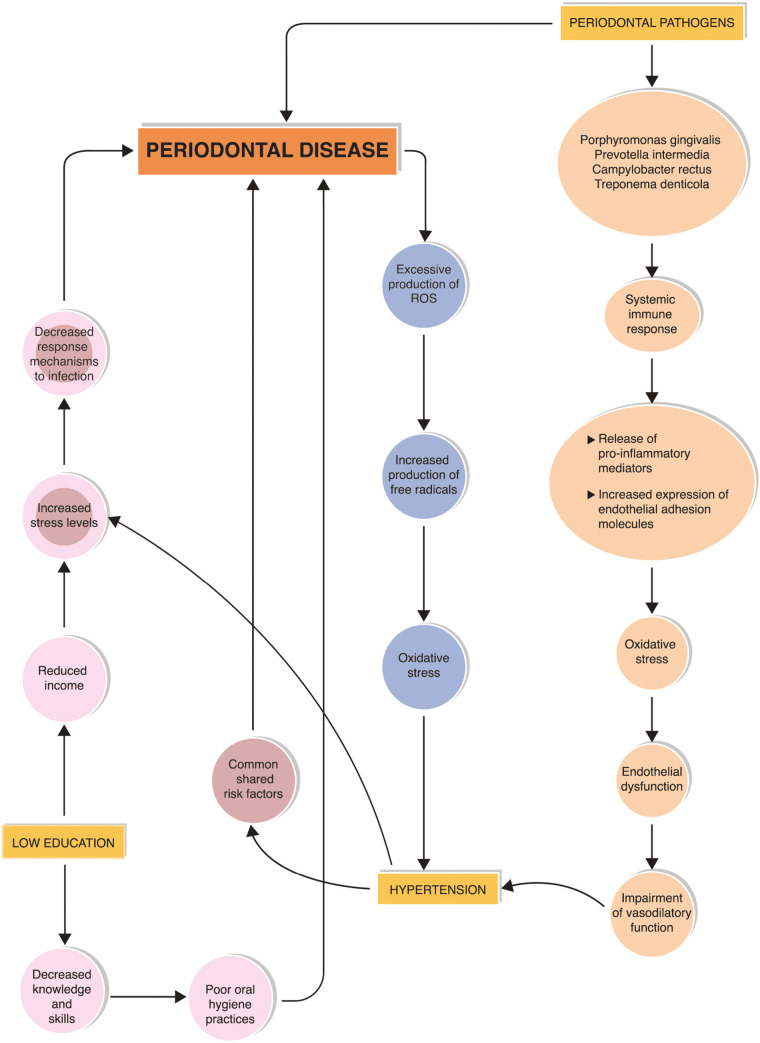
Numerous observational and interventional studies have found a substantial relationship between PD and hypertension.[43,44,45,46,47,48] PD and chronic inflammation induce excessive production of reactive oxygen species and free radicals, oxidative stress, immune response, the release of proinflammatory mediators, increased expression of endothelial adhesion molecules, endothelial dysfunction, and impaired vasodilatory function, which are widely accepted pathophysiological mechanisms explaining its association with hypertension.[49,50,51] Common genetic alleles have been identified with PD and hypertension, which explains the genetic predisposition factors associated with these diseases.[52] Similarly, a relationship between hypertension and PD could be debated based on commonly shared risk factors such as increased stress, elevated cortisol levels, inadequate dietary supplements, altered immune mechanisms, and genetic factors. In the present study, PD was significantly related to hypertension in both populations as per the univariate analysis. But on the contrary, in rural populations, the significance was noticed even in multivariate analysis.
According to recent research, the association between periodontitis and hypertension is not affected by common risk factors such as advanced age, male gender, non-Caucasian ethnicity, smoking, overweight/obesity, diabetes, low socioeconomic level, and inadequate education.[53] Nevertheless, in the present study, the authors presume that low EL and SES, deprivation of resources, and being in an underprivileged area could have added to the innate stress among individuals of this predominantly fishermen community. Dietary intake of sources rich in antioxidants, vitamins, polyphenols, fibers, and nitrates is important for maintaining a healthy periodontium. Vitamins A, C, and E are free radical scavengers that reduce oxidative stress.[49] Green vegetables and fruits are rich in sources of nitrates, polyphenols, fibers, and antioxidants, which help in the modulation of blood pressure, synthesis of collagen, and epithelial cell homeostasis. Commensal bacteria also play a role in the regulation of blood pressure by mediating the production of vasodilators such as nitric oxide from dietary nitrates.[24,54] The substantial relationship that exists between PD and hypertension in rural regions may be attributable to poor daily consumption of fruits and vegetables.
As stress is considered a common denominator for PD and hypertension,[55] its role in the interaction between PD and hypertension needs further exploration. Consistent stress levels in hypertension may lead to sustained release of steroid hormones and decreased response mechanisms to infections. An illustration explains the interrelationship between hypertension, low EL, and PD in [Figure 2].
Figure 2.
Illustration demonstrating the interrelationship between hypertension, low educational level and periodontal disease
Many studies revealed that men are more likely than women to develop PD, assuming poor oral hygiene and less professional care as the cause of plaque and calculus deposition.[56,57] In the present study, although males were more prone to PD in the rural areas, a significant connection could not be found in either population.
The role of obesity as a mediator for PD has been well studied. Many studies have reported increased periodontal pocket depth in patients with a higher BMI.[58,59,60,61,62] The role of proinflammatory adipocytokines released from adipocytes, such as adiponectin,[63] resistin,[64] and leptin,[65] has been proposed as a possible link between PD and obesity. However, there was no significant relationship between PD and obesity in either urban or rural populations in multivariate analysis.
STRENGTH
Only a few studies have compared the various risk factors of PD in urban and rural settings using a cross-sectional community survey. This study is the first of its kind to explain the mediating effects of poor oral hygiene and tobacco use on low EL and PD. A significant relationship between PD and hypertension was observed in this study, thus asserting the importance of a common risk factor approach.
LIMITATIONS
As with any cross-sectional study, causality could not be shown. Some of the risk factors reported as significant in previous studies were not found to be related to PD in this study.
CONCLUSIONS
In this study, the overall prevalence of PD was 50%, with the rural population being more afflicted. Aging, poor oral hygiene, and low EL were significant risk factors for PD in both urban and rural settings, with hypertension only being significant in the latter. Poor oral hygiene and tobacco use have been demonstrated to be mediators of the strong link between low EL and PD. Poor oral hygiene carried the highest risk for PD in both populations. These points to the need to create better oral health awareness and to provide more treatment facilities to minimize the impact of poor oral hygiene on periodontium. The necessity to take an integrated strategy to public health by incorporating oral health as part of NCD preventive and intervention programs is highlighted by a shared risk relationship between PD and hypertension.
CONFLICT OF INTEREST
There is no conflict of interest to declare.
FINANCIAL SUPPORT AND SPONSORSHIP
The study was funded by the Cardiological Society of India (Kerala Chapter). The funding source had no role in the design, data collection, analysis, interpretation, or writing of the article.
AUTHOR CONTRIBUTIONS
Conceptualization: CPD, SG, ZG, and SH. Data curation: CPD, SG, ZG, and SH. Formal Analysis: JVA. Methodology: CPD, SG, ZG, and SH. Funding: ZG. Resources: SG, ZG, and SH. Supervision: SG, ZG, and SH. Software: JVA. Visualization: CPD, SG, ZG, and SH. Writing original draft: CPD and AR. Writing review and editing: CPD, SG, ZG, SH, and AR.
ETHICAL POLICY AND INSTITUTIONAL REVIEW BOARD STATEMENT
The CSI Kerala CRP Study was approved by the Ethics Committee of the Cardiological Society of India (Kerala Chapter). The clearance number for the study (01/CSI/IEC/2011/dated 20/01/2011). All participants provided written informed consent. The methods used adhered to the Helsinki Declaration of 1975, as amended in 2000, and the regional ethics committee’s guidelines on human testing.
PATIENT DECLARATION OF CONSENT
I hereby confirm my willingness to participate in the oral examination and periodontal health assessment survey, which is conducted by the Cardiological Society of India, Kerala Chapter, and is part of the Coronary Artery Disease and Its Risk Factors Prevalence Study (CSI Kerala CRP Study) in Thiruvananthapuram district. I understand that my participation in the research is voluntary, and I am free to withdraw at any time, even after recruitment. I will be informed about any dental conditions or diseases affecting my oral health and will receive guidance on proper medical treatment. I understand that the principal investigator is available to answer my research-related queries at any time. I have read and fully understood the research information above. I hereby sign this document voluntarily and without any duress.
DATA AVAILABILITY STATEMENT
The data supporting this study’s findings are available from the corresponding author, [CPD], upon reasonable request.
ACKNOWLEDGMENT
We thank the office bearers and staff members of the Cardiological Society of India, Kerala Chapter, Dr. N. O. Varghese, former Principal, staff members, and residents, Government Dental College, Thiruvananthapuram, Kerala, India, and for their support in conducting this study. We also thank Mr. Manas Chacko, Former Scientist-B, Sree Chitra Tirunal Institute for Medical Sciences and Technology, Thiruvananthapuram, Kerala, India, for the statistical work. We also thank Mr. Shylendra Nath S, First Stroke, Thiruvananthapuram, for the graphic illustrations.
REFERENCES
- 1.GBD 2019 Diseases and Injuries Collaborators. Global burden of 369 diseases and injuries in 204 countries and territories, 1990–2019: A systematic analysis for the Global Burden of Disease Study 2019. Lancet. 2020;396:1204–22. doi: 10.1016/S0140-6736(20)30925-9. Erratum in: Lancet 2020;396:1562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ramanarayanan V, Janakiram C, Joseph J, Krishnakumar K. Oral health care system analysis: A case study from India. J Family Med Prim Care. 2020;9:1950–7. doi: 10.4103/jfmpc.jfmpc_1191_19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.FDI World Dental Federation. Editorial: The 2018 FDI policy statements. Int Dent J. 2019;69:3–4. doi: 10.1111/idj.12474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Srivastava MC, Srivastava R, Verma PK, Gautam A. Metabolic syndrome and periodontal disease: An overview for physicians. J Family Med Prim Care. 2019;8:3492–5. doi: 10.4103/jfmpc.jfmpc_866_19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sanz M, D’Aiuto F, Deanfield J, Fernandez-Aviles F. European workshop in periodontal health and cardiovascular disease-scientific evidence on the association between periodontal and cardiovascular diseases: A review of the literature. Eur Heart J Suppl. 2010;12:B3–B12. [Google Scholar]
- 6.Boillot A, El Halabi B, Batty GD, Rangé H, Czernichow S, Bouchard P. Education as a predictor of chronic periodontitis: A systematic review with meta-analysis population-based studies. PLoS One. 2011;6:e21508. doi: 10.1371/journal.pone.0021508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Genco RJ, Borgnakke WS. Risk factors for periodontal disease. Periodontol 2000. 2013;62:59–94. doi: 10.1111/j.1600-0757.2012.00457.x. [DOI] [PubMed] [Google Scholar]
- 8.Van Dyke TE, Sheilesh D. Risk factors for periodontitis. J Int Acad Periodontol. 2005;7:3–7. [PMC free article] [PubMed] [Google Scholar]
- 9.Chandra A, Yadav OP, Narula S, Dutta A. Epidemiology of periodontal diseases in Indian population since last decade. J Int Soc Prev Community Dent. 2016;6:91–6. doi: 10.4103/2231-0762.178741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bhat M, Do LG, Roberts-Thomson K. Risk indicators for prevalence, extent and severity of periodontitis among rural Indian population aged 35–54 years. Int J Dent Hyg. 2018;16:492–502. doi: 10.1111/idh.12351. [DOI] [PubMed] [Google Scholar]
- 11.Janakiram C, Mehta A, Venkitachalam R. Prevalence of periodontal disease among adults in India: A systematic review and meta-analysis. J Oral Biol Craniofac Res. 2020;10:800–6. doi: 10.1016/j.jobcr.2020.10.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.FDI World Dental Federation. FDI policy statement on non-communicable diseases adopted by the FDI General Assembly: 30 August 2013—Istanbul, Turkey. Int Dent J. 2013;63:285–6. doi: 10.1111/idj.12078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sheiham A, Watt RG. The common risk factor approach: a rational basis for promoting oral health. Community Dent Oral Epidemiol. 2000;28:399–406. doi: 10.1034/j.1600-0528.2000.028006399.x. [DOI] [PubMed] [Google Scholar]
- 14.Varenne B. Integrating oral health with non-communicable diseases as an essential component of general health: WHO’s strategic orientation for the African Region. J Dent Educ. 2015;79:S32–7. [PubMed] [Google Scholar]
- 15.Janakiram C, Dye BA. A public health approach for prevention of periodontal disease. Periodontol 2000. 2020;84:202–14. doi: 10.1111/prd.12337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sarma PS, Sadanandan R, Thulaseedharan JV, Soman B, Srinivasan K, Varma RP, et al. Prevalence of risk factors of non-communicable diseases in Kerala, India: Results of a cross-sectional study. BMJ Open. 2019;9:e027880. doi: 10.1136/bmjopen-2018-027880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.World Health Organization. WHO STEPS Manual, WHO STEPS Surveillance, Part 2: Planning and set up: Preparing the sample. 2008:2-2-24, 25. [Google Scholar]
- 18.Zachariah G, Harikrishnan S, Krishnan MN, Mohanan PP, Sanjay G, Venugopal K, et al. Prevalence of coronary artery disease and coronary risk factors in Kerala, South India: A population survey-design and methods. Indian Heart J. 2013;65:243–9. doi: 10.1016/j.ihj.2013.04.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Krishnan MN, Zachariah G, Venugopal K, Mohanan PP, Harikrishnan S, Sanjay G, et al. Prevalence of coronary artery disease and its risk factors in Kerala, South India: A community-based cross-sectional study. BMC Cardiovasc Disord. 2016;16:12–23. doi: 10.1186/s12872-016-0189-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.von Elm E, Altman DG, Egger M, Pocock SJ, Gøtzsche PC, Vandenbroucke JP STROBE Initiative. STROBE initiative: Strengthening the reporting of Observational Studies in Epidemiology (STROBE) statement: Guidelines for reporting observational studies. Int J Surg. 2014;12:1495–9. [Google Scholar]
- 21.Dain CP, Ganapathi S, Geevar Z, Harikrishnan S, Ammu JV, Chacko M. The traditional and modifiable risk factors of coronary artery disease—A community-based cross-sectional study among 2 populations. Medicine (Baltimore) 2021;100:e27350. doi: 10.1097/MD.0000000000027350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Misra A, Chowbey P, Makkar BM, Vikram NK, Wasir JS, Chadha D, et al. Concensus Group. Consensus statement for diagnosis of obesity, abdominal obesity and the metabolic syndrome for Asian Indians and recommendations for physical activity, medical and surgical management. J Assoc Physicians India. 2009;57:163–70. [PubMed] [Google Scholar]
- 23.Nazir M, Al-Ansari A, Al-Khalifa A, Alhareky M, Gaffar B, Almas K. Global prevalence of periodontal disease and lack of its surveillance. Sci World J. 2020;2020:2146160. doi: 10.1155/2020/2146160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Surma S, Romańczyk M, Witalińska-Łabuzek J, Czerniuk MR, Łabuzek K, Filipiak KJ. Periodontitis, blood pressure, and the risk and control of arterial hypertension: Epidemiological, clinical, and pathophysiological aspects—Review of the literature and clinical trials. Curr Hypertens Rep. 2021;23:27. doi: 10.1007/s11906-021-01140-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lertpimonchai A, Rattanasiri S, Arj-Ong Vallibhakara S, Attia J, Thakkinstian A. The association between oral hygiene and periodontitis: A systematic review and meta-analysis. Int Dent J. 2017;67:332–43. doi: 10.1111/idj.12317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Asif SM, Naheeda S, Assiri KI, Almubarak HM, Kaleem SM, Zakirulla M, et al. Oral hygiene practice and periodontal status among two tribal population of Telangana state, India—An epidemiological study. BMC Oral Health. 2019;19:8. doi: 10.1186/s12903-018-0705-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kassebaum NJ, Bernabé E, Dahiya M, Bhandari B, Murray CJ, Marcenes W. Global burden of severe periodontitis in 1990–2010: A systematic review and meta-regression. J Dent Res. 2014;93:1045–53. doi: 10.1177/0022034514552491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Mathur LK, Manohar B, Shankarapillai R, Pandya D. Obesity and periodontitis: A clinical study. J Indian Soc Periodontol. 2011;15:240–4. doi: 10.4103/0972-124X.85667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Eke PI, Borgnakke WS, Genco RJ. Recent epidemiologic trends in periodontitis in the USA. Periodontol 2000. 2020;82:257–67. doi: 10.1111/prd.12323. [DOI] [PubMed] [Google Scholar]
- 30.Hugoson A, Laurell L, Lundgren D. Frequency distribution of individuals aged 20–70 years according to severity of periodontal disease experience in 1973 and 1983. J Clin Periodontol. 1992;19:227–32. doi: 10.1111/j.1600-051x.1992.tb00458.x. [DOI] [PubMed] [Google Scholar]
- 31.Mack F, Mojon P, Budtz-Jørgensen E, Kocher T, Splieth C, Schwahn C, et al. Caries and periodontal disease of the elderly in Pomerania, Germany: Results of the study of health in Pomerania. Gerodontology. 2004;21:27–36. doi: 10.1046/j.1741-2358.2003.00001.x. [DOI] [PubMed] [Google Scholar]
- 32.Beck JD, Koch GG, Rozier RG, Tudor GE. Prevalence and risk indicators for periodontal attachment loss in a population of older community-dwelling blacks and whites. J Periodontol. 1990;61:521–8. doi: 10.1902/jop.1990.61.8.521. [DOI] [PubMed] [Google Scholar]
- 33.Locker D, Leake JL. Periodontal attachment loss in independently living older adults in Ontario, Canada. J Public Health Dent. 1993;53:6–11. doi: 10.1111/j.1752-7325.1993.tb02664.x. [DOI] [PubMed] [Google Scholar]
- 34.Trindade D, Carvalho R, Machado V, Chambrone L, Mendes JJ, Botelho J. Prevalence of periodontitis in dentate people between 2011 and 2020: A systematic review and meta-analysis of epidemiological studies. J Clin Periodontol. 2023;50:604–26. doi: 10.1111/jcpe.13769. [DOI] [PubMed] [Google Scholar]
- 35.Chen MX, Zhong YJ, Dong QQ, Wong HM, Wen YF. Global, regional, and national burden of severe periodontitis, 1990–2019: An analysis of the Global Burden of Disease Study 2019. J Clin Periodontol. 2021;48:1165–88. doi: 10.1111/jcpe.13506. [DOI] [PubMed] [Google Scholar]
- 36.Celeste RK, Oliveira SC, Junges R. Threshold-effect of income on periodontitis and interactions with race/ethnicity and education. Rev Bras Epidemiol. 2019;22:e190001. doi: 10.1590/1980-549720190001. [DOI] [PubMed] [Google Scholar]
- 37.Genco RJ, Borgnakke WS. Diabetes as a potential risk for periodontitis: Association studies. Periodontol 2000. 2020;83:40–5. doi: 10.1111/prd.12270. [DOI] [PubMed] [Google Scholar]
- 38.Liccardo D, Cannavo A, Spagnuolo G, Ferrara N, Cittadini A, Rengo C, et al. Periodontal disease: A risk factor for diabetes and cardiovascular disease. Int J Mol Sci. 2019;20:1414. doi: 10.3390/ijms20061414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Darby I. Risk factors for periodontitis and peri-implantitis. Periodontol 2000. 2022;90:9–12. doi: 10.1111/prd.12447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Martinez-Canut P, Lorca A, Magán R. Smoking and periodontal disease severity. J Clin Periodontol. 1995;22:743–9. doi: 10.1111/j.1600-051x.1995.tb00256.x. [DOI] [PubMed] [Google Scholar]
- 41.Chu YH, Tatakis DN, Wee AG. Smokeless tobacco use and periodontal health in a rural male population. J Periodontol. 2010;81:848–54. doi: 10.1902/jop.2010.090310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Goel K, Sharma S, Baral DD, Agrawal SK. Current status of periodontitis and its association with tobacco use amongst adult population of Sunsari district, in Nepal. BMC Oral Health. 2021;21:66. doi: 10.1186/s12903-021-01416-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Rivas-Tumanyan S, Campos M, Zevallos JC, Joshipura KJ. Periodontal disease, hypertension, and blood pressure among older adults in Puerto Rico. J Periodontol. 2013;84:203–11. doi: 10.1902/jop.2012.110748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Muñoz Aguilera E, Suvan J, Buti J, Czesnikiewicz-Guzik M, Barbosa Ribeiro A, Orlandi M, et al. Periodontitis is associated with hypertension: A systematic review and meta-analysis. Cardiovasc Res. 2020;116:28–39. doi: 10.1093/cvr/cvz201. [DOI] [PubMed] [Google Scholar]
- 45.Martin-Cabezas R, Seelam N, Petit C, Agossa K, Gaertner S, Tenenbaum H, et al. Association between periodontitis and arterial hypertension: A systematic review and meta-analysis. Am Heart J. 2016;180:98–112. doi: 10.1016/j.ahj.2016.07.018. [DOI] [PubMed] [Google Scholar]
- 46.Macedo Paizan ML, Vilela-Martin JF. Is there an association between periodontitis and hypertension? Curr Cardiol Rev. 2014;10:355–61. doi: 10.2174/1573403X10666140416094901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Arowojolu MO, Oladapo O, Opeodu OI, Nwhator SO. An evaluation of the possible relationship between chronic periodontitis and hypertension. J West Afr Coll Surg. 2016;6:20–38. [PMC free article] [PubMed] [Google Scholar]
- 48.Pietropaoli D, Del Pinto R, Ferri C, Ortu E, Monaco A. Definition of hypertension-associated oral pathogens in NHANES. J Periodontol. 2019;90:866–76. doi: 10.1002/JPER.19-0046. [DOI] [PubMed] [Google Scholar]
- 49.Najeeb S, Zafar MS, Khurshid Z, Zohaib S, Almas K. The role of nutrition in periodontal health: An update. Nutrients. 2016;8:530. doi: 10.3390/nu8090530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Leong XF, Ng CY, Badiah B, Das S. Association between hypertension and periodontitis: possible mechanisms. ScientificWorldJ. 2014;2014:768237. doi: 10.1155/2014/768237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Harrison DG, Guzik TJ, Lob HE, Madhur MS, Marvar PJ, Thabet SR, et al. Inflammation, immunity, and hypertension. Hypertension. 2011;57:132–40. doi: 10.1161/HYPERTENSIONAHA.110.163576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Czesnikiewicz-Guzik M, Osmenda G, Siedlinski M, Nosalski R, Pelka P, Nowakowski D, et al. Causal association between periodontitis and hypertension: Evidence from Mendelian randomization and a randomized controlled trial of non-surgical periodontal therapy. Eur Heart J. 2019;40:3459–70. doi: 10.1093/eurheartj/ehz646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Del Pinto R, Pietropaoli D, Munoz-Aguilera E, D’Aiuto F, Czesnikiewicz-Guzik M, Monaco A, et al. Periodontitis and hypertension: Is the association causal? High Blood Press Cardiovasc Prev. 2020;27:281–9. doi: 10.1007/s40292-020-00392-z. [DOI] [PubMed] [Google Scholar]
- 54.Pignatelli P, Fabietti G, Ricci A, Piattelli A, Curia MC. How periodontal disease and presence of nitric oxide reducing oral bacteria can affect blood pressure. Int J Mol Sci. 2020;21:7538. doi: 10.3390/ijms21207538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Coelho JMF, Miranda SS, da Cruz SS, Trindade SC, Passos-Soares JS, Cerqueira EMM, et al. Is there association between stress and periodontitis? Clin Oral Investig. 2020;24:2285–94. doi: 10.1007/s00784-019-03083-9. [DOI] [PubMed] [Google Scholar]
- 56.Meisel P, Reifenberger J, Haase R, Nauck M, Bandt C, Kocher T. Women are periodontally healthier than men, but why don’t they have more teeth than men? Menopause. 2008;15:270–5. doi: 10.1097/gme.0b013e31811ece0a. [DOI] [PubMed] [Google Scholar]
- 57.Shiau HJ, Reynolds MA. Sex differences in destructive periodontal disease: A systematic review. J Periodontol. 2010;81:1379–89. doi: 10.1902/jop.2010.100044. [DOI] [PubMed] [Google Scholar]
- 58.Gaio EJ, Haas AN, Rösing CK, Oppermann RV, Albandar JM, Susin C. Effect of obesity on periodontal attachment loss progression: A 5-year population-based prospective study. J Clin Periodontol. 2016;43:557–65. doi: 10.1111/jcpe.12544. [DOI] [PubMed] [Google Scholar]
- 59.Jepsen S, Suvan J, Deschner J. The association of periodontal diseases with metabolic syndrome and obesity. Periodontol 2000. 2020;83:125–53. doi: 10.1111/prd.12326. [DOI] [PubMed] [Google Scholar]
- 60.Khan S, Bettiol S, Kent K, Barnett T, Peres M, Crocombe LA. Obesity and periodontitis in Australian adults: A population-based cross-sectional study. Int Dent J. 2020;70:53–61. doi: 10.1111/idj.12514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Nascimento GG, Leite FR, Do LG, Peres KG, Correa MB, Demarco FF, et al. Is weight gain associated with the incidence of periodontitis? A systematic review and meta-analysis. J Clin Periodontol. 2015;42:495–505. doi: 10.1111/jcpe.12417. [DOI] [PubMed] [Google Scholar]
- 62.Palle AR, Reddy CM, Shankar BS, Gelli V, Sudhakar J, Reddy KK. Association between obesity and chronic periodontitis: A cross-sectional study. J Contemp Dent Pract. 2013;14:68–73. doi: 10.5005/jp-journals-10024-1294. [DOI] [PubMed] [Google Scholar]
- 63.Wang Z, Chen Z, Fang F, Qiu W. The role of adiponectin in periodontitis: Current state and future prospects. Biomed Pharmacother. 2021;37:11358. doi: 10.1016/j.biopha.2021.111358. [DOI] [PubMed] [Google Scholar]
- 64.Kang SK, Park YD, Kang SI, Kim DK, Kang KL, Lee SY, et al. Role of resistin in the inflammatory response induced by nicotine plus lipopolysaccharide in human periodontal ligament cells in vitro. J Periodontal Res. 2015;50:602–13. doi: 10.1111/jre.12240. [DOI] [PubMed] [Google Scholar]
- 65.Chaffee BW, Weston SJ. Association between chronic periodontal disease and obesity: A systematic review and meta-analysis. J Periodontol. 2010;81:1708–24. doi: 10.1902/jop.2010.100321. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data supporting this study’s findings are available from the corresponding author, [CPD], upon reasonable request.