Abstract
Helicobacter pylori colonizes the gastric mucosa, and the infection is related to the development of diverse gastric pathologies, possibly by directly or indirectly affecting epithelial-cell function. We analyzed the influence of the bacteria on transepithelial electrical resistance (TER) on a model tight epithelium, T84, grown to confluence in permeable filters. H. pylori sonicates produced a dramatic decrease in TER after 1 to 2 h of exposure, while sonicates from other bacteria did not induce a significant reduction of TER. The effect induced by sonicates was mimicked by a water-soluble fraction from the bacterial surface, was not reproducible with isolated lipopolysaccharide, and was concomitant with a significant increase in the paracellular permeability of the marker molecule [14C]mannitol. Furthermore, H. pylori sonicates also provoked a significant increase in permeability to [14C]mannitol across rat gastric mucosa in vitro. The sonicate-induced decrease in TER in T84 monolayers was inhibited by the protein kinase C (PKC) activator phorbol myristate acetate. As PKC is directly involved in tight junction regulation, we suggest that H. pylori may induce intracellular signalling events counteracting PKC effects. Following long-term H. pylori stimulation, epithelial monolayers regained baseline resistance values slowly after 24 h. The resistance recovery process was inhibited by cycloheximide, indicating its dependency upon protein synthesis. No association between resistance variation and E-cadherin protein levels was observed. These results indicate that H. pylori alters in vitro the barrier properties of the epithelium, probably by generating cell signalling events counteracting the normal function of PKC. This increased permeability may provide a potential mechanism by which H. pylori antigens can reach the gastric lamina propria, thereby activating the mucosal immune system.
Helicobacter pylori is a bacterial pathogen that infects the stomach, exhibiting specific tropism for human gastric epithelium (18), and can also bind to a large range of epithelial cell types in vitro (9). The bacterium has been identified as the major etiological agent in the development of chronic gastritis and duodenal ulcer (11), and recent studies suggest that it plays a role in the development of gastric carcinoma (44). The infection begins by mucus colonization, followed by attachment of the bacteria to the underlying epithelial cells (9). The mechanisms of tissue damage are still not clear, although several virulence factors have been identified, such as the vacuolating toxin (12), urease (13), and some bacterial adhesins (18). Despite nonspecific protection factors, such as mucus, tight junctions, or acid secretion (40), H. pylori can colonize the stomach and become chronic in the absence of treatment (25). Once located between the mucus and the epithelial layers, H. pylori exerts a pathological influence over the epithelial cells and their environment.
Gastric epithelial cells are tightly joined to each other, forming a continuous barrier that selectively restricts the movement of substances between the external and internal compartments. Any disruption or modification of the epithelial architecture can lead to an alteration of this barrier function. Therefore, the epithelium requires cellular mechanisms to seal the paracellular space, thus preventing the passive diffusion of macromolecules (30). Tight junctions, which are located at the apical poles of epithelial cells, restrict the paracellular flux and are responsible for increasing or decreasing the permeability in the intestine (32), although the permeability properties of the tight junction also depend upon the integrity of the adherens junctions. These junctions are localized immediately adjacent to the tight junction, and their basic constituent is the transmembrane protein E-cadherin, which associates with a number of intracellular proteins, called catenins, that link it with some components of the cytoskeleton (2).
In the present study we have investigated the influence of H. pylori on the permeability of cultured epithelial-cell monolayers. Human gastric epithelial-cell lines that have traditionally been used in adherence studies with H. pylori are highly permeable, as they do not display a tight phenotype in vitro, and therefore they are not suitable for the determination of epithelial-permeability changes. Moreover, it is well known that freshly isolated human gastric epithelial cells do not proliferate in primary cultures and do not form uniform confluent monolayers (41, 47). Hence, the epithelial-cell line T84, originally derived from a colon adenocarcinoma, was chosen for the performance of epithelial-barrier function studies, as it represents a very well established tight epithelium with excellent retention of functional phenotype in cultures in which polarity is maintained (33). Thus, T84 monolayers grown on permeable supports represent a model epithelium in which epithelial performance may be studied experimentally. Moreover, many of the mechanisms involved in tight-junction regulation are shared by different types of epithelium, such as, for example, the dependence of tight-junction permeability on extracellular Ca2+ concentration, whose effect is mediated via the Ca2+-dependent adhesion molecule E-cadherin, present in all epithelia (23, 24). In this study we report the effect of H. pylori on the epithelial-barrier function of T84 monolayers and show preliminary data of the intracellular processes occurring and the bacterial protein fraction involved. We also used isolated rat gastric mucosa to examine the influence of H. pylori on the permeability of a native acid-secreting epithelium from a species which is not colonized by the bacteria under natural conditions (6).
MATERIALS AND METHODS
Bacterium processing.
The following H. pylori strains were used: two reference strains, NCTC 11638 and NCTC 11637 (both VacA+ CagA+), and two clinical isolates kindly provided and typed by W. Dundon (Trinity College, Dublin, Ireland), one from a patient with gastritis (VacA− CagA−) and the other from a patient with a duodenal ulcer (VacA+ CagA−). Bacteria were grown on blood agar for 48 h in a microaerobic and humidified atmosphere at 37°C. Cells were harvested in phosphate-buffered saline (PBS) and centrifuged at 13,000 × g for 10 min at 4°C. The pellet obtained in this way was sonicated on ice by using 6 15-s 100-W pulses, with 30-s cooling intervals. The supernatant was then filtered through a 0.22-μm-pore-size hydrophilic cellulose acetate membrane for sterilization and was stored at −20°C. Haemophilus influenzae (NCTC 11931), Escherichia coli, and Campylobacter jejuni (clinical isolates) were used as controls, and sonicates were obtained as described above. In all experiments, bacterial sonicates were used at a final protein concentration of 15 μg/ml.
Whole-bacterium preparations were obtained by harvesting cells in PBS and were centrifuged at 13,000 × g. The pellet was then stored at −20°C until use. To obtain the soluble surface proteins from H. pylori, bacteria were harvested in Dulbecco’s modified Eagle medium (MEM)–nutrient mix F-12 medium (Gibco Laboratories, Grand Island, N.Y.), vortexed for 10 s, and then centrifuged at 13,000 × g. The supernatant containing the soluble fraction was separated and stored at −20°C. Heat-inactivated H. pylori sonicates were prepared by heating the sonicate preparation at 70°C for 15 min. Purified lipopolysaccharide (LPS) was prepared as previously described (36). All preparations were stored at −20°C until use.
Epithelial-cell cultures.
The epithelial-cell line T84, obtained from the American Type Culture Collection, Manassas, Va., was used in this study. T84 cells, originally derived from a colonic crypt cell carcinoma, were chosen for electrophysiological studies because they represent a very well established tight epithelium with excellent retention of functional phenotype in culture (33).
T84 cells were grown in Dulbecco’s MEM–nutrient mix F-12 medium, supplemented with l-glutamine (2 mM), penicillin (100 U/ml), streptomycin (100 μg/ml), and 10% fetal calf serum (Gibco). They were incubated in flasks at 37°C in a humidified atmosphere of 5% CO2 in air and were split by using trypsin-EDTA solution (Gibco).
TER analysis.
Experiments were carried out as described by Taylor et al. (45). Basically, T84 cells were seeded on semipermeable support membrane cell culture inserts (area, 1.13 cm2; pore diameter, 0.45 μm; Falcon; Becton Dickinson, Paramus, N.J.) at a concentration of 106/filter. Growth of the T84 epithelial monolayers on the semipermeable supports was monitored by measuring transepithelial electrical resistance (TER) with the Endohm apparatus (World Precision Instruments, Sarasota, Fla.) at 24-h intervals. TER increased progressively during approximately 10 to 14 days until confluence, when cells formed high-resistance monolayers with stable TER values of ∼1,400 Ω/cm2 (mean, 1,389 ± 287; n = 24).
Once confluence was reached, T84 cells were exposed to bacterium preparations (15 μg/ml) in culture medium at the apical or basolateral surface, as required. Following treatment, TER across the monolayers was monitored over a range of 2, 4, 6, and 24 h in a sterile flow hood with the Endohm apparatus, and cells were replaced in incubation chambers between readings. Resistance values of cells in medium alone were used as a negative control. Every experiment was performed in triplicate.
When cycloheximide (protein synthesis inhibitor), herbimycin A (protein tyrosine kinase inhibitor), staurosporine (protein kinase C [PKC] inhibitor) or phorbol myristate acetate (PMA) (PKC activator) was used, it was freshly added together with the bacterial preparations or alone at concentrations of 1 μg/ml, 2 μg/ml, 0.1 μg/ml, and 10 ng/ml, respectively.
Electrophysiological response to established secretagogues.
Confluent T84 monolayers growing in Snapwell filters with semipermeable membranes (area, 1.13 cm2; pore diameter, 0.45 μm; Falcon) were stimulated for 2 h with 15 μg of sonicated H. pylori NCTC 11638/ml. Then H. pylori was removed, and filters were mounted on Ussing chambers at 37°C. Nonstimulated T84 monolayers were used as a negative control. Both compartments of the Ussing chambers were bathed with 10 ml of Krebs-Heinsleit solution (118 mM NaCl, 4.7 mM KCl, 1.2 mM MgSO4 · 7H2O, 1.2 mM KH2PO4, 25 mM NaHCO3, 2.5 mM CaCl2, 11.1 mM d-glucose, adjusted to pH 7.4 at 37°C with HCl), and solutions were stirred and oxygenated by continuous gassing with 95% O2–5% CO2. The monolayers were clamped to a potential difference of 0 V between apical and basolateral compartments, and the short-circuit current (SCC) and TER were continuously monitored. After 1 h of equilibration, the monolayers were basolaterally exposed to the chloride secretagogues forskolin (3 and 10 μM) and carbachol (10 and 100 μM), and changes in SCC and TER were recorded. Changes in SCC are indicative of the relative ability of control and H. pylori-treated monolayers to secrete chloride ions.
Paracellular flux of the marker molecule [14C]mannitol in T84 confluent monolayers.
T84 monolayers grown to confluence in Snapwell filters and incubated for 2 h with H. pylori NCTC 11638 sonicates were mounted in Ussing chambers. After 1 h of equilibration, a sample was collected from each one of the compartments to determine background radioactivity. At time zero, 0.5 μCi of [14C]mannitol (molecular weight, 182.2) was added to the 10-ml basolateral compartment, and samples were taken immediately and during the following 20 min from the hot and cold compartments (basolateral and apical, respectively). The apparent permeability coefficient (Papp) was determined according to the equation Papp = KVr/A · 60, where Vr is the volume (in milliliters) of the receiver compartment, A is the surface of the membrane (in square centimeters), and K is the slope of the cumulative fraction absorbed (FAcum) versus time (in minutes). FAcum = ΣCri/Cdi, where Cri is counts per minute of [14C]mannitol in the receiver compartment at the end of interval i and Cdi is counts per minute in the donor compartment at the start of interval i. [14C]mannitol was analyzed in a liquid scintillation counter (LKB Wallac 1217, Rackbeta; Wallac Oy, Turku, Finland). In each case the slope (K) for mannitol transfer was calculated from the relationship between counts per 10 ml of fluid per square centimeter of T84 cells and time. Papp values (in centimeters per second) were calculated and compared for control cells versus treated cells.
Paracellular flux of the marker molecule [14C]mannitol in rat gastric mucosa.
The technique for determining the paracellular flux of [14C]mannitol in rat gastric mucosa was previously described by Curtis and Gall (14). Briefly, adult male Wistar rats were sacrificed by stunning followed by decapitation. Their stomachs were immediately removed, opened along the lesser curvature, and dissected free of smooth muscle. Tissues from opposite sides of the corpus, used as matched experimental pairs, were mounted on Ussing chambers that exposed 0.63 cm2 of tissue to 10 ml of Krebs-Heinsleit solution. [14C]mannitol was added in the basolateral compartment, and samples were taken at intervals as described above for T84 monolayers.
Immunofluorescence staining for E-cadherin.
For immunofluorescence microscopy studies, 3 × 105 cells/well were seeded in plastic chamber slides (Nunc Inc., Naperville, Ill.) and cultured until confluence. Then the medium was replaced with 15 μg of H. pylori NCTC 11638 sonicate/ml in Dulbecco’s MEM, and treatment was stopped at 2 h by washing out the medium with PBS, followed by a single wash with distilled water. Cells were allowed to air dry for 24 h and then were fixed with 1% paraformaldehyde. Subsequently, they were permeabilized by a 30-min incubation with 0.2% saponin in PBS–1% bovine serum albumin–0.02% sodium azide containing anti-E-cadherin monoclonal antibody (MAb) (1:100). Then cells were carefully washed and incubated with fluorescein isothiocyanate goat anti-mouse antibody (1:50) for 15 min. Finally, cells were washed twice in PBS and mounted in Dabco fluorescence mounting medium (DAKO) for microscopic examination. An isotype-matched antibody (murine hybridoma anti-HLA-IE; American Type Culture Collection) was used as a negative control.
Cell extraction, electrophoresis, and immunoblotting.
Total-cell extracts from control cells and from cells pretreated with H. pylori for 2, 6, 24, and 48 h were prepared as previously described (31). Briefly, total-cell extracts were prepared by solubilizing the cells in 0.5% (wt/vol) Nonidet P-40 (NP-40) in ice-cold PBS containing 1 mM phenylmethylsulfonyl fluoride (PMSF) and 10 μg of leupeptin/ml. Proteins (50 μg) were separated by sodium dodecyl sulfate–10% polyacrylamide gel electrophoresis and transferred to a polyvinylidene difluoride membrane. Nonspecific binding sites were blocked with BLOTTO-Tween for 1 h. The membrane was then incubated with an anti-E-cadherin MAb, an antiphosphotyrosine MAb (Upstate Biotechnology, Lake Placid, N.Y.) or a control antibody (anti-HLA-IE) for 18 h, washed, and then incubated with biotinylated sheep anti-mouse antibody for 1 h, followed by streptavidin-biotinylated alkaline phosphatase complex for 30 min. Immunoreactive bands were visualized with nitroblue tetrazolium and 5-bromo-4-chloro-3-indolylphosphate (BCIP). Molecular weight marker proteins were localized by staining with 1.25% Coomassie blue in 40% methanol–7% glacial acetic acid.
Immunoprecipitation.
Immunoprecipitation experiments followed by Western blotting were designed to assess tyrosine phosphorylation on E-cadherin of H. pylori-treated cells versus control cells.
(i) Preparation of the cell lysates.
Confluent T84 monolayers pretreated for 2 h with H. pylori and the corresponding control monolayers were washed three times with ice-cold 0.2 mM sodium vanadate in PBS. Cells were then scraped from the filters and lysed by addition of 150 μl of immunoprecipitation buffer (1% Triton X-100, 150 mM NaCl, 10 mM Tris [pH 7.4], 1 mM EDTA, 1 mM EGTA, 0.2 mM sodium vanadate, 0.2 mM PMSF, 0.5% NP-40/well, and constant agitation was maintained for 30 min at 4°C. Cells were passed several times through a 26-gauge needle to disperse any large aggregates. Insoluble material was removed by centrifugation.
(ii) Immunoprecipitation.
Previous to the immunoprecipitation step, 200 μl of protein G-Sepharose beads (Pharmacia LKB Biotechnology Inc., Uppsala, Sweden) were washed three times with 0.5% NP-40 in PBS. Half of the protein G-Sepharose beads were then incubated for 2 h end over end at 4°C with anti-E-cadherin MAb or control antibody in order to allow the formation of protein G-MAb complexes. Simultaneously, the other half of the beads were used to preclear the cell preparations (to avoid nonspecific binding) and were also incubated with mixing for 2 h at 4°C. MAb-coated protein G-Sepharose beads were then washed twice with 0.5% NP-40 and twice with washing buffer (20 mM Tris–150 mM NaCl) and were incubated with the precleared cell fractions for 2 h end over end at 4°C in order to allow the formation of immune complexes. Finally, protein G-Sepharose bead-bound immune complexes were washed three times with immunoprecipitation buffer. Immune complexes were then eluted by boiling in sodium dodecyl sulfate sample buffer.
Cytotoxicity assay.
A neutral red cytotoxicity assay was performed by following a modified version of the protocol described by Borenfreund and Puerner (5). Basically, T84 cell monolayers growing on 24-well plates were washed twice with Hanks’ balanced salt solution and incubated for 30 min at 37°C with neutral red solution (50 μg of neutral red/ml in Dulbecco’s MEM, 1 ml/well). Neutral red solution was then removed, and wells were rapidly washed with a formalin-calcium solution (4% [wt/vol] formaldehyde and 1% [wt/vol] anhydrous calcium chloride). Then the wells were incubated with a glacial acetic acid-ethanol solution (1% [vol/vol] glacial acetic acid–50% [vol/vol] ethanol) for 15 min at room temperature. After gentle agitation, absorbance was measured at 540 nm. Absorbance values are inversely proportional to the cellular damage, since damaged cells cannot retain the dye after the washing-fixation step with the formalin-calcium solution.
Data analysis.
Results are expressed as mean ± standard error of the mean (SEM) or standard deviation (SD) as indicated. For statistical comparison, Student’s t test and the Mann-Whitney U test were performed on raw data to analyze TER and permeability data, respectively. For clarity, TER data are expressed in time course format graphics as percentages of control values at the beginning of each experiment. Neutral red assay absorbance values of H. pylori-treated cells versus control cells were also compared by Student’s t test.
RESULTS
H. pylori effects on TER of epithelial monolayers.
To investigate the effect of the gastric pathogen H. pylori in epithelial-barrier function, we measured the TER of confluent cell monolayers grown on filters after exposure to sonicates from four different strains of H. pylori in the apical bathing medium. Sonicates (15 μg/ml) from strain NCTC 11638 caused a large decrease in TER, to 41.5% ± 13.3% (mean ± SD) of the pretreatment value (P < 0.001; n = 18), at 1 to 2 h after exposure (Fig. 1). The magnitude of this effect is concentration dependent for concentrations below 15 μg/ml. Concentrations higher than 15 μg/ml (up to 500 μg/ml) failed to increase the observed drop in TER (data not shown). The initial decrease in TER was subsequently reversed, reestablishing 55 to 100% of the pretreatment value at 24 h (Fig. 1). Sonicates from strain NCTC 11637 (VacA+ CagA+) and the VacA+ CagA− and VacA− CagA− strains were also able to induce a significant reduction of TER when applied to the apical surfaces of the cells (data not shown), and no TER variation was observed when sonicates of any strain were applied in the basolateral bathing medium (Fig. 1, inset).
FIG. 1.
TER variation at different time intervals. TER was monitored in T84 monolayers treated with 5 (open circles), 10 (solid triangles), and 15 (solid squares) μg of sonicated NCTC 11638 H. pylori/ml versus control cells in medium alone (open squares). The effect is concentration dependent, and the maximum decrease in TER occurred between 1 and 2 h in monolayers treated with 15 μg of H. pylori/ml. The graph shown is from a representative experiment; P = 0.008; n = 3). (Inset) TER of control monolayers (open bar) and of monolayers basolaterally treated with H. pylori (striped bar). No significant difference was found.
As a control, parallel experiments were carried out with sonicates prepared in the same fashion from two gastrointestinal bacteria, E. coli and C. jejuni, and a nongastrointestinal pathogen, H. influenzae. Sonicates (15 μg/ml) were also applied in the apical bathing medium, producing a nonsignificant TER decrease to values between 75 and 90% of the pretreatment value after 2 h of exposure. However, all these bacteria failed to mimic the TER drop produced by H. pylori (Fig. 2).
FIG. 2.
TER variation of T84 monolayers exposed to 15 μg of E. coli (solid circles), C. jejuni (open triangles), or H. influenzae (solid square) sonicate/ml compared to cells in medium alone (open squares) and cells treated with H. pylori NCTC 11638 sonicate (open circles). H. pylori produced the most significative TER at 2 h. Data from a representative experiment are shown (n = 6).
Determination of H. pylori components involved in reduction of TER.
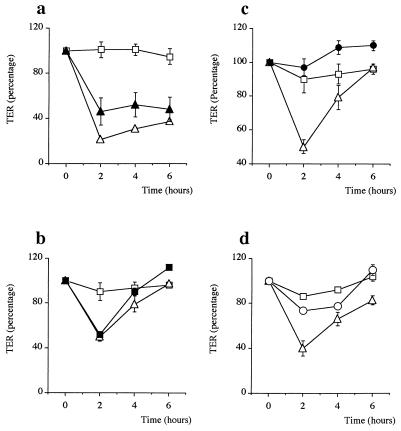
In an attempt to determine which component(s) of H. pylori is involved in this process and to define its biological nature, a series of experiments were performed. First, TER was monitored after exposure of monolayers to whole bacteria. Whole bacteria (15 μg/ml) from strain NCTC 11638 also produced a significant drop in TER at 2 h (Fig. 3a), indicating that the molecule(s) involved could be localized at the bacterial surface. Then a water-soluble surface bacterial fraction from the same strain (15 μg/ml), added at the apical surfaces of epithelial monolayers, also perfectly mimicked the effect of the sonicates, producing the maximum TER decrease at 2 h (Fig. 3b). To exclude the possibility of water-soluble LPS producing this effect, purified H. pylori LPS was added to the epithelial cells and failed to produce any reduction in TER (Fig. 3c). Finally, H. pylori sonicates that had been heat inactivated at 70°C for 15 min lost the capacity to elicit a significant decrease in TER (Fig. 3d). These results suggest that the bacterial component(s) which induces TER reduction is located on the bacterial surface and is likely to be proteinic in nature.
FIG. 3.
Variation of TER of T84 monolayers exposed to whole NCTC 11638 bacteria (solid triangles) (a), H. pylori NCTC 11638 surface proteins (solid squares) (b), H. pylori LPS (solid circles) (c), or heat-inactivated H. pylori NCTC 11638 sonicate (open circles) (d) compared to TERs of control cells in medium alone (open squares) and cells exposed to NCTC 11638 sonicate (open triangles). Whole bacteria and surface proteins mimicked the effect of H. pylori sonicate, while LPS had no effect. When H. pylori sonicate is heated at 70°C for 15 min, the characteristic TER drop at 2 h does not occur, which indicates that the molecule(s) involved in this process is proteinic in nature. Data shown are from four representative experiments.
Analysis of molecular intracellular events mediating TER decrease.
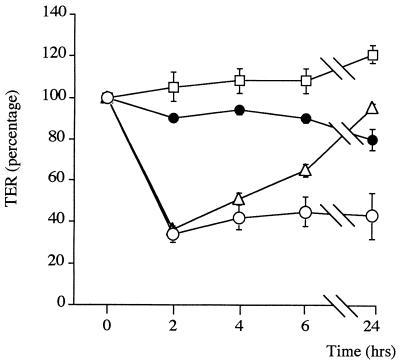
We investigated the intracellular molecular events occurring in epithelial monolayers after incubation with H. pylori. Specifically, we examined the effects of inhibition of protein tyrosine kinase and PKC, as both may be involved in tight-junction regulation (2). The H. pylori-induced TER decrease at 2 h is not blocked by coincubation with cycloheximide (Fig. 4), a protein synthesis inhibitor, or with herbimycin A (Fig. 5a), a protein tyrosine kinase inhibitor. However, cycloheximide completely inhibited the subsequent TER recovery (Fig. 4), indicating that the recovery process is protein synthesis dependent, while herbimycin A blocked it only partially (Fig. 5a).
FIG. 4.
Variation of TER in T84 monolayers stimulated with 15 μg of sonicated H. pylori NCTC 11638/ml in the presence of the protein synthesis inhibitor cycloheximide (open circles) versus T84 monolayers with cycloheximide alone (solid circles), medium alone (open squares), and H. pylori sonicates alone (open triangles). Cycloheximide did not prevent the decrease in TER produced by H. pylori sonicate at 2 h but did block the subsequent reestablishment of the pretreatment TER baseline, indicating that the process is dependent upon protein synthesis. Data shown are from a representative experiment (n = 3).
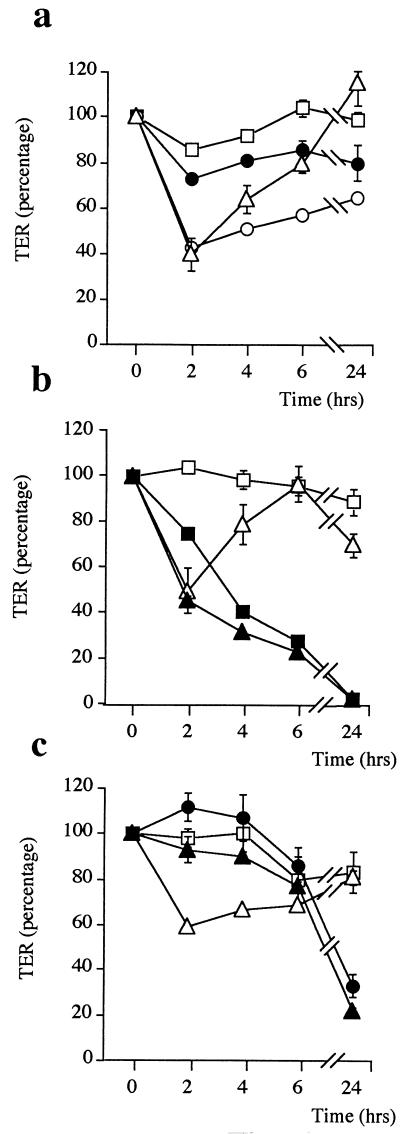
FIG. 5.
Variation of TER in T84 monolayers stimulated with H. pylori sonicate plus the protein tyrosine kinase inhibitor herbimycin A (open circles) versus herbimycin alone (solid circles) (a), the PKC inhibitor staurosporine (solid triangles) versus staurosporine alone (solid squares) (b), or the PKC activator PMA (solid triangles) versus PMA alone (solid circles) (c). Open squares, TER of control cells in medium alone; open triangles, TER of cells in the presence of H. pylori sonicate alone. Herbimycin A did not prevent the decrease in TER produced by H. pylori sonicate at 2 h, but it affected the subsequent reestablishment of TER. Staurosporine by itself produced the abolishment of the resistance at 24 h. In contrast, in the presence of PMA, the characteristic early drop in TER produced by H. pylori sonicate did not occur. Data shown are from four representative experiments (n = 3 to 6).
In experiments designed to examine a possible involvement of PKC, staurosporine (a PKC inhibitor) alone produced a progressive reduction in TER up to the complete abolition of epithelial resistance at 24 h (Fig. 5b). On the other hand, the PKC activator PMA inhibited the TER decrease produced by H. pylori in the first 2 h (Fig. 5c), which indicates that the bacteria could alter the epithelial-barrier properties by somehow inhibiting PKC activity.
Permeability assays.
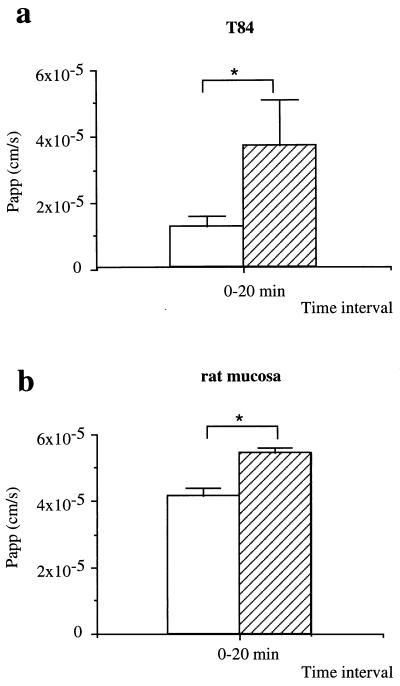
TER measurements give an indication of the ionic permeability of tight junctions but can also reflect changes in membrane conductance (37). As an independent measure of paracellular flux, we examined the passage of a membrane-impermeant molecule, radiolabelled mannitol, across cell monolayers previously treated for 2 h with 15 μg of H. pylori sonicates/ml. Mannitol flux across the epithelial monolayers was significantly increased in H. pylori-treated monolayers (Fig. 6a) over that in nontreated monolayers (mean Papp ± SEM, 1.27 × 10−5 ± 0.32 × 10−5 cm/s for control T84 monolayers versus 3.79 × 10−5 ± 1.39 × 10−5 cm/s for H. pylori-pretreated T84 monolayers; n = 6; P < 0.05), indicating that the bacteria increase tight-junction permeability. These data are consistent with the TER measurements, which are therefore unlikely to be due to increased membrane conductance. Furthermore, H. pylori 11638 sonicates also enhanced in a significant manner the [14C]mannitol flux across the H. pylori-pretreated rat gastric mucosa samples in vitro compared to that for the matched control nontreated mucosa samples (mean Papp [cm/s] ± SEM, 4.14 × 10−5 ± 0.27 × 10−5 for the control samples versus 5.47 × 10−5 ± 0.13 × 10−5 for the H. pylori-pretreated mucosa samples; n = 4; P < 0.05), as shown in Fig. 6b.
FIG. 6.
H. pylori-induced increase in permeability. (a) T84 confluent monolayers. The Papp of [14C]mannitol flux in non-H. pylori-treated control cells (open bar) versus that for H. pylori-pretreated cells (striped bar) is shown. (b) Rat mucosa. The Papp of [14C]mannitol flux in control nontreated rat mucosa (open bar) versus that for H. pylori-pretreated rat mucosa (striped bar) is shown. In paired experiments, basolateral-to-apical [14C]mannitol flux measurements were made over a single 20-min period following 2-h exposure of T84 cells or rat mucosa to H. pylori sonicates (15 μg/ml). Graphs show Papp means from six (a) and four (b) experiments. P < 0.05 for panel a and P < 0.05 for panel b by the Mann-Whitney U test.
Physical and functional viability assessment.
The physical and functional viability of H. pylori-treated T84 monolayers compared to that of control T84 monolayers was subsequently assessed. Control and H. pylori-pretreated monolayers were able to respond at the two tested concentrations of the chloride secretagogues forskolin and carbachol, which indicates that the functional (secretory) capacity of the treated cells is unaltered (Fig. 7).
FIG. 7.
Response of T84 monolayers to established secretagogues. Control (open circles) and H. pylori-pretreated (solid circles) confluent T84 monolayers mounted on Ussing chambers were stimulated with 3 and 10 μM forskolin and 10 and 100 μM carbachol at the indicated times. Treated and control monolayers were able to respond to both secretagogues, and the highest stimulation was obtained with 10 μM carbachol. Changes in SCC are indicative of the relative abilities of the monolayers to secrete chloride ions.
Further evidence that the observed changes in TER were not due to a cytotoxic effect of the organism were obtained with the neutral red cytotoxicity assay: mean optical density ± SD was 0.74 ± 0.16 for control monolayers versus 0.75 ± 0.14 for H. pylori-pretreated monolayers; n = 24; not significant).
Influence of H. pylori on the expression of E-cadherin.
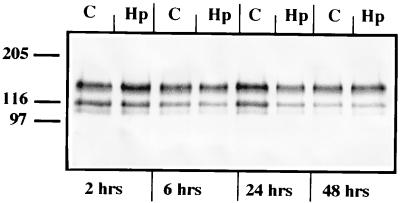
The functional organization of polarized epithelia depends mostly on the adhesion of molecules belonging to the cadherin family, which form intercellular junctions via homotypic interactions. Therefore, we analyzed the influence of H. pylori on the expression of E-cadherin, a member of a family of Ca2+-dependent cell adhesion molecules which are localized in the zonula adherens (1). To determine whether the levels of E-cadherin were altered in H. pylori-treated cells compared to those in controls, E-cadherin cell levels were detected by Western blotting after 4, 24, and 48 h of cell treatment with H. pylori sonicates. E-cadherin expression in treated and control cells was also analyzed by flow cytometry (data not shown).
Western blot analysis detected no change in the basal expression of E-cadherin after H. pylori treatment at any of the times at which expression was examined (Fig. 8). E-cadherin expression in treated and control cells was also analyzed by flow cytometry, and the results confirmed the Western blot data (data not shown). Western blot experiments revealed an expected band of about 120 kDa plus two smaller bands (approximately 105 and 100 kDa) of unknown nature, presumably corresponding to a soluble form of E-cadherin that has been detected in other types of cancerous epithelium and which does not exist in normal epithelial cells (42).
FIG. 8.
Western blot analysis of E-cadherin expression in T84 cells treated with H. pylori (Hp) for 2, 6, 24, and 48 h and in control cells (C) in medium alone. Besides the classical 120-kDa band, two smaller bands (∼100 and ∼105 kDa) were detected in all cases. Western blot analysis detected no change in the basal levels of E-cadherin after H. pylori treatment at any of the times at which they were examined.
The functions of cadherins are regulated from the cytoplasmic side, and tyrosine phosphorylation appears to be an important regulatory signal for these proteins (43). Hence we analyzed the tyrosine phosphorylation state of E-cadherin in H. pylori-treated cells and compared it to that in control cells by immunoprecipitation with an anti-E-cadherin MAb followed by immunoblotting with an anti-phosphotyrosine MAb. Results indicated that there was no difference in tyrosine phosphorylation status between H. pylori-treated and control cells (Fig. 9). In all cases, phosphotyrosine immunoblotting of the E-cadherin immunoprecipitates revealed that E-cadherin (120 kDa) was not tyrosine phosphorylated, but tyrosine phosphorylation was detected on two proteins that coprecipitated with it: a 94-kDa band, likely to be β-catenin (43), and a 143-kDa band of unknown nature. Phosphorylation of these proteins was not altered by H. pylori exposure.
FIG. 9.
Immunoprecipitation-Western blot analysis of tyrosine phosphorylation of E-cadherin in control and H. pylori-treated T84 monolayers. Western blots were probed with anti-E-cadherin antibody (a) and antiphosphotyrosine antibody (b). Lanes: A, control T84 whole-cell preparation; B, control T84 cells immunoprecipitated with anti-E-cadherin MAb; C, H. pylori-treated T84 cells immunoprecipitated with anti-E-cadherin MAb. E-cadherin (120 kDa) was not tyrosine phosphorylated in any case, but tyrosine phosphorylation was detected on two coprecipitated proteins (94 kDa, likely to be β-catenin, and 147 kDa). Phosphorylation of these proteins was not altered by exposure to H. pylori.
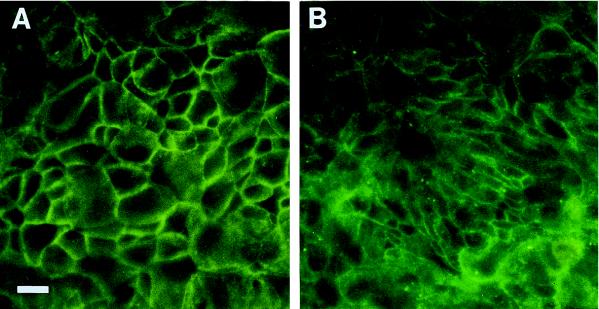
Finally, to visualize the expression of E-cadherin in vitro, cells were treated with H. pylori sonicates, stained with anti-E-cadherin MAb by immunofluorescence, and microscopically analyzed. Analysis was performed at 2 h to correspond temporally with major changes in TER. Immunofluorescence images showed that pretreated cells displayed enlarged cytoplasm (Fig. 10A) compared to that of cells incubated in medium alone (Fig. 10B). Computer image analysis indicated that there was a significant difference between the average size of control cells and that of H. pylori-treated cells (mean size ± SD, 7.79 ± 5.75 μm2 for control cells and 19.80 ± 7.71 μm2 for H. pylori-treated cells; P < 0.001; n = 20).
FIG. 10.
Anti-E-cadherin immunofluorescence staining of T84 monolayers grown in plastic chamber slides of cells treated with H. pylori sonicates for 2 h (A) versus control cells in medium alone (B). Treated cells appeared bigger than controls at 2 h. Changes in morphology and loss of adherence to plastic are maximum at 48 h. Bar, 40 μm.
DISCUSSION
This study has revealed the ability of the human gastric pathogen H. pylori to disrupt the barrier function of a model epithelium by producing a rapid increase in the permeability of tight junctions and a dramatic decrease in the TER. Furthermore, we show that bacterial sonicates induced a significant increase in the permeability of rat gastric mucosa in vitro, which indicates that H. pylori-induced barrier alterations can occur in a native, complex, and acid-secretory tissue and are not simply an artifact observed only with model epithelia.
It is known that H. pylori produces physical alterations of epithelial cells. Destruction of villi at the site of adhesion, disruption of the intercellular junctions, and the presence of vacuoles in the cell have been reported (7, 46), but to date there are no data revealing a pathophysiological relationship between this parasitic organism and the gastroenteric epithelium. We used an in vitro model to determine the physiological effects of H. pylori sonicates in the colonic cell line T84, which represents a polarized epithelium with high resistance (33). We chose this model firstly because all human gastric epithelial cell lines commercially available organize leaky epithelial monolayers in vitro. Primary cultures of human gastric epithelial cells can reach a polarized state, but they do not proliferate in vitro and therefore do not form confluent monolayers (41, 47). Secondly, many epithelia share similar mechanisms involved in tight-junction regulation. Thus, the formation and breakdown of these structures in cultured cells can be affected by protein kinase A, protein kinase C, heterotrimeric G proteins, and Ca2+ and by E-cadherin dysfunction (3, 4, 8, 27). Lastly, this study was concerned with regulation of tight-junction integrity rather than cell adhesion to specific bacterial adhesins. Hence sonicates were used to reproduce the effects of whole bacteria.
To analyze the effect of H. pylori sonicates on the epithelial-barrier function of T84 monolayers, we monitored TER at different time intervals. TER changes reflect variation in tight junction integrity as shown by electron microscopy (22, 32), although they may also indicate changes in permeability at the membrane level (30). Therefore we also analyzed the paracellular permeability of H. pylori-treated monolayers by measuring the flux of [14C]mannitol, which moves principally through the paracellular route (30). [14C]mannitol flux through samples of H. pylori-pretreated rat gastric mucosa was also assessed in an attempt to determine if the observed effects could be reproduced in a gastric epithelium. Our results showed that H. pylori sonicates produced a dramatic decrease in TER in T84 monolayers and induced a rapid increase in [14C]mannitol flux not only across the T84 model epithelium but also across the rat mucosa. These data suggest that the bacterium can alter the barrier function of the epithelium without adhering to it, as it is known that H. pylori does not bind to rat gastric epithelial cells (29) and that the adhesion of this bacterium to T84 cells is maximal at pH 5.4 (10), while our experiments were carried out under neutral pH conditions. Bacterial sonicates fail to produce any effect in the TER of MDCK cells (data not shown), which are also able to establish monolayers with physiological resistance. This may indicate that although bacterial adherence may not be essential to alter the tightness of the epithelial barrier, a recognition step between bacterial molecules and specific receptors of gastrointestinal epithelial cells may take place.
These effects could suggest one potential mechanism by which H. pylori antigens reach the underlying tissue and therefore become available for interaction with the immune cell populations in the lamina propria (16, 35). The bacterial molecule(s) originating this change in permeability is likely to be a protein(s) localized upon the external surface, as the effect caused by H. pylori sonicates is mimicked by entire microorganisms as well as by the water-soluble heat-sensitive molecules from the bacterial surface, and purified LPS had no effect. Gram-negative bacteria have a thin peptidoglycan layer surrounded by the outer membrane, which contains LPS and proteins (26). The H. pylori molecule(s) that causes the increase in paracellular flux could include some of the proteins with high antigenic capacity situated on the microorganism surface, such as proteases (48), porins (17), or others (urease was not responsible for the observed changes [data not shown]). In parallel experiments, TER was not significantly affected by coculturing T84 cells with sonicates from other gram-negative gastrointestinal bacteria, such as E. coli or C. jejuni, or from the gram-positive bacterium H. influenzae, which indicates that the findings obtained were specific to H. pylori.
Neutral red cytotoxicity assays also revealed that the H. pylori effect on epithelial resistance was not due to cell necrosis and exfoliation, which could create holes on the monolayer, thus inducing alterations of normal permeability and TER. However, fluorescence microscopy analysis showed that treated cells display a different, enlarged morphology compared to control cells. These changes in morphological structure in the epithelial cells may be related to the functionality changes observed, since they exhibit a similar time course. It has been reported that intact microorganisms are able to induce significant cytoskeletal rearrangements in AGS cells (39). We observed that TER reduction was also induced by intact bacteria in addition to sonicates, which may suggest that such changes could be due to similar cytoskeletal rearrangements.
Infection of polarized monolayers of epithelial cells (MDCK and Caco-2) with Salmonella species has also been shown to elicit a decrease in TER (19, 20). Coincident with our result, short-term infection of MDCK monolayers with Salmonella typhimurium induces a decrease in TER concomitant with an increase in the transepithelial flux of inulin (28). However, the mechanisms underlying the two types of infection are different in that S. typhimurium-induced TER changes are associated with bacterial invasion and membrane ruffling, which are shown to occur within minutes of infection in MDCK cells (21), while H. pylori is not an invasive bacterium. Moreover, H. pylori effects were produced by bacterial sonicates, which may indicate interaction between bacterial proteins and apical receptors on the surfaces of the epithelial cells.
To obtain a preliminary insight into the intracellular molecular events mediated by the bacteria in T84 epithelial monolayers, a series of pharmacological agents was used. We observed that the H. pylori-associated decrease in TER after 2 h of exposure was inhibited in the presence of the phorbol ester PMA. It is well known that one major effect of PMA in eukaryotic cells is the activation of the enzyme PKC (22, 34), which may influence, together with all the classic second-messenger and signalling pathways, both epithelial assembly and barrier properties. It is believed that PKC may act downstream from cadherin-mediated cell-to-cell contacts, but not directly on tight-junction proteins. Instead, cadherin-mediated interactions may act to influence the actin cytoskeleton through a PKC-mediated pathway, which then is involved in regulation of tight-junction assembly (2). We observed that the PKC inhibitor staurosporine failed to prevent the decrease in TER induced by H. pylori; moreover, staurosporine by itself produced a nonreversible abrogation of the resistance. Therefore, the H. pylori-induced decrease in TER may occur as a consequence of the activation of some intracellular pathways counteracting the effects of PKC. On the other hand, the early reduction of TER induced by the sonicate was slowly reversed over a 24-h course in a process which requires protein synthesis, as the baseline recovery does not occur in the presence of the protein synthesis inhibitor cycloheximide. Recovery of TER always occurred, even in the presence of sonicates, but, interestingly, recovery was not a feature in preparations treated with staurosporine, which raises the possibility that TER recovery is an active process dependent on PKC function. Soluble factors derived from H. pylori induce an increase in inositol phosphates in a wide variety of epithelial cells (HeLa, Henle 407, HEP-2, and AGS) which is maximal after 2 to 3 h of infection (38). It is known that phosphatidylinositols play an important role in the regulation of some isoforms of PKC. In the presence of Ca2+ these agents activate PKC (34), which then may phosphorylate cytoskeletal proteins, inducing a final reorganization of tight junctions, thus recovering TER and reducing permeability. Herbimycin A also partially inhibited the subsequent TER recovery, which may suggest a role for protein tyrosine kinases in the TER process, possibly through tyrosine phosphorylation of cytoskeletal elements (39). All these data indicate that the interaction between the epithelial cells and H. pylori sonicates triggers a series of intracellular signal transduction pathways in which PKC is very likely to be involved. The elucidation of this pathway will be the subject of further study.
Cadherin-mediated adhesion directly affects the assembly and maintenance of tight junctions (2, 22). As recent evidence has implicated the participation of various cell-to-cell adhesion molecules in signal transduction (1), we investigated whether the physiological alterations in epithelial cells induced by H. pylori were related to any change in the levels of the transmembrane Ca2+-dependent adhesion molecule E-cadherin. Analysis of the levels of this molecule in H. pylori-treated T84 cells by flow cytometry and Western blotting revealed no changes at any of the times of exposure. These findings suggest that permeability changes are not related to differences in the level of E-cadherin. These observations are supported by the finding that H. pylori did not alter the tyrosine phosphorylation status of E-cadherin or associated proteins.
In conclusion, the present study presents evidence that H. pylori disrupts the epithelial-barrier function in T84 epithelial monolayers. The process may involve activation of cell signalling pathways, including PKC and possibly tyrosine kinases. Our data suggest a pathophysiological mechanism of interaction between the bacteria and the epithelium, which may initiate the degenerative changes observed in H. pylori-associated pathologies.
ACKNOWLEDGMENTS
We thank Denise Hyde and William Dundon for culturing of bacterial strains.
This study was supported in part by a grant from the Fundación Madrileña de Enfermedades Digestivas y Hepáticas (Spain). D. Kelleher was a Wellcome Senior Fellow in Clinical Science.
REFERENCES
- 1.Aghib D, McCrea P. The E-cadherin complex contains the src substrate p120. Exp Cell Res. 1995;218:359–369. doi: 10.1006/excr.1995.1167. [DOI] [PubMed] [Google Scholar]
- 2.Anderson J, Itallie C V. Tight junctions and the molecular basis for regulation of paracellular permeability. Am J Physiol. 1995;269:G467–G475. doi: 10.1152/ajpgi.1995.269.4.G467. [DOI] [PubMed] [Google Scholar]
- 3.Balda M, Gonzales-Mariscal L, Contreras R, Macias-Silva M, Torres-Markez M, Garcia-Sainz J, Cereijido M. Assembly and sealing of tight junctions: possible participation of G-proteins, phospholipase C, protein kinase C and calmodulin. J Membr Biol. 1991;122:193–202. doi: 10.1007/BF01871420. [DOI] [PubMed] [Google Scholar]
- 4.Balda M, Gonzalez M, Matter K, Cereijido M, Anderson J. Assembly of the tight junction: the role of diacylglycerol. J Cell Biol. 1993;123:293–302. doi: 10.1083/jcb.123.2.293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Borenfreund E, Puerner J. A simple quantitative procedure using monolayer cultures for cytotoxicity assays (9HTD/NR-90) J Tissue Cult Methods. 1984;9:7–9. [Google Scholar]
- 6.Cantorna M, Balish E. Inability of human clinical isolates of Helicobacter pylori to colonize the alimentary tract of germfree rodents. Can J Microbiol. 1990;36:237–241. doi: 10.1139/m90-041. [DOI] [PubMed] [Google Scholar]
- 7.Chen X, Correa P, Offerhaus J, Rodriguez E, Janney F, Hoffmann E, Fox J, Hunter F, Diavolitsis S. Ultrastructure of the gastric mucosa harbouring Campylobacter like organisms. Am J Clin Pathol. 1986;86:575–582. doi: 10.1093/ajcp/86.5.575. [DOI] [PubMed] [Google Scholar]
- 8.Citi S. Protein kinase inhibitors prevent junction dissociation induced by low extracellular calcium in MDCK epithelial cells. J Cell Biol. 1992;117:169–178. doi: 10.1083/jcb.117.1.169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Clyne M, Drumm B. Adherence of Helicobacter pylori to primary human gastrointestinal cells. Infect Immun. 1993;61:4051–4057. doi: 10.1128/iai.61.10.4051-4057.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Corthesy-Theulaz I, Porta N, Pringault E, Racine L, Bogdanova A, Draehenbuhl J, Blum A, Michetti P. Adhesion of Helicobacter pylori to polarized T84 human intestinal cell monolayers is pH dependent. Infect Immun. 1996;64:3827–3832. doi: 10.1128/iai.64.9.3827-3832.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cover T, Blaser M. Helicobacter pylori and gastroduodenal disease. Annu Rev Med. 1992;43:135–145. doi: 10.1146/annurev.me.43.020192.001031. [DOI] [PubMed] [Google Scholar]
- 12.Cover T L, Dooley C P, Blaser M J. Characterization of and human serologic response to proteins in Helicobacter pylori broth culture supernatants with vacuolizing cytotoxin activity. Infect Immun. 1990;58:603–610. doi: 10.1128/iai.58.3.603-610.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cover T L, Puryear W, Perez-Perez G I, Blaser M J. Effect of urease on HeLa cell vacuolation induced by Helicobacter pylori cytotoxin. Infect Immun. 1991;59:1264–1270. doi: 10.1128/iai.59.4.1264-1270.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Curtis G, Gall D. Macromolecular transport by rat gastric mucosa. Am J Physiol. 1992;262:G1033–G1040. doi: 10.1152/ajpgi.1992.262.6.G1033. [DOI] [PubMed] [Google Scholar]
- 15.Dunn B, Campbell G F, Pérez-Pérez G. Purification and characterization of Helicobacter pylori urease. J Biol Chem. 1990;265:9464–9469. [PubMed] [Google Scholar]
- 16.Ernst, P., and S. Pecquet. 1991. Interaction between H. pylori and the local mucosal immune system. Scand. J. Gastroenterol. 187(Suppl.):56–64. [PubMed]
- 17.Exner M, Doig P, Trust T, Hancock R. Isolation and characterization of a family of porin proteins from Helicobacter pylori. Infect Immun. 1995;63:1567–1572. doi: 10.1128/iai.63.4.1567-1572.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Falk P, Roth K, Borén T, Westblom T, Gordon J, Normark S. An in vitro adherence assay reveals that Helicobacter pylori exhibits cell lineage-specific tropism in the human gastric epithelium. Proc Natl Acad Sci USA. 1993;90:2035–2039. doi: 10.1073/pnas.90.5.2035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Finlay B, Falkow S. Salmonella interactions with polarized human intestinal Caco-2 epithelial cells. J Infect Dis. 1990;162:1096–1106. doi: 10.1093/infdis/162.5.1096. [DOI] [PubMed] [Google Scholar]
- 20.Finlay B, Gumbiner B, Falkow S. Penetration of Salmonella through a polarized Madin-Darby canine kidney epithelial cell monolayer. J Cell Sci. 1988;107:221–230. doi: 10.1083/jcb.107.1.221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Francis C, Starnbach M, Falkow S. Morphological and cytoskeletal changes in epithelial cells occur immediately upon interaction with Salmonella typhimurium grown under low-oxygen conditions. Mol Microbiol. 1992;6:3077–3087. doi: 10.1111/j.1365-2958.1992.tb01765.x. [DOI] [PubMed] [Google Scholar]
- 22.Garrod D, Collins J. Intercellular junctions and cell adhesion in epithelial cells. In: Fleming T P, editor. Epithelial organization and development. London, United Kingdom: Chapman & Hall; 1992. pp. 1–53. [Google Scholar]
- 23.Gumbiner B. Structure, biochemistry and assembly of epithelial tight junctions. Am J Physiol. 1987;253:C749–758. doi: 10.1152/ajpcell.1987.253.6.C749. [DOI] [PubMed] [Google Scholar]
- 24.Gumbiner B. Cell adhesion: the molecular basis of tissue architecture and morphogenesis. Cell. 1996;84:345–357. doi: 10.1016/s0092-8674(00)81279-9. [DOI] [PubMed] [Google Scholar]
- 25.Hatz R, Brooks W, Kramling H, Enderes G. Stomach immunology and Helicobacter pylori infection. Curr Opin Gastroenterol. 1992;8:993–1001. [Google Scholar]
- 26.Henderson B, Wilson M. Cytokine induction by bacteria: beyond lipopolysaccharide. Cytokine. 1996;8:269–282. doi: 10.1006/cyto.1996.0036. [DOI] [PubMed] [Google Scholar]
- 27.Howarth A, Singer K, Stevenson B. Analysis of the distribution and phosphorylation state of ZO-1 in MDCK and nonepithelial cells. J Cell Biol. 1994;137:261–270. doi: 10.1007/BF00232594. [DOI] [PubMed] [Google Scholar]
- 28.Jepson M A, Collares-Buzato C B, Clark M A, Hirst B H, Simmons N L. Rapid disruption of epithelial barrier function by Salmonella typhimurium is associated with structural modification of intercellular junctions. Infect Immun. 1995;63:356–359. doi: 10.1128/iai.63.1.356-359.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kobayashi Y, Okazaki D, Murakami K. Adhesion of Helicobacter pylori to gastric epithelial cells in primary cultures obtained from stomachs of various animals. Infect Immun. 1993;61:4058–4063. doi: 10.1128/iai.61.10.4058-4063.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lewis S, Berg J, Kleine T. Modulation of epithelial permeability by extracellular macromolecules. Physiol Rev. 1995;75:561–589. doi: 10.1152/physrev.1995.75.3.561. [DOI] [PubMed] [Google Scholar]
- 31.Long A, Kelleher D. Conventional protein kinase C isoforms are not essential for cellular proliferation of a T cell lymphoma line. FEBS Lett. 1993;333:243–247. doi: 10.1016/0014-5793(93)80662-e. [DOI] [PubMed] [Google Scholar]
- 32.Madara J, Pappenheimer J. Structural basis for physiological regulation of paracellular pathways in intestinal epithelia. J Membr Biol. 1987;100:149–164. doi: 10.1007/BF02209147. [DOI] [PubMed] [Google Scholar]
- 33.Madara J, Stafford J, Dharmsathaphorn K, Carlson S. Structural analysis of a human intestinal epithelial cell line. Gastroenterology. 1987;92:1133–1145. doi: 10.1016/s0016-5085(87)91069-9. [DOI] [PubMed] [Google Scholar]
- 34.Mahoney C, Huang K. Catalytic properties of protein kinase C. In: Kuo J, editor. Protein kinase C. New York, N.Y: Oxford University Press; 1994. pp. 27–37. [Google Scholar]
- 35.Mai U, Pérez-Pérez G, Wahl L, Wahl S, Blaser M, Smith P. Soluble surface proteins from Helicobacter pylori activate monocytes/macrophages by lipopolysaccharide-independent mechanism. J Clin Invest. 1991;87:894–900. doi: 10.1172/JCI115095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Moran A P, Helander I M, Kosunen T U. Compositional analysis of Helicobacter pylori rough-form lipopolysaccharides. J Bacteriol. 1992;174:1370–1377. doi: 10.1128/jb.174.4.1370-1377.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Parkos C, Coglan S, Delp C, McArnout M A, Madara J. Neutrophil migration across a cultured epithelial monolayer elicits a biphasic resistance response representing sequential effects on transcellular and paracellular pathways. J Cell Biol. 1992;117:757–764. doi: 10.1083/jcb.117.4.757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Pucciarelli M, Ruschkowski S, Trust T, Finlay B. Helicobacter pylori induces an increase in inositol phosphates in cultured epithelial cells. FEMS Microbiol Lett. 1995;129:293–300. doi: 10.1111/j.1574-6968.1995.tb07595.x. [DOI] [PubMed] [Google Scholar]
- 39.Segal E, Falkow S, Tompkins L. Helicobacter pylori attachment to gastric cells induces cytoskeletal rearrangements and tyrosine phosphorylation of host cell proteins. Proc Natl Acad Sci USA. 1996;93:1259–1264. doi: 10.1073/pnas.93.3.1259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Slomiany B, Bilski J, Sarosiek J, Murthy V L, Dworkin B, Van Horn K, Zielenski J, Slomiany A. Campylobacter pyloridis degrades mucin and undermines gastric mucosal integrity. Biochem Biophys Res Commun. 1987;144:307–314. doi: 10.1016/s0006-291x(87)80511-9. [DOI] [PubMed] [Google Scholar]
- 41.Smoot D T, Resau J H, Naab T, Desbordes B C, Gilliam T, Bull-Henry K, Curry S B, Nidiry J, Sewchand J, Mills-Robertson K, Frontin K, Abebe E, Dillon M, Chippendale G R, Phelps P C, Scott V F, Mobley H L T. Adherence of Helicobacter pylori to cultured human gastric epithelial cells. Infect Immun. 1993;61:350–355. doi: 10.1128/iai.61.1.350-355.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Swami S, Kumble S, Triadafilopoulos G. E-cadherin expression in gastroesophageal reflux disease, Barrett’s esophagus, and esophageal adenocarcinoma: an immunohistochemical and immunoblot study. Am J Gastroenterol. 1995;90:1808–1813. [PubMed] [Google Scholar]
- 43.Takeda H, Nagafuchi A, Yonemura S, Tsukita S, Behrnes J, Birchmeier W, Tsukita S. V-src kinase shifts the cadherin-based cell adhesion from the strong to the weak state and β-catenin is not required for the shift. J Cell Biol. 1995;131:1839–1847. doi: 10.1083/jcb.131.6.1839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Talley N, Zinsmeister A, Weaver A, DiMagno E, Carpenter H, Perez-Perez G, Blaser M. Gastric adenocarcinoma and Helicobacter pylori infection. J Natl Cancer Inst. 1991;83:1734–1739. doi: 10.1093/jnci/83.23.1734. [DOI] [PubMed] [Google Scholar]
- 45.Taylor C, Murphy A, Kelleher D, Baird A. Changes in barrier function of a model intestinal epithelium by intraepithelial lymphocytes require new protein synthesis by epithelial cells. Gut. 1997;40:634–640. doi: 10.1136/gut.40.5.634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Tricottet V, Bruneval P, Vire O, Camilleri J. Campylobacter-like organisms and surface epithelium abnormalities in active, chronic gastritis in humans: an ultrastructural study. Ultrastruct Pathol. 1986;10:113–122. doi: 10.3109/01913128609014587. [DOI] [PubMed] [Google Scholar]
- 47.Wagner S, Beil W, Mai U, Bokemeyer C, Meyer J, Manns M. Interactions between Helicobacter pylori and human gastric epithelial cells in culture: effect of antiulcer drugs. Pharmacology. 1994;49:226–237. doi: 10.1159/000139238. [DOI] [PubMed] [Google Scholar]
- 48.Windle H, Kelleher D. Identification and characterization of a metalloprotease activity from Helicobacter pylori. Infect Immun. 1997;65:3132–3137. doi: 10.1128/iai.65.8.3132-3137.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]