Abstract
The results of this study provide the first evidence that two completely separate vaccine approaches, one based on a subunit vaccine consisting of a mild adjuvant admixed with purified culture filtrate proteins and enhanced by the cytokine interleukin-2 and the second based on immunization with DNA encoding the Ag85A protein secreted by Mycobacterium tuberculosis, could both prevent the onset of caseating disease, which is the hallmark of the guinea pig aerogenic infection model. In both cases, however, the survival of vaccinated guinea pigs was shorter than that conferred by Mycobacterium bovis BCG, with observed mortality of these animals probably due to consolidation of lung tissues by lymphocytic granulomas. An additional characteristic of these approaches was that neither induced skin test reactivity to commercial tuberculin. These data thus provide optimism that development of nonliving vaccines which can generate long-lived immunity approaching that conferred by the BCG vaccine is a feasible goal.
Tuberculosis is the leading worldwide cause of death from infectious disease, with a recent report on the global epidemiology of tuberculosis predicting that without worldwide control measures it could be responsible for 30 million deaths between the years 1990 and 2000 (21).
Humans exhibit a wide range of responses to tuberculosis infection. The majority are resistant, dealing with exposure to the bacillus by killing it via innate mechanisms of immunity or by generating a strong state of acquired cellular immunity that usually leads to control of the infection. In these latter individuals, the only visible symptom of exposure is conversion to a positive state of skin test reactivity to tuberculin purified protein derivative (PPD). In a small percentage of this latter group of people, however, the initial infection is not contained but instead gives rise to progressive pulmonary infection.
These two ends of the spectrum of disease in humans can be modeled in mice, which are resistant to tuberculosis, and in guinea pigs, which are susceptible to the disease. Mice generate a strong cellular immune response against tuberculosis, which controls bacterial growth and limits damage to the lungs. Guinea pigs also initially develop strong immunity, but this is eventually associated with considerable tissue damage, leading to extensive caseation and tissue necrosis that eventually kills the animal. As such, this is a useful model of events in infected humans, which follow a similar pattern.
The attenuated BCG strain of Mycobacterium bovis has been extensively used as a vaccine against tuberculosis for the past several decades. The vaccine has several virtues, including the fact that it can be safely given to young children, is cheap to produce, and gives rise to a long-lived state of host resistance (4). It has been comprehensively evaluated in a relatively large number of controlled vaccine trials, and in various populations and geographic regions the calculated protective efficacy of the vaccine has unfortunately varied between 0 and 80% (24, 29). This extreme variability has prompted new research into replacing BCG with a more effective vaccine against tuberculosis (18, 23).
In this regard, we have hypothesized that proteins produced and secreted by the metabolizing M. tuberculosis bacilli (which are present in culture filtrate), rather than constitutive or stress proteins, are the key antigens recognized by the protective immune response (16, 17). This hypothesis has received increasing support in the field, and reports from four separate laboratories have shown variable degrees of success in vaccinating mice or guinea pigs against experimental tuberculosis by using culture filtrate proteins (CFP) (2, 10, 20, 22). To date, however, no information has been presented about the ability of these vaccines to prevent the development of lethal pathologic changes, such as pulmonary caseous necrosis, over a sustained period. This information would be important, given that BCG vaccination limits the production of caseation within the lungs of guinea pigs. Naive guinea pigs infected via the aerosol route show evidence of caseation and necrosis a few weeks after exposure. This gradually leads to caseous necrosis within lesions, which can either mineralize or cavitate. Outward signs of these events are shallow breathing, sudden significant weight loss, and death occurring 8 to 20 weeks postinfection.
For a new vaccine to have any credence as a potential replacement for BCG, it is imperative to demonstrate that (i) it can prevent this caseating disease and instead induce a cellular response in the lungs similar to that induced by BCG, and (ii) it can protect the animal over the long term, at least to the extent provided by BCG (classical studies [13] show that BCG can protect guinea pigs for about 200 to 400 days postinfection).
In the present study these questions were investigated in the guinea pig model after a series of comprehensive pilot studies in the mouse. The results indicate that two vaccine types, one based upon a mixture of monophosphoryl lipid A (MPL) adjuvant, CFP, and the cytokine interleukin-2 (IL-2) and the other based upon a DNA plasmid vaccine encoding a major culture filtrate protein from M. tuberculosis (Ag85A), could prolong the survival of infected guinea pigs and prevent them from developing caseating disease. In these animals, a lymphocytic granulomatous response ensued, similar to that seen in animals vaccinated with BCG.
MATERIALS AND METHODS
Animals.
Specific-pathogen-free female C57BL/6 mice and female outbred Hartley guinea pigs were purchased from Charles River Laboratories (North Wilmington, Mass.) and held under barrier conditions in an ABL-3 biohazard laboratory at Colorado State University. Mice were 6 to 8 weeks old at the beginning of the experiments and were housed four to a cage. The mice were sacrificed 30 days after infection with M. tuberculosis Erdman. Guinea pigs weighed approximately 500 to 600 g at the beginning of the experiment and were housed two to a cage. Guinea pigs in the first study were sacrificed 30 days after aerogenic infection with M. tuberculosis H37Rv. A second set of vaccinated guinea pigs was monitored for several months after aerogenic infection with M. tuberculosis H37Rv. Because of expense, we were limited to four animals per group for these studies. The animals were weighed every few days, and those showing evidence of sudden significant weight loss were euthanized. Animals were allowed free access to water and standard mouse or guinea pig chow, respectively.
Bacterial infections.
M. tuberculosis Erdman and H37Rv and M. bovis BCG Pasteur were previously grown to early mid-log phase in Proskauer Beck medium containing 0.05% Tween 80. Cultures were aliquoted into 1-ml tubes and stored at −70°C until used. Thawed aliquots were diluted in double-distilled sterile water to the desired inoculum concentrations. An aerosol generation device (Glas-Col, Terre Haute, Ind.) was used to expose animals to an aerosol of M. tuberculosis and was calibrated to deliver approximately 20 to 50 bacilli into each guinea pig lung. Mice were aerogenically infected with approximately 100 bacilli.
CFP.
Purified CFP from M. tuberculosis were kindly provided by John Belisle, Colorado State University.
Cytokines.
Recombinant murine IL-12 (rIL-12) was kindly provided by Genetics Institute, Cambridge, Mass. Polyethylene glycol recombinant human IL-2 was kindly provided by Chiron Corp., Emeryville, Calif.
Adjuvant.
Adjuvant formulations based upon MPL were kindly provided by Ribi ImmunoChem Research, Inc., Hamilton, Mont. MPL is a nontoxic derivative of the lipid A from Salmonella minnesota. MPL was solubilized in triethanolamine (TeoA) by sonication; stock solutions contained 0.02% TeoA and 0.4% dextrose.
Vaccinations.
For mice, vaccines containing a total of 100 μg of M. tuberculosis CFP were emulsified in MPL-TeoA adjuvant. In addition, some preparations contained rIL-12 (500 ng per mouse) or rIL-2 (100 μg per mouse). Vaccines were given twice, subcutaneously, 3 weeks apart. In mice which received both cytokines, rIL-12 was included in the first immunization and rIL-2 was included in the second. BCG at 106 bacilli was injected subcutaneously and was given once at the same time as the second immunizations. Animals were aerogenically challenged with approximately 100 M. tuberculosis bacilli 30 days after vaccination.
Guinea pigs were immunized with 150 μg of CFP in MPL-TeoA. In addition, some vaccines contained rIL-12 (1 μg) and/or rIL-2 (20 μg). Vaccines were injected subcutaneously three times, at 3-week intervals. The vaccine containing CFP and IL-2 was given only twice due to a slight hypersensitivity reaction in some animals. BCG (103 bacilli/guinea pig) was injected intradermally (i.d.) once at the same time as the third immunizations. The animals were aerogenically challenged with approximately 50 M. tuberculosis bacilli 6 weeks later.
DNA vaccines, consisting of the control plasmid vector V1Jns (DNA-vector) and V1Jns containing the genes encoding the secreted and nonsecreted forms of M. tuberculosis Ag85A protein (DNA-Ag85A), were kindly provided by the Merck Research Laboratories (West Point, Pa.). Vaccines were given intramuscularly three times at 3-week intervals. Each guinea pig was given 200 μg of plasmid DNA in saline per quadricep muscle (400 μg total per immunization). The animals were then infected aerogenically as above.
Delayed-type hypersensitivity (DTH) measurements.
Tuberculin PPD (lot CT68) was purchased from the Connaught Laboratories (Toronto, Canada). Mice were injected in the left hind footpad with 5 μg of PPD in 50 μl of sterile saline via a 30-gauge needle. Phosphate-buffered saline (PBS) alone was injected into the contralateral footpad as a negative control. Footpad thickness was measured 48 h later with calipers capable of measuring 0.05-mm increments in thickness.
Guinea pigs were shaved on the back, and 1 μg of each skin test reagent suspended in 50 μl of sterile saline was injected i.d. into different sites via a 30-gauge needle. The skin test reagents consisted of saline and bovine serum albumin, as negative controls, PPD, M. tuberculosis H37Rv lipoarabinomannan-free purified CFP, and purified 19-kDa, 45-kDa, and Ag85A proteins from M. tuberculosis. Induration was measured 48 h after injection by using a dial gauge caliper.
Histological analysis.
Tissues were fixed in 10% neutral buffered formalin for routine microscopic processing. All tissues were stained with hematoxylin and eosin. In each case, the left lower lobe was sagittally sectioned through the middle of the lobe. The tissues were coded and evaluated by a board-certified pathologist without knowledge of the treatment groups.
The following parameters were subjectively assessed in tissue sections: severity (degree of parenchymal involvement), size of typical granulomas, amount of caseous necrosis, relative number of neutrophils and lymphocytes, degree to which lymphocytes were organized in the granuloma, and extent to which the granulomas were organized (sharp demarcation from surrounding tissue, often with lymphocytes and/or fibrosis at the periphery). Sections were evaluated at least twice without knowledge of treatment or previous grading, and the results were reproducible. Although there was some lesion variability within vaccination groups, presumably due to the use of outbred animals, this variability was much less pronounced than was lesion variability between vaccine groups.
RESULTS
Protection studies with mice.
Because of the expense of the guinea pig model, a comprehensive series of pilot experiments was conducted with mice. Two inoculations were given, 3 weeks apart, followed by an aerosol challenge with M. tuberculosis Erdman 4 weeks later, and bacterial numbers in the lungs were determined 30 days postchallenge. It was decided to avoid strong adjuvants in these studies because (i) it would be unlikely that such preparations could be used in humans, and (ii) they also induce very strong DTH reactions. As a result, studies were performed with the adjuvant MPL-TeoA, which is known to enhance both cell-mediated and humoral responses. This adjuvant, which is currently undergoing human clinical safety trials (8, 12), has many of the immunomodulatory properties of lipopolysaccharide without the toxicity typically associated with endotoxins. MPL induces macrophages to secrete several cytokines including IL-1, IL-6, IL-8, granulocyte-macrophage colony-stimulating factor, and tumor necrosis factor (14) and enhance antigen uptake, processing, and presentation (25).
In initial studies, CFP given in alum or in MPL was not effective (data not shown). As a result, several further experiments were conducted in which the vaccine formulation was supplemented with IL-12, given the property of this cytokine to enhance the secretion of the protective cytokine gamma interferon by TH1-type CD4 T cells in several infectious-disease models (1, 5–7, 15, 26, 27), and with IL-2 in an attempt to expand the number of useful clones of mycobacterium-specific T cells. (A long-lived form of IL-2 conjugated to polyethylene glycol was used; this material has been reported to exhibit a 15-fold decrease in plasma IL-2 clearance compared with that exhibited by unmodified IL-2 [28].)
The results of one of several representative experiments are shown in Table 1. The BCG control-vaccinated mice had a 1.34-log reduction in bacterial numbers in the lungs, compared to PBS controls. None of the vaccine formulations containing various combinations of MPL, CFP, and cytokines had any protective effect, except when both IL-2 and IL-12 were administered, resulting in a statistically significant 0.91-log reduction in bacterial load. Combinations of adjuvant and cytokines had no effect alone (data not shown). Interestingly, despite this protective effect, these animals showed no evidence of DTH reactivity to PPD injection.
TABLE 1.
Vaccine-induced protection in the mouse model
| Immunization group | Log10 mean viable bacteria in the lung ± SEa | Log10 protection | Mean DTH response (mm) ± SDb |
|---|---|---|---|
| PBS | 4.77 ± 0.07 | 0 | 0 ± 0 |
| BCG | 3.43 ± 0.22* | 1.34 | 0.4 ± 0.28 |
| MPL | 4.88 ± 0.08 | −0.12 | 0 ± 0 |
| MPL-CFP | 5.03 ± 0.21 | −0.27 | 0 ± 0 |
| MPL–IL-12 | 4.81 ± 0.08 | −0.05 | 0 ± 0 |
| MPL–CFP–IL-12 | 4.46 ± 0.11 | 0.31 | 0.025 ± 0 |
| MPL–CFP–IL-2 | 4.58 ± 0.16 | 0.18 | 0 ± 0 |
| MPL–CFP–IL-12–IL-2 | 3.86 ± 0.08* | 0.91 | 0 ± 0 |
Protective efficacy in mice of a subunit vaccine containing MPL–CFP–IL-12–IL-2 4 weeks after an aerogenic infection with M. tuberculosis. Data are presented as log10 CFU ± standard error (SE), where n = 4. Asterisks represent statistical significance (P < 0.05) based on the unpaired, two-tailed Student t test.
DTH response measured 48 h after injection of PPD into the left footpads of vaccinated mice 2 days before infection with M. tuberculosis. Data are presented as the difference in footpad thickness (left footpad thickness [PPD] minus right footpad thickness [saline]).
Short-term protection responses in guinea pigs.
Experiments were then performed with guinea pigs to determine if similar conditions of cytokine enhancement were necessary for protection in this susceptible animal model. Vaccines were given three times, 3 weeks apart, to groups of outbred guinea pigs, which were then challenged via aerosol infection with approximately 50 viable M. tuberculosis H37Rv bacilli six weeks following the last injection.
The results of this study (Table 2) showed a 1.35-log reduction in the lungs of guinea pigs that had received intradermal (i.d.) BCG vaccination. A marginal but statistically significant (P = 0.048) reduction in bacterial counts within the lungs was also seen in guinea pigs given the MPL-CFP vaccine which had been supplemented with IL-12 and IL-2. None of the other vaccine groups exhibited any statistically significant reduction in lung bacterial counts.
TABLE 2.
Vaccine-induced protection in the guinea pig model
| Immunization group | Log10 mean viable bacteria (right lung lobe) ± SEa | Log10 protection (lungs) | Log10 mean viable bacteria (spleen) ± SEb | Log10 protection (spleen) |
|---|---|---|---|---|
| PBS | 5.75 ± 0.16 | 0 | 5.58 ± 0.22 | 0 |
| BCG | 4.40 ± 0.10* | 1.35 | 2.30 ± 0.00* | 3.28 |
| MPL | 5.49 ± 0.22 | 0.26 | 5.10 ± 0.29 | 0.48 |
| MPL/CFP | 5.79 ± 0.25 | −0.03 | 5.16 ± 0.48 | 0.42 |
| MPL/CFP/IL-12 | 6.14 ± 0.19 | −0.39 | 5.36 ± 0.30 | 0.23 |
| MPL/CFP/IL-2 | 6.06 ± 0.17 | −0.31 | 5.90 ± 0.26 | −0.32 |
| MPL/CFP/IL-12/IL-2 | 5.21 ± 0.05* | 0.54 | 4.69 ± 0.23* | 0.89 |
| DNA-vector | 5.82 ± 0.07 | −0.07 | 5.80 ± 0.31 | −0.22 |
| DNA-Ag85A | 5.68 ± 0.20 | 0.07 | 5.00 ± 0.12 | 0.59 |
Bacterial counts in the right lower lung lobe of guinea pigs immunized with the indicated vaccines and aerogenically infected with M. tuberculosis 6 weeks after vaccination. Data are represented as the mean CFP/right lung lobe ± standard error (n = 4). Asterisks represent statistical significance (P < 0.05) based on the unpaired, two-tailed Student t test.
Bacterial counts in the spleens of guinea pigs immunized with the indicated vaccines and aerogenically infected with M. tuberculosis 6 weeks after vaccination. Data are represented as the mean CFP/spleen ± standard error (n = 4).
All the guinea pigs used in these studies were tested for DTH against a panel of mycobacterial antigens just before aerosol challenge. Animals were injected i.d. on the back, and induration was measured 48 h later. As shown in Fig. 1, animals vaccinated with MPL formulations gave weak responses to purified CFP but not to PPD. Only BCG-vaccinated guinea pigs gave strong responses to most of the antigen panel.
FIG. 1.
Development of DTH reactions (induration) 48 h after i.d. injection with a panel of mycobacterial antigens in guinea pigs previously immunized with the indicated vaccines. Data are presented as the mean diameter of induration ± standard deviation (n = 4).
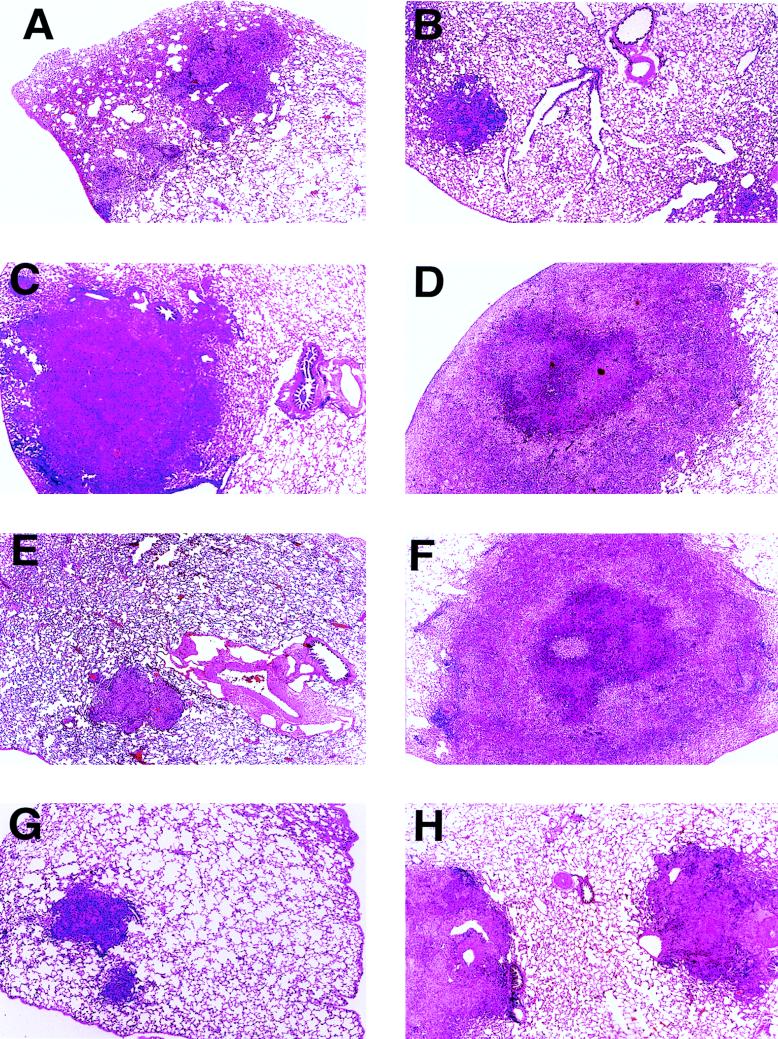
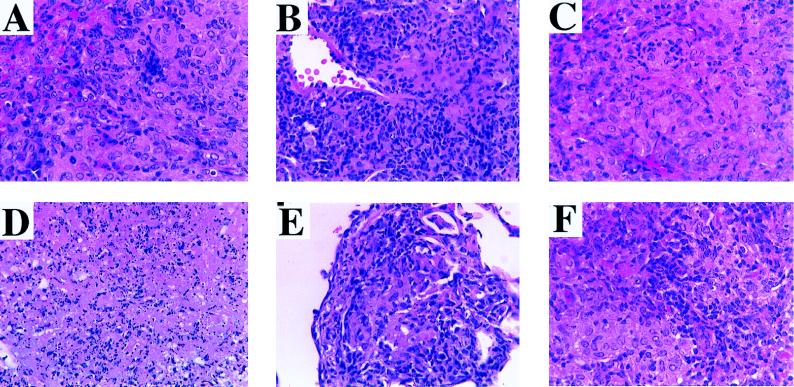
Representative histological appearances of the lung tissues of each group of infected guinea pigs 30 days after aerosol infection are shown in Fig. 2. Cellular details of the granulomatous lesions are depicted in Fig. 3. In the animals injected with PBS, the lung lesions tended to be moderately large (approximately 50% of the pulmonary parenchyma was involved [Fig. 2A]), with scattered infiltrates of lymphocytes admixed with epithelioid macrophages (Fig. 3A) and only modest organization. In contrast, granulomas in the lungs of BCG-vaccinated animals affected a smaller portion of the pulmonary parenchyma (approximately 25 to 33%), were small and compact with sharp lines of demarcation to the surrounding parenchyma (Fig. 2B), and had increased numbers of lymphocytes throughout the lesions (Fig. 3B). No caseation was observed in the BCG group, and caseation was minimal in the saline control group. In animals inoculated with MPL alone, MPL-CFP, or MPL–CFP–IL-12, large portions of the lungs were affected (≥50%), with individual granulomas characterized by large size, modest demarcation from the surrounding parenchyma (Fig. 2C, D, and F), occasional small areas of central caseation (Fig. 3D), and generally scant, unorganized lymphocyte infiltrations (Fig. 3C). Animals vaccinated with MPL–CFP–IL-2 (Fig. 2E) and MPL–CFP–IL-12–IL-2 (Fig. 2G) developed granulomas that were small and of limited extent, similar to the BCG group. Lesion size and extent were intermediate in the lungs of DNA-Ag85A-vaccinated animals (Fig. 2H). The lesions in the last three groups contained lymphocytic infiltrates varying from scattered to heavier infiltrates (Fig. 3E and F). Lesion demarcation was sharp, and caseation was not observed.
FIG. 2.
Representative photomicrographs of lung tissue sections harvested from vaccinated guinea pigs 30 days after an aerosol infection with M. tuberculosis H37Rv. (A) PBS control. Approximately 50% of the parenchyma is replaced by multiple, moderately sized granulomas. (B) BCG. The limited area of affected parenchyma contains small, focal granulomas. (C) MPL control. There is extensive parenchymal destruction by a large, poorly demarcated granuloma. (D) MPL-CFP. This lesion is similar to that in the MPL control (C), except that this extensive granuloma has central caseation (see Fig. 3D). (E) MPL–CFP–IL-2. A small, sharply demarcated granuloma affects a minimal amount of parenchyma. (F) MPL–CFP–IL-12. This lesion is essentially identical to that in the MPL-CFP animal (D). (G) MPL–CFP–IL-12–IL-2. The limited parenchymal involvement is characterized by small granulomas similar to those found with BCG treatment (B) and MPL–CFP–IL-2 treatment (E). (H) DNA-Ag85A. An intermediate amount of parenchyma is affected by moderately sized, well-demarcated granulomas. Magnification, ×20.
FIG. 3.
Higher-magnification photomicrographs of some of the lesions depicted in Fig. 2, demonstrating cytological details. (A) PBS control. Scattered lymphocytes are admixed with epithelioid macrophages. (B) BCG. Numerous lymphocytes are present throughout the section. There is no caseation. (C) MPL control. Note the relative paucity of lymphocytes amid numerous foamy macrophages. (D) MPL-CFP. This section demonstrates an area of central caseation, the same lesion seen in the lungs of MPL–CFP–IL-12-vaccinated animals (higher magnification not included). (E) MPL–CFP–IL-2. Note the similarity to the lesion in the BCG control (B). This is essentially the same lesion observed in MPL–CFP–IL-2–IL-12-vaccinated animals (higher magnification not included). (F) DNA-Ag85A. Relatively numerous lymphocytes are admixed with fields of epithelioid macrophages. Hematoxylin and eosin stain; magnifications, ×156.
Long-term responses in guinea pigs.
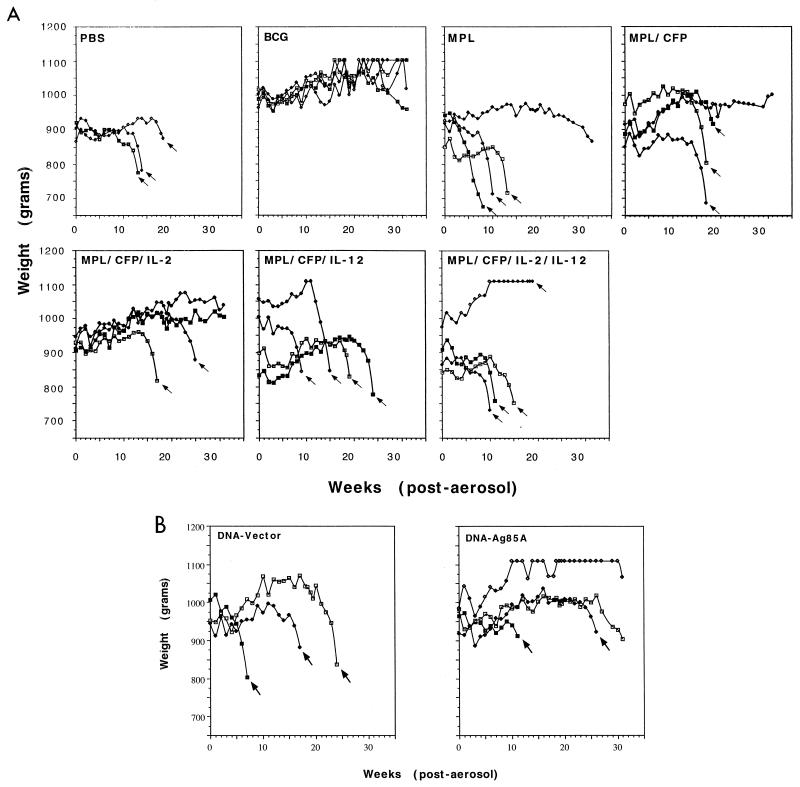
The results of the study of long-term responses in guinea pigs are shown in Fig. 4. Placebo control animals (PBS or MPL alone) began to lose weight about 2 weeks after aerosol challenge, after which time their weight plateaued for several weeks. Then, 8 to 18 weeks postchallenge, these animals died. One animal in the MPL group began to lose weight at 15 weeks, but not quite at a rate at which euthanasia was deemed appropriate.
FIG. 4.
Body weights of individual guinea pigs, given subunit vaccines (A) or DNA vaccines (B), after aerogenic infection with M. tuberculosis. Arrows indicate the last weight measurement before death. All remaining animals were euthanized at 31 weeks postinfection.
Animals given MPL-CFP increased in weight past 10 weeks and appeared relatively healthy, but then three of the four died around week 16. One guinea pig with significantly lower lung bacterial counts (see below) survived to the end of the experiment (212 days).
A very surprising result was seen in the MPL–CFP–IL-12–IL-2 group, which was the only group to show significant protection at day 30 after aerosol challenge. Three of the four animals did not thrive and died between 11 and 16 weeks. The fourth animal gained weight but also died 20 weeks post challenge. A similar result was seen in animals given MPL–CFP–IL-12.
Somewhat better results were seen when only IL-2 was added to the MPL-CFP vaccine. One guinea pig began to lose weight 17 weeks postinfection and died shortly thereafter, and another guinea pig in this group died after 27 weeks. The remaining two guinea pigs, however, continued to thrive and still appeared healthy when the study was terminated.
In the BCG-vaccinated group, three of four animals appeared healthy throughout, while one animal began to lose weight and was found to have higher bacterial counts in the lungs than the others did (Table 3).
TABLE 3.
Bacterial load data for long-term survivors
| Group | Time to death (days)/bacterial load (log) in animal:
|
|||
|---|---|---|---|---|
| 1 | 2 | 3 | 4 | |
| PBS | 98/7.84 | 103/7.45 | 138/5.87 | NDa,b/NAg |
| MPL | 61/8.01 | 74/8.46 | 94/7.04 | 221d/4.84 |
| MPL-CFP | 126/7.46 | 126/7.62 | 136/7.07 | 221d/4.45 |
| MPL–CFP–IL-2–IL-12 | 79/NDc | 82/6.43 | 109/NDc | 140/5.93 |
| MPL–CFP–IL-12 | 70/7.05 | 109/7.41 | 139/6.71 | 172/NDc |
| MPL–CFP–IL-2 | 131/8.24 | 181/NDc | 221d/6.58 | 221d/4.79 |
| DNA-Ag85A | 89/NDc | 194e/7.82 | 221d/6.69 | 221d/6.16 |
| DNA-vector | NDb/NA | 55/7.61 | 127/7.15 | 177/NDc |
| BCG | 221d/3.31 | 221d/4.19 | 221d/6.54f | 221d/4.77 |
ND, not determined.
Animal died before aerosol challenge for unknown reasons.
Animal died overnight and the tissues autolysed.
Animal survived length of experiment [221 days].
Animal had extensive lymphadenopathy or lymphoma.
Animal was starting to lose weight.
NA, not applicable.
In a parallel experiment testing the vaccine efficacy of DNA-Ag85A, one animal died after 11 weeks and another died at 26 weeks (this animal had signs of lymphadenopathy, which may have contributed to its death). Two others, however, appeared healthy at the end of the study (although one was starting to show evidence of weight loss). Vector control guinea pigs died between 6 and 23 weeks.
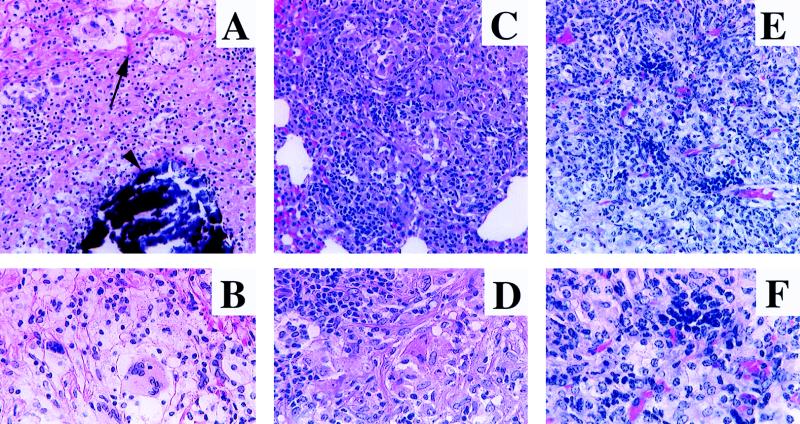
Histological examination of survivors and animals that died during the experiment revealed dramatic differences (representative examples are shown in Fig. 5, with cytological details given in Fig. 6). Lungs from placebo control groups (PBS, MPL, DNA-vector) as well as from the CFP-MPL group exhibited extensive (>80%) granulomatous pneumonia throughout the vast majority of their tissue (Fig. 5A to C), with dystrophic calcification observed within the centers of caseation (Fig. 6A). Granulomas within the lungs of BCG and surviving MPL–CFP–IL-2 vaccinated animals were smaller and more compact, involving less than 50% of the lung tissue (Fig. 5D and E), whereas granulomatous pneumonia involving about 75% of the lung parenchyma was observed in animals vaccinated with the DNA-Ag85A vaccine (Fig. 5F). Granulomas in each of the surviving animals, primarily those from the BCG, MPL–CFP–IL-2, and DNA-Ag85A groups, were highly lymphocytic (Fig. 6C to F), whereas only scattered lymphocytes were seen in groups of animals dying of infection (Fig. 6A and B). Significantly, even animals from the DNA-Ag85A group, with extensive granulomatous pneumonia, lacked pulmonary necrosis and caseation and had a high percentage of lymphocytes within the lesions (Fig. 5F and 6E and F).
FIG. 5.
Representative photomicrographs of lungs from vaccinated guinea pigs infected via the aerosol route with M. tuberculosis H37Rv at least 15 weeks previously. (A) PBS control euthanized due to rapid weight loss after 15 weeks. Granulomatous pneumonia replaces the vast majority of the parenchyma. (B) MPL-CFP-vaccinated animal that died 18 weeks postinfection. The lesions are similar to those seen in the PBS control (A). (C) DNA-vector control animal that died 18 weeks postinfection. The lesions are similar to those seen in the PBS control (A). (D) BCG-vaccinated animal euthanized at 31 weeks postinfection (study termination). Smaller, discrete granulomas affect approximately 40 to 50% of the pulmonary parenchyma. (E) MPL–CFP–IL-2-vaccinated animal euthanized at 31 weeks (study termination). The extent of the lesions is very similar to that in the BCG-vaccinated animal (D). (F) DNA-Ag85A-vaccinated animal euthanized at 31 weeks (study termination). The lesions are extensive, involving 75 to 80% of the parenchyma. Hematoxylin and eosin stain; magnifications, ×3.5.
FIG. 6.
Higher-magnification photomicrographs of some of the lesions depicted in Fig. 5, demonstrating cytological detail. (A) PBS control (same animal as in Fig. 5A). Areas of fibrosis (arrow) surround a zone of necrosis, characterized by cytolysis (indistinct cells and cell margins) and karyolysis, with a central core of dystrophic calcification (deeply basophilic deposits [arrowhead]). Magnification, ×103.5. (B) Higher magnification of the specimen in panel A. Note the vast predominance of epithelioid macrophages and a multinucleated giant cell. Magnification, ×172.5. (C) BCG-vaccinated animal (same animal as in Fig. 5D). Note the decreased fibrosis, increased number of lymphocytes, lack of necrosis, and excellent granuloma organization compared to PBS controls (A). Magnification, ×103.5. (D) Higher magnification of the specimen in panel C. A typical section with abundant lymphocytes and numerous macrophages is shown. Magnification, ×172.5. (E) DNA-Ag85A-vaccinated animal (same animal as in Fig. 5F). Note the extensive granulomatous pneumonia, similar in cytological makeup to that in the BCG-vaccinated animal (C) and the MPL–CFP–IL-2-vaccinated animal (higher magnification not shown). Magnification, ×121. (F) Higher magnification of the specimen in panel E. A typical section similar to that for the BCG-vaccinated animal is shown. Magnification, ×224. All panels stained with hematoxylin and eosin.
In general, there was good correlation between mortality of individual animals and bacterial counts (Table 3). Given that the bacterial load 30 days after aerosol inoculation was in the 5.5- to 6.0-log range, some surviving animals showed evidence of reduction (<5 log) whereas mortality was invariably associated with counts in the 7- to 8-log range.
DISCUSSION
The results of this study provide the first evidence that two nonliving vaccine formulations, one based on the CFP of M. tuberculosis and the other consisting of a DNA vaccine encoding a major immunogenic antigen of CFP (Ag85; mycolyl transferase [3]), can confer in the definitive guinea pig model increased survival compared to appropriate controls and prevention of caseating disease that gradually develops 10 to 20 weeks after aerosol infection. Instead, infected animals immunized with both vaccines developed lung disease similar to that seen in BCG-vaccinated control animals, characterized by a lymphocytic form of granulomatous response. Hence, while these new vaccines did not prevent mortality in a manner comparable to that conferred by BCG, these results generate optimism that a new generation of nonliving vaccines can be developed in the near future.
It was noticeable that with one exception other than BCG, bacterial counts in the lungs of both animal models were not reduced below those of placebo controls 30 days after aerosol challenge (the “gold standard” in most studies published to date). However, at least two vaccines showed evidence of causing prolonged survival compared to controls. This may be providing an important lesson, namely, that short-term reduction in bacterial counts may not, in fact, be the most important criterion and that survival/pathology data in the guinea pig model may in fact give a better picture of the long-term effectiveness of a vaccine. We believe our results suggest that the survival data in this model is influenced by the type of lesion produced. While lesion severity is undoubtedly a critical component of survival, the cytological character of the lesion within the DNA-Ag85A group, particularly the degree of lymphocytic infiltration, may have a better correlation with survival in this model.
This concept was best illustrated by the results obtained with formulations containing IL-12. When IL-12 was given with IL-2, day 30 protection was seen in both models. However, 8 to 10 weeks after aerosol challenge of guinea pigs, these animals all began to die. The reason for this is not known, but our speculation at present is that IL-12 enhances an initial gamma interferon response by T cells but in the process drives these antigen-reactive cells into a short-lived mode. As a result, these cells are absent when the infection begins to progress or reactivate a few weeks later.
Much better survival and less severe lung disease (characterized by increased lymphocyte infiltration) were seen if IL-2 alone was added to the MPL-CFP vaccine. These data suggest the hypothesis, therefore, that IL-2 was sufficient to drive a long-lived memory T-cell response to this vaccine formulation. This is certainly an approach worthy of further investigation, especially considering the almost identical lung disease seen in these animals and BCG controls (Fig. 5D and E).
Similar disease, although with more lung consolidation, was seen in animals given the DNA vaccine. In contrast to earlier studies in the mouse in which some degree of protection was observed (11), the DNA-Ag85A did not reduce bacterial numbers at day 30 in the guinea pig; nevertheless, there was long-term lymphocytic granulomatous disease similar to (but more extensive than in) the BCG controls. Whether this observation reflects strong generation of memory immunity or prolonged stimulation of the immune system through expression of Ag85 antigen by host muscle cells is not currently known.
There was no apparent association between an increase in bacterial numbers in the surviving groups and lung consolidation; if anything, bacterial numbers were reduced. This implies, therefore, that it was the continuing host granulomatous response to the infection, rather than the infection per se, that was responsible for the progressive infiltration of lung tissue and the eventual death of the animals, a process which would probably eventually kill the BCG-vaccinated group. This in turn implies, therefore, that dampening the inflammatory response in the lungs (without reactivating the remaining bacteria) should further lengthen survival times.
To the very limited extent to which they can be compared, the findings of this study are similar to those of Horwitz et al. (9), who showed that various components of CFP delivered in a different (Syntex) adjuvant resulted in small reductions in bacterial load in the lungs and protected these animals for 10 weeks after aerosol challenge. An appropriate BCG control was not included in that study, but even so we would argue that the experiments were curtailed long before any pulmonary caseation had fully developed, hence preventing demonstration of this central tenet of the guinea pig model.
Our different tactic, to use a mild adjuvant rather than a potent one and then enhance it by the use of IL-2, not only was successful in the guinea pig model but had the additional benefit of not inducing a skin test reaction to PPD. As a result, this vaccine should not disable the clinical diagnosis test. (We should emphasize, however, that these animals still gave small reactions to purified CFP, indicating that they are not actually anergic.)
Turning to practical matters, how could these new vaccines be used in the clinical setting? The majority of the worldwide population has been given BCG or is otherwise sensitized by exposure to environmental mycobacteria. As we have discussed elsewhere (18), perhaps a more realistic use of these new vaccines (given the fact that their capacity to confer survival was less than that conferred by BCG) would be to boost individuals already previously vaccinated or those who may be at risk of reactivation disease due to latent tuberculosis or drug-resistant tuberculosis that is refractory to treatment. To address these questions, we are conducting experiments with guinea pigs vaccinated in early life with BCG to see if boosting with the new vaccines in midlife will prolong survival over that obtained with BCG alone, as well as experiments with inbred strains of mice prone to reactivation tuberculosis to see if this event can be prevented or delayed.
ACKNOWLEDGMENTS
This work was supported by NIH grant AI-75320, by a grant from the Colorado Institute for Biotechnology, and by generous contributions from Merck, Chiron Corp., and Ribi Immunochem.
We thank Elisa French for performing necropsies on the long-term-infected guinea pigs and the staff at the CSU Laboratory of Animal Resources for their excellent care and monitoring of the mice and guinea pigs. We are very grateful to J. Terry Ulrich, and Marty Giedlin for their invaluable advice and to Mike Jessen for technical assistance.
REFERENCES
- 1.Afonso L C C, Scharton T M, Vieira L Z, Wysocka M, Trinchieri G, Scott P. The adjuvant effect of interleukin-12 in a vaccine against Leishmania major. Science. 1994;263:235–237. doi: 10.1126/science.7904381. [DOI] [PubMed] [Google Scholar]
- 2.Andersen P. Effective vaccination of mice against Mycobacterium tuberculosis infection with a soluble mixture of secreted mycobacterial proteins. Infect Immun. 1994;62:2536–2544. doi: 10.1128/iai.62.6.2536-2544.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Belisle J T, Vissa V D, Sievert T, Takayama K, Brennan P J, Besra G S. Role of the major antigen of Mycobacterium tuberculosis in cell wall biogenesis. Science. 1997;276:1420–1422. doi: 10.1126/science.276.5317.1420. [DOI] [PubMed] [Google Scholar]
- 4.Bloom B R, Fine P E M. The BCG experience: implications for future vaccines against tuberculosis. In: Bloom B R, editor. Tuberculosis: pathogenesis, protection, and control. Washington, D.C: ASM Press; 1994. pp. 531–557. [Google Scholar]
- 5.Castro A G, Silva R A, Appelberg R. Endogenously produced IL-12 is required for the induction of protective T cells during Mycobacterium avium infections. J Immunol. 1995;155:2013–2019. [PubMed] [Google Scholar]
- 6.Cooper A M, Roberts A D, Rhoades E R, Callahan J E, Getzy D M, Orme I M. The role of interleukin-12 in acquired immunity to Mycobacterium tuberculosis infection. Immunology. 1995;84:423–432. [PMC free article] [PubMed] [Google Scholar]
- 7.Flynn J L, Goldstein M M, Triebold K J, Sypek J, Wolf S, Bloom B R. IL-12 increases resistance of BALB/c mice to Mycobacterium tuberculosis infection. J Immunol. 1995;155:2515–2524. [PubMed] [Google Scholar]
- 8.Fries L F, Gordon D M, Richards R L, Egan J E, Hollingdale M R, Gross M, Silverman C, Alving C R. Liposomal malaria vaccine in humans: a safe and potent adjuvant strategy. Proc Natl Acad Sci USA. 1992;89:358–362. doi: 10.1073/pnas.89.1.358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Horwitz M A, Lee B-W E, Dillon B J, Harth G H. Protective immunity against tuberculosis induced by vaccination with major extracellular proteins of Mycobacterium tuberculosis. Proc Natl Acad Sci USA. 1995;92:1530–1534. doi: 10.1073/pnas.92.5.1530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hubbard R D, Flory C M, Collins F M. Immunization of mice with mycobacterial culture filtrate proteins. Clin Exp Immunol. 1992;87:94–98. doi: 10.1111/j.1365-2249.1992.tb06419.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Huygen K, Content J, Denis O, Montgomery D L, Yawman A M, Deck R R, DeWitt C, Orme I M, Baldwin S, D’Souza C, Drowart A, Lozes E, Vandenbussche P, Van Vooren J-P, Liu M A, Ulmer J B. Immunogenicity and protective efficacy of a tuberculosis DNA vaccine. Nat Med. 1996;2:893–898. doi: 10.1038/nm0896-893. [DOI] [PubMed] [Google Scholar]
- 12.Koutsoukos M, Leroux G, Vandepapeliere P, Slaoui M, Pala P. Program and abstracts of the 34th Interscience Conference on Antimicrobial Agents and Chemotherapy. Washington, D.C: American Society for Microbiology; 1994. Induction of cell mediated immune responses in man with vaccines against herpes simplex virus based on glycoprotein D; p. 217. [Google Scholar]
- 13.McMurray D N. Guinea pig model of tuberculosis. In: Bloom B R, editor. Tuberculosis: pathogenesis, protection, and control. Washington, D.C: ASM Press; 1994. pp. 113–134. [Google Scholar]
- 14.Mitchell M S, Harel W, Kempf R A, Hu E, Kan-Mitchell J, Boswell W D, Dean G, Stevenson L. Active specific immunotherapy for melanoma: phase I trial of allogeneic lysates and a novel adjuvant. Cancer Res. 1990;48:5883–5893. [PubMed] [Google Scholar]
- 15.Mountford A P, Anderson S, Wilson R A. Induction of Th1 cell-mediated protective immunity to Schistosoma mansoni by co-administration of larval antigens and IL-12 as an adjuvant. J Immunol. 1996;156:4739–4745. [PubMed] [Google Scholar]
- 16.Orme I M. Induction of nonspecific acquired resistance and delayed-type hypersensitivity, but not specific acquired resistance, in mice inoculated with killed mycobacterial vaccines. Infect Immun. 1988;56:3310–3312. doi: 10.1128/iai.56.12.3310-3312.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Orme I M. Progress in the development of new vaccines against tuberculosis. Int J Tuberc Lung Dis. 1997;1:95–100. [PubMed] [Google Scholar]
- 18.Orme I M. Prospects for new vaccines against tuberculosis. Trends Microbiol. 1995;3:401–404. doi: 10.1016/s0966-842x(00)88987-8. [DOI] [PubMed] [Google Scholar]
- 19.Orme I M, Andersen P, Boom W H. T cell response to Mycobacterium tuberculosis. J Infect Dis. 1993;167:1481–1497. doi: 10.1093/infdis/167.6.1481. [DOI] [PubMed] [Google Scholar]
- 20.Pal P G, Horwitz M A. Immunization with extracellular proteins of Mycobacterium tuberculosis induces cell-mediated immune responses and substantial protective immunity in a guinea pig model of pulmonary tuberculosis. Infect Immun. 1992;60:4781–4792. doi: 10.1128/iai.60.11.4781-4792.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Raviglione M C, Snider D E, Kochi A. Global epidemiology of tuberculosis. JAMA. 1995;273:220–226. [PubMed] [Google Scholar]
- 22.Roberts A D, Sonnenberg M J, Ordway D J, Furney S K, Brennan P J, Belisle J T, Orme I M. Characteristics of protective immunity engendered by vaccination of mice with purified culture filtrate protein antigens of Mycobacterium tuberculosis. Immunology. 1995;85:502–508. [PMC free article] [PubMed] [Google Scholar]
- 23.Roche P W, Triccas J A, Winter N. BCG vaccination against tuberculosis: past disappointments and future hopes. Trends Microbiol. 1995;3:397–401. doi: 10.1016/s0966-842x(00)88986-6. [DOI] [PubMed] [Google Scholar]
- 24.Rodrigues L, Smith P G. Tuberculosis in developing countries and methods for its control. Trans R Soc Trop Med Hyg. 1990;84:739–744. doi: 10.1016/0035-9203(90)90172-b. [DOI] [PubMed] [Google Scholar]
- 25.Rudbach J A, Johnson D A, Ulrich J T. RIBI adjuvants: chemistry, biology and utility in vaccines for human and veterinary medicine. In: Stewart-Tull D E S, editor. The theory and practical application of adjuvants. New York, N.Y: John Wiley & Sons, Inc.; 1995. pp. 287–313. [Google Scholar]
- 26.Saunders B M, Zhan Y, Cheers C. Endogenous interleukin-12 is involved in resistance of mice to Mycobacterium avium complex infection. Infect Immun. 1995;63:4011–4015. doi: 10.1128/iai.63.10.4011-4015.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sypek J P, Chung C L, Mayor S E H, Subramanyam J M, Goldman S L, Sieburth D S, Wolf S F, Schaub R G. Resolution of cutaneous leishmaniasis: interleukin 12 initiates a protective T helper type 1 immune response. J Exp Med. 1993;177:1797–1802. doi: 10.1084/jem.177.6.1797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Teppler H, Kaplan G, Smith K A, Montana A L, Meyn P, Cohn Z A. Prolonged immunostimulatory effect of low-dose polyethylene glycol interleukin-2 in patients with human immunodeficiency virus type 1 infection. J Exp Med. 1993;177:483–492. doi: 10.1084/jem.177.2.483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.World Health Organization. Trial of BCG vaccines in South India for tuberculosis prevention. Bull W H O. 1979;57:810–827. [PMC free article] [PubMed] [Google Scholar]