Abstract
Background:
Tumour budding has been recognized as a morphologic marker of tumour invasion. Invasive characteristics such as depth of invasion, mode of invasion and worst pattern of invasion are potentially powerful parameters predicting the regional metastasis.
Aim:
This study was done to understand the significance of tumour budding and various characteristics of invasion and their impact on grading of oral squamous cell carcinoma.
Materials and Methods:
An immunohistochemical study was performed on tissue sections obtained from 34 paraffin-embedded blocks of clinically and histologically diagnosed cases of oral squamous cell carcinoma. The sections were stained with pan cytokeratin and observed under high power magnification.
Results:
Tumour budding and the invasive patterns were found to be significant in OSCC. A proposed grading system based on tumour budding and cell nest was found to have a significant correlation with the WHO grading system.
Conclusion:
This study demonstrated the importance of using tumour buds as an additional parameter in the grading system and also assessed the importance of invasive patterns, cellular atypia and stromal contents in OSCC.
Keywords: Depth of invasion, mode of invasion, oral squamous cell carcinoma, tumour budding
INTRODUCTION
Oral squamous cell carcinoma (OSCC) is the most common oral cancer, accounting for more than 90% of the cases that showed varying degrees of histological differentiation with high invasive and metastatic potential.[1] The overall survival rate of OSCC has remained less than 50% for more than a decade, despite advances in diagnosis and therapy.[2] Treatment plan and prognosis of OSCC are primarily based on the clinical staging and histologic grading system. The American Joint Committee on Cancer (AJCC) in cooperation with the TNM Committee of the Union for International Cancer Control (UICC) created the TNM staging system, which is used as the international standards for cancer recording, staging, prognosis, treatment planning and in therapeutics.[3] Although the system has been widely accepted, several studies have shown that the stratification of each patient prognosis lacks predictive accuracy to differentiate between high-risk groups and low-risk groups based on lymph node metastasis, local recurrence and patient survival.[4,5] A few studies have suggested that the major disadvantage in the use of TNM staging system is in the early-stage tongue squamous carcinoma, because of its high tendency for occult loco-regional metastasis.[6]
The WHO grading system is the most commonly used histologic grading system. According to the degree of differentiation, the tumours have been graded histologically as well, moderately and poorly differentiated OSCC.[7] Even though the WHO grading system has been used commonly, several studies have shown an inadequate correlation with the outcome and treatment response in individual patients. Small incisional biopsy tissues taken from a tumour that has diverse histological heterogeneity, sampling inadequacy, tumour structural characteristics dependency instead of functional ones, limited tumour cell assessment in host tissues and the surrounding stroma were the suggested reasons for the inaccuracy in the grading system.[8] To improve on this, in the recently updated eighth edition of the AJCC staging manual, the depth of invasion (DOI) was added to the T category, and the worst pattern of invasion (WPOI) was also added as an additional factor.
Tumour budding was initially designated as tumour sprouting to the stroma and was first reported in 1954 by Imai.[9] Recent studies have shown the presence of tumour budding as a promising prognosticator in OSCC. Elseragy et al. examined a series of early tongue SCC and reported that the addition of tumour budding to the WHO differentiation criteria has a better prognostic value than the conventional WHO system.[10] Tumour budding has been reported to be an independent prognostic factor for several cancers such as colorectal, pancreas, oesophageal, pulmonary and gastric cancer.[11,12,13,14,15,16]
This study is aimed to assess the various invasion patterns and tumour budding in OSCC and to compare a newer grading system that uses tumour budding, with that of the WHO grading system. In addition to these, cellular atypia (nuclear diameter, mitotic count and degree of keratinization) and stromal content were also assessed which reflects the behavioural pattern of tumour cells.
MATERIALS AND METHODS
Paraffin-embedded archival blocks of 34 cases of OSCC patients who underwent incisional biopsies were taken from the Department of Oral and Maxillofacial Pathology and were graded according to WHO criteria. Twelve cases of well-differentiated OSCC, 12 cases of moderately differentiated OSCC and 10 cases of poorly differentiated OSCC were taken for the study. All specimens were obtained with prior informed consent, and the study protocol was reviewed and approved by Institutional Ethics Committee of PMS College of Dental Science and Research, Thiruvananthapuram (IEC No. PMS/IEC/2022/REGULAR/APR/45).
Immunohistochemistry (IHC) procedure was used for the detection of tumour cells, wherein PAN cytokeratin (AE1/AE3) was used as the primary antibody and Poly Excel Poly HRP (pre-diluted, PEH002) was used as secondary antibody. Sections of 3μm thickness were mounted on APES-coated slides. These sections were deparaffinized in xylene and rehydrated through graded alcohol solutions, followed by wash with distilled water. For antigen retrieval, the slides were immersed in Tris/EDTA buffer and then subjected to steam pressure for 10-15 minutes. The slides were cooled and incubated with hydrogen peroxide for 10 minutes to block the endogenous peroxidase activity. The slides were subsequently incubated with the primary antibody (CK AE1/AE3-PathnSitu) for 45 minutes at room temperature. This was followed by incubation with secondary antibody for 20 minutes. The reaction was visualized with diaminobenzidine (DAB). All slides were counterstained with Mayer’s haematoxylin, dehydrated and mounted.
ASSESSMENT OF TUMOUR BUDDING AND INVASIVE PATTERNS
Immunohistochemically stained sections were evaluated using light microscopy. Tumour budding was assessed in areas showing maximal budding activity (BA) and was scored in both one high power field (HPF) and in 10 HPFs. In 1 HPF, low BA was defined as 1-4 budding nests and high BA as five budding nests. In 10 HPFs, low BA was defined as 1-14 nests and high BA if ≥15 budding nests were detected. Cell nests were defined as clustered tumour cells, surrounded by stroma and were classified based on the size of the smallest invasive cell nest. Clusters of >15 tumour cells were classified as large cell nests, 5-15 tumour cells as intermediate cell nests, 2-4 tumour cells as small cell nests, and single-cell invasion were stated for single tumour cell. Cell nest size (CNS) was assessed both at the invasive margin and within the tumour core region.[17]
Mode and pattern of invasion were graded based on criteria by Jakobsson et al[18]. DOI is measured by first finding the “horizon” of the basement membrane of the adjacent squamous mucosa. A perpendicular “plumb line” is established from this horizon to the deepest point of tumour invasion, which represents DOI. DOI is recorded in millimetres.[19,20,21]
Nuclear diameter of tumour cells was assessed within the tumour core region, which harbours the largest nuclei. The scoring was given by comparing the nuclei of small tumour-associated lymphocytes as the reference. If the largest nuclei of tumour cells measures ≤3 lymphocytes, then the score given was small, nuclei roughly matching the diameter of four lymphocytes were scored as intermediate, and nuclei with a diameter of >4 lymphocytes were classified as large. Tumour area with the highest mitotic activity was taken, and its frequency was counted in 10 HPFs (40x) and scored as <10, 10-20, 20-30, 30-40 and >40 in each field. The degree of keratinization was scored as weak (focal or single-cell keratinization), intermediate and strong (keratinization covering >30% of the whole tumour cell area). The amount of tumour-stromal content was classified as very low (<10% of the whole tumour area), low (>10% to 25%), moderate (>25% to 50%) or high (>50%).[17]
In analogy to Boxberg et al., scores for BA (1: no budding/10 HPFs; 2: <15 budding foci/10 HPFs; 3: ≥15 budding foci/10 HPFs) and CNS within the tumour core region (1: >15 cells; 2: 5-15 cells; 3: 2-4 cells; 4: single-cell invasion) were assigned. These two variables were summed up to obtain a grading score ranging from 2 to 7. Based on this analysis of sum scores, tumours with scores 2-3 were graded as well-differentiated (G0), with scores 4-6 as moderately differentiated (G1) and with score 7 as poorly differentiated (G2).[17] Table 1 shows the proposed grading system for OSCC using BA and CNS.
Table 1.
Proposed grading system for oral squamous cell carcinoma (OSCC) using tumour budding[17]
| Score | |
|---|---|
| Tumour budding activity (BA)/10 HPF | |
| No budding | 1 |
| <15 budding foci | 2 |
| >15 budding foci | 3 |
| Smallest cell nest size (CNS)/10 HPF | |
| >15 cells | 1 |
| 5-15 cells | 2 |
| 2-4 cells | 3 |
| Single-cell invasion | 4 |
| Tumour Grading | |
| Well-differentiated (G0) | 2-3 |
| Moderately differentiated (G1) | 4-6 |
| Poorly differentiated (G2) | 7 |
Statistical analysis
Fisher’s exact test was used to evaluate the correlations among tumour budding, mode of invasion, the worst pattern of invasion, depth of invasion and the histologic parameters such as cellular atypia and stromal contents. Multiple regression equation is used to find the significant linear relationship between the parameters and also BA and CNS with the proposed tumour grading. Agreement between the WHO and proposed grading system that considers BA and CNS was done with Cohen Kappa statistics. P value <0.05 was considered statistically significant.
RESULTS
According to the current WHO grading system, 35.29% (12/34) were well-differentiated OSCC, 35.29% (12/34) were moderately differentiated OSCC and 29.41% (10/34) were poorly differentiated OSCC. A small nuclear diameter of tumour cells was seen in 12% of OSCC (4/34), intermediate nuclear size in 15% (5/34) and large nuclear size in 73% (25/34) of cases. A weak degree of keratinization was noticed in 23.5% (8/34), intermediate keratinization in 23.5% (8/34), and strong keratinization in 53% (18/34) of cases. Very low stromal content was seen in 26% (9/34), low in 6% (2/34), moderate in 15% (5/34) and heavy stroll content in 53% (18/34) of cases. Type 1 worst pattern of invasion was seen in 15% (5/34) of cases, type 2 in 9% (3/34), type 3 in 15% (5/34), type 4 in 32% (11/34) and type 5 in 3% (10/34) of cases. Grade I mode of invasion was seen in 17.64% (6/34), grade 2 in 3% (1/34), grade 3 in 23% (8/34), grade 4C in 18% (7/34) and grade 4D in 35% (12/34) of cases. The depth of invasion was assessed only in three cases due to unavailability of adequate tissue specimens, which has the values between 5 and 10 mm [Figures 1 and 2].
Figure 1.
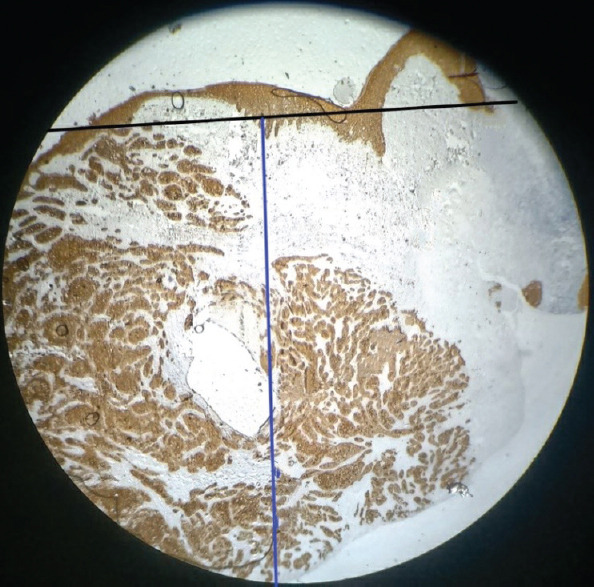
Representation of depth of invasion (7 mm); black line indicates the horizon of the basement membrane and blue line indicates the perpendicular plumb line established from the horizon
Figure 2.
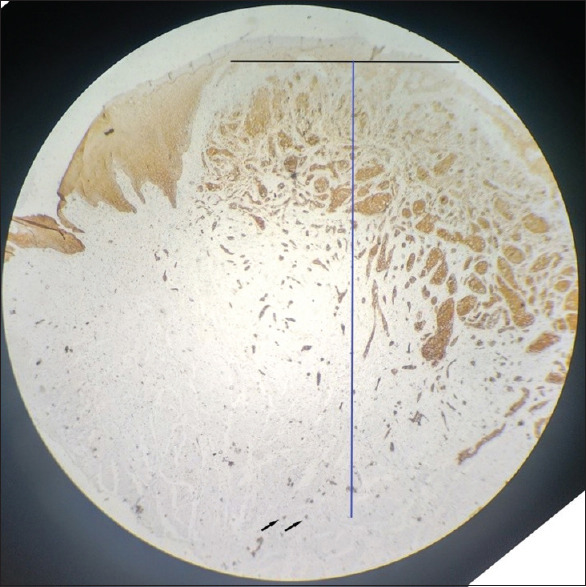
Representation of depth of invasion (6 mm). Black line indicates the horizon of the basement membrane and blue line indicates the perpendicular plumb line established from the horizon. Black arrows indicates tumour buds
A significant correlation was noted between tumour budding and nuclear diameter, degree of keratinization, stromal content and mitotic count (P < 0.05). Tumour budding also showed significant positive correlation with mode of invasion and worst pattern of invasion (P < 0.05). Mode of invasion showed high correlation value (0.9416) and exhibited significant linear regression relationship with WPOI [Table 2].
Table 2.
Multiple regression equation showing the significant linear relationship between the parameters
| Variable | Correlation | P | F calculated | F significant |
|---|---|---|---|---|
| Budding activity versus | ||||
| DOK | 0.53 | 0.0013 | 12.34 | 0.0013 |
| Stromal | 0.497 | 0.0028 | 10.51 | 0.0028 |
| WPOI | 0.412 | 0.0155 | 6.53 | 0.0155 |
| MOI | 0.45 | 0.0074 | 8.17 | 0.007446 |
| Nuclear | 0.42 | 0.0118 | 7.13 | 0.01183 |
| DOK versus | ||||
| Stromal | 0.7128 | 0.000002244 | 33.0569 | 0.000002244 |
| WPOI | 0.7186 | 0.000001701 | 34.1637 | 0.000001701 |
| MOI | 0.7121 | 0.000002322 | 32.9224 | 0.000002322 |
| Nuclear | 0.7190 | 0.000001664 | 34.2514 | 0.000001664 |
| Stromal versus | ||||
| WPOI | 0.6174 | 0.000100337 | 19.7126 | 0.0001003 |
| MOI | 0.6862 | 0.000007482 | 28.4693 | 0.000007482 |
| Nuclear | 0.7095 | 0.000002625 | 32.4398 | 0.000002625 |
| WPOI versus | ||||
| MOI | 0.9416 | 1.09×10-15 | 250.4299 | 1.09×10-15 |
| Nuclear | 0.7429 | 4.9×10-6 | 39.4156 | 4.9×10-6 |
| MOI versus | ||||
| Nuclear | 0.7403 | 5.6×10-6 | 38.8117 | 5.6×10-6 |
DOK=Degree of keratinization, Stromal=Stromal content, WPOI=Worst pattern of invasion, MOI=Mode of invasion, Nuclear=Nuclear diameter
Tumour buds were assessed in both low power magnification (10x) and high power magnification (40x) [Figures 3 and 4]. Budding activity (BA) and cell nest size (CNS) were significantly associated with tumour grading (P < 0.05). According to our proposed grading system in this study, 32.35% (11/34) of cases were well-differentiated OSCC, 35.29% (12/34) were moderately differentiated OSCC and 32.35% (11/34) were poorly differentiated OSCC. Cohen Kappa statistics revealed good agreement (72%) between WHO and the newly proposed grading system that considers BA and CNS [Figure 5].
Figure 3.
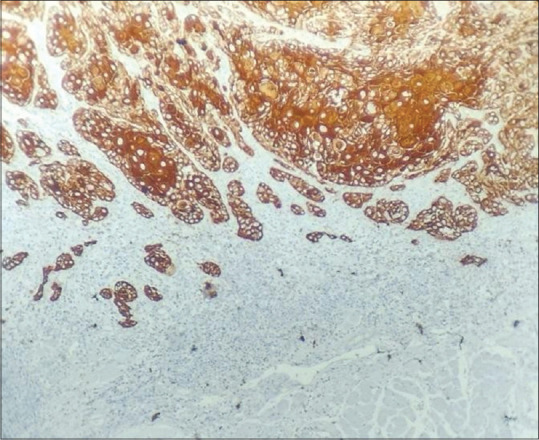
Tumour budding in oral squamous cell carcinoma (OSCC) in 10x magnification
Figure 4.
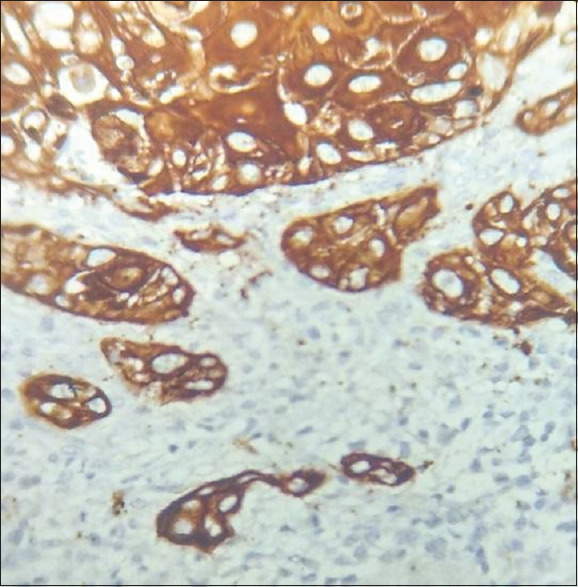
Tumour budding in oral squamous cell carcinoma (OSCC) in 40x magnification
Figure 5.
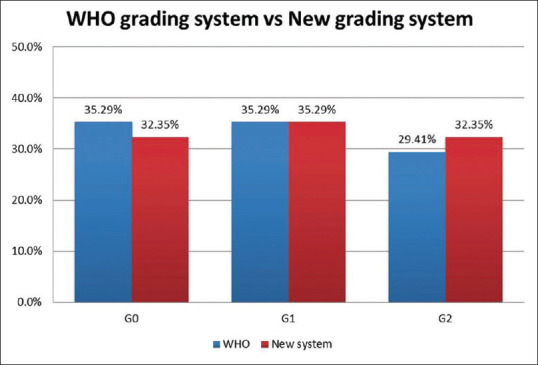
Graph showing the correlation between the WHO grading system and the proposed new grading system
In this study, low-grade tumours showed small and intermediate nuclear diameter, while high-grade tumours showed intermediate and large nuclear diameters. Majority of low-grade tumours showed very low and low stromal content. The degree of keratinization was weak in all the well-differentiated OSCC, while the moderately differentiated and poorly differentiated tumours showed both intermediate and strong degree of keratinization. Type 1 and 2 WPOI were seen only in low-grade tumours, while types 3, 4 and 5 were seen in high-grade tumours. Among the 10 cases, which shows type 5 WPOI, six cases showed more tumour buds with single-cell invasion, which indicates the aggressive behaviour of the tumour cells.
DISCUSSION
The first quantitative histopathological grading system for OSCC was initiated by Broders, which is still accepted by the WHO.[22] However, several studies show lack of correlation between Broders’ degree of differentiation and prognosis.[23,24,25] The main reason suggested for the poor correlation was the relative heterogeneity of the tumour cell population with the differences in degree of differentiation. Later, Jakobsson et al. (1973) developed a multi-factorial malignancy grading system.[18] Anneroth et al. (1984) modified Jakobsson’s grading system of SCCs in the tongue and the floor of the mouth.[26] Bryne et al. (1989) observed that the cells in the deep invasive margin, termed as ‘invasive front’ frequently showed a lower degree of differentiation and higher grade of cellular dissociation.[27,28,29]
Invasive front (IF), which is considered to be particularly important for tumour progression, is the zone of active invasion and important crosstalk between tumour and stroma.[30] Tumour budding is a non-proliferating, non-apoptotic, and highly aggressive subpopulation of tumour cells that display migratory and invasive capacities.[31] Tumour budding is defined as the presence of single carcinoma cell or small clusters of cells (≤5 cells) located at the IF of epithelial tumours. Tumour budding exhibits two characteristic features of malignancy such as: loss of epithelial cell cohesion and active invasion potential. The presence of tumour buds at the invasive front reflects the aggressive behaviour of OSCC.[32] The presence and prognostic significance of tumour budding in OSCC were identified in numerous studies.[33,34,35] A high tumour bud count at the invasive front has been linked to a poor prognosis and a high risk of metastases in several cancer types, including OSCC.[36,37,38,39,40,41,42,43,44] A few studies have shown a significant correlation between WPOI and lymph node (LN) metastasis.[45,46] Numerous studies have found correlation between depth of invasion and lymph node metastasis.[47,48,49,50,51,52,53]
In this study, we investigated the histopathologic features that were previously incorporated in a novel grading scheme in oral SCC, pulmonary SCC and oesophageal squamous cell carcinoma.[54,55] In analogy to these studies, a simple grading scheme incorporating the two histomorphological characteristics, such as BA and CNS, were used in our study. Although tumour budding and cell nest size are closely related, they influence the malignant potential of a tumour from different angles. Cell nest size within the tumour core captures the tumour’s overall capability of cellular discohesion, and BA represents the invasive potential of an OSCC into surrounding tissue within the region of the highest aggressiveness. In our study, tumour budding activity was more with high-grade tumours than in well-differentiated OSCC. Additionally, in a few well-differentiated tumours, single tumour cell invasion was evident, suggesting a more aggressive behavioural nature of the tumour. A proposed grading system based on tumour budding and cell nest has a significant corre64lation with the WHO grading system.
CONCLUSION
The prognosis and treatment plan of OSCC can be predicted by evaluating the histological parameters like tumour budding and the invasive characters such as the mode of invasion, worst pattern of invasion and depth of invasion, all of which can be assessed on routine haematoxylin and eosin stains. This study has evaluated the importance of using tumour budding in the grading system along with other parameters. Thus, based on previous studies, and through our study, we suggest the importance of using tumour budding as an important parameter in the tumour grading system. Along with this, we also recommend the minimum requirements of preoperative biopsy specimen, to evaluate the depth of invasion, which can help in the treatment modality of OSCC.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
REFERENCES
- 1.Leão PLR, Marangon Junior H, Melo VVM, Caixeta ÂB, Souza PEA, de Aguiar MCF, et al. Reproducibility, repeatability, and level of difficulty of two methods for tumor budding evaluation in oral squamous cell carcinoma. J Oral Pathol Med. 2017;46:949–55. doi: 10.1111/jop.12578. [DOI] [PubMed] [Google Scholar]
- 2.Warnakulasuriya S. Global epidemiology of oral and oropharyngeal cancer. Oral Oncol. 2009;45:309–16. doi: 10.1016/j.oraloncology.2008.06.002. [DOI] [PubMed] [Google Scholar]
- 3.Amin MB, Greene FL, Edge SB, Compton CC, Gershenwald JE, Brookland RK, et al. The eighth edition AJCC cancer staging manual:Continuing to build a bridge from a population-based to a more “personalized”approach to cancer staging. CA Cancer J Clin. 2017;67:93–9. doi: 10.3322/caac.21388. [DOI] [PubMed] [Google Scholar]
- 4.Helliwell TR. Molecular markers of metastasis in squamous carcinomas. J Pathol. 2001;194:289–93. doi: 10.1002/1096-9896(200107)194:3<289::AID-PATH912>3.0.CO;2-L. [DOI] [PubMed] [Google Scholar]
- 5.O-charoenrat P, Pillai G, Patel S, Fisher C, Archer D, Eccles S, et al. Tumour thickness predicts cervical nodal metastases and survival in early oral tongue cancer. Oral Oncol. 2003;39:386–90. doi: 10.1016/s1368-8375(02)00142-2. [DOI] [PubMed] [Google Scholar]
- 6.Bello IO, Soini Y, Salo T. Prognostic evaluation of oral tongue cancer:Means, markers and perspectives (II) Oral Oncol. 2010;46:636–43. doi: 10.1016/j.oraloncology.2010.06.008. [DOI] [PubMed] [Google Scholar]
- 7.El-Naggar AK, Chan JK, Grandis JR, Takata T, Slootweg PJ. International Agency for Research on Cancer (IARC) press;WHO Classification of Head and Neck Tumors. WHO/IARC Classification of Tumours. (4th ed) 2017;9 [Google Scholar]
- 8.Woolgar JA. Histopathological prognosticators in oral and oropharyngeal squamous cell carcinoma. Oral Oncol. 2006;42:229–39. doi: 10.1016/j.oraloncology.2005.05.008. [DOI] [PubMed] [Google Scholar]
- 9.Imai T. The growth of human carcinoma:A morphological analysis. Fukuoka Igaku Zasshi. 1954;45:72–102. [Google Scholar]
- 10.Elseragy A, Salo T, Coletta RD, Kowalski LP, Haglund C, Nieminen P, et al. A proposal to revise the histopathologic grading system of early oral tongue cancer incorporating tumor budding. Am J Surg Pathol. 2019;43:703–9. doi: 10.1097/PAS.0000000000001241. [DOI] [PubMed] [Google Scholar]
- 11.Almangush A, Salo T, Hagström J, Leivo I. Tumour budding in head and neck squamous cell carcinoma-A systematic review. Histopathology. 2014;65:587–94. doi: 10.1111/his.12471. [DOI] [PubMed] [Google Scholar]
- 12.Sakata J, Yamana K, Yoshida R, Matsuoka Y, Kawahara K, Arita H, et al. Tumor budding as a novel predictor of occult metastasis in cT2N0 tongue squamous cell carcinoma. Hum Pathol. 2018;76:1–8. doi: 10.1016/j.humpath.2017.12.021. [DOI] [PubMed] [Google Scholar]
- 13.Rogers AC, Winter DC, Heeney A, Gibbons D, Lugli A, Puppa G, et al. Systematic review and meta-analysis of the impact of tumour budding in colorectal cancer. Br J Cancer. 2016;115:831–40. doi: 10.1038/bjc.2016.274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Miyata H, Yoshioka A, Yamasaki M, Nushijima Y, Takiguchi S, Fujiwara Y, et al. Tumor budding in tumor invasive front predicts prognosis and survival of patients with esophageal squamous cell carcinomas receiving neoadjuvant chemotherapy. Cancer. 2009;115:3324–34. doi: 10.1002/cncr.24390. [DOI] [PubMed] [Google Scholar]
- 15.O’Connor K, Li-Chang HH, Kalloger SE, Peixoto RD, Webber DL, Owen DA, et al. Tumor budding is an independent adverse prognostic factor in pancreatic ductal adenocarcinoma. Am J Surg Pathol. 2015;39:472–8. doi: 10.1097/PAS.0000000000000333. [DOI] [PubMed] [Google Scholar]
- 16.Taira T, Ishii G, Nagai K, Yoh K, Takahashi Y, Matsumura Y, et al. Characterization of the immunophenotype of the tumor budding and its prognostic implications in squamous cell carcinoma of the lung. Lung Cancer. 2012;76:423–30. doi: 10.1016/j.lungcan.2011.11.010. [DOI] [PubMed] [Google Scholar]
- 17.Boxberg M, Jesinghaus M, Dorfner C, Mogler C, Drecoll E, Warth A, et al. Tumour budding activity and cell nest size determine patient outcome in oral squamous cell carcinoma:Proposal for an adjusted grading system. Histopathology. 2017;70:1125–37. doi: 10.1111/his.13173. [DOI] [PubMed] [Google Scholar]
- 18.Jakobsson PA, Eneroth CM, Killander D, Moberger G, Mårtensson B. Histologic classification and grading of malignancy in carcinoma of the larynx. Acta Radiol Ther Phys Biol. 1973;12:1–8. doi: 10.3109/02841867309131085. [DOI] [PubMed] [Google Scholar]
- 19.Yamamoto E, Kohama G, Sunakawa H, Iwai M, Hiratsuka H. Mode of invasion, bleomycin sensitivity, and clinical course in squamous cell carcinoma of the oral cavity. Cancer. 1983;51:2175–80. doi: 10.1002/1097-0142(19830615)51:12<2175::aid-cncr2820511205>3.0.co;2-m. [DOI] [PubMed] [Google Scholar]
- 20.Brandwein-Gensler M, Teixeira MS, Lewis CM, Lee B, Rolnitzky L, Hille JJ, et al. Oral squamous cell carcinoma:Histologic risk assessment, but not margin status, is strongly predictive of local disease-free and overall survival. Am J Surg Pathol. 2005;29:167–78. doi: 10.1097/01.pas.0000149687.90710.21. [DOI] [PubMed] [Google Scholar]
- 21.Berdugo J, Thompson LDR, Purgina B, Sturgis CD, Tuluc M, Seethala R, et al. Measuring depth of invasion in early squamous cell carcinoma of the oral tongue:Positive deep margin, extratumoral perineural invasion, and other challenges. Head Neck Pathol. 2019;13:154–61. doi: 10.1007/s12105-018-0925-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Broders AC. Squamous-cell epithelioma of the lip:A study of five hundred and thirty-seven cases. JAMA. 1920;74:656–64. [Google Scholar]
- 23.Arthur JF, Fenner ML. The influence of histological grading on prognosis in carcinoma of the tongue (a computer analysis of 299 cases) Clin Radiol. 1966;17:384–96. doi: 10.1016/s0009-9260(66)80060-0. [DOI] [PubMed] [Google Scholar]
- 24.Bethmann W, Heinrich C. [Comparative histological and clinical studies on squamous cell carcinoma of the jaw region. Stoma (Heidelb) 1965;18:5–23. [PubMed] [Google Scholar]
- 25.Stoddart TG. Conference of cancer of the lip (based on a series of 3166 cases) Can Med Assoc J. 1964;90:666–70. [PMC free article] [PubMed] [Google Scholar]
- 26.Anneroth G, Hansen LS. A methodologic study of histologic classification and grading of malignancy in oral squamous cell carcinoma. Scand J Dent Res. 1984;92:448–68. doi: 10.1111/j.1600-0722.1984.tb00915.x. [DOI] [PubMed] [Google Scholar]
- 27.Bryne M, Koppang HS, Lilleng R, Stene T, Bang G, Dabelsteen E. New malignancy grading is a better prognostic indicator than Broders'grading in oral squamous cell carcinomas. J Oral Pathol Med. 1989;18:432–7. doi: 10.1111/j.1600-0714.1989.tb01339.x. [DOI] [PubMed] [Google Scholar]
- 28.Bryne M, Koppang HS, Lilleng R, Kjærheim Å. Malignancy grading of the deep invasive margins of oral squamous cell carcinomas has high prognostic value. J Pathol. 1992;166:375–81. doi: 10.1002/path.1711660409. [DOI] [PubMed] [Google Scholar]
- 29.Bryne M. Is the invasive front of an oral carcinoma the most important area for prognostication? Oral Dis. 1998;4:70–7. doi: 10.1111/j.1601-0825.1998.tb00260.x. [DOI] [PubMed] [Google Scholar]
- 30.Christofori G. New signals from the invasive front. Nature. 2006;441:444–50. doi: 10.1038/nature04872. [DOI] [PubMed] [Google Scholar]
- 31.Zlobec I, Lugli A. Epithelial mesenchymal transition and tumor budding in aggressive colorectal cancer:Tumor budding as oncotarget. Oncotarget. 2010;1:651–61. doi: 10.18632/oncotarget.199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wang C, Huang H, Huang Z, Wang A, Chen X, Huang L, et al. Tumor budding correlates with poor prognosis and epithelial-mesenchymal transition in tongue squamous cell carcinoma. J Oral Pathol Med. 2011;40:545–51. doi: 10.1111/j.1600-0714.2011.01041.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Cho SJ, Kakar S. Tumor budding in colorectal carcinoma:Translating a morphologic score into clinically meaningful results. Arch Pathol Lab Med. 2018;142:952–7. doi: 10.5858/arpa.2018-0082-RA. [DOI] [PubMed] [Google Scholar]
- 34.Hong KO, Oh KY, Shin WJ, Yoon HJ, Lee JI, Hong SD. Tumor budding is associated with poor prognosis of oral squamous cell carcinoma and histologically represents an epithelial-mesenchymal transition process. Hum Pathol. 2018;80:123–9. doi: 10.1016/j.humpath.2018.06.012. [DOI] [PubMed] [Google Scholar]
- 35.Shimizu S, Miyazaki A, Sonoda T, Koike K, Ogi K, Kobayashi JI, et al. Tumor budding is an independent prognostic marker in early stage oral squamous cell carcinoma:With special reference to the mode of invasion and worst pattern of invasion. PLoS One. 2018;13:e0195451. doi: 10.1371/journal.pone.0195451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Angadi PV, Patil PV, Hallikeri K, Mallapur MD, Hallikerimath S, Kale AD. Tumor budding is an independent prognostic factor for prediction of lymph node metastasis in oral squamous cell carcinoma. Int J Surg Pathol. 2015;23:102–10. doi: 10.1177/1066896914565022. [DOI] [PubMed] [Google Scholar]
- 37.Jensen DH, Dabelsteen E, Specht L, Fiehn AM, Therkildsen MH, Jønson L, et al. Molecular profiling of tumour budding implicates TGF?-mediated epithelial-mesenchymal transition as a therapeutic target in oral squamous cell carcinoma. J Pathol. 2015;236:505–16. doi: 10.1002/path.4550. [DOI] [PubMed] [Google Scholar]
- 38.Almangush A, Bello IO, Keski-Säntti H, Mäkinen LK, Kauppila JH, Pukkila M, et al. Depth of invasion, tumor budding, and worst pattern of invasion:Prognostic indicators in early-stage oral tongue cancer. Head Neck. 2014;36:811–8. doi: 10.1002/hed.23380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Attramadal CG, Kumar S, Boysen ME, Dhakal HP, Nesland JM, Bryne M. Tumor budding, EMT and cancer stem cells in T1-2/N0 oral squamous cell carcinomas. Anticancer Res. 2015;35:6111–20. [PubMed] [Google Scholar]
- 40.Xie N, Wang C, Liu X, Li R, Hou J, Chen X, et al. Tumor budding correlates with occult cervical lymph node metastasis and poor prognosis in clinical early-stage tongue squamous cell carcinoma. J Oral Pathol Med. 2015;44:266–72. doi: 10.1111/jop.12242. [DOI] [PubMed] [Google Scholar]
- 41.Seki M, Sano T, Yokoo S, Oyama T. Histologic assessment of tumor budding in preoperative biopsies to predict nodal metastasis in squamous cell carcinoma of the tongue and floor of the mouth. Head Neck. 2016;38(Suppl 1):E1582–90. doi: 10.1002/hed.24282. [DOI] [PubMed] [Google Scholar]
- 42.Koike M, Kodera Y, Itoh Y, Nakayama G, Fujiwara M, Hamajima N, et al. Multivariate analysis of the pathologic features of esophageal squamous cell cancer:Tumor budding is a significant independent prognostic factor. Ann Surg Oncol. 2008;15:1977–82. doi: 10.1245/s10434-008-9901-6. [DOI] [PubMed] [Google Scholar]
- 43.Lugli A, Karamitopoulou E, Zlobec I. Tumour budding:A promising parameter in colorectal cancer. Br J Cancer. 2012;106:1713–7. doi: 10.1038/bjc.2012.127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Bronsert P, Enderle-Ammour K, Bader M, Timme S, Kuehs M, Csanadi A, et al. Cancer cell invasion and EMT marker expression:A three-dimensional study of the human cancer-host interface. J Pathol. 2014;234:410–22. doi: 10.1002/path.4416. [DOI] [PubMed] [Google Scholar]
- 45.Chatterjee D, Bansal V, Malik V, Bhagat R, Punia RS, Handa U, et al. Tumor budding and worse pattern of invasion can predict nodal metastasis in oral cancers and associated with poor survival in early-stage tumors. Ear Nose Throat J. 2019;98:E112–9. doi: 10.1177/0145561319848669. [DOI] [PubMed] [Google Scholar]
- 46.Hiratsuka H, Miyakawa A, Nakamori K, Kido Y, Sunakawa H, Kohama G. Multivariate analysis of occult lymph node metastasis as a prognostic indicator for patients with squamous cell carcinoma of the oral cavity. Cancer. 1997;80:351–6. [PubMed] [Google Scholar]
- 47.Arora A, Husain N, Bansal A, Neyaz A, Jaiswal R, Jain K, et al. Development of a new outcome prediction model in early-stage squamous cell carcinoma of the oral cavity based on histopathologic parameters with multivariate analysis:The aditi-nuzhat lymph-node prediction score (ANLPS) system. Am J Surg Pathol. 2017;41:950–60. doi: 10.1097/PAS.0000000000000843. [DOI] [PubMed] [Google Scholar]
- 48.Lundqvist L, Stenlund H, Laurell G, Nylander K. The importance of stromal inflammation in squamous cell carcinoma of the tongue. J Oral Pathol Med. 2012;41:379–83. doi: 10.1111/j.1600-0714.2011.01107.x. [DOI] [PubMed] [Google Scholar]
- 49.Kurokawa H, Yamashita Y, Takeda S, Zhang M, Fukuyama H, Takahashi T. Risk factors for late cervical lymph node metastases in patients with stage I or II carcinoma of the tongue. Head Neck. 2002;24:731–6. doi: 10.1002/hed.10130. [DOI] [PubMed] [Google Scholar]
- 50.Tan WJ, Chia CS, Tan HK, Soo KC, Iyer NG. Prognostic significance of invasion depth in oral tongue squamous cell carcinoma. ORL J Otorhinolaryngol Relat Spec. 2012;74:264–70. doi: 10.1159/000343796. [DOI] [PubMed] [Google Scholar]
- 51.Fukano H, Matsuura H, Hasegawa Y, Nakamura S. Depth of invasion as a predictive factor for cervical lymph node metastasis in tongue carcinoma. Head Neck. 1997;19:205–10. doi: 10.1002/(sici)1097-0347(199705)19:3<205::aid-hed7>3.0.co;2-6. [DOI] [PubMed] [Google Scholar]
- 52.Li QL, Chen FJ, Zeng ZY, Yang AK, Wu QL, Zhang HZ, et al. [Clinical and pathological related factors of occult cervical lymph node metastasis in squamous cell carcinoma of tongue. Ai Zheng. 2003;22:66–70. [PubMed] [Google Scholar]
- 53.Ling W, Mijiti A, Moming A. Survival pattern and prognostic factors of patients with squamous cell carcinoma of the tongue:A retrospective analysis of 210 cases. J Oral Maxillofac Surg. 2013;71:775–85. doi: 10.1016/j.joms.2012.09.026. [DOI] [PubMed] [Google Scholar]
- 54.Weichert W, Kossakowski C, Harms A, Schirmacher P, Muley T, Dienemann H, et al. Proposal of a prognostically relevant grading scheme for pulmonary squamous cell carcinoma. Eur Respir J. 2016;47:938–46. doi: 10.1183/13993003.00937-2015. [DOI] [PubMed] [Google Scholar]
- 55.Jesinghaus M, Boxberg M, Konukiewitz B, Slotta-Huspenina J, Schlitter AM, Steiger K, et al. A novel grading system based on tumor budding and cell nest size is a strong predictor of patient outcome in esophageal squamous cell carcinoma. Am J Surg Pathol. 2017;41:1112–20. doi: 10.1097/PAS.0000000000000865. [DOI] [PubMed] [Google Scholar]


