Abstract
Tumor necrosis factor receptor p55 (TNFRp55) mediates host resistance to several pathogens by allowing microbicidal activities of phagocytes. In the studies reported here, TNFRp55−/− mice infected with the intracellular parasite Trypanosoma cruzi showed clearly higher parasitemia and cumulative mortality than wild-type (WT) controls did. However, gamma interferon (IFN-γ)-activated macrophages from TNFRp55−/− mice produced control levels of nitric oxide and killed the parasite efficiently in vitro. Trypanocidal mechanisms of nonphagocytic cells (myocardial fibroblasts) from both TNFRp55−/− and WT mice were also activated by IFN-γ in a dose-dependent way. However, IFN-γ-activated TNFRp55−/− nonphagocytes showed less effective killing of T. cruzi than WT control nonphagocytes, even when interleukin 1β (IL-1β) was added as a costimulator. In vivo, T. cruzi-infected TNFRp55−/− mice and WT mice released similar levels of NO and showed similar levels of IFN-γ mRNA and inducible nitric oxide synthase mRNA in their tissues. Instead, increased susceptibility to T. cruzi of TNFRp55−/− mice was associated with reduced levels of parasite-specific immunoglobulin G (IgG) (but not IgM) antibodies during infection, which is probably linked to abnormal B-cell differentiation in secondary lymphoid tissues of the mutant mice. Surprisingly, T. cruzi-infected TNFRp55−/− mice showed increased inflammatory and necrotic lesions in several tissues, especially in skeletal muscles, indicating that TNFRp55 plays an important role in controlling the inflammatory process. Accordingly, levels of Mn2+ superoxide dismutase mRNA, a TNF-induced enzyme which protects the cell from the toxic effects of superoxide, were lower in mutant than in WT infected mice.
Tumor necrosis factor (TNF) is a multifunctional cytokine effective in in vivo resistance to a variety of microorganisms. TNF mediates macrophage microbicidal activity and, at the tissue level, acute and chronic inflammation. TNF activity is regulated by two homodimeric receptors with molecular sizes of 55 and 75 kDa (25). These two receptors are usually coexpressed in most tissues but contain very different transmembrane and cytoplasmatic domains, thereby involving different intracellular signal transducers (21, 34) related to distinct cell functions. Thus, TNF receptor p75 (TNFRp75) has been implicated in thymocyte proliferation and apoptosis (15, 50), whereas TNFRp55 appears responsible for most of the biological functions of TNF. Also important, TNFRp55 acts in synergy with signals elicited by gamma interferon (IFN-γ), leading to the production of NO, a major microbicidal factor (26, 43). Thus, TNFRp55 mediates resistance to parasites, fungi, and intracellular bacteria, as shown by the increased susceptibility of TNFRp55−/− mice to infections with Leishmania major (56), Listeria monocytogenes (33), Candida albicans (45), and Mycobacterium tuberculosis (9). Conversely, such mice are resistant to shock induced by lipopolysaccharide and galactosamine (33). Moreover, TNFRp55−/− mice appeared to be protected from myosin-induced autoimmune myocarditis (31) and from formation of inflammatory granuloma induced by infection with Mycobacterium bovis or Corynebacterium parvum (39). Correspondingly, TNFR signaling has been implicated in the pathogenesis of experimental allergic encephalomyelitis and human multiple sclerosis, arthritis, diabetes mellitus, and lupus erythematosus (2).
Trypanosoma cruzi, the etiologic agent of Chagas’ disease, infects 18 to 20 million people in South and Central America (29). The infection induces cellular and humoral immune responses which play a vital role in the control of parasite growth in humans as also shown in experimental T. cruzi infections. Activation of parasite-specific immune cells results in the release of cytokines, which are important in regulating the T. cruzi load. However, morbidity is also mediated by the immune response, as indicated by the presence of inflammatory lesions and autoreactive cells and antibodies (48). TNF alpha (TNF-α) has been shown to be produced during infection with T. cruzi (5, 6, 36, 37, 49, 59), but studies on its role have led to contradictory interpretations. Thus, mice transgenic for soluble TNFR or treated with anti-TNF-α antibodies have shown either increased (38, 43) or reduced susceptibility to infection (54). Moreover, administration of recombinant TNF-α exacerbated mortality (3), and increased levels of endogenous TNF were associated with increased susceptibility (36) and shown to mediate cachexia and inflammatory damage during infection with T. cruzi (54). In vitro, TNF was shown to be microbicidal to T. cruzi by itself (7, 54) or in synergy with lipopolysaccharide (57) or IFN-γ (30, 43) but was also shown to have no effect at all (13).
Here we present the results of studies of infection of TNFRp55−/− mice with T. cruzi. Whereas control of the infection is defective in such mice, this is not associated with defective NO release and/or defective trypanocidal ability of macrophages. However, a less efficient killing of T. cruzi by IFN-γ-activated TNFRp55−/− nonphagocytic cells and markedly diminished levels of anti-T. cruzi immunoglobulin G (IgG) antibodies may explain the increased susceptibility of mutant mice to infection. Interestingly, TNFRp55−/− mice also showed more severe inflammatory lesions concomitantly with decreased levels of transcripts for Mn2+ superoxide dismutase (MnSOD), a central component in the protective cellular antioxidant cascade.
MATERIALS AND METHODS
Mice and parasites.
TNFRp55−/− mice were generated by using embryonic stem cell technology (33). The mutant mice were backcrossed for nine generations with C57Bl/6 mice, and mice of the latter strain were used as wild-type (WT) controls. Groups of mice were infected intraperitoneally (i.p.) with 104 CA-I or 15 Tulahuén strain trypomastigotes obtained from peripheral blood of infected mice. The CA-I strain was isolated from a patient with chronic myocardiopathy and characterized as having low virulence and being myotropic (14), while the Tulahuén strain (47) is reticulotropic and virulent. Parasitemia was measured periodically, and mortality was recorded.
For in vitro experiments, T. cruzi trypomastigotes (Tulahuén strain) collected from supernatants of L-929 cell monolayers 7 days after infection were used.
Competitive PCR assay.
The accumulation of inducible nitric oxide synthase (iNOS), IFN-γ, MnSOD, and β-actin mRNA in freshly extracted organs from infected mice was measured by competitive PCR assays as previously described (35). Competitor fragments with a different length but using the same primers as the target DNA were constructed by using composite primers and an exogenous DNA fragment as described previously (42). Competitors were amplified by PCR, purified (Qiagen, Studio City, Calif.), and quantified in a spectrophotometer. The primer sequences for the amplification of the cDNA were as follows: sense iNOS, 5′ CCC TTC CGA AGT TTC TGG CAG CAG CAG C 3′; antisense iNOS, 5′ GGC TGT CAG AGC CTC GTG GCT TTG G 3′; sense MnSOD, 5′ CCC AGA CCT GCC TTA CGA CT 3′; antisense MnSOD, 5′ CGA CCT TGC TCC TTA TTG AA 3′; sense IFN-γ, 5′ AAC GCT ACA CAC TGC ATC TTG G 3′; antisense IFN-γ, 5′ GAC TTC AAA GAG TCT GAG G 3′; sense β-actin, 5′ GTG GGC CGC TCT AGG CAC CAA 3′; antisense β-actin, 5′ CTC TTT GAT GTC ACG CAC GAT TTC 3′.
Ten- or threefold serial dilutions of the competitor were amplified in the presence of a constant amount of cDNA. Reactions were carried out for 28 to 45 cycles in a thermal cycler (Perkin-Elmer Cetus, Norwalk, Conn.) with an annealing step at 60°C (except 55°C was used for MnSOD).
Histopathological and immunohistochemical studies.
Various organs were fixed in 4% neutral buffered formalin and processed for conventional paraffin embedding. Two samples each of skeletal muscle (hind leg) and heart, spleen, and liver tissue were randomly selected. Sections were cut at 4-μm widths, deparaffinized, and stained with hematoxylin and eosin. A blinded microscopic evaluation of the two sets of serial sections from each organ was performed on precoded slides.
The grade of inflammatory reaction on hematoxylin-eosin-stained paraffin sections was scored in 10 microscopic fields (MF) (magnification, ×400) as follows. Fewer than 10 inflammatory cells in no more than two MF was scored as 0. The occasional presence of inflammatory cells (<10 cells per high-powered field) in no more than five MF was scored as 1. The presence of occasional inflammatory cells in at least six MF, small inflammatory cell clusters (10 to 100 cells) in no more than two MF, or large inflammatory cell clusters (>100 cells) in one MF was scored as 2. The presence of small inflammatory cell clusters in at least three MF or large inflammatory cell clusters in two MF was scored as 3. A score of 4 was assigned when large inflammatory cell clusters were observed in three or more MF.
Histological analysis was also performed by histocytometry with the aid of an Integrationsplate eyepiece (Zeiss) with 100 hits. The presence or absence of parasite nests for every hit was scored.
Immunostaining of skeletal muscle tissue sections was performed with polyclonal rabbit anti-mouse iNOS (Affinity Bioreagents, Falkenberg, Sweden) or monoclonal rat anti-mouse CD45 (clone 30F11.1; Pharmingen, San Diego, Calif.) antibodies or control rabbit or normal rat serum. Slides were developed by an ABC peroxidase method using 3,3-diaminobenzidine as a chromogen, as previously described (35).
NO measurement.
We assayed NO in vivo by serum nitrate measurements after reducing nitrate to nitrite with Aspergillus sp. nitrate reductase (12). NO released by cultured peritoneal cells was measured by the concentration of nitrite. Cells were adjusted to 106 per ml in Dulbecco minimal essential medium (DMEM) without phenol red containing 5% fetal calf serum and distributed in triplicate in V-shaped 96-well plates. A 0.5 mM concentration of l-N6-imino-ethyl-lysine (l-NIL) (Sigma, St. Louis, Mo.), a relatively specific inhibitor of iNOS, was added to test wells. Supernatants were sampled after 24 h for determination of nitrite concentration by the Griess reaction (8). Nitrite concentration in culture supernatants was determined by using a standard curve with NaNO2 in DMEM. The nitrate concentration in serum was determined by using a standard curve with NaNO3 in fetal calf serum.
In vitro infection of macrophages and fibroblasts.
Fibroblasts were obtained by enzymatic digestion of hearts from 4-week-old TNFRp55−/− or WT mice. In brief, the hearts were minced in Iscove’s modified Dulbecco medium supplemented with 10% fetal calf serum, penicillin, and streptomycin (IMDM) and incubated in IMDM containing 1 mg of collagenase per ml at 37°C for 10 min. The supernatant containing released cells was collected, the washed pellets were further enzymatically digested for two cycles, and recovered free cells were pooled. The pooled cells were plated and highly enriched cultures of adherent cardiac, nonmyocyte cells were recovered during the plating procedure. After the third passage, nearly all cells appeared to be fibroblasts by morphology and by negative immunostaining for muscle-specific α-actin (Sigma). Such cultures were trypsinized, and 1 × 104 to 3 × 104 cells were resuspended in IMDM and seeded on a 24-well plate, each well containing a 13-mm-diameter coverslide. Activation and infection were performed 48 h after plating as described below.
Peritoneal cavity cells from WT and TNFRp55−/− mice were suspended in RPMI 1640 medium containing 5% fetal calf serum, penicillin, and streptomycin and seeded into 24-well tissue culture plates containing a 13-mm-diameter coverslide in each well. A total of 2 × 105 cells per well was allowed to adhere for 4 h at 37°C in a humidified CO2 incubator and washed twice with phosphate-buffered saline (PBS) to remove nonadherent cells.
Plated cells (macrophages or myocardial cells) were cultured in the presence or absence of recombinant murine IFN-γ, interleukin 1β (IL-1β), TNF-α, anti-TNF-α (all from Pharmingen), and/or l-NIL for 24 h at 37°C. The monolayers were then infected with 106 tissue culture trypomastigote forms of T. cruzi obtained from the supernatant of L-929 cells. After 4 h for macrophages and 6 h for fibroblasts, the monolayers were washed four times with PBS. The wells were then replenished with fresh medium, containing cytokines and/or l-NIL. Two or three days later, the cultures were washed three times with PBS, fixed with methanol, and stained with Giemsa. All conditions were set up in triplicate. The percentage of infected cells and the number of parasites were determined for at least 200 cells per culture.
Antibody determinations.
The anti-T. cruzi antibody contents in the sera from infected mice were measured in an enzyme-linked immunosorbent assay (ELISA). The plates were coated overnight with a 200-μg/ml concentration of whole homogenate from T. cruzi epimastigotes prepared by pressure-depressure. The plates were blocked with 1% bovine serum albumin in PBS before the sera were added. The plates were subsequently developed with alkaline phosphatase-conjugated goat anti-mouse IgG (γ chain specific) (Sigma) or anti-mouse IgM (μ chain specific) (Sigma). The assay was standardized between plates by including the titration of a serum sample from a mouse chronically infected with T. cruzi.
Neutralization assay.
A volume containing 106 parasites per ml was incubated with 1 volume of serum (pooled from four individuals) from TNFRp55−/− or WT mice at 0 or 28 days after infection for 1 h at 37°C. SCID mice (six animals per group) were subsequently inoculated i.p. with 104 treated parasites. Parasitemia was determined 16 days after infection.
RESULTS
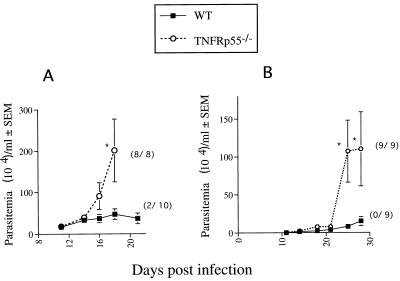
TNFRp55−/− mice were infected with the Tulahuén or CA-I strain of T. cruzi, and the course of infection in these mice was compared with that in WT mice. WT mice showed low levels of parasitemia which peaked 3 to 5 weeks after infection and were controlled thereafter. No such control was observed in mutant mice (Fig. 1). All mutant mice died, whereas 80 or 100% of the WT mice survived when infected with strain Tulahuén or CA-I, respectively (Fig. 1).
FIG. 1.
Parasitemia and mortality of TNFRp55−/− and WT mice infected i.p. with 50 Tulahuén (A) or 104 CA-I (B) organisms. The mean number of organisms per milliliter ± standard error of the mean for one of two representative experiments with each parasite strain is depicted. The cumulative mortality at 20 (A) or 35 (B) days after infection is indicated in parentheses. Differences in cumulative mortality between WT and TNFRp55−/− mice were significant (P < 0.02, Fisher’s exact chi-square test). ∗, significantly different from the value for WT infected controls (P < 0.05, Mann-Whitney-U Wilcoxon test).
To determine if the defective control of T. cruzi could be due to an impaired capacity of TNFRp55−/− macrophages to kill parasites, macrophages from noninfected mutant or WT mice were incubated with different concentrations of IFN-γ before in vitro infection with T. cruzi. When incubated with low (5 U/ml) doses of IFN-γ, macrophages from both genotypes killed the parasite and released similar levels of NO (Table 1).
TABLE 1.
Trypanocidal activity in and NO release of IFN-γ-activated macrophages from WT and TNFRp55−/− micea
| IFN-γ added | WT
|
TNFRp55−/−
|
||||
|---|---|---|---|---|---|---|
| No. of parasites/100 cells (% infected cells) | NO2− release (μM)
|
No. of parasites/100 cells (% infected cells) | NO2− release (μm)
|
|||
| Medium | Medium + l-NIL | Medium | Medium + l-NIL | |||
| No | 273 ± 50 (28 ± 7) | 2 ± 2 | 3 ± 2 | 351 ± 58 (35 ± 7) | 11 ± 9 | 5 ± 1 |
| Yes | 10 ± 6b (5 ± 3)c | 49 ± 1c | 3 ± 2 | 36 ± 2b (3 ± 1)c | 79 ± 16c | 6 ± 3 |
Recombinant murine IFN-γ (5 U/ml) and/or 0.5 mM l-NIL was added to macrophages 24 h before infection with T. cruzi. Four hours after infection, free parasites were washed and reagents were replenished. The nitrite concentration in the supernatant was measured by the Griess assay 3 days after infection. Supernatants from noninfected cells contained nondetectable levels of nitrite after 4 days in culture. The mean numbers of infected cells and the numbers of parasites per 100 cells ± standard errors of the means from triplicate determinations as recorded 48 h after infection are shown.
Difference from the nontreated infected control value is significant (P < 0.05, Mann-Whitney-U Wilcoxon test).
Difference from the nontreated infected control value is significant (P < 0.05, Pearson chi-square test).
Addition of l-NIL, a selective iNOS inhibitor, blocked both NO release (Table 1) and parasite killing (data not shown). On the other hand, parasiticidal activity could not be blocked by adding high levels of neutralizing anti-TNF-α antibodies in either the WT or mutant strains, suggesting that none of the TNF receptors are necessary for IFN-γ-mediated killing of T. cruzi (data not shown). On the contrary, NO was released by WT but not by TNFRp55−/− macrophages when murine TNF-α and IFN-γ were used as stimuli in the absence of parasites (Table 2). Upon such stimulation, 20 μg of anti-TNF-α antibodies per ml blocked accumulation of nitrites in the supernatant of WT macrophages (data not shown).
TABLE 2.
TNFRp55 is necessary for NO release after IFN-γ and TNF-α stimulationa
| IFN-γ added | NO2− release (μM)
|
|||
|---|---|---|---|---|
| Medium + TNF-α
|
Medium
|
|||
| WT | TNFRp55−/− | WT | TNFRp55−/− | |
| No | <2 | <4 | <3 | <3 |
| Yes | 57 | 7 | <4 | <3 |
Peritoneal cells were obtained from mice and plated at 106 per well in a 96-well plate. After nonadherent cells were washed, murine recombinant IFN-γ (5 U/ml) and TNF-α (500 U/ml) were added. Supernatant was collected after 48 h, and the nitrite concentration was measured by the Griess assay.
Since T. cruzi invades both professional and nonprofessional phagocytes, we investigated whether a decreased capacity of nonphagocytic TNFRp55−/− cells to kill T. cruzi could explain the enhanced susceptibility of mutant mice. Primary cultures of both WT and TNFRp55−/− myocardial fibroblasts hampered T. cruzi growth when activated with IL-1β (a potent iNOS inducer for nonphagocytes (11, 41) (Table 3). This effect probably occurred in the absence of parasite killing since reduction of the number of parasites per cell, but not of the number of infected cells, was observed. In contrast, IFN-γ-activated cells killed T. cruzi in a dose-dependent fashion (Table 3). Moreover, IFN-γ-activated myocardial cells from TNFRp55−/− mice showed lower parasite killing than WT mouse cells (Table 3). Coincubation of TNFRp55−/− or WT mouse myocardial cells with both IL-1β and IFN-γ increased their trypanocidal activity, but comparatively less in mutant cells, suggesting the involvement of TNFRp55 in trypanocidal activation of nonphagocytic cells (Table 4). The IFN-γ-activated trypanocidal mechanism(s) of both TNFRp55−/− and WT myocardial fibroblasts was mediated by NO, since killing was inhibited by l-NIL (Table 5).
TABLE 3.
Trypanocidal activity of IFN-γ or IL-1β-activated myocardial cells from WT and TNFRp55−/− micea
| Cytokine and concn | No. of parasites/100 cells (% infected cells)
|
|
|---|---|---|
| WT | TNFRp55−/− | |
| IFN-γ (U/ml) | ||
| 0 | 315 ± 60 (15 ± 2) | 337 ± 32 (17 ± 3) |
| 5 | 77 ± 25b (5 ± 1)c | 219 ± 60 (10 ± 2) |
| 100 | 37 ± 7b (5 ± 1)c | 169 ± 50b (10 ± 1)c |
| IL-1β (ng/ml) | ||
| 0 | 933 ± 76 (14 ± 1) | 677 ± 160 (10 ± 1) |
| 0.1 | 693 ± 15 (12 ± 2) | 529 ± 210 (8 ± 2) |
| 1 | 557 ± 55b (14 ± 1)c | 413 ± 37 (12 ± 1) |
| 10 | 384 ± 150b (11 ± 0)c | 388 ± 78b (9 ± 1) |
Recombinant murine IFN-γ or IL-1β was added to cells which were infected 24 h later with tissue culture trypomastigotes. Six hours after infection, the cells were washed and IFN-γ or IL-1β was added again in the concentrations indicated. Mean numbers of infected cells and numbers of parasites per 100 cells from triplicate cells ± standard errors of the means were recorded 72 h after infection.
Difference from the nontreated infected control value is significant (P < 0.05, Mann-Whitney-U Wilcoxon test).
Difference from the nontreated infected control value is significant (P < 0.05, Pearson chi-square test).
TABLE 4.
Trypanocidal activity of IFN-γ- and IL-1β-activated myocardial fibroblastsa
| Cytokine | No. of parasites/100 cells (% infected cells)
|
|
|---|---|---|
| WT | TNFRp55−/− | |
| −IL-1β | ||
| IFN-γ (U/ml) | ||
| 0 | 606 ± 198 (10 ± 2) | 828 ± 151 (15 ± 3) |
| 5 | 340 ± 45b (5 ± 3) | 356 ± 53b (9 ± 3) |
| 100 | 147 ± 31b (4 ± 2)c | 318 ± 51b,d (8 ± 3) |
| +IL-1β (10 ng/ml) | ||
| IFN-γ (U/ml) | ||
| 0 | 274 ± 31b,e (7 ± 1) | 381 ± 47b,e (11 ± 2) |
| 5 | 144 ± 24b,e (4 ± 3) | 283 ± 59b,d (9 ± 3) |
| 50 | 19 ± 3b,e (3 ± 1)c | 187 ± 31b,d,e (5 ± 1)c |
| −IFN-γ | ||
| IL-1β (ng/ml) | ||
| 0 | 472 ± 71 (14 ± 4) | 537 ± 89 (14 ± 3) |
| 0.5 | 233 ± 35b (7 ± 3) | 233 ± 19b (8 ± 2) |
| 10 | 124 ± 14b (7 ± 2) | 133 ± 26b (5 ± 1)c |
| +IFN-γ (5 U/ml) | ||
| IL-1β (ng/ml) | ||
| 0 | 213 ± 31b,e (6 ± 2) | 312 ± 62b,e (10 ± 2) |
| 0.5 | 99 ± 11b,e (4 ± 1)c | 149 ± 25b,e (7 ± 2) |
| 10 | 67 ± 15b,e (2 ± 1)c | 120 ± 13b,d (5 ± 1)c |
Various concentrations of recombinant murine IFN-γ or IL-1β were added to WT or TNFRp55−/− myocardial cells in the presence (+) or absence (−) of a constant concentration of IL-1β or IFN-γ. Six hours after infection, free parasites were washed and cytokines were added again. Mean numbers of infected cells and numbers of parasites per 100 cells ± standard errors of the means from triplicate cultures were recorded 72 h after infection.
Difference from the untreated control value is significant (P < 0.05, Mann-Whitney-U Wilcoxon test).
Difference from the untreated control value is significant (P ≤ 0.05, Pearson chi-square test).
Difference from the value for the similarly treated WT control is significant (P < 0.05, Mann-Whitney-U Wilcoxon test).
Difference from the value for the control in the absence of IL-1β (no IL-1β) or IFN-γ (no IFN-γ) is significant (P < 0.05, Mann-Whitney-U Wilcoxon test).
TABLE 5.
Effect of a NOS inhibitor on the trypanocidal activation of myocardial cellsa
| IFN-γ (5 U/ml) | l-NIL (0.5 mm) | No. of parasites/100 cells (% infected cells)
|
|
|---|---|---|---|
| WT | TNFRp55−/− | ||
| − | − | 86 ± 24 (9.0 ± 3.0) | 72 ± 21 (7.4 ± 2.6) |
| + | − | 26 ± 3b (7.6 ± 0.4) | 12 ± 1b (5.2 ± 2.1) |
| + | + | 69 ± 14 (8.4 ± 2.7) | 51 ± 18 (6.6 ± 0.6) |
Recombinant murine IFN-γ was or was not added to myocardial cells in the presence or absence of l-NIL. Six hours after infection, free parasites were washed and IFN-γ and l-NIL were added again. Mean numbers of infected cells and numbers of parasites per 100 cells ± standard errors of the means were recorded from triplicate wells 72 h after infection. −, absence; +, presence.
Difference from the nontreated control value is significant (Mann-Whitney-U Wilcoxon test).
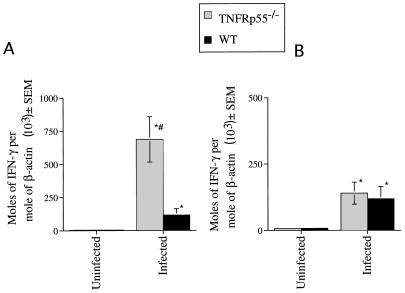
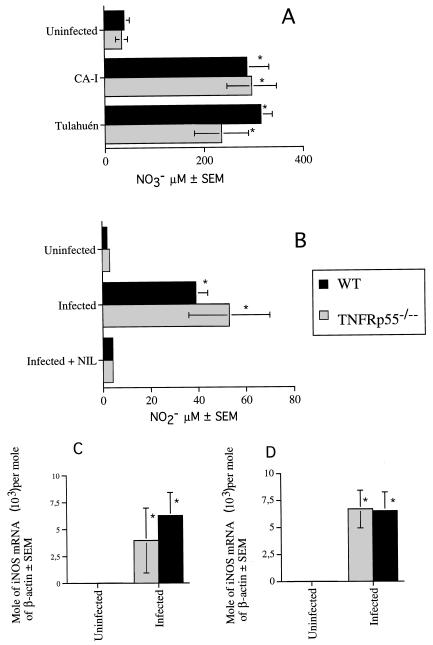
Since TNF-α plays a role in IFN-γ release (1, 40), we measured IFN-γ mRNA in tissues from infected WT and TNFRp55−/− mice. TNFRp55 was not necessary for in vivo accumulation of IFN-γ mRNA, since increased accumulation of cytokine transcripts was detected in tissues from WT or TNFRp55−/− mice infected with strain CA-I (Fig. 2) or Tulahuén (data not shown). Also, no association between NO release and increased susceptibility of TNFRp55−/− mice was found, since the levels of nitrite in cell culture supernatants, nitrate in sera, and iNOS mRNA (Fig. 3) and of protein (data not shown) in tissues were similar in TNFRp55−/− and WT mice during infection with T. cruzi.
FIG. 2.
IFN-γ mRNA accumulation was determined in skeletal muscle (A) and spleens (B) from TNFRp55−/− and WT mice at 0 or 30 days after infection with T. cruzi (CA-I). Total RNA was obtained from spleens or skeletal muscle of individual mice, and IFN-γ and β-actin mRNA were measured in a competitive PCR assay. The mean moles of IFN-γ mRNA per mole of β-actin mRNA (used as a housekeeping gene) from four cDNA samples per group, of one of two independent experiments, are depicted. ∗, significantly different from the value for noninfected WT or mutant controls (P < 0.001, Mann-Whitney-U Wilcoxon test). #, significantly different from the value for WT infected mice (P < 0.05, Mann-Whitney-U Wilcoxon test).
FIG. 3.
Nitrate concentration in serum (A), nitrite content in cell supernatants (B), and iNOS mRNA accumulation in the spleen (C) and skeletal muscle (D) were determined in TNFRp55−/− and WT mice after infection with T. cruzi. Samples were obtained 20 and 30 days after infection with strains Tulahuén and CA-I, respectively. (A) The nitrate level was determined in serum from five individual mice for each group. The mean NO3− concentrations are depicted. ∗, significantly different from values for uninfected controls (P < 0.05, Mann-Whitney-U Wilcoxon test). (B) To measure NO release, peritoneal cells from individual mice (six per group) were cultured for 24 h in the presence or absence of 0.5 mM l-NIL. NO production was measured 24 h later by the Griess assay, and mean NO2− concentrations are depicted. The data of one of two independent experiments are shown. ∗, significantly different from value for uninfected mice (P < 0.05, Mann-Whitney-U Wilcoxon test). Differences between values for WT and TNFRp55−/− infected mice are not significant. (C and D) Total RNA was obtained from spleens (C) and skeletal muscle (D) of individual mice. iNOS and β-actin mRNA were measured in a competitive PCR assay. The mean moles of iNOS mRNA per mole of β-actin mRNA from four cDNA samples per group, of one of two independent experiments, are depicted. ∗, significantly different from value for uninfected controls (P < 0.05, Mann-Whitney-U Wilcoxon test).
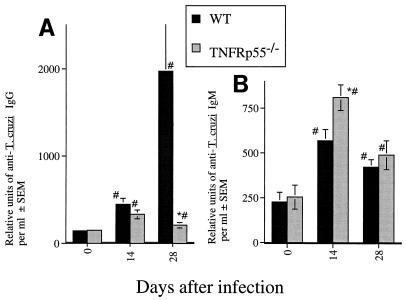
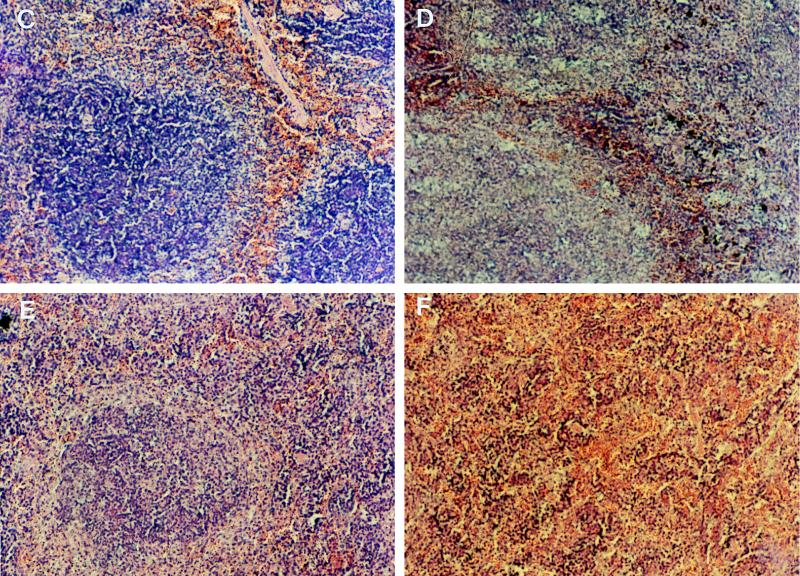
Immunoglobulin isotype switch and levels of IgM-specific response did not differ in TNFRp55−/− and WT infected mice (Fig. 4). However, a decrease in anti-T. cruzi IgG level was observed between days 14 and 28 after infection in TNFRp55−/− mice whereas a further increase was observed in WT mice (Fig. 4). Confirming previous observations (16, 27), spleens of TNFRp55−/− mice showed no germinal center formation during infection with T. cruzi, possibly explaining the low specific IgG response (Fig. 4).
FIG. 4.
(A and B) Titers of anti-T. cruzi IgG (A) and IgM (B) in sera from individual mice (six mice per group) at 17 and 28 days after infection with strain CA-I. The mean numbers of arbitrary units of specific IgG and IgM per milliliter obtained from one of two independent experiments are depicted. ∗, significantly different from values for WT infected mice (P < 0.05, F-test, analysis of variance). #, significantly different from values for uninfected WT or TNFRp55−/− controls (P < 0.05, F-test, analysis of variance). (C to F) Hematoxylin-eosin staining of spleen tissue sections from WT (C and D) and TNFRp55−/− mice (E and F) at 0 (C and E) or 15 (D and F) days after infection with T. cruzi. It is important to note the lack of primary follicles (E) and germinal center formation (F) in tissues from uninfected or infected TNFRp55−/− mice.
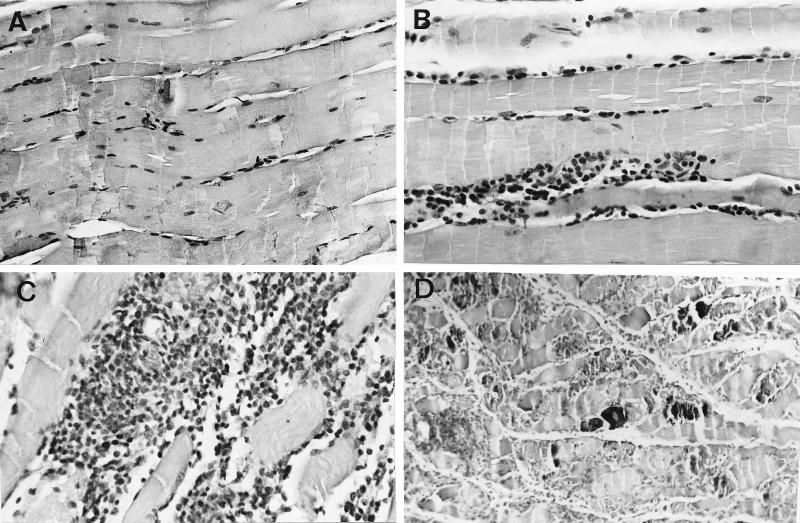
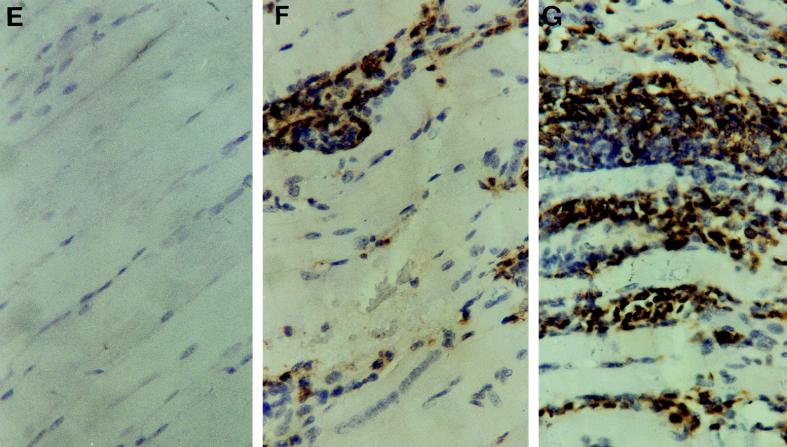
To determine whether the absence of TNFRp55 influenced the cellular infiltrates associated with acute infection with T. cruzi, the histology of heart, skeletal muscle, and liver tissue sections from infected mice was analyzed. Skeletal muscles from TNFRp55−/− mice showed markedly enhanced density of mononuclear inflammatory infiltrates 30 days after infection with the myotropic CA-I strain in comparison to that of WT infected controls (Fig. 5). A higher density of CD45+ cellular infiltrates and the presence of calcification and necrosis were observed (Fig. 5; Table 6). However, the increased severity of lesions in TNFRp55−/− mice was not associated with a higher number of parasite nests (Table 6). Inflammatory reactions in heart and liver tissues were more intense in TNFRp55−/− than WT mice, although the lesions in such tissues were less severe than those in skeletal muscles (Table 6). No differences in the severity of tissue lesions between WT and TNFRp55−/− mice could be detected at an earlier time point (14 days after CA-I infection).
FIG. 5.
Hematoxylin-eosin stainings (A to D) and immunohistochemical study of CD45 expression (E to G) of skeletal muscles from TNFRp55−/− (A, C, D, and G) and WT (B, E, and F) mice at 0 (A and E) or 30 (B, C, D, F, and G) days after infection with T. cruzi (strain CA-I). Negative controls for immunohistochemical stainings done by incubation with normal rat serum on tissues from T. cruzi-infected mice showed no peroxidase staining (data not shown). It is important to note the dramatically increased density of inflammatory infiltrates (C and G) and the presence of necrosis and calcification (D) in tissues obtained from infected TNFRp55−/− mice.
TABLE 6.
Inflammatory lesions in tissues of WT and TNFRp55−/− mice during T. cruzi infectiona
| Mouse group | Inflammatory reaction score (n = 5)
|
Immunostained tissue results (n = 7)
|
||||
|---|---|---|---|---|---|---|
| Liver | Heart | Skeletal muscle | % CD45 cellsb | Inflammatory reaction score | No. of nests/mm2c | |
| WT | 1.4 ± 0.2 | 0.2 ± 0.2 | 2.3 ± 0.4 | 3.4 ± 0.7 | 2.1 ± 0.5 | 3.2 ± 0.9 |
| TNFRp55−/− | 2.1 ± 0.1d | 1 ± 0.3 | 3.9 ± 0.1d | 7.4 ± 0.6d | 3.8 ± 0.2d | 2.0 ± 1.0 |
Tissue samples were obtained from mice 30 days after infection with the CA-I strain of T. cruzi. The arbitrary inflammatory reaction scoring is described in Materials and Methods. n, number of mice analyzed. Values are means ± standard errors of the means.
The percentage of area stained for CD45 (Ly5)+ cells in skeletal muscle sections was measured with an image analyzer (Q500MC; Leica, Cambridge, United Kingdom).
The number of parasite nests was quantified in 20 fields covering an area of 3.5 mm2.
Difference from the value for the WT is statistically significant (P < 0.05, Mann-Whitney-U Wilcoxon test).
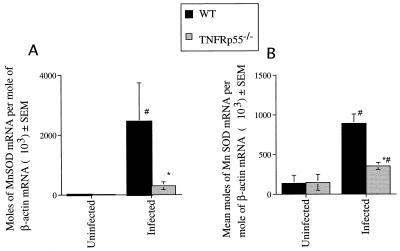
Cell culture studies have shown that TNF can induce the expression of the antioxidant MnSOD, thereby protecting tissues against oxidative insults. We therefore quantified levels of MnSOD mRNA in tissues from TNFRp55−/− and WT infected mice. T. cruzi infection induced a marked increase in the MnSOD mRNA levels in the spleen tissue and skeletal muscles of WT mice but not or only marginally in tissues from TNFRp55−/− mice (Fig. 6).
FIG. 6.
MnSOD mRNA accumulation was determined in skeletal muscle (A) and spleens (B) from individual mice at 0 and 30 days after infection with T. cruzi (CA-I). MnSOD and β-actin mRNA were measured in a competitive PCR assay. The mean moles of MnSOD mRNA per mole of β-actin mRNA from four cDNA samples per group, of one of two independent experiments, are depicted. ∗, significantly different from value for infected WT mice (P < 0.05, Mann-Whitney-U Wilcoxon test). #, significantly different from value for uninfected controls (P < 0.05, Mann-Whitney-U Wilcoxon test).
DISCUSSION
Our observations suggest that TNFRp55 signaling is involved in both control of parasite proliferation and protection against tissue pathology during infection with T. cruzi. TNFRp55−/− mice showed an exacerbated susceptibility to infection with virulent and less virulent strains of T. cruzi. These findings extend and corroborate previous results with mice treated with anti-TNF-α antibodies (43) or mice transgenic for soluble TNFR (38).
Macrophages play an important role in the control of infection with T. cruzi, as shown by the increased susceptibility of mice depleted of such cells (20, 24, 53). However, the increased susceptibility of TNFRp55−/− mice to infection was not related to any in vitro demonstrable decreased ability of IFN-γ-activated macrophages to kill T. cruzi and to produce NO. Furthermore, this microbicidal effect could not be attributed to the action of TNFRp75, since T. cruzi killing was not blocked by neutralizing anti-TNF-α antibodies. However, in contrast to normal macrophages, TNF did not act in synergy with IFN-γ for NO production when T. cruzi was not included in the coculture with TNFRp55−/− cells, in agreement with previous observations (9). Thus, parasites provide a second signal for NO production and killing that may overcome the absence of signaling via the TNFRp55 and probably that of TNFRp75, since killing is not abolished by neutralizing anti-TNF-α antibodies. We also showed that IFN-γ and IL-1β (which induces iNOS expression in nonprofessional phagocytes [11, 41]) activated trypanocidal activity in both WT and TNFRp55−/− mouse myocardial cells. However, the trypanocidal activity of TNFRp55−/− myocardial cells was lower than that of WT cells after IFN-γ (but not IL-1β) in vitro activation, suggesting the involvement of TNFRp55 signaling in IFN-γ-mediated (thugh not in IL-1β-mediated) parasite killing by nonprofessional phagocytes. Our data also suggest that IL-1β does not operate downstream of IFN-γ-mediated TNFRp55-dependent killing, since TNFRp55−/− cells also displayed a lower T. cruzi killing than WT cells after stimulation with both cytokines. Trypanocidal mechanisms of TNFRp55−/− and WT nonphagocytic cells were NO dependent, as previously shown for macrophages (10, 28, 30, 35).
TNF-α induces secretion of IFN-γ (40, 51), but we could not demonstrate decreased IFN-γ mRNA accumulation in infected TNFRp55−/− mice. The increased levels of IFN-γ mRNA detected in the skeletal muscle of infected mutant mice as compared to that in the WT counterparts is likely due to the increased inflammatory infiltrates in such tissues. Enhanced levels of IFN-γ transcripts in tissues from infected TNFRp55−/− mice were paralleled by increased iNOS expression at the mRNA and protein levels and NO release. Thus, although displaying a crucial role in resistance against T. cruzi (32, 55), IFN-γ and high levels of NO are not sufficient by themselves to provide protection. Instead, susceptibility appeared clearly related to diminished levels of specific IgG, but not IgM, in TNFRp55−/− mice. This defect in the mature antibody response was related to a lack of primary follicles and germinal centers in the spleens of TNFRp55−/− mice during T. cruzi infection, in agreement with other studies (16, 27). This is in line with the protective role of specific IgG, shown by protection of naive mice against infection with T. cruzi upon passive transfer of IgG antibodies and by the distinct susceptibility of mice genetically selected for their low or high production of antibodies (23, 46, 52). Moreover, the infectivity of T. cruzi parasites in SCID mice was neutralized by preincubation with sera from WT but not from TNFRp55−/− infected mice (data not shown). Thus, increased parasitemia in TNFRp55−/− mice might be due to a defective antibody-dependent lysis of extracellular parasites.
TNFRp55 has previously been shown to mediate the pathologic effects of TNF, which include inflammation and tissue damage in autoimmune and infectious diseases. It was therefore surprising to find dramatically exacerbated rather than decreased inflammation after infection of TNFRp55−/− mice with T. cruzi, a feature not related to an increased number of amastigote nests. This observation contrasts with the reduced inflammatory lesions observed in T. cruzi-infected in soluble-TNFRp55 transgenic mice (38). The possible presence of low levels of nonblocked TNF-α in such transgenic mice (which might also explain the unaltered levels of specific antibodies in this model) might account for the difference in pathology between these transgenic mice and infected TNFRp55−/− mice. TNF has been shown to reduce inflammatory reactions in some autoimmune diseases (22, 60) but, to our knowledge, in no infectious disease model. TNFRp55 mediates apoptosis of peripheral lymphocytes and might thereby control inflammatory damage (44). However, we found comparable numbers of apoptotic cells in inflammatory lesions from TNFRp55−/− and WT infected mice as measured by the terminal deoxynucleotidyltransferase-mediated dUTP-biotin nick end labeling staining method (data not shown).
Reactive oxygen species (ROS), implicated as important pathological mediators in various disorders including inflammation, are released during T. cruzi infection (4, 36). TNF has been shown to induce the expression of the mitochondrial MnSOD (50, 58). SOD triggers the crucial first step of the antioxidant cascade by catalysis of the dismutation of superoxide radicals (O2·) to oxygen (O2) and hydrogen peroxide (H2O2). Consequently, induction of MnSOD protects cells against ROS-mediated damage, as well as lethal radiation, and against IL-1- and TNF-induced cytotoxicity (19). Interestingly, O2· has been indicated to play a role in coxsackievirus B3 myocarditis in mice, and SOD has a favorable effect when administered therapeutically during this infection (17, 18). Our studies demonstrate that infection with T. cruzi induced an increase in MnSOD mRNA accumulation in WT mice that was markedly impaired in TNFRp55−/− mice. We speculate that ROS are produced in the absence of TNF signaling. We could thus explain the observed anti-inflammatory activity mediated by TNFRp55.
In conclusion, our observations indicate that TNFRp55 controls the parasite load possibly by its involvement in the maturation of B-cell compartments in secondary lymphoid organs and thereby the IgG antibody production and/or inactivation of trypanocidal mechanisms of nonphagocytes. We suggest that TNFRp55 also plays a protective role against pathological consequences of infection by induction of antioxidative mechanisms in TNF targets.
ACKNOWLEDGMENTS
This work was supported by Sida/SAREC and Cancerfonden, Stockholm, Sweden, and the Biomedical Concerted Action BMH1-CT94-0947.
The TNFRp55−/− mice were kindly provided by Tak Mak (Department of Medical Biophysics and Immunology, University of Toronto, Toronto, Ontario, Canada). We thank Clas Une for his critical comments on the manuscript and Joseph Lawrence (Department of Pathology, Karolinska Hospital) for his technical assistance.
REFERENCES
- 1.Bancroft G J, Sheehan K C F, Shreiber R D, Unanue E R. Tumour necrosis factor is involved in the T-cell independent pathway of macrophage activation in scid mice. J Immunol. 1989;143:127–135. [PubMed] [Google Scholar]
- 2.Bazzoni F, Beutler B. The tumor necrosis factor ligand and receptor families. N Engl J Med. 1996;334:1717–1725. doi: 10.1056/NEJM199606273342607. [DOI] [PubMed] [Google Scholar]
- 3.Black C M, Israelski D M, Suzuki Y, Remington J S. Effect of recombinant tumour necrosis factor on acute infection in mice with Toxoplasma gondii or Trypanosoma cruzi. Immunology. 1989;68:570–574. [PMC free article] [PubMed] [Google Scholar]
- 4.Cardoni R L, Rottenberg M E, Segura E L. Increased production of reactive oxygen species by spleen cells from mice acutely infected with Trypanosoma cruzi. Cell Immunol. 1990;128:11–21. doi: 10.1016/0008-8749(90)90002-9. [DOI] [PubMed] [Google Scholar]
- 5.Chandrasekar B, Melby P, Troyer D, Freeman G. Induction of proinflammatory cytokine expression in experimental acute chagasic cardiomyopathy. Biochem Biophys Res Commun. 1996;223:365–371. doi: 10.1006/bbrc.1996.0900. [DOI] [PubMed] [Google Scholar]
- 6.D’Avila-Reis D, Jones E, Jostes S T, Jr, Lopes E, Gazzinelli G, Colley D, McCurley T. Characterization of inflammatory infiltrates in chronic chagasic myocardial lesions: presence of tumor necrosis factor-α+ cells and dominance of granzyme A CD8+ lymphocytes. Am J Trop Med Hyg. 1993;48:637–644. doi: 10.4269/ajtmh.1993.48.637. [DOI] [PubMed] [Google Scholar]
- 7.De Titto E H, Catterall J R, Remington J S. Activity of recombinant tumor necrosis factor on Toxoplasma gondii and Trypanosoma cruzi. J Immunol. 1986;137:1342–1346. [PubMed] [Google Scholar]
- 8.Ding A H, Nathan C F, Stuher D J. Release of reactive nitrogen intermediates and reactive oxygen intermediates from mouse peritoneal macrophages: comparison of activating cytokines and evidence for independent production. J Immunol. 1988;141:2407–2411. [PubMed] [Google Scholar]
- 9.Flynn J, Goldstein M, Chan J, Triebold K, Pfeffer K, Lowestein C, Schreiber R, Mak T, Bloom B. Tumor necrosis factor-α is required in the protective immune response against Mycobacterium tuberculosis in mice. Immunity. 1995;2:561–572. doi: 10.1016/1074-7613(95)90001-2. [DOI] [PubMed] [Google Scholar]
- 10.Gazzinelli R T, Oswald I P, Hieny S, James S L, Sher A. The microbicidal activity of interferon-γ treated macrophages against Trypanosoma cruzi involves an l-arginine-dependent, nitric oxide-mediated mechanism inhibitable by interleukin-10 and transforming growth factor-β. Eur J Immunol. 1992;22:2501–2506. doi: 10.1002/eji.1830221006. [DOI] [PubMed] [Google Scholar]
- 11.Gilbert R S, Herschman H R. “Macrophage” nitric oxide synthase is a glucocorticoid-inhibitable primary response gene in 3T3 cells. J Cell Physiol. 1993;157:128–132. doi: 10.1002/jcp.1041570117. [DOI] [PubMed] [Google Scholar]
- 12.Gilliam M, Sherman M, Griscavage J, Ignarro L. A spectrophotometric assay for nitrate using NADPH oxidation by Aspergillus nitrate reductase. Anal Biochem. 1993;212:359–365. doi: 10.1006/abio.1993.1341. [DOI] [PubMed] [Google Scholar]
- 13.Golden J, Tarleton R L. Trypanosoma cruzi: cytokine effects on macrophage trypanocidal activity. Exp Parasitol. 1991;72:391–402. doi: 10.1016/0014-4894(91)90085-b. [DOI] [PubMed] [Google Scholar]
- 14.Gonzalez Cappa S M, Chiale P, del Prado G E, Katzin A M, de Martini G W, de Isola E D, Orrego L A, Segura E L. Aislamiento de una cepa de Trypanosoma cruzi de un paciente con miocardiopatía chagásica crónica y su caracterización biológica. Medicina (Buenos Aires) 1980;40:63–68. [PubMed] [Google Scholar]
- 15.Heller R, Song K, Fan N, Chang D. The p70 tumor necrosis factor receptor mediates cytotoxicity. Cell. 1992;70:47–56. doi: 10.1016/0092-8674(92)90532-h. [DOI] [PubMed] [Google Scholar]
- 16.Hir M L, Bluethmann H, Bluethmann M, Kosko-Vilbois M, Müller M, de Padova F, Moore M, Ryffel B, Eugster H. Differentiation of follicular dendritic cells and full antibody responses require tumor necrosis factor receptor-1 signaling. J Exp Med. 1996;183:2367–2372. doi: 10.1084/jem.183.5.2367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hiraoka Y, Kishimoto C, Kurokawa M, Ochiai H, Sasayama S. Effects of polyethylene glycol conjugated superoxide dismutase on coxsackievirus B3 myocarditis in mice. Cardiovasc Res. 1992;26:956–961. doi: 10.1093/cvr/26.10.956. [DOI] [PubMed] [Google Scholar]
- 18.Hiraoka Y, Kishimoto C, Takada H, Kurokawa M, Ochiai H, Shiraki K, Sasayama S. Role of oxygen derived free radicals in the pathogenesis of coxsackievirus B3 myocarditis in mice. Cardiovasc Res. 1993;27:957–961. doi: 10.1093/cvr/27.6.957. [DOI] [PubMed] [Google Scholar]
- 19.Hirose K, Lomgo D, Oppenheim J, Matsushima K. Overexpression of mitochondrial manganese superoxide dismutase promotes the survival of tumor cells exposed to interleukin-1, tumor necrosis factor, selected anti-cancer drugs and ionizing radiation. FASEB J. 1993;7:361–368. doi: 10.1096/fasebj.7.2.8440412. [DOI] [PubMed] [Google Scholar]
- 20.Hoff R. Killing in vitro of Trypanosoma cruzi by macrophages from mice immunized with T. cruzi or BCG, and absence of cross-immunity on challenge in vivo. J Exp Med. 1975;142:299–315. doi: 10.1084/jem.142.2.299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hsu H, Xiong J, Goeddel D. The TNF-receptor 1-associated protein TRADD signals cell death and NF-κβ activation. Cell. 1995;81:495–504. doi: 10.1016/0092-8674(95)90070-5. [DOI] [PubMed] [Google Scholar]
- 22.Jacob C, Devitt M. Tumor necrosis factor alpha in murine autoimmune “lupus” nephritis. Nature. 1988;331:356–358. doi: 10.1038/331356a0. [DOI] [PubMed] [Google Scholar]
- 23.Kierszenbaum F, Howard J. Mechanisms of resistance against Trypanosoma cruzi infection: the importance of antibodies and antibody forming capacity in the Biozzi high and low responder mice. J Immunol. 1976;116:1208–1211. [PubMed] [Google Scholar]
- 24.Kierszenbaum F, Knetch E, Budzko D B, Pizziment M C. Phagocytosis: a defense mechanism against infection with Trypanosoma cruzi. J Immunol. 1974;112:1839–1844. [PubMed] [Google Scholar]
- 25.Lewis M, Tartaglia L, Lee A, Bennet G, Rcie F, Wong G, Chen E, Goeddel D. Cloning and expression of cDNAs for two distinct tumor necrosis factor receptors demonstrate that one receptor is species specific. Proc Natl Acad Sci USA. 1991;88:2830–2839. doi: 10.1073/pnas.88.7.2830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.MacMicking J, Nathan C, Hom G, Chartrain N, Fletcher D, Trumbauer M, Stevens K, Xie Q, Sokol K, Hutchinson N. Altered responses to bacterial infection and endotoxic shock in mice lacking inducible nitric oxide synthase. Cell. 1995;81:641–650. doi: 10.1016/0092-8674(95)90085-3. [DOI] [PubMed] [Google Scholar]
- 27.Matsumoto M, Mariathasan S, Nahm M, Baranyay F, Peschon J, Chaplin D. Role of lymphotoxin and the type I TNF receptor in the formation of germinal centers. Science. 1996;264:703–707. doi: 10.1126/science.271.5253.1289. [DOI] [PubMed] [Google Scholar]
- 28.Metz G, Carlier Y, Vray B. Trypanosoma cruzi upregulates nitric oxide release by IFN-γ-preactivated macrophages, limiting cell infection independently of the respiratory burst. Parasite Immunol. 1993;15:693–699. doi: 10.1111/j.1365-3024.1993.tb00584.x. [DOI] [PubMed] [Google Scholar]
- 29.Moncayo A. Tropical disease research. Eleventh Program Report. Geneva, Switzerland: World Health Organization; 1993. Chagas’ disease; pp. 67–75. [Google Scholar]
- 30.Muñoz-Fernandez M A, Fernandez M A, Fresno M. Synergism between tumor necrosis factor and interferon-γ on macrophage activation for the killing of intracellular Trypanosoma cruzi through a nitric oxide-dependent mechanism. Eur J Immunol. 1992;22:301–307. doi: 10.1002/eji.1830220203. [DOI] [PubMed] [Google Scholar]
- 31.Penninger J, Neu N, Bachmaier K. A genetic map of autoimmune heart disease. The Immunologist. 1996;4:131–141. [Google Scholar]
- 32.Petray P, Velez E C, Grinstein S, Örn A, Rottenberg M E. Role of nitric oxide in resistance and histopathology during experimental infection with Trypanosoma cruzi. Immunol Lett. 1995;47:121–126. doi: 10.1016/0165-2478(95)00083-h. [DOI] [PubMed] [Google Scholar]
- 33.Pfeffer K, Matsuyama T, Kündig T, Wakeham A, Kishihara K, Shahinian A, Wiegmann K, Ohashi P, Krönke M, Mak T. Mice deficient for the 55 kd tumor necrosis factor receptor are resistant to endotoxin shock, yet succumb to L. monocytogenes infection. Cell. 1993;73:457–467. doi: 10.1016/0092-8674(93)90134-c. [DOI] [PubMed] [Google Scholar]
- 34.Rothe M, Wong S, Henzel W, Goeddel D. A novel family of putative signal transducers associated with the cytoplasmatic domain of the 75 kDa tumor necrosis factor receptor. Cell. 1994;78:681–692. doi: 10.1016/0092-8674(94)90532-0. [DOI] [PubMed] [Google Scholar]
- 35.Rottenberg M, Castaños-Velez E, Mesquita R, Goñi Laguardia O, Biberfeld P, Örn A. Intracellular colocalization of inducible nitric oxide synthase and Trypanosoma cruzi: evidence for a dual pathway of iNOS induction. Eur J Immunol. 1996;26:3203–3213. doi: 10.1002/eji.1830261254. [DOI] [PubMed] [Google Scholar]
- 36.Russo M, Starobinas N, Santos R R-D, Minoprio P, Eisen H, Hontebeyrie-Joskowicz M. Susceptible mice present higher macrophage activation than resistant mice during infection with myotropic strains of Trypanosoma cruzi. Parasite Immunol. 1989;11:385–395. doi: 10.1111/j.1365-3024.1989.tb00675.x. [DOI] [PubMed] [Google Scholar]
- 37.Russo M, Starobinas N. Macrophage activation and resistance to Trypanosoma cruzi. Res Immunol. 1991;142:144–146. doi: 10.1016/0923-2494(91)90026-f. [DOI] [PubMed] [Google Scholar]
- 38.Santos-Lima E, Gracia I, Vicentelli M, Vasalli P, Minoprio P. Evidence for a protective role of tumor necrosis factor in the acute phase of Trypanosoma cruzi infection in mice. Infect Immun. 1997;65:457–465. doi: 10.1128/iai.65.2.457-465.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Senaldi G, Yin S, Shaklee C, Piguet P, Mak T, Ulrich T. Corynebacterium parvum and Mycobacterium bovis bacillus Calmette-Guerin-induced granuloma formation is inhibited in TNF receptor I (TNFR-I) knockout mice and by treatment with soluble TNF-RI. J Immunol. 1996;157:5022–5026. [PubMed] [Google Scholar]
- 40.Sher A, Oswald I, Hieny S, Gazzinelli R T. Toxoplasma gondii induces a T-cell independent interferon-γ response in NK cells which requires both adherent accessory cells and TNF-α. J Immunol. 1993;150:3982. [PubMed] [Google Scholar]
- 41.Shindo T, Ikeda U, Ohkawa F, Kawahara Y, Yokohama M, Shimada K. Nitric oxide synthesis in cardiac myocytes and fibroblasts by inflammatory cytokines. Cardiovasc Res. 1995;29:813–819. [PubMed] [Google Scholar]
- 42.Siebert P D, Larrick J W. Competitive PCR. Nature. 1992;359:557–559. doi: 10.1038/359557a0. [DOI] [PubMed] [Google Scholar]
- 43.Silva J, Vespa G, Cardoso M, Aliberti J, Cunha F. Tumor necrosis factor alpha mediates resistance to Trypanosoma cruzi infection in mice by inducing nitric oxide production in infected gamma-interferon-activated macrophages. Infect Immun. 1995;63:4862–4867. doi: 10.1128/iai.63.12.4862-4867.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Speicer D, Sebzda E, Ohteki T, Bachmann M, Pfeffer K, Mak T, Ohashi P. Tumor necrosis factor receptor p55 mediates deletion of peripheral cytotoxic T lymphocytes in vivo. Eur J Immunol. 1996;26:3055–3060. doi: 10.1002/eji.1830261235. [DOI] [PubMed] [Google Scholar]
- 45.Steinshamn S, Bemelmans M, van Tits L, Bergh K, Burman W, Waage A. TNF receptors in murine Candida albicans infection. J Immunol. 1996;157:2155–2159. [PubMed] [Google Scholar]
- 46.Takehara H, Perini A, Da Silva M, Mota I. Trypanosoma cruzi: role of different antibody classes in protection against infection in mice. Exp Parasitol. 1981;52:137–146. doi: 10.1016/0014-4894(81)90069-2. [DOI] [PubMed] [Google Scholar]
- 47.Taliaferro W H, Pizzi T. Connective tissue reactions in normal and immunized mice to a reticulotropic strain of Trypanosoma cruzi. J Infect Dis. 1955;96:199–226. doi: 10.1093/infdis/96.3.199. [DOI] [PubMed] [Google Scholar]
- 48.Tarleton R L. Pathology of American trypanosomiasis. In: Warren K S, editor. Immunology and molecular biology of parasitic infections. 3rd ed. Oxford, United Kingdom: Blackwell Scientific Publications Ltd.; 1993. pp. 64–71. [Google Scholar]
- 49.Tarleton R L. Tumour necrosis factor (cachectin) production during experimental Chagas’ disease. Clin Exp Immunol. 1988;73:186–191. [PMC free article] [PubMed] [Google Scholar]
- 50.Tartaglia L, Weber R, Figari I, Reynolds C, Palladino M, Goeddel D. The two different receptors for tumor necrosis factor mediate distinct cellular responses. Proc Natl Acad Sci USA. 1991;88:9292–9296. doi: 10.1073/pnas.88.20.9292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Tripps C, Wolf S, Unanue E. Interleukin 12 and tumor necrosis factor alpha are costimulators of interferon gamma production by natural killer cells in severe combined immunodeficiency mice with listeriosis, and interleukin 10 is a physiological antagonist. Proc Natl Acad Sci USA. 1993;90:3725–3729. doi: 10.1073/pnas.90.8.3725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Trischmann T M, Bloom B R. Trypanosoma cruzi: ability of T cell enriched and depleted lymphocyte populations to passively protect mice. Exp Parasitol. 1980;49:225–232. doi: 10.1016/0014-4894(80)90119-8. [DOI] [PubMed] [Google Scholar]
- 53.Trischmann T M, Tanowitz H, Wittner M, Bloom B R. Trypanosoma cruzi: role of the immune response in natural resistance of inbred strains of mice. Exp Parasitol. 1978;45:160–168. doi: 10.1016/0014-4894(78)90055-3. [DOI] [PubMed] [Google Scholar]
- 54.Truyens C, Torrico F, Angelo-Barrios A, Lucas R, Heremans H, de Batselier P, Carlier Y. The cachexia associated with Trypanosoma cruzi acute infection in mice is attenuated by anti-TNF-α, but not by anti-IL-6 or anti-IFN-γ antibodies. Parasite Immunol. 1995;17:561–568. doi: 10.1111/j.1365-3024.1995.tb00999.x. [DOI] [PubMed] [Google Scholar]
- 55.Vespa G, Cunha F, Silva J. Nitric oxide is involved in control of Trypanosoma cruzi-induced parasitemia and directly kills the parasite in vitro. Infect Immun. 1994;62:5177–5182. doi: 10.1128/iai.62.11.5177-5182.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Vieira L, Goldschmidt M, Nashleanas M, Pfeffer K, Mak T, Scott P. Mice lacking the TNF receptor p55 fail to resolve lesions caused by infection with Leishmania major, but control parasite replication. J Immunol. 1996;157:827–835. [PubMed] [Google Scholar]
- 57.Wirth J, Kierszenbaum F. Recombinant tumor necrosis factor enhances macrophage destruction of Trypanosoma cruzi in the presence of bacterial endotoxin. J Immunol. 1988;141:286–288. [PubMed] [Google Scholar]
- 58.Wong G, Goeddel D. Induction of manganous superoxide dismutase by tumor necrosis factor: possible protective mechanisms. Science. 1988;242:941–944. doi: 10.1126/science.3263703. [DOI] [PubMed] [Google Scholar]
- 59.Zhang L, Tarleton R. Persistent production of inflammatory and anti-inflammatory cytokines and associated MHC and adhesion molecule expression at the site of infection and disease in experimental Trypanosoma cruzi infection. Exp Parasitol. 1996;84:203–213. doi: 10.1006/expr.1996.0106. [DOI] [PubMed] [Google Scholar]
- 60.Zhou T, Edwards C, Yang P, Wang Z, Bluethmann H, Mountz J. Greatly accelerated lymphoadenopathy and autoimmune disease in lpr mice lacking tumor necrosis factor receptor I. J Immunol. 1996;156:2661–2665. [PubMed] [Google Scholar]