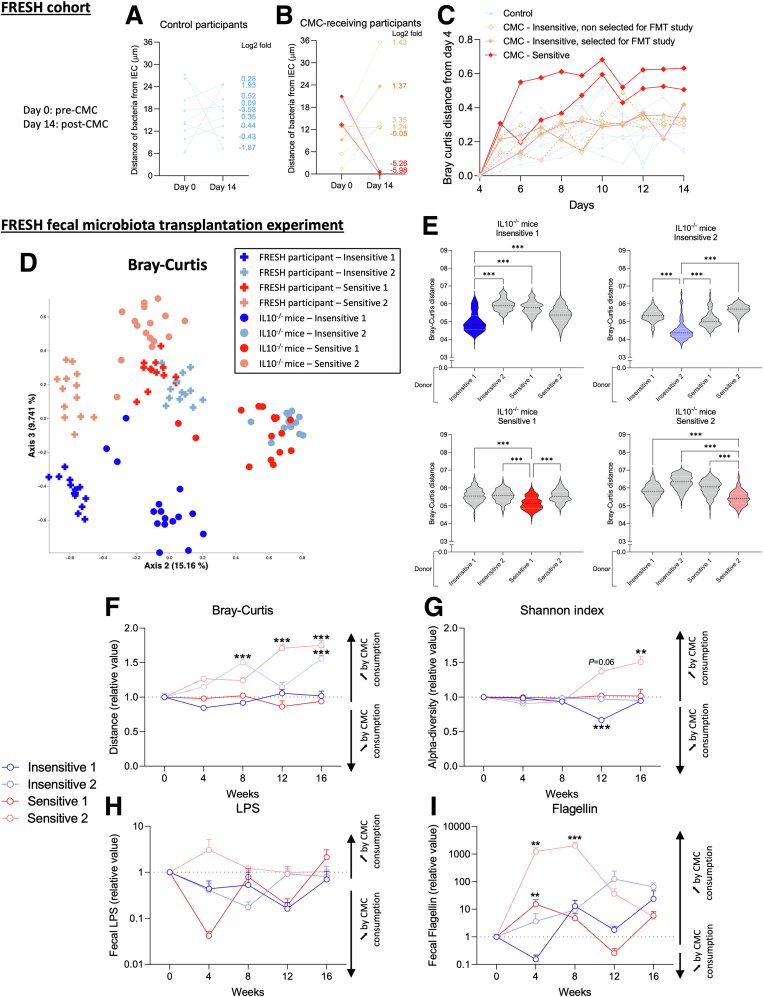
Carboxymethylcellulose (CMC) thickener/emulsifier is used commonly by the food industry to enhance texture and extend shelf life.1 Preclinical work has shown that its consumption detrimentally impacts the intestinal microbiota in a way that promotes chronic inflammation.2, 3, 4, 5 We recently reported results from the Functional Research of Emulsifiers in Humans Corrected (FRESH) study, a randomized, double-blind, controlled-feeding assay.6 After a washout period, half of the healthy recruited participants were assigned randomly to a CMC-supplemented diet (Supplementary Figure 1A). Those subjects showed significant alterations in microbiota composition and fecal metabolome relative to control subjects.6 However, the response to CMC was highly heterogenous. Specifically, 2 subjects were highly CMC sensitive in that they showed stark alterations in microbiota composition and developed microbiota encroachment, whereas other subjects were relatively insensitive to CMC (Figure 1A–C). Such CMC sensitivity was not associated with overt signs of intestinal inflammation but nonetheless might mark proneness to chronic inflammation, compelling us to better understand mechanisms that mediate CMC sensitivity.
Figure 1.
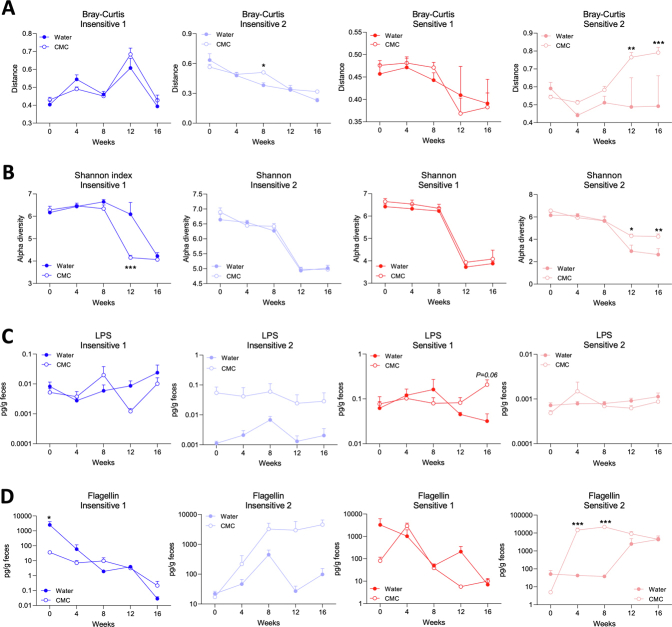
Human sensitivity to CMC consumption influences alterations of microbiota composition and function in recipient interleukin (IL)10-/-mice. (A–C) Functional Research of Emulsifiers in Humans participants were categorized as CMC-insensitive (N = 5) or CMC-sensitive (N = 2). Microbiota localization in (A) controls and (B) CMC-treated participants between day 14 (post-CMC) and day 0 (pre-CMC). (C) Evolution of the microbiota composition (Bray–Curtis distance). (D–I) Germfree IL10-/- mice were transplanted with fecal suspension from CMC-insensitive or CMC-sensitive participants, then treated with either water or CMC for 16 weeks. (D and E) Principal coordinate analysis of the Bray–Curtis distance matrix from participants and recipient mice. (F) Bray–Curtis distance, (G) Shannon index, (H) fecal levels of lipopolysaccharide (LPS), and (I) flagellin over time in transplanted mice. Data were normalized compared with the water-treated group and week 0, both defined as 1. Data are means ± SEM. N = 3–4. ∗∗P < .01, ∗∗∗P < .001. (A–C) Data from Chassaing et al.6 FMT, fecal microbiota transplantation.
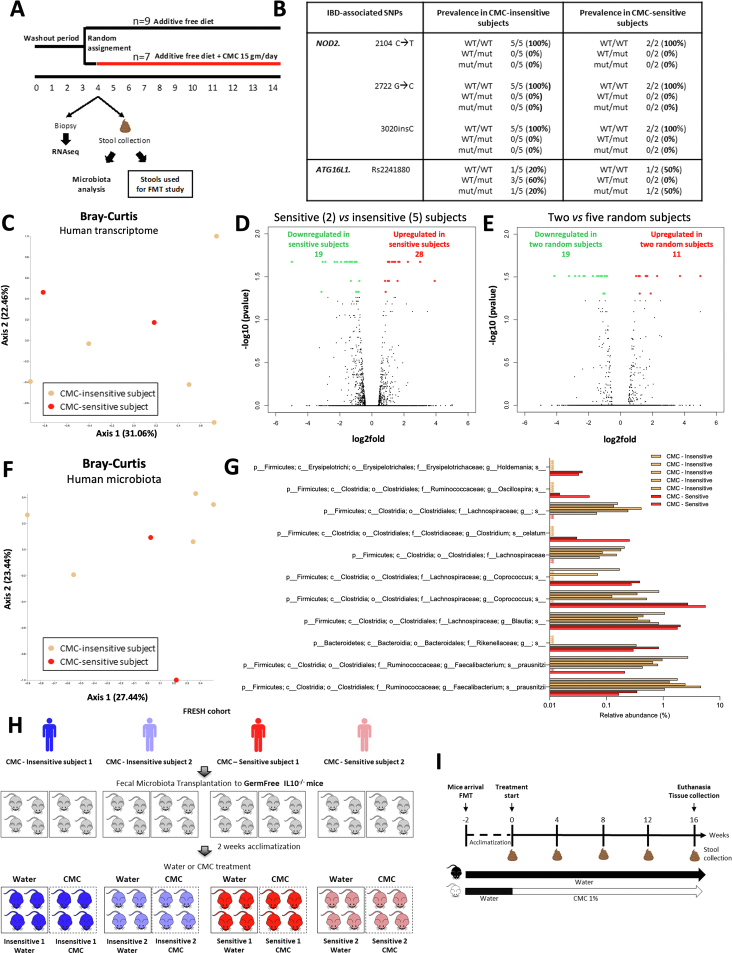
We first hypothesized that, although healthy, CMC-sensitive subjects might have genetic polymorphisms associated with inflammatory bowel disease prevalence.7,8 Our probing showed that CMC-treated participants did not harbor any NOD2 mutations, whereas ATG16L1 variants were distributed regardless of CMC sensitivity (Supplementary Figure 1B). We next considered that basal intestinal gene expression might determine CMC sensitivity, and we subjected colonic biopsy specimens to a RNA sequencing approach. Principal coordinates analysis and volcano plots showed similar colonic transcriptomes between CMC-sensitive and CMC-insensitive participants (Supplementary Figure 1C and D). Moreover, the number of differentially expressed genes discriminating CMC-sensitive and CMC-insensitive participants was similar to the number of genes obtained when comparing randomly selected participants (Supplementary Figure 1D and E), suggesting false discoveries. Hence, neither inflammatory bowel disease–associated mutations nor basal gene expression are associated with CMC sensitivity.
We next investigated a potential role for basal (pre-CMC) microbiota composition via 16S ribosomal RNA gene sequencing. Although principal coordinates analysis of Bray–Curtis distances found no clear difference between sensitive and insensitive participants (Supplementary Figure 1F), microbiome multivariable association with linear models analysis identified 11 discriminating Amplicon Sequence Variants between these 2 groups (Supplementary Figure 1G). This algorithm did not detect differences between randomly selected subjects, arguing that Amplicon Sequence Variants that are associated with CMC sensitivity were not false discoveries but rather had marked, and perhaps contributed to, CMC sensitivity status.
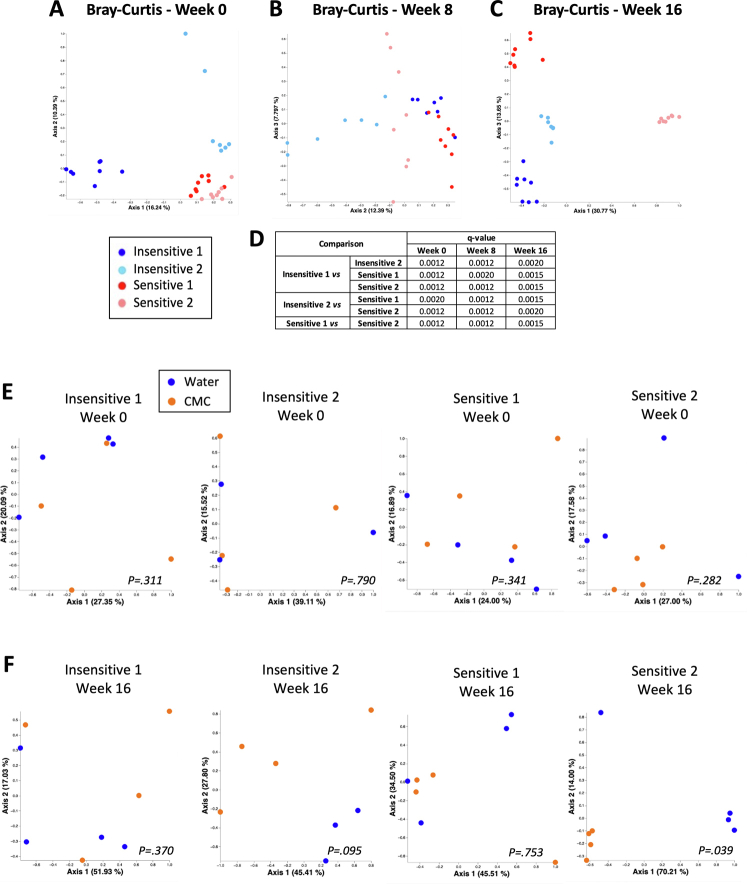
We next investigated the extent to which microbiota composition mediates CMC sensitivity. We selected 2 CMC-sensitive and 2 CMC-insensitive participants and transplanted their pre-CMC fecal samples into germ-free, colitis-prone, interleukin 10-/- mice (Supplementary Figure 1H and I). After microbiota stabilization, mice were assigned to CMC or water treatment for 16 weeks (Supplementary Figure 2B). Faithful transfer of donors’ microbiota into recipient mice was assessed by 16S ribosomal RNA gene sequencing, revealing the expected differential clustering between mouse and human samples. Nonetheless, each group of recipients was more similar to its own donor compared with other donors (Figure 1D and E, Supplementary Figure 2), indicating successful transfer. We observed significant alterations in microbiota composition between CMC-treated and water-treated groups in a way that was not clearly associated with sensitivity status (Figure 1F and G, Supplementary Figures 2 and 3). Analysis of the level of microbiota-derived proinflammatory markers importantly revealed that CMC consumption transiently increased flagellin levels in both groups of CMC-sensitive receiving mice (Figure 1H and I and Supplementary Figure 3C and D).
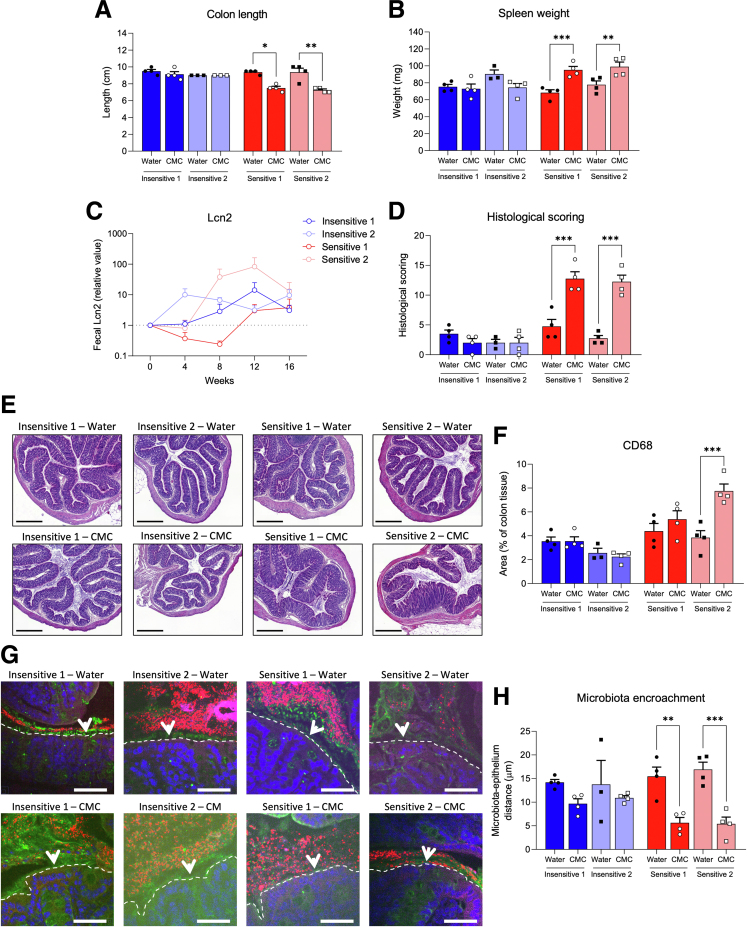
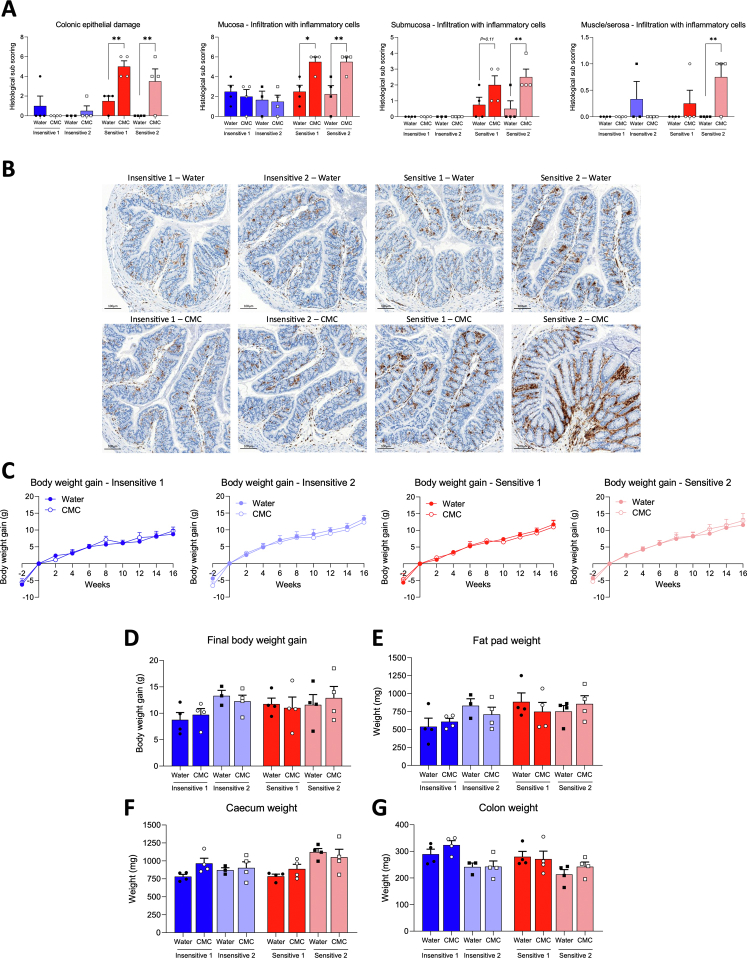
We next investigated the impact of CMC consumption on intestinal inflammation. We observed that mice harboring microbiota from either CMC-insensitive donor lacked signs of intestinal inflammation after CMC consumption,* while mice harboring microbiota from either sensitive donor showed stark intestinal inflammation after CMC consumption, as highlighted by a significant decrease in colon length and an increase in spleen weight (Figure 2A and B). Furthermore, although CMC-induced increases in fecal lipocalin-2 were modest, mice recipients of either CMC-sensitive microbiota displayed a marked CMC-induced increase in colonic histopathologic inflammatory score (Figure 2C–E and Supplementary Figure 4A). This was shown further by increased colonic CD68+ macrophages (Figure 2F and Supplementary Figure 4B), although metabolism markers were not impacted (Supplementary Figure 4C–G).2 Assessment of microbiota localization revealed that the extent of CMC-induced inflammation was paralleled by the degree of microbiota encroachment. More specifically, CMC consumption had only modest impacts on microbiota-epithelium distance in mice colonized with CMC-insensitive microbiotas, although it resulted in stark microbiota encroachment upon CMC consumption in mice harboring microbiotas from either CMC-sensitive donor (Figure 2G and H). These indicate a role for basal microbiotas in influencing CMC impact on this cardinal feature of intestinal inflammation and suspected driver of chronic diseases.2,3,6,9,10
Figure 2.
Microbiota from CMC-sensitive Functional Research of Emulsifiers in Humans Corrected participants are sufficient to drive intestinal inflammation and microbiota encroachment. Gnotobiotic mice were killed after 16 weeks of water or CMC treatment. (A) Colon length and (B) spleen weight. (C) Fecal lipocalin-2 levels over time, normalized compared with the water-treated group and week 0, both defined as 1. (D and E) Histopathologic scoring, individual subscores are shown in Supplementary Figure 4A. Scale bars: 300 μm. (F) Quantification of colonic CD68+ cells. (H) Microbiota encroachment and (G) representative images. Mucus, green; actin, purple; bacteria, red; and DNA, blue. Dashed lines delimit the epithelium; Arrowheads indicate the mucus layer. Scale bars: 50 μm. Data are means ± SEM. N = 3–4. ∗P < .05, ∗∗P < .01, and ∗∗∗P < .001.
Our findings suggest that the microbiota participate in the extent to which an individual harbors proneness to CMC-induced inflammatory diseases. Accordingly, CMC consumption may be one trigger of chronic inflammation in genetically prone individuals colonized with a given microbial ecosystem. Future work using a larger number of participants appear needed to substantiate this observation and decipher the exact microbiota contributor(s) driving CMC sensitivity.
Acknowledgments
The authors thank Hanh Tong and Sabrine Naimi for their help with protocol and animal care. The authors thank Lillian Chau, Brittaney Bonhomme, and Lisa Nessel for their help in implementing the Functional Research of Emulsifiers in Humans Corrected study. The authors also thank the Hist’IM and the Genom’IC platforms (Institut Cochin, INSERM U1016, Paris, France) for their expertise. Finally, the authors acknowledge the Molecular Evolution core at the Parker H. Petit Institute for Bioengineering and Bioscience at Georgia Institute of Technology for the use of their shared equipment, services, and expertise regarding the RNA sequencing approach.
Footnotes
Conflicts of interest The authors disclose no conflicts.
Funding Supported by a starting grant from the European Research Council under the European Union’s Horizon 2020 research and innovation program (ERC-2018-StG-804135 INVADERS); a Chaire d’Excellence from IdEx Université de Paris (ANR-18-IDEX-0001); an award from the Fondation de l'Avenir (AP-RM-21-032); Agence Nationale de la Rechercher (ANR) grants EMULBIONT (ANR-21-CE15-0042-01) and DREAM (ANR-20-PAMR-0002); and the national program Microbiote from INSERM (B.C.). Also supported by National Institutes of Health grants DK115180 (A.T.G., J.D.L and G.D.W.), 5UL1TR001878 (G.D.W.), and P30-DK050306 (G.D.W.); the Penn Center for Nutritional Science and Medicine; and the Max Planck Society.
Note: To access the supplementary material accompanying this article, go to the full text version at https://doi.org/10.1016/j.jcmgh.2023.11.001.
Supplementary Material
Supplementary Figure 1.
Supplementary Figure 2.
Supplementary Figure 3.
Supplementary Figure 4.
References
- 1.Chazelas E., et al. Sci Rep. 2020;10:3980. doi: 10.1038/s41598-020-60948-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Chassaing B., et al. Nature. 2015;519:92–96. doi: 10.1038/nature14232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chassaing B., et al. Gut. 2017;66:1414–1427. doi: 10.1136/gutjnl-2016-313099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Viennois E., et al. Cell Rep. 2020;33 doi: 10.1016/j.celrep.2020.108229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Rousta E., et al. Nutrients. 2021;13:3565. doi: 10.3390/nu13103565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chassaing B., et al. Gastroenterology. 2022;162:743–756. doi: 10.1053/j.gastro.2021.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hugot J.P., et al. Nature. 2001;411:599–603. doi: 10.1038/35079107. [DOI] [PubMed] [Google Scholar]
- 8.Hampe J., et al. Nat Genet. 2007;39:207–211. doi: 10.1038/ng1954. [DOI] [PubMed] [Google Scholar]
- 9.Chassaing B., et al. Gut. 2014;63:1069–1080. doi: 10.1136/gutjnl-2013-304909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chassaing B., et al. PLoS One. 2018;13 doi: 10.1371/journal.pone.0195310. [DOI] [PMC free article] [PubMed] [Google Scholar]