Abstract
Aim:
SGLT2 inhibitors provide multiple benefits to patients with type 2 diabetes – including improved glycemic control and decreased risks of cardiorenal disease. Because drug responses vary among individuals, we initiated investigations to identify genetic variants associated with the magnitude of drug responses.
Methods:
Canagliflozin (300 mg) was administered to 30 healthy volunteers. Several endpoints were measured to assess clinically relevant responses – including drug-induced increases in urinary excretion of glucose, sodium, and uric acid.
Results:
This pilot study confirmed that canagliflozin (300 mg) triggered acute changes in mean levels of several biomarkers: fasting plasma glucose (−4.1 mg/dL; p=6x10−5), serum creatinine (+0.05 mg/dL; p=8x10−4), and serum uric acid (−0.90 mg/dL; p=5x10−10). The effects of sex on glucosuria depended upon how data were normalized. Whereas males’ responses were ~60% greater when data were normalized to body surface area, males and females exhibited similar responses when glucosuria was expressed as grams of urinary glucose per gram-creatinine. The magnitude of glucosuria was not significantly correlated with fasting plasma glucose, estimated GFR, or age in these healthy non-diabetic individuals with estimated GFR>60 mL/min/1.73m2.
Conclusions:
Normalizing data relative to creatinine excretion will facilitate including data from males and females in a single analysis. Furthermore, because our ongoing pharmacogenomic study (NCT02891954) is conducted in healthy individuals, this will facilitate detection of genetic associations with limited confounding by other factors such as HbA1c and renal function.
Registration:
Funding:
Research grants from the National Institute of Diabetes and Digestive and Kidney Diseases: R21DK105401, R01DK108942, T32DK098107, and P30DK072488.
Keywords: canagliflozin, diabetes, kidney, pharmacogenomics, precision medicine, sex as a biological variable, sodium-glucose cotransporter-2, type 2 diabetes, uric acid
1 ∣. INTRODUCTION
Although head-to-head comparative effectiveness studies demonstrate that diabetes drugs are not strongly differentiated with respect to mean HbA1c-lowering 1, there is wide variation in individual responses to the same therapy. A recent head-to-head study reported HbA1c-lowering efficacies (mean ± SEM) of 0.89% ± 0.24% for canagliflozin and 1.44% ± 0.39% for liraglutide 2. This corresponds to standard deviations of ~0.9% and ~1.5%, respectively. While some patients experienced little if any decrease in HbA1c, others experienced >2.5% HbA1c-lowering. This inter-individual variation results from a combination of genetic and environmental factors. Pharmacogenetics has potential to provide insights enabling physicians to prescribe therapies for individual patients based on predictors of individual responses and risks of adverse effects 1.
Pharmacogenomics has identified genetic variants contributing to inter-individual variation in responses to diabetes drugs 3-7. Some genetic variants alter pharmacokinetics by altering function of drug transporters or drug metabolizing enzymes 3,8. Other genetic variants alter functions of proteins that mediate drug responses, thereby altering pharmacodynamics 4,6,9. This pilot study represents one step toward identifying genetic variants contributing to inter-individual variation in responses to SGLT2 inhibitors – an increasingly important class of diabetes drugs 10,11. Glucuronidation is the principal pathway whereby SGLT2 inhibitors are metabolized. Loss-of-function variants in glucuronidation enzymes alter pharmacokinetics of SGLT2 inhibitors by increasing drug exposure 8, but these variants are unlikely to cause major alterations in drug responses in routine clinical use because SGLT2 inhibitors are usually administered at maximally effective doses. Increasing drug exposure may not alter responses to drugs that are administered at maximally effective doses.
Several approaches have been applied in pharmacogenomic research – including acute studies in healthy volunteers assessing pharmacodynamic endpoints 3,12 or chronic studies in disease patients assessing clinical endpoints such as HbA1c or cardiovascular outcomes 9,12. Short-term studies in healthy volunteers offer methodological advantages – including minimization of confounding factors due to co-existing diseases and effects of changing co-medications during the study. This pilot study provided an opportunity to validate pharmacodynamic endpoints for our ongoing GWAS of pharmacodynamic responses to canagliflozin (NCT02891954). Specifically, we have selected drug-induced glucosuria as the primary end-point because this provides the mechanism mediating two important clinical benefits of SGLT2 inhibitors: HbA1c-lowering and weight loss. Our approach was inspired by high priority NIH initiatives: (a) scientific rigor and reproducibility 13; (b) investigation of sex as a biological variable 14; and (c) precision medicine 15.
Research participants collected two separate 24-hour urine collections. We confirmed the reproducibility of urine collections as indicated by absence of a statistically significant difference in creatinine content between the two separate urine collections – a critical prerequisite for our twin objectives of minimizing the impact of random variation and maximizing the impact of genetic variation. This pilot study also investigated the impact of participants’ self-designated sex on pharmacodynamic responses. Mean canagliflozin-induced glucosuria was substantially greater in males than females when expressed as grams of glucose per 1.73 m2 of body surface area or per kg of body weight. In contrast, mean magnitudes of canagliflozin-induced glucosuria were similar in males and females when expressed as grams of glucose per gram of creatinine. Accordingly, our ongoing pharmacogenomic study will normalize drug-induced glucosuria data relative to urinary creatinine excretion so that the magnitude of canagliflozin-induced glucosuria would be relatively independent of sex (“sex-agnostic”).
2 ∣. METHODS
2. 1 ∣. Study population
The Old Order Amish population of Lancaster County, PA immigrated to the Colonies from Central Europe in the early 1700’s. There are currently ~40,000 Old Order Amish individuals in Lancaster County, PA – nearly all of whom trace their ancestry back ~15 generations to ~750 founders 16-18. Investigators at the University of Maryland Baltimore have investigated genetic determinants of cardiometabolic health in this population since 1993. These studies generated a genotype database used to compile a list of individuals to be invited to participate in this clinical trial. Individuals with any of four homozygous genotypes were eligible to participate: (a) nonsense mutation in SLC5A4 (rs62239058); (b) nonsense mutation in SLC5A9 (rs850763); (c) missense variant in SLC2A9 (rs1689079); and (d) “wild type” major alleles of SLC5A4, SLC5A9, and SLC2A9.
2.2 ∣. Conduct of clinical trial (NCT02462421).
This clinical trial was reviewed and approved by the University of Maryland Baltimore’s Institutional Review Board. A research nurse accompanied by a member of the Amish community made home visits to invite selected individuals to participate in the study. Potential participants were invited to provide informed consent after nurses explained the study in detail. Additional methodological details are summarized in the Supplementary Appendix.
2.3 ∣. Statistical analyses
Student’s t-tests as implemented in Microsoft Excel and GraphPad Prism software were used to assess treatment effects (paired t-test) and differences between groups (unpaired t-tests). Nominal p-values are presented without correcting for multiple comparisons. A nominal p-value of <0.05 was taken as the threshold for statistical significance.
3 ∣. RESULTS
3. 1 ∣. Disposition and baseline characteristics of participants
This clinical trial assessed pharmacodynamic responses of thirty healthy Old Order Amish participants to a single dose of canagliflozin (300 mg). Disposition of the participants is summarized in Fig. S1. The study population (17 females/13 males) had a mean age of 57.8 ± 2.5 years and a mean BMI of 28.0 ± 0.9 kg/m2 (Table S1). Baseline laboratory data are summarized in Table S1.
3.2 ∣. Responses to canagliflozin: ↓ fasting plasma glucose, ↓ serum uric acid, and ↑ serum creatinine
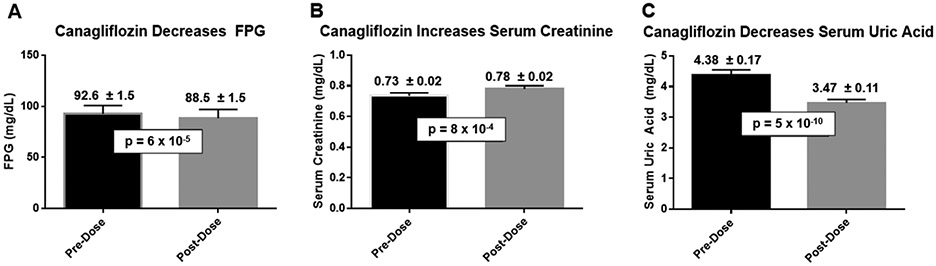
Canagliflozin inhibits SGLT2, thereby decreasing proximal tubular reabsorption of glucose and sodium 10. Drug-induced glucosuria triggered a ~4.5% decrease in mean fasting plasma glucose levels (p=0.00006; Fig. 1A). The decrease in proximal tubular sodium reabsorption induced natriuresis (Table 1, Fig. S2), which triggered a modest volume contraction as reflected by an ~8% increase in serum creatinine (p=0.0008; Fig. 1B). Canagliflozin also increased urinary excretion of uric acid, leading to a ~21% decrease in mean serum uric acid levels (p=5 x 10−10; Fig. 1C) 10,19-21. Thus, administration of a single dose of canagliflozin exerted the expected pharmacodynamic effects on circulating biomarkers in healthy volunteers – thereby serving as positive controls for our clinical trials (NCT02462421 and NCT02891954).
Figure 1. Acute effects of canagliflozin on circulating biomarkers.
Baseline blood samples were obtained for measurement of fasting plasma glucose (panel A), serum creatinine (panel B), and serum uric acid (panel C). Canagliflozin (300 mg, p.o.) was administered 24 hours later to each of the participants. Twenty-four hours after administration of canagliflozin, fasting blood samples were obtained to assess the impact of canagliflozin on fasting plasma glucose, serum creatinine, and serum uric acid. Data are presented as mean ± SEM (N=30).
Table 1.
Canagliflozin increases urinary sodium excretion (UNa).
| Mean ± SEM (range) | Males | Females | Total |
|---|---|---|---|
| N (sample size) | 13 | 17 | 30 |
| Baseline UNa (total mEq per 24 hrs) | 252 ± 19 | 175 ± 11 | 208 ± 12 |
| Post-cana UNa (total mEq per 24 hrs) | 293 ± 191 (p=0.13) |
239 ± 291 (p=0.03) |
263 ± 191 (p=0.007) |
| Δ UNa (mEq per g-creatinine) | 31 ± 14 | 55 ± 22 | 45 ± 14 |
| Δ UNa (mEq per m2 body surface area) | 19 ± 12 | 35 ± 15 | 28 ± 10 |
| Δ UNa (mEq per kg body weight) | 0.46 ± 0.28 | 0.87 ± 0.36 | 0.68 ± 0.24 |
p-values calculated using Student’s t-test for paired data in comparison to baseline values for UNa
Our primary efficacy endpoint is based on measurements of biomarkers in 24-hour urine collections. We, therefore, investigated the reproducibility of 24-hour urine collections. On average, participants excreted 1286 ± 81 mg of creatinine during the 24 hours before canagliflozin as compared to 1252 ± 74 mg of creatinine during the 24 hours after canagliflozin administration (Fig. S3A). The quantities of 24-hour urinary creatinine before and after canagliflozin administration were highly correlated with one another (r=0.95; p=10−15). When we compared creatinine contents of the two specimens collected by each individual participant, differences were <5% for ~75% of paired urine collections (Fig. S3B). These data demonstrate that most participants successfully obtained complete collections of urine produced during a 24-hour time period.
3. 3 ∣. A sex-agnostic approach to normalizing urinary biomarker data.
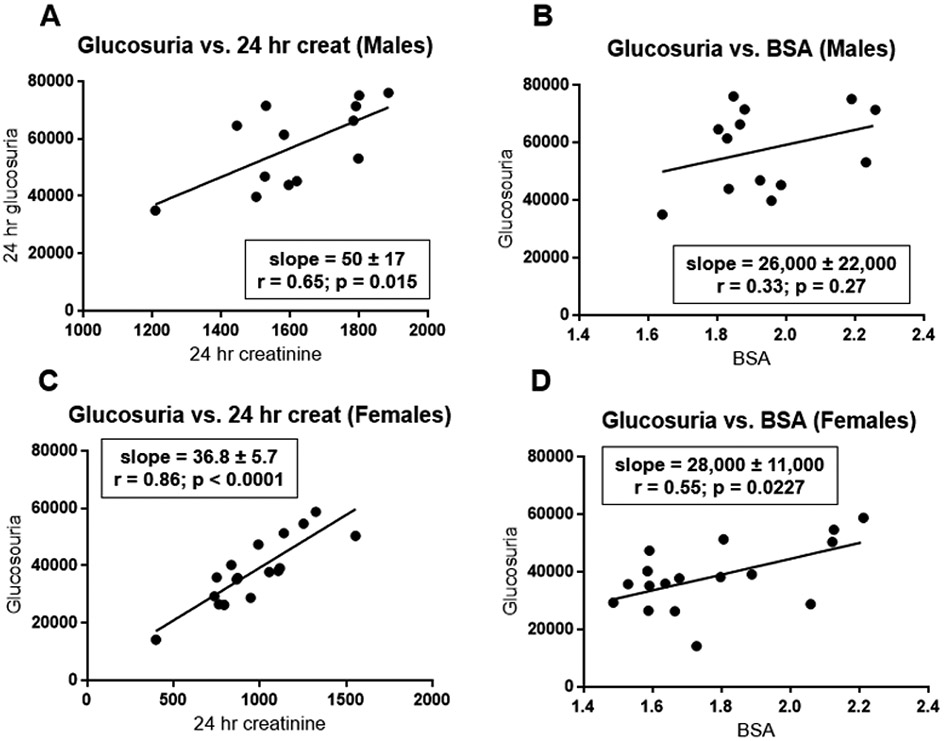
Urinary excretion of glucose provides a quantitative biomarker for efficacy of canagliflozin 22-27. Furthermore, enhanced urinary glucose excretion plays a critical role in mediating two important benefits provided by SGLT2 inhibitors: improved glycemic control in diabetic patients and weight loss in overweight/obese patients 10. To enable pharmacogenomic investigation, it is critical to optimize the approach to normalize data in order to compare glucosuric responses among participants. We considered two standard approaches: (a) normalizing urinary glucose excretion relative to body size (i.e., body weight or body surface area); or (b) normalizing glucosuria relative to 24-hour urinary creatinine excretion. Body weight was tightly correlated with body surface area (r=0.99) in both males and females (Fig. S4A), suggesting that these two parameters provide comparable indices for body size. Although 24-hour urinary creatinine was correlated with body surface area (r=0.68 for males and r=0.65 for females), males on average excreted ~60% more creatinine than females (Fig. S4B) when compared at the same body surface area.
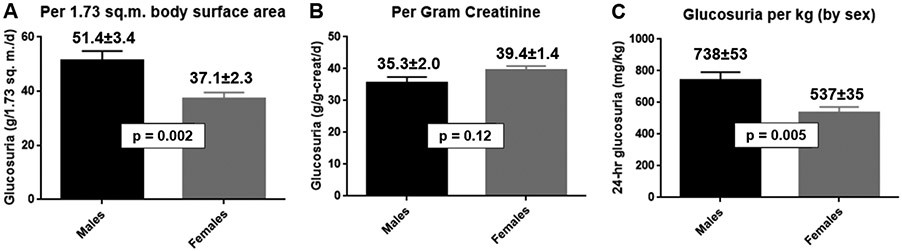
Glucosuria was correlated with urinary creatinine excretion in both males (r=0.65; Fig. 2A) and females (r=0.86; Figs. 2C). Glucosuria was also correlated with body surface area (Figs. 2B and 2D) or body weight (Fig. S5) – albeit with smaller correlation coefficients (0.29-0.33 in males and 0.54-0.55 in females). The closer correlations with urinary creatinine excretion favors the approach of normalizing glucosuria relative to urinary creatinine excretion. Furthermore, this approach yielded similar mean values for glucosuria in both sexes: 35.4 ± 2.0 in males versus 39.4 ± 1.4 g-glucose per g-creatinine in females (p=0.12; Fig. 3B). In contrast, mean values for glucosuria were 37-39% larger in males than females when expressed as a function of body surface area (Fig. 3A) or body weight (Fig. 3C). Thus, because mean values of glucosuria are similar in both sexes when expressed on a per g-creatinine basis, this will greatly facilitate including data from both sexes in the same analysis.
Figure 2. Correlations of glucosuria with urinary creatinine excretion and body surface area.
Glucosuria (expressed as mg/day) is plotted as a function of either 24-hour urinary creatinine excretion (mg/day) (panels A and C) or body surface area (m2) (panels B and D). Data are presented separately for males (panels A and B) and females (panels C and D). Using data analysis programs provided in Excel, we estimated slopes for the least-square lines and correlation coefficients. P-values were calculated using GraphPad Prism software.
Figure 3. Sex differences with respect to the magnitude of canagliflozin-induced glucosuria.
Using data presented in Figure 3, we calculated mean ± SEM for canagliflozin-induced 24-hour urinary glucose excretion normalized in one of two ways: grams of glucose per 1.73 m2 body surface area (Panel A) or grams of glucose per gram of creatinine (Panel B). Data for male and female participants are represented as either black or gray columns, respectively.
3.4 ∣. Inter-individual variation in canagliflozin-induced glucosuria.
We observed substantial inter-individual variation in the magnitude of canagliflozin-induced increases in urinary glucose excretion. The magnitude of glucosuria varied over an approximately twofold range: from ~25 to ~50 g-glucose/g-creatinine (Fig. S6). Two critical factors contribute to determining the quantity of glucose filtered at the glomerulus (the “filtered glucose load”): (a) plasma glucose levels and (b) glomerular filtration rates. Thus, we investigated correlations of glucosuria with fasting plasma glucose levels (FPG), creatinine clearance rates, and age (Fig. S7). This clinical trial focused on healthy volunteers – excluding patients with HbA1c ≥ 6.5% or eGFR < 60 mL/min/1.73 m2. In this population, we did not observe statistically significant correlations of 24-hour urinary excretion of glucose with fasting plasma glucose, measured creatinine clearance rates, or age (Fig. S7). In addition to being statistically insignificant, the correlation coefficients were quite small; variances in fasting plasma glucose, creatinine clearance, and age accounted for only ~1.4%, 0.5%, and 4.8% of the total variance in canagliflozin-induced glucosuria, respectively. Measured creatinine clearance was closely correlated with eGFR in both males and females (Fig. S8). However, we focused on the measured creatinine clearance because it is likely to be a more valid index of each individual’s glomerular filtration rate.
3.5 ∣. Canagliflozin-induced natriuresis
Because SGLT2 functions as a co-transporter for glucose plus Na+, canagliflozin inhibits proximal tubular reabsorption of both glucose and Na+ 10. Drug-induced natriuresis was calculated by subtracting 24-hour urinary Na+ excretion observed at baseline from 24-hour urinary Na+ excretion observed in the 24 hours after administration of canagliflozin. Canagliflozin increased mean urinary Na+ excretion by ~25% in the total population (N=30; p=0.007) (Table 1). Research participants were free to eat ad libitum and to engage in their usual daily activities. As no effort was made to control sodium intake, there may have been substantial day-to-day variation in the sodium content of participants’ diets and/or the quantity of sodium lost through sweating. Such day-to-day variations represent probable sources of unmeasured confounders with respect to estimates of drug-induced natriuresis. Notwithstanding these caveats, estimates of drug-induced natriuresis appeared to be larger for females than males – regardless of whether the magnitude of 24-hour Na+ urinary excretion was normalized relative to urinary creatinine excretion, body surface area, or body weight (Table 2). Although inhibition of SGLT2 directly blocks reabsorption of one ion of sodium per molecule of glucose, canagliflozin indirectly triggers homeostatic mechanisms that have substantial impact on the total quantity of sodium actually excreted in the urine – e.g., activation of the renin-angiotensin-aldosterone axis and decreased secretion of atrial natriuretic peptides 10. It is possible that an individual’s sex might impact the magnitude of homeostatic regulation.
3.6 ∣. Canagliflozin-induced uricosuria
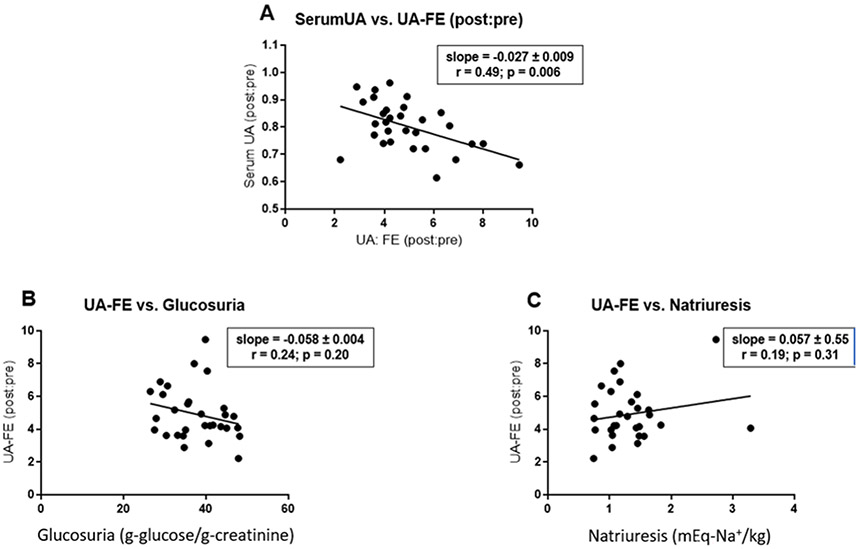
SGLT2 inhibitors enhance urinary excretion of uric acid and decrease serum levels of uric acid 10,19-21. Consistent with these previous publications, we observed that canagliflozin induced a 2- to 10-fold increase in fractional excretion of uric acid. The magnitude of the increase in fractional excretion of uric acid was correlated with the magnitude of the decrease in serum uric acid levels (r=0.48; p=0.006) (Fig. 4A). Although mechanisms mediating SGLT2 inhibitor-induced uricosuria have not been established, the effect may be an indirect consequence of the increase in glucose concentrations in the renal tubular fluid 19. Accordingly, we investigated whether the increase in fractional excretion of uric acid was correlated with canagliflozin-induced glucosuria. However, we did not detect statistically significant correlation between the drug-induced increase in fractional excretion of uric acid and canagliflozin-induced glucosuria or natriuresis (Fig. 4BC).
Figure 4. Canagliflozin-induced changes in serum uric acid and fractional excretion of uric acid.
Panel A represents a plot of the effect of canagliflozin on serum uric acid level as a function of the canagliflozin-induced increase in fractional excretion of uric acid. Both parameters are presented as ratios of post-canagliflozin levels divided by pre-canagliflozin levels. Panels B and C represents a plot of canagliflozin-induced fractional change in fractional excretion of uric acid as a function of canagliflozin-induced glucosuria (grams of glucose per gram of creatinine; panel B) or canagliflozin-induced natriuresis (expressed as a ratio of post-canagliflozin values divided by pre-canagliflozin values; panel C). We estimated slopes for the least-square lines and correlation coefficients using data analysis programs provided in Excel. p-values were calculated using GraphPad Prism software.
3.7 ∣. Pilot pharmacogenomic investigation of genetic variants in three candidate genes (SLC2A9, SLC5A4, and SLC5A9).
This pilot study was originally designed to investigate possible association of three candidate genetic variants with pharmacodynamic responses to an SGLT2 inhibitor: nonsense variants in SLC5A4 (rs62239058; p.E139X) and SLC5A9 (rs850763; p.E593X) and a missense variant in SLC2A9 (rs1689079; p.V253I). SLC5A4 and SLC5A9 encode two homologs of SGLT2 (SGLT3 and SGLT4, respectively) 28,29. SLC2A9 encodes GLUT9, a transporter reported to exchange glucose for uric acid 30.
Because the clinical trial was terminated early when it became apparent that the clinical trial would not meet its recruitment targets within the available time and budget, the study was underpowered to detect association of genetic variants with drug responses. In any case, none of the three genetic variants was associated with a statistically significant difference when compared to the control group. (Table S2). We cannot, however, exclude the possibility that these variants might exhibit significant associations in a substantially larger study with greater statistical power.
DISCUSSION
Following NIH’s initiative to improve scientific rigor and reproducibility 13, we leveraged this pilot study to validate the experimental protocol supporting our ongoing genome-wide association study (GWAS) to identify genetic variants associated with pharmacodynamic responses to SGLT2 inhibitors (NCT02891954). Based on quantitative comparisons of creatinine content in independent 24-hour urine collections, we confirmed that research participants successfully provided highly reproducible 24-hour urine collections. We did, nevertheless, observe a statistically insignificant trend toward a decreased mean creatinine content (−2.6%) in the second 24-hour urine collection (Fig. S3). Although not statistically significant, we suspect that this small difference may be real and reproducible. Canagliflozin-induced natriuresis leads to a modest volume contraction as reflected in the 6.4% increase in serum creatinine levels (Fig. 1B). The modestly decreased creatinine excretion during the second urine collection is consistent with the expected transient decrease in urinary creatinine excretion characterizing the transition between two steady-states (before and after administration of canagliflozin). Thus, even the small, statistically insignificant difference between the two 24-hour urine collections probably reflects the known pharmacological effects of canagliflozin rather than experimental variation.
Furthermore, we confirmed that canagliflozin elicited the expected pharmacodynamic responses and obtained information on the magnitude of inter-individual variation, which has informed power calculations for our ongoing GWAS. As expected, a single dose of canagliflozin triggered statistically significant responses in several parameters: fasting plasma glucose, serum uric acid, serum creatinine as well as urinary excretion of sodium, glucose, and uric acid (Figs. 1-4; Table 1). These data are reassuring with respect to the feasibility of our ongoing GWAS for pharmacodynamic responses to canagliflozin.
In 2016, the NIH established an expectation for NIH-supported research to include investigations of sex as a biological variable 14,31-33. While there has been debate about the optimal approach to implement this requirement, there is general agreement that sex-related variables may emerge as relevant within the context of a specific research program 34. Although some traits (e.g., karyotype sex) may be dichotomous for most individuals, there are many biological traits (e.g., height) that represent continuous variables with considerable overlap between sexes 34. We used participants’ self-reported sex as a proxy for karyotype sex. We applied this binary classification as the basis to conduct an exploratory investigation of whether an individual’s sex was associated with pharmacodynamic responses to canagliflozin. In parallel, we compared three approaches to normalizing our data – i.e., expressing drug-induced glucosuria relative to body surface area, body weight, or urinary excretion of creatinine. When expressed as grams of glucose per gram of creatinine, mean data were very similar in both males and females (Fig. 3B). In contrast, our data revealed substantial sex differences in mean quantities of canagliflozin-induced glucosuria when expressed relative to body surface area (Fig. 3A) or body weight (Fig. 3C). For example, canagliflozin induced an average of 37% more glucosuria in males when expressed on the basis of grams of glucose per kg of body weight (Fig. 3C). These data are consistent with weight loss data reported by the sponsors of dapagliflozin at the FDA’s Advisory Committee meeting on July 19, 2011 35. Specifically, 24 weeks of dapagliflozin therapy induced greater placebo-subtracted weight loss in males than in females (2.76 kg versus 1.22 kg). The FDA’s analysis concluded that the differential effect of sex was statistically significant (p=0.048) – albeit there was considerable overlap between males and females with respect to the magnitude of weight loss 35. Also, many factors contribute to determining the magnitude of weight loss in individual patients – including, for example, the magnitude of SGLT2 inhibitor-induced compensatory increase in food intake 36. Nevertheless, taken together, these data with dapagliflozin are consistent with the hypothesis that the larger magnitude of SGLT2 inhibitor-induced glucosuria in men (expressed per gram body weight) contributes to a larger magnitude of mean drug-induced weight loss.
Creatinine is produced by a non-enzymatic chemical reaction – i.e., the conversion of phosphocreatine to creatinine 37. Because phosphocreatine is located primarily in muscle, the rate of creatinine production implicitly reflects lean tissue mass. When glucosuria is expressed relative to urinary excretion of creatinine, this implicitly indexes glucosuria relative to lean body mass. These conclusions are consistent with a previous report 38 that mean measured glomerular filtration rate (mGFR) was lower in females than males when normalized relative to body surface area. This sex-based difference was eliminated when mGFR was normalized relative to lean body mass. Inasmuch as the kidney’s filtered glucose load is proportional to mGFR, the filtered glucose load would be predicted to be independent of sex if compared in people with similar lean body masses and similar levels of plasma glucose. Because SGLT2 inhibitors inhibit renal tubular reabsorption of glucose, this provides a strong rationale to normalize glucosuria relative to lean body mass, which implicitly normalizes relative to filtered glucose load.
We acknowledge that our approach of normalizing glucosuria relative to urinary creatinine excretion may not eliminate 100% of possible sex-associated differences. Nevertheless, by minimizing the quantitative contribution of sex-associated differences in the primary endpoint (glucosuria), this approach enables us to create an inclusive database containing data obtained in all participants – regardless of their self-reported sex. Accordingly, this case study exemplifies how routine study of sex as a biological variable does not necessarily conflict with the aims of precision medicine as suggested by DiMarco et al. 34. Our experience demonstrates that investigations of sex as a biological variable may offer opportunities for investigators to learn whether sex-related variables emerge as being relevant in the context of their particular research programs.
This pilot study confirms the feasibility of conducting a pharmacogenomic study focused on pharmacodynamic effects of canagliflozin in healthy volunteers. Based on assessment of creatinine content of 24-hour urine collections, we conclude that participants in our study provided complete collections of the urine produced during a 24-hour time period. Reproducibility and completeness of urine collections are critical elements of a scientifically rigorous clinical trial. We observed substantial inter-individual variation of our primary outcome (i.e., canagliflozin-induced glucosuria) – varying over a twofold range from ~25 to ~50 grams of glucose per gram of creatinine. By restricting our study to non-diabetic individuals with relatively normal estimated glomerular filtration rates, we limited the contributions of two important potential confounders. Inter-individual variation in fasting plasma glucose and creatinine clearance accounted for ~1.4% and ~0.5% of the observed variance in canagliflozin-induced glucosuria. Although our participants spanned a wide range of ages (35-82 years old), the variation in age accounted for only ~5% of the variance in canagliflozin-induced glucosuria. Finally, our studies of sex as a biological variable demonstrated that the magnitudes of canagliflozin-induced glucosuria were similar in males and females when expressed on a per gram-creatinine basis. Based on our observations, we conclude that >90% of the observed variance in our primary endpoint is unexplained after accounting for contributions of age, sex, renal function, and fasting plasma glucose. Accordingly, our ongoing pharmacogenomic study (NCT02891954) is well positioned to define the contribution of genetic factors to the relatively large residual variation in the pharmacodynamic effect of canagliflozin.
Our ongoing pharmacogenomic study focuses on pharmacodynamic responses to canagliflozin in non-diabetic healthy individuals. This represents one step toward identifying genetic variants associated with the magnitude of canagliflozin’s effects on clinical endpoints (e.g., HbA1c) in individuals with type 2 diabetes. Such follow-up studies can be conducted in the future to determine whether genetic variants associated with pharmacodynamic responses in healthy individuals will also be associated with clinical responses in individuals with diabetes. We are encouraged by the fact that this clinical trial demonstrated a substantial degree of inter-individual variation (twofold) in pharmacodynamic response. Furthermore, this degree of variation in pharmacodynamic response is likely to be predictive of clinically meaningful differences in clinically relevant endpoints in individuals with diabetes. The prescribing information for canagliflozin states that canagliflozin monotherapy decreases mean HbA1c by 1.03% (300 mg dose) and 0.77% (100 mg dose). Sha et al. 39 reported that the 300 mg dose of canagliflozin induced ~33% more glucosuria than the 100 mg dose. When we compare the efficacies of those two approved doses of canagliflozin, a 33% increase in glucosuria is associated with 0.26% larger HbA1c-lowering. This suggests that a twofold range in drug-induced glucosuria might be able to account for a clinically meaningful range among individuals with respect to drug-induced HbA1c lowering – from a low of 0.4% to a high of 1.2%.
Supplementary Material
ACKNOWLEDGEMENTS
We gratefully acknowledge the National Institute of Diabetes and Digestive and Kidney Disease for the following research grants: R21DK105401, R01DK108942, T32DK098107, and P30DK072488. We also gratefully acknowledge contributions of the research participants and the skilled staff at the Amish Research Clinic for their critical roles in making this study possible. We are grateful to Mary Pavlovich, Melanie Daue, and Kathy Ryan for their help with database management. Dr. Laura Yerges-Armstrong provided genotype data enabling us to identify individuals to be invited to participate in this genotype-guided recruitment study.
Footnotes
Competing Interests
SIT serves as a consultant for Ionis Pharmaceuticals and receives an inventor’s share of royalties from NIDDK for metreleptin as a treatment for generalized lipodystrophy. ARS is an employee of Regeneron Genetics Center. BDM and MEM receive grant support from Regeneron Genetics Center. BDM, MEM, EAS, and HBW have received partial salary support from funds provided by RGC. ALB, ZSY, and HRC declare no competing interests.
REFERENCES
- 1.Inzucchi SE, Bergenstal RM, Buse JB, et al. Management of hyperglycemia in type 2 diabetes: a patient-centered approach: position statement of the American Diabetes Association (ADA) and the European Association for the Study of Diabetes (EASD). Diabetes Care. 2012;35(6):1364–1379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ali AM, Martinez R, Al-Jobori H, et al. Combination Therapy With Canagliflozin Plus Liraglutide Exerts Additive Effect on Weight Loss, but Not on HbA1c, in Patients With Type 2 Diabetes. Diabetes Care. 2020;43(6):1234–1241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Pawlyk AC, Giacomini KM, McKeon C, Shuldiner AR, Florez JC. Metformin pharmacogenomics: current status and future directions. Diabetes. 2014;63(8):2590–2599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Pearson ER. Dorothy Hodgkin Lecture 2021: Drugs, genes and diabetes. Diabet Med. 2021;38(12):e14726. [DOI] [PubMed] [Google Scholar]
- 5.Rathmann W, Bongaerts B. Pharmacogenetics of novel glucose-lowering drugs. Diabetologia. 2021;64(6):1201–1212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chedid V, Vijayvargiya P, Carlson P, et al. Allelic variant in the glucagon-like peptide 1 receptor gene associated with greater effect of liraglutide and exenatide on gastric emptying: A pilot pharmacogenetics study. Neurogastroenterol Motil. 2018;30(7):e13313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mashayekhi M, Wilson JR, Jafarian-Kerman S, et al. Association of a glucagon-like peptide-1 receptor gene variant with glucose response to a mixed meal. Diabetes Obes Metab. 2021;23(1):281–286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Francke S, Mamidi RN, Solanki B, et al. In vitro metabolism of canagliflozin in human liver, kidney, intestine microsomes, and recombinant uridine diphosphate glucuronosyltransferases (UGT) and the effect of genetic variability of UGT enzymes on the pharmacokinetics of canagliflozin in humans. J Clin Pharmacol. 2015;55(9):1061–1072. [DOI] [PubMed] [Google Scholar]
- 9.Zhou K, Yee SW, Seiser EL, et al. Variation in the glucose transporter gene SLC2A2 is associated with glycemic response to metformin. Nat Genet. 2016;48(9):1055–1059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Beitelshees AL, Leslie BR, Taylor SI. Sodium-Glucose Cotransporter 2 Inhibitors: A Case Study in Translational Research. Diabetes. 2019;68(6):1109–1120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Taylor SI, Yazdi ZS, Beitelshees AL. Pharmacological treatment of hyperglycemia in type 2 diabetes. J Clin Invest. 2021;131(2). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Shuldiner AR, O'Connell JR, Bliden KP, et al. Association of cytochrome P450 2C19 genotype with the antiplatelet effect and clinical efficacy of clopidogrel therapy. JAMA. 2009;302(8):849–857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Collins FS, Tabak LA. Policy: NIH plans to enhance reproducibility. Nature. 2014;505(7485):612–613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Rich-Edwards JW, Kaiser UB, Chen GL, Manson JE, Goldstein JM. Sex and Gender Differences Research Design for Basic, Clinical, and Population Studies: Essentials for Investigators. Endocr Rev. 2018;39(4):424–439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Denny JC, Collins FS. Precision medicine in 2030-seven ways to transform healthcare. Cell. 2021;184(6):1415–1419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hsueh WC, Mitchell BD, Aburomia R, et al. Diabetes in the Old Order Amish: characterization and heritability analysis of the Amish Family Diabetes Study. Diabetes Care. 2000;23(5):595–601. [DOI] [PubMed] [Google Scholar]
- 17.Agarwala R, Biesecker LG, Tomlin JF, Schaffer AA. Towards a complete North American Anabaptist genealogy: A systematic approach to merging partially overlapping genealogy resources. Am J Med Genet. 1999;86(2):156–161. [DOI] [PubMed] [Google Scholar]
- 18.Agarwala R, Schaffer AA, Tomlin JF. Towards a complete North American Anabaptist Genealogy II: analysis of inbreeding. Hum Biol. 2001;73(4):533–545. [DOI] [PubMed] [Google Scholar]
- 19.Chino Y, Samukawa Y, Sakai S, et al. SGLT2 inhibitor lowers serum uric acid through alteration of uric acid transport activity in renal tubule by increased glycosuria. Biopharm Drug Dispos. 2014;35(7):391–404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Novikov A, Fu Y, Huang W, et al. SGLT2 inhibition and renal urate excretion: role of luminal glucose, GLUT9, and URAT1. Am J Physiol Renal Physiol. 2019;316(1):F173–F185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Suijk DLS, van Baar MJB, van Bommel EJM, et al. SGLT2 Inhibition and Uric Acid Excretion in Patients with Type 2 Diabetes and Normal Kidney Function. Clin J Am Soc Nephrol. 2022;17(5):663–671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Komoroski B, Vachharajani N, Boulton D, et al. Dapagliflozin, a novel SGLT2 inhibitor, induces dose-dependent glucosuria in healthy subjects. Clin Pharmacol Ther. 2009;85(5):520–526. [DOI] [PubMed] [Google Scholar]
- 23.Sha S, Polidori D, Farrell K, et al. Pharmacodynamic differences between canagliflozin and dapagliflozin: results of a randomized, double-blind, crossover study. Diabetes Obes Metab. 2015;17(2):188–197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Busse D, Tang W, Scheerer M, et al. Comparison of pharmacokinetics and the exposure-response relationship of dapagliflozin between adolescent/young adult and adult patients with type 1 diabetes mellitus. Br J Clin Pharmacol. 2019;85(8):1820–1828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sokolov V, Yakovleva T, Chu L, et al. Differentiating the Sodium-Glucose Cotransporter 1 Inhibition Capacity of Canagliflozin vs. Dapagliflozin and Empagliflozin Using Quantitative Systems Pharmacology Modeling. CPT Pharmacometrics Syst Pharmacol. 2020;9(4):222–229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Blau JE, Bauman V, Conway EM, et al. Canagliflozin triggers the FGF23/1,25-dihydroxyvitamin D/PTH axis in healthy volunteers in a randomized crossover study. JCI Insight. 2018;3(8). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Blau JE, Taylor SI. Adverse effects of SGLT2 inhibitors on bone health. Nat Rev Nephrol. 2018;14(8):473–474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bianchi L, Diez-Sampedro A. A single amino acid change converts the sugar sensor SGLT3 into a sugar transporter. PLoS One. 2010;5(4):e10241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Tazawa S, Yamato T, Fujikura H, et al. SLC5A9/SGLT4, a new Na+-dependent glucose transporter, is an essential transporter for mannose, 1,5-anhydro-D-glucitol, and fructose. Life Sci. 2005;76(9):1039–1050. [DOI] [PubMed] [Google Scholar]
- 30.Witkowska K, Smith KM, Yao SY, et al. Human SLC2A9a and SLC2A9b isoforms mediate electrogenic transport of urate with different characteristics in the presence of hexoses. Am J Physiol Renal Physiol. 2012;303(4):F527–539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Clayton JA, Collins FS. Policy: NIH to balance sex in cell and animal studies. Nature. 2014;509(7500):282–283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Legato MJ, Johnson PA, Manson JE. Consideration of Sex Differences in Medicine to Improve Health Care and Patient Outcomes. JAMA. 2016;316(18):1865–1866. [DOI] [PubMed] [Google Scholar]
- 33.Woitowich NC, Beery A, Woodruff T. A 10-year follow-up study of sex inclusion in the biological sciences. Elife. 2020;9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.DiMarco M, Zhao H, Boulicault M, Richardson SS. Why "sex as a biological variable" conflicts with precision medicine initiatives. Cell Rep Med. 2022;3(4):100550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.FDA. Advisory Committee Meeting for Dapagliflozin (July 19, 2011). 2011.
- 36.Ferrannini G, Hach T, Crowe S, Sanghvi A, Hall KD, Ferrannini E. Energy Balance After Sodium-Glucose Cotransporter 2 Inhibition. Diabetes Care. 2015;38(9):1730–1735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Iyengar MR, Coleman DW, Butler TM. Phosphocreatinine, a high-energy phosphate in muscle, spontaneously forms phosphocreatine and creatinine under physiological conditions. J Biol Chem. 1985;260(12):7562–7567. [PubMed] [Google Scholar]
- 38.Abraham AG, Shafi T, Tighiouart H, et al. Effects of Body Size and Composition on Sex Differences in Measured GFR in a US Community-Based Older Cohort (MESA-Kidney). Am J Kidney Dis. 2018;72(5):767–770. [DOI] [PubMed] [Google Scholar]
- 39.Sha S, Devineni D, Ghosh A, et al. Pharmacodynamic effects of canagliflozin, a sodium glucose co-transporter 2 inhibitor, from a randomized study in patients with type 2 diabetes. PLoS One. 2014;9(9):e110069. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.