Abstract
Germinal centers (GCs) are key microanatomical sites in lymphoid organs where responding B cells mature and undergo affinity-based selection. The duration of the GC reaction has long been assumed to be relatively brief, but recent studies in humans, nonhuman primates, and mice indicate that GCs can last for weeks to months after initial antigen exposure. This review examines recent studies investigating the factors that influence GC duration, including antigen persistence, T-follicular helper cells, and mode of immunization. Potential mechanisms for how persistent GCs influence the B-cell repertoire are considered. Overall, these studies provide a blueprint for how to design better vaccines that elicit persistent GC responses.
Introduction
Protection against many pathogens relies on developing an effective humoral immune response. One characteristic feature of this response is the continued increase in antibody affinity for an antigen over time [1]. This affinity maturation occurs in germinal centers (GCs), which are distinct microanatomical structures that form primarily in the follicles of secondary lymphoid organs (SLOs) [2]. Activated B cells that enter the GC undergo iterative rounds of somatic hypermutation (SHM) and proliferation while affinity-maturing their B-cell receptor (BCR) against the inciting antigen [2,3]. Graduates of the GC differentiate into long-lived bone marrow plasma cells (BMPCs) and circulating memory B cells (MBCs) [reviewed in 4]. Additionally, long-lived plasma cells can be retained in the tissue of origin (e.g. spleen) 5] or gut-associated lymphoid tissue [6,7], and tissue-resident MBCs serve important defense roles at sites such as surface barriers (reviewed in [8]). Forming these effector B-cell types is critical for mediating long-term protection against a pathogen and is a major goal of vaccine development.
GC formation begins when antigen-experienced cognate B and T cells interact at the interface between the T-cell zone and the border of the follicle [9,10]. Activated B cells that receive survival and co-stimulatory signals from cognate T cells can migrate to the center of the follicle to seed the GC, which is divided into two distinct compartments — the dark zone (DZ) and the light zone (LZ) [11,12]. CXC motif chemokine ligand 12 (CXCL12)-producing stromal cells in the DZ attract CXC motif chemokine receptor 4 (CXCR4)-expressing GC B cells, which proliferate and undergo SHM [11]. After they have undergone SHM, these GC B cells downregulate CXCR4 and migrate into the LZ through CXC motif chemokine receptor 5 (CXCR5) sensing of its ligand CXC motif chemokine ligand 13 (CXCL13), which is produced by follicular dendritic cells (FDCs) in the LZ [11,13]. FDCs are critical sites of antigen deposition in the follicle, retaining and presenting antigen to GC B cells to regulate their affinity maturation [14,15]. FDCs also work with T-follicular helper (Tfh) cells in the LZ to send necessary survival signals to GC B cells [16-18]. Positively selected GC B cells can return to the DZ to undergo additional SHM and proliferation or exit the GC as BMPCs or MBCs [19].
The generation of long-lived effector cells is critical for lasting protection against pathogens. While MBCs and, less frequently, BMPCs, can be produced in a GC-independent manner, those arising from the GC typically have a higher affinity for antigen [20-23]. GC-dependent MBCs are poised to rapidly differentiate into short-lived, extrafollicular plasmablasts (PBs) in the event of pathogen re-exposure, producing high-affinity antibodies that can work in concert with matured antibodies from BMPCs to help clear pathogens quickly [22,24]. Some evidence in mice and humans suggests that upon restimulation with antigen, MBCs can differentiate to PCs that potentially contribute to the BMPC pool [25,26], although direct differentiation to long-lived PCs remains undetermined. MBCs can also re-enter the GC and undergo additional rounds of SHM and affinity maturation, thus enhancing subsequent responses [27].
Since the mutational load of GC B cells increases over time, and high levels of mutation enhance antibody affinity, extending the duration of GCs can enhance the production of high-affinity antibodies [21,28]. In this review, we will summarize what is currently known about GC duration, as well as explore multiple factors that may influence GC persistence, including immune complex formation, antigen persistence in the follicle, the ability of GC B cells to acquire T-cell help, and the proliferative capacity of GC B cells. Additionally, we will highlight how the method and context of antigen exposure alter the duration of the GC and how vaccination strategies may be optimized to enhance GC persistence.
How long do germinal centers last?
The ultimate duration of the GC reaction varies depending on the model system studied. Most studies assessing GC kinetics have been done in murine models of vaccination in which an antigen and adjuvant are given as a bolus. These models frequently use a hapten conjugated to a carrier protein, adjuvanted with alum, and track the hapten-specific GC response in the SLOs. With this immunization method, the antigen-specific mouse GC declines between 2 and 5 weeks after immunization. However, small numbers of antigen-specific GC B cells have been detected in the spleen as late as 21 weeks post immunization [21,29,30]. Using alternate adjuvants may extend GC magnitude and duration, but immunization with other proteins in alum leads to a similar GC kinetics [31-34]. The GC response after injection of sheep red blood cells has also been extensively studied and frequently declines between 2 and 4 weeks post injection in mice [35-37]. Alternate vaccine design and delivery can increase the magnitude and duration of the mouse GC response. Antigens packaged in nanoparticles, particularly those including innate immune system agonists, result in GCs persisting up to 8 weeks post immunization in mice and frequently induce a larger GC compared with a bolus immunization [38,39].
In contrast to vaccination, infection consistently produces more persistent GCs in mice, especially in the SLOs draining the affected tissues. In mice infected with influenza virus, splenic GCs persist until 6–9 weeks post infection, whereas GCs in the lung-draining mediastinal lymph node last between 18 and 24 weeks post infection [40-43]. Notably, later GCs had higher levels of SHM than early GCs and continuously graduated MBCs, highlighting the importance of GC persistence in increasing the affinity of the subsequent antibody response [40,42]. This phenomenon is also observed when comparing GCs generated from acute or chronic infection using the lymphocytic choriomeningitis virus (LCMV) model. Chronically infected mice exhibit more robust GCs at later time points [44,45]. These GCs facilitate higher levels of SHM that lead to an increase in neutralizing antibodies in chronically infected animals compared with acute infection [44].
GC persistence has also been studied in nonhuman primate (NHP) models. Different methods of antigen packaging, adjuvanting, and dosing have also been compared in NHPs. An alum-adjuvanted bolus induces a GC that persists up to 10 weeks post immunization [46]. In contrast, using a saponin-like adjuvant extends GC persistence to 14 weeks post immunization in rhesus monkeys [47]. Escalating the immunization dose over several weeks or delivering antigen through a slow-release osmotic pump also increases the magnitude of the NHP GC response [46,47].
In humans, indirect evidence that GC responses can persist for extended periods is demonstrated by peripheral B-cell SHM rates in antigen-specific BCRs. In subjects vaccinated with a replication-competent adenovirus type-4 recombinant virus expressing influenza H5 hemagglutinin, increases in H5-specific antibody SHM were detected up to 12 months after vaccination [48]. This ongoing SHM persisted beyond the period of active viral replication (2–4 weeks after vaccination) [48]. Similarly, in a longitudinal study, two flavivirus-naive donors vaccinated with the yellow fever 17D vaccine exhibited increased antibody SHM and affinity maturation for 6–9 months post immunization [49]. Ongoing SHM in peripheral B cells has also been observed in humans after influenza H7N9 vaccination [50] and Ebola virus infection [51].
In a study of survivors of infection with severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), the causative agent of the coronavirus disease-2019 (COVID-19) pandemic, increases in SHM and antibody affinity were observed over several months [52]. This was corroborated by other studies of SARS-CoV-2 infection based on peripheral blood samples [53,54]. However, because postmortem samples of spleen and lymph nodes from severely infected COVID-19 patients showed disrupted GCs, these studies presented no direct evidence of persisting GCs [52].
Other studies have utilized ultrasound-guided fine-needle aspiration to detect GC B cells in lymph nodes after vaccination in humans [55,56]. Notably, the use of mRNA vaccines in response to the COVID-19 pandemic has highlighted the importance of vaccine design in influencing GC magnitude and persistence. In humans, two doses of BNT162b2 (Pfizer-BioNTech), an mRNA vaccine encoding the spike protein (S) of SARS-CoV-2, produced an S-specific GC response that lasted up to 6 months post immunization [56-58]. In contrast, immunization with the unadjuvanted quadrivalent inactivated vaccine for influenza virus induces an antigen-specific GC that lasts between 2 and 4 months post vaccination [55; unpublished data]. While exposure history and pre-existing memory may complicate the comparison between these vaccines in humans, the strength of the GC response to mRNA vaccination suggests that it could be a useful strategy for increasing GC persistence after immunization.
These studies in murine and NHP models and humans demonstrate that GCs can persist for extended periods. Classical dogma based on hapten immunizations in mice would suggest that B-cell responses are rapidly generated, affinity-matured, and GCs are only maintained for a few weeks. However, the studies above indicate that GC responses can persist for months following infection or vaccination and that the antigen-specific B-cell repertoire can continue to mutate and affinity-mature over this period. To design vaccines that elicit these persistent GC reactions, multiple factors should be considered that may influence the duration of the GC.
Factors influencing germinal center persistence
Antigen persistence
After immunization, the antigen is rapidly delivered to the draining lymph nodes [59-61], but only retained for days to weeks within the B-cell follicles [62]. Opsonization of antigens by complement or immunoglobulins generates immune complexes, which can be efficiently trafficked into follicles by subcapsular sinus macrophages [63,64]. These immune complexes are then captured and retained on the surface of FDCs, which present membrane-bound antigens to B cells for selection within the GC [15,16]. B-cell clones will test the affinity of their BCRs in a mechanosensory process [65,66], interacting with FDCs [67] to extract antigen from their surface [68]. FDCs are unique antigen-presenting cells in that they recycle and retain immune complexes without degradation on their surface for extended periods [14,69], thus acting as a source of intact antigen to fuel ongoing selection in the GC.
Extended antigen persistence may contribute to the durability of GC responses. In silico modeling suggests that while T-cell help is necessary for generating GCs, antigen consumption by B cells governs the duration of the GC [70]. Computational modeling also predicts that immune complex formation and antigen concentration on the surface of FDCs dictate GC kinetics and that increased antigen availability results in increased numbers of GC B cells in mice [39]. In vivo, persistent antigen has been found to influence GC B-cell numbers and Tfh cells. In vesicular stomatitis virus (VSV)-infected mice that exhibited GCs up to 100 days post infection, antigen persisted on FDCs for the duration of the GC [71]. Mice immunized with a continuous supply of antigen exhibit prolonged antigen retention on the surface of FDCs and a greater number of GC B cells compared with the bolus immunization [39]. A constant supply of antigens also positively correlates with the magnitude of the Tfh-cell response and the maintenance of the Tfh phenotype [72]. This finding agrees with the result that viral persistence drives CD4 T cells toward a Tfh phenotype [73], suggesting that persistent antigen influences both GC B- and Tfh-cell levels. Additionally, the lack of antigen presentation by B cells reduces Tfh-cell differentiation [74]; thus, a constant source of antigen from FDCs to B cells is likely necessary to sustain cognate B–Tfh interactions.
Limited evidence in human studies also suggests antigen persistence may contribute to GC duration. GC responses that lasted up to 6 months have been detected in humans that received SARS-CoV-2 mRNA vaccines [56]. Röltgen et al. separately demonstrated that SARS-CoV-2 vaccine S-protein is present in lymph node GCs of vaccinees up to 60 days post second dose [75]. If such mRNA vaccine-derived antigen is indeed driving long-lasting GCs in human vaccine recipients, it would support the hypothesis that GC persistence is dependent on antigen availability.
Other computational modeling experiments demonstrate that while increased antigen availability extends the length of the GC reaction, it can also influence the degree of affinity maturation the B-cell repertoire undergoes [76], as lower-affinity B-cell clones can compete for help with increased antigen availability. These results have been confirmed in vivo, where sequential immunization with antigen variants has been shown to broaden the reactivity of the B-cell response [77], suggesting that increased antigen persistence may not only lengthen GC responses but also influence repertoire diversity and affinity [78].
T-follicular helper cells
Tfh cells play an important role in the GC reaction by providing ‘help’ to antigen-specific B- cell clones in the form of CD40 ligand (CD40L), interleukin (IL)-21, and IL-4 [79]. B cells that do not receive co-stimulation after binding antigen likely die [80], and Tfh-cell impairment in mice results in reduced or absent GCs [81-83]. While Tfh cells are not only involved in initiating GC responses, they likely contribute to determining GC duration, as GCs stimulated by T-independent antigens such as 4-Hydroxy-3-nitrophenylacetic (NP)-Ficoll do not persist as long as conventional T-dependent GCs [84]. T-cell help is considered a possible limiting factor in GC selection, with GC B cells competing for survival signals by presenting antigen to Tfh cells to enter the DZ and clonally expand [18]. However, some data suggest that T-cell help may not be necessary for LZ–DZ cycling [85]. Instead, increased Tfh-cell help ‘refuels’ GC B cells for improved survival and proliferation in the DZ, promoting selection and polyclonal responses [85]. This model demonstrates how cognate B–T-cell interactions may not be necessary for B-cell GC cycling, but are required to sustain prolonged GC responses. Without Tfh-cell help, GC B cells cannot receive growth and proliferation signals [85,86] necessary for long-lived GCs.
In addition to controlling selection and proliferative capacity, Tfh cells also influence the terminal differentiation of GC B cells to plasma cells or MBCs, in part by regulating GC persistence. Increased plasma cell differentiation due to constitutive CD40L expression in mouse B cells results in premature termination of GCs [87]. The Tfh-cell repertoire also likely plays a role in determining GC persistence. Experiments suggest low-affinity T-cell antigens result in early GC collapse and reduced memory B-cell output [88]. The length of the GC reaction may also be determined by the differentiation of Foxp3+ Tfh cells, also known as Tfh-regulatory cells [89]. These cells have been found to expand in mice before GC contraction, with late-stage GC B cells displaying prolonged interactions with Foxp3+ Tfh cells [90]. Tfh-regulatory cells have also been shown to suppress Tfh numbers [89]. Disruption of B-cell interactions with Tfh-regulatory cells results in elevated GC responses [91]. Thus, Tfh phenotypic changes likely influence the length of GCs. These studies demonstrate that by influencing GC entry, proliferation of GC B cells, and GC termination, Tfh cells are critical in determining GC length by controlling many of the ‘on’ and ‘off’ switches for sustained B-cell responses.
Proliferative capacity and B-cell receptor signaling
As GCs are derived from the clonal expansion of proliferating B cells, the persistence of GC responses is influenced by the divisional capacity and internal signaling of B cells. The formation and maintenance of GCs are dependent on the expression of the cell cycle regulator c-Myc in B cells [92,93]. Downstream of BCR signaling, c-Myc controls the expression of D-type cyclins necessary for the DZ GC B-cell proliferation [94]. The levels of c-Myc expression proportionally dictate the amount of cellular expansion in the DZ [95], and proliferative capacity is controlled by the Tfh-cell help [85] and BCR/CD40 signaling [96]. Blocking these signals or knocking out cyclin D3 reduces proliferation in the GC [97]. Inhibition of c-Myc also results in reduced GC size [93]. Thus, the degree to which B-cell clones continuously receive proliferative signals likely influences the overall duration of the GC response.
Type of infection or antigen exposure
How long GCs persist is influenced by whether a pathogen generates an acute or chronic infection. Mouse models utilizing LCMV, which can produce acute or chronic infections depending on the strain used and infection dose, demonstrate that chronic viral infections drive more extended GC responses. Mice chronically infected with LCMV had GC B cells that persisted up to 60 days post infection [44]. Chronic LCMV infection generates higher numbers of GC B cells and increased antiviral antibody titers over time compared with acute infection [44,45]. While B cells accumulate mutations in acute and chronic infections, long-term chronic infections generate higher-neutralizing antibody titers dependent on SHM of antibody clones, and sustain clonal diversity throughout the response [44,45]. Additionally, for some pathogens, the generation of a potent neutralizing antibody response is dependent on appreciable levels of affinity maturation, as evidenced by longitudinal analysis of responses to Ebola virus in humans [51]. These results in mice strongly suggest that persistent GCs are more likely to be established by chronic pathogen infections. However, evidence for persisting GCs in mice in response to influenza [42] or VSV [71] infection indicates that the persistence of antigen may continue to drive GC responses even after viral infection has been resolved.
Infection or vaccination with pathogens (live or inactivated) is more likely to generate long-lived GC responses than vaccination with protein antigens. A longitudinal study in humans found that half-lives for antibody responses to viral antigens were far greater (50–200 years) than for responses to nonreplicating protein antigens (11–19 years) [98]. Mice immunized with protein antigens typically do not generate GCs that persist for more than a few weeks [21,99]. Still, modulation of the delivery method of protein antigen to better stimulate pattern recognition receptors of the innate immune system can increase GC duration to over one year [38]. The method of vaccination used to stimulate GC responses likely greatly influences whether GCs are maintained for long periods.
Method of vaccination
Modulating the vaccine delivery method beyond bolus injection or the adjuvant used has been shown to contribute to the development of prolonged GC responses. An escalating dose regimen of antigen delivery, in which mice were immunized with exponentially increasing doses of a protein antigen, was shown to increase both the magnitude of the antibody response and the numbers of GC B cells [39]. It is hypothesized that this method of antigen delivery improves the GC response by providing a continuous supply of intact antigens rather than degraded epitopes for diverse B-cell selection [100]. Another possibility is that greater antigen supply increases the probability that antigen–antibody immune complexes form due to early PB induction. FDCs can then capture and retain these immune complexes for GC B-cell selection. Increased antigen dosing may also overcome pre-existing high-affinity antibody titers that clear antigen before it can be presented in GCs, thus limiting the GC response.
Multiple studies have validated the continuous supply of antigens as a robust method for optimizing antibody responses. Osmotic pump immunization utilized in NHPs demonstrated that extended immunogen release could improve neutralizing antibody titers to human immunodeficiency virus (HIV) versus bolus injection of antigen [101]. Osmotic pump delivery of antigen [102] and mini collagen pellets [103] have also been shown to modulate mouse antibody responses. Finally, slow antigen release devices have been shown to induce high-titer antibody responses and long duration in sheep [104]. In addition to the method of antigen delivery, modulating the site of booster vaccination may contribute to improving GC persistence and MBC recall. Data in mice indicate that ipsilateral boosting with antigen rather than contralateral boosting more efficiently recalls MBC to secondary GC reactions [105]. This result may be observed because ipsilateral boosting better coordinates cognate antigen delivery with persistent GCs in draining lymph nodes, thus helping to expand the MBC repertoire. In agreement with the studies supporting increased antigen availability contributing to persisting GCs, altering vaccination strategies to supply antigen continuously will likely extend GC response duration.
Insights from ‘naturally’ persisting germinal centers — gut and tertiary lymphoid structures
In addition to the studies cited above contributing evidence regarding the factors that extend GC duration, some information may be gleaned by studying two scenarios where GCs persist naturally for extended periods. In contrast to the typically assumed transient GCs of SLOs, GCs persist chronically in the Peyer’s patches of the murine gut due to continuous stimulation by commensal bacteria [reviewed in 106,107]. However, there is underdeveloped gut-associated lymphoid tissue and an absence of GCs in the gut of germ-free mice [108,109], suggesting a lack of antigen results in an abrogation of the persistent GC response. Murine gut GCs have been shown to robustly select antigen-specific clones and facilitate SHM and affinity maturation of BCRs [110], despite a lack of immunization or infection. The rate of B-cell selection is tunable based on the presence or absence of microbiota, with faster clonal dominance occurring in germ-free mice [110]. Collectively, data from gut GCs indicate that persistent GC reactions likely require continuous antigen stimulation, but also that long-lived GCs can support complex clonal selection. Thus, stimulating persisting GCs in SLOs will likely support diverse B-cell repertoires.
Further support for the benefit of persisting GCs comes from tertiary lymphoid structures (TLSs). TLSs are ectopic lymphoid structures that develop outside of SLOs at sites of inflammation or chronic antigen stimulation and are associated with both autoimmune diseases and tumors [reviewed in 111,112]. TLSs associated with tumors can generate mature GCs and support antigen-driven B-cell selection [reviewed in 111]. In several forms of cancer, the presence of TLSs with GCs has been associated with improved patient prognosis. Patients with pancreatic ductal adenocarcinoma that develops TLS GCs show increased rates of B-cell SHM and improved survival [113]. Improved prognosis was also associated with TLS GCs in patients with lung squamous cell carcinoma [114] and colorectal cancer [115]. B-cell signatures and GCs in tumors are enriched in patients that respond positively to immune checkpoint blockade therapy [116], and CXCL13-producing Tfh cells have been found to be a positive biomarker for survival in patients with breast cancer (presumably indicative of GC activity) [117]. The development of TLS GCs in the presence of chronic antigen stimulation (tumors) and the improved association of these GCs with patient survival provides another ‘natural’ example of persistent GCs that require a continuous source of antigen and generate enhanced humoral effector output.
Why should we care about persistent germinal centers?
The goal of understanding the factors that influence the GC reaction is to design better vaccines that produce long-lasting humoral immunity. This is contingent on the ability of a vaccine to generate effective MBC repertoires and long-lived BMPCs. Part of the difficulty in generating vaccines against certain infectious agents is the high degree of antigenic variability that allows the pathogen to evade the humoral immune system. This is seen with HIV, malaria, influenza, and, most recently, SARS-CoV-2. Generating more persistent GC responses may facilitate protection against such pathogens by boosting antibody titers and generating greater SHM in MBCs and BMPCs [46,57]. Extended GC responses may contribute to generating a repertoire of responding B cells that more broadly bind pathogen strains [56], potentially generating MBC pools that can anticipate pathogen mutants. Persistent GCs can support a complex polyclonal selection environment, with ‘invader’ clones continuously entering the ongoing GC reactions [31,41]. This likely contributes to the ‘permissive’ selection of a diversity of clones spanning various affinities [118], and thus is more likely to produce highly mutated B-cell clones while preventing responses from being biased toward a limited repertoire.
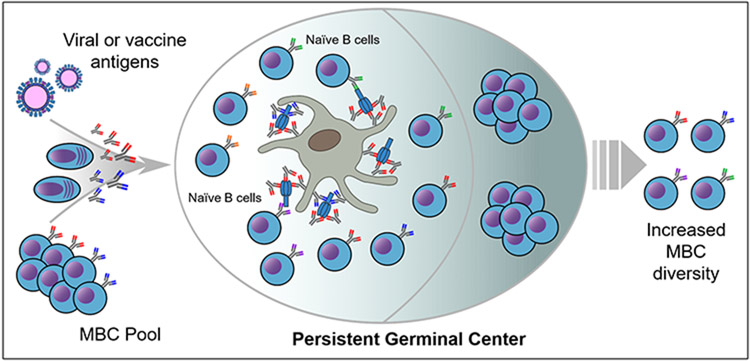
One potential mechanism for how persistent GCs benefit the B-cell repertoire could be extended access to novel antigen epitopes (Figure 1). If early PB responses generated by MBCs produce secreted antibodies to conserved epitopes, these antibodies could form immune complexes or bind antigens on the surface of FDCs. These early secreted antibodies would block access by GC B-cell clones to the conserved epitopes unless BCRs were of sufficiently high affinity to outcompete the MBC clonotypes. This would drive B-cell selection toward clonotypes targeting nonconserved or novel epitopes, thus ensuring a more diverse repertoire while simultaneously supporting the development of high-affinity clones. In a scenario where GC duration is relatively short, this process could be ‘shut down’ early by secreted antibodies reducing intact antigen persistence. The result would be limited B-cell selection and maintenance of a MBC pool targeting conserved, previously experienced epitopes (i.e. ‘antigenic imprinting’ phenomenon observed with influenza). However, if GC reactions were extended for long periods, it would allow for B-cell selection against the exposed epitopes and increase the diversity of exported MBC and BMPC clones. Greater SHM loads and increased diversity would facilitate broader binding to pathogen strains. Thus, persistent GCs would yield the best ‘fruit’ for future humoral responses.
Figure 1.
Potential mechanism for how persistent GCs could increase memory B-cell repertoire diversity. Upon encountering antigens, MBCs rapidly generate PB responses that produce antibodies to previously encountered epitopes. These antibodies bind antigen to form immune complexes, which are then deposited on the surface of FDCs within GCs. These immune complexes may impede repertoire diversification in short-lived GCs by blocking access to epitopes or reducing the duration of antigen presentation. However, persistent GCs could allow for sufficient time for prolonged access of naive B cells to exposed novel epitopes. These clones could be selected and exported as MBCs, expanding the repertoire and driving future responses away from conserved epitopes or antigenic imprinting.
Evidence for such a mechanism has been identified by Tas and colleagues in mice. Broadly binding, low-affinity primary antibody responses can enhance recruitment of naive cells to GCs upon secondary challenge, while high-titer, high-affinity primary responses focused on specific epitopes reduce later cognate B-cell recruitment to GCs [119]. This inhibition of naive B-cell recruitment can be overcome with excess antigen doses [119], lending credence to the hypothesis that antigen availability influences GC responses. High-affinity primary responses may result in increased antigen clearance upon subsequent rechallenge, limiting GC duration. These data demonstrate the importance of considering how primary responses influence subsequent GC reactions when designing vaccine regimens, particularly when antigenic variability is a concern.
Conclusion
This review summarizes how GCs, once previously believed to last only a few weeks, are structures that can be persistently maintained in SLOs for months after the pathogen has been cleared. Many factors likely contribute to whether a GC subsides or is continuously renewed, including antigen persistence, ongoing Tfh-cell help, B-cell proliferative capacity, and the mode of infection or immunization. While GCs are perceived as transient in the spleen and lymph nodes, studying persistent GCs under chronic stimulation conditions, such as the gut and TLSs, may provide evidence for how to prime long-term reactions. The development of new strategies for vaccination will permit GC responses that persist long after the initial immunization. Such GCs may better generate MBCs and BMPCs that protect against antigenically variable pathogens.
Acknowledgements
This work was supported in part with funding from the US National Institutes of Health (NIH) and Moderna, Inc. The Ellebedy laboratory was supported by National Institutes of Health grants U01AI141990, 1U01AI150747, 5U01AI144616-02, and R01AI168178-01. Hanover C. Matz is supported by a National Institutes of Health Institutional Training Grant (5T32AI007172). Katherine M. McIntire is a supported by a National Institutes of Health Institutional Training Grant (5T32AI7163).
Footnotes
Declaration of Competing Interest
The Ellebedy laboratory received funding under sponsored research agreements from Moderna. The Ellebedy laboratory received funding from Emergent BioSolutions and AbbVie. AHE has received consulting and speaking fees from InBios International, Inc, Fimbrion Therapeutics, RGAX, Mubadala Investment Company, Moderna, Pfizer, GSK, Danaher, Third Rock Ventures, Goldman Sachs, and Morgan Stanley and is the founder of ImmuneBio Consulting. AHE is the recipient of a licensing agreement with Abbvie. The content of this paper is solely the responsibility of the authors and does not necessarily represent the official view of NIAID or NIH.
Data Availability
No data were used for the research described in the article.
References and recommended reading
Papers of particular interest, published within the period of review, have been highlighted as:
• of special interest
•• of outstanding interest
- 1.Eisen HN, Siskind GW: Variations in affinities of antibodies during the immune response. Biochemistry 1964, 3:996–1008. [DOI] [PubMed] [Google Scholar]
- 2.Berek C, Berger A, Apel M: Maturation of the immune response in germinal centers. Cell 1991, 67:1121–1129. [DOI] [PubMed] [Google Scholar]
- 3.Oprea M, Perelson AS: Somatic mutation leads to efficient affinity maturation when centrocytes recycle back to centroblasts. J Immunol 1997, 158:5155–5162. [PubMed] [Google Scholar]
- 4.Mesin L, Ersching J, Victora GD: Germinal center B cell dynamics. Immunity 2016, 45:471–482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sze DM-Y, Toellner K-M, de Vinuesa CG, Taylor DR, MacLennan ICM: Intrinsic constraint on plasmablast growth and extrinsic limits of plasma cell survival. J Exp Med 2000, 192:813–822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mesin L, Di Niro R, Thompson KM, Lundin KEA, Sollid LM: Long-lived plasma cells from human small intestine biopsies secrete immunoglobulins for many weeks in vitro. J Immunol 2011, 187:2867–2874. [DOI] [PubMed] [Google Scholar]
- 7.Bemark M, Hazanov H, Strömberg A, Komban R, Holmqvist J, Köster S, Mattsson J, Sikora P, Mehr R, Lycke NY: Limited clonal relatedness between gut IgA plasma cells and memory B cells after oral immunization. Nat Commun 2016, 7:1–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Allie SR, Randall TD: Resident memory B cells. Viral Immunol 2020, 33:282–293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Garside P, Ingulli E, Merica RR, Johnson JG, Noelle RJ, Jenkins MK: Visualization of specific B and T lymphocyte interactions in the lymph node. Science 1998, 281:96–99. [DOI] [PubMed] [Google Scholar]
- 10.Okada T, Miller MJ, Parker I, Krummel MF, Neighbors M, Hartley SB, O’Garra A, Cahalan MD, Cyster JG: Antigen-engaged B cells undergo chemotaxis toward the T zone and form motile conjugates with helper T cells. PLoS Biol 2005, 3:e150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Allen CDC, Ansel KM, Low C, Lesley R, Tamamura H, Fujii N, Cyster JG: Germinal center dark and light zone organization is mediated by CXCR4 and CXCR5. Nat Immunol 2004, 5:943–952. [DOI] [PubMed] [Google Scholar]
- 12.Pereira JP, Kelly LM, Xu Y, Cyster JG: EBI2 mediates B cell segregation between the outer and centre follicle. Nature 2009, 460:1122–1126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bannard O, Horton RM, Allen CDC, An J, Nagasawa T, Cyster JG: Germinal center centroblasts transition to a centrocyte phenotype according to a timed program and depend on the dark zone for effective selection. Immunity 2013, 39:912–924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Heesters BA, Chatterjee P, Kim Y-A, Gonzalez SF, Kuligowski MP, Kirchhausen T, Carroll MC: Endocytosis and recycling of immune complexes by follicular dendritic cells enhances B cell antigen binding and activation. Immunity 2013, 38:1164–1175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Suzuki K, Grigorova I, Phan TG, Kelly LM, Cyster JG: Visualizing B cell capture of cognate antigen from follicular dendritic cells. J Exp Med 2009, 206:1485–1493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Allen CDC, Okada T, Tang HL, Cyster JG: Imaging of germinal center selection events during affinity maturation. Science 2007, 315:528–531. [DOI] [PubMed] [Google Scholar]
- 17.Garin A, Meyer-Hermann M, Contie M, Figge MT, Buatois V, Gunzer M, Toellner K-M, Elson G, Kosco-Vilbois MH: Toll-like receptor 4 signaling by follicular dendritic cells is pivotal for germinal center onset and affinity maturation. Immunity 2010, 33:84–95. [DOI] [PubMed] [Google Scholar]
- 18.Victora GD, Schwickert TA, Fooksman DR, Kamphorst AO, Meyer-Hermann M, Dustin ML, Nussenzweig MC: Germinal center dynamics revealed by multiphoton microscopy with a photoactivatable fluorescent reporter. Cell 2010, 143:592–605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Schwickert TA, Lindquist RL, Shakhar G, Livshits G, Skokos D, Kosco-Vilbois MH, Dustin ML, Nussenzweig MC: In vivo imaging of germinal centres reveals a dynamic open structure. Nature 2007, 446:83–87. [DOI] [PubMed] [Google Scholar]
- 20.Bortnick A, Chernova I, Quinn WJ, Mugnier M, Cancro MP, Allman D: Long-lived bone marrow plasma cells are induced early in response to T cell-independent or T cell-dependent antigens. J Immunol 2012, 188:5389–5396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kaji T, Ishige A, Hikida M, Taka J, Hijikata A, Kubo M, Nagashima T, Takahashi Y, Kurosaki T, Okada M, et al. : Distinct cellular pathways select germline-encoded and somatically mutated antibodies into immunological memory. J Exp Med 2012, 209:2079–2097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Pape KA, Taylor JJ, Maul RW, Gearhart PJ, Jenkins MK: Different B cell populations mediate early and late memory during an endogenous immune response. Science 2011, 331:1203–1207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Taylor JJ, Pape KA, Jenkins MK: A germinal center-independent pathway generates unswitched memory B cells early in the primary response. J Exp Med 2012, 209:597–606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Dogan I, Bertocci B, Vilmont V, Delbos F, Mégret J, Storck S, Reynaud C-A, Weill J-C: Multiple layers of B cell memory with different effector functions. Nat Immunol 2009, 10:1292–1299. [DOI] [PubMed] [Google Scholar]
- 25.Mesin L, Schiepers A, Ersching J, Barbulescu A, Cavazzoni CB, Angelini A, Okada T, Kurosaki T, Victora GD: Restricted clonality and limited germinal center reentry characterize memory B cell reactivation by boosting. Cell 2020, 180:92–106 e11.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Alsoussi WB, Malladi SK, Zhou JQ, Liu Z, Ying B, Kim W, Schmitz AJ, Lei T, Horvath SC, Sturtz AJ, et al. : SARS-CoV-2 Omicron boosting induces de novo B cell response in humans. Nature, 2023, doi: 10.1038/s41586-023-06025-4. [DOI] [PubMed] [Google Scholar]
- 27.McHeyzer-Williams LJ, Milpied PJ, Okitsu SL, McHeyzer-Williams MG: Class-switched memory B cells remodel BCRs within secondary germinal centers. Nat Immunol 2015, 16:296–305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Jacob J, Przylepa J, Miller C, Kelsoe G : In situ studies of the primary immune response to (4-hydroxy-3-nitrophenyl)acetyl. III. The kinetics of V region mutation and selection in germinal center B cells. J Exp Med 1993, 178:1293–1307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Good-Jacobson KL, Chen Y, Voss AK, Smyth GK, Thomas T, Tarlinton D: Regulation of germinal center responses and B-cell memory by the chromatin modifier MOZ. Proc Natl Acad Sci USA 2014, 111:9585–9590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Jacob J, Kassir R, Kelsoe G: In situ studies of the primary immune response to (4-hydroxy-3-nitrophenyl)acetyl. I. The architecture and dynamics of responding cell populations. J Exp Med 1991, 173:1165–1175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.•. Hägglöf T, Cipolla M, Loewe M, Chen ST, Mesin L, Hartweger H, ElTanbouly MA, Cho A, Gazumyan A, Ramos V, et al. : Continuous germinal center invasion contributes to the diversity of the immune response. Cell 2023, 186:147–161 e15. This paper demonstrates how invasion of ongoing GCs by naïve B cells can diversify the immune response. It simultaneously shows that high affinity antibodies lower the affinity threshold for GC entry.
- 32.Olafsdottir TA, Lindqvist M, Nookaew I, Andersen P, Maertzdorf J, Persson J, Christensen D, Zhang Y, Anderson J, Khoomrung S, et al. : Comparative systems analyses reveal molecular signatures of clinically tested vaccine adjuvants. Sci Rep 2016, 6:39097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Pedersen GK, Wørzner K, Andersen P, Christensen D: Vaccine adjuvants differentially affect kinetics of antibody and germinal center responses. Front Immunol 2020, 11:579761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Turner JS, Benet ZL, Grigorova I: Transiently antigen primed B cells can generate multiple subsets of memory cells. PLoS One 2017, 12:e0183877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Rahman ZSM, Rao SP, Kalled SL, Manser T: Normal induction but attenuated progression of germinal center responses in BAFF and BAFF-R signaling-deficient mice. J Exp Med 2003, 198:1157–1169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Shinall SM, Gonzalez-Fernandez M, Noelle RJ, Waldschmidt TJ: Identification of murine germinal center B cell subsets defined by the expression of surface isotypes and differentiation antigens. J Immunol 2000, 164:5729–5738. [DOI] [PubMed] [Google Scholar]
- 37.Wang Y, Carter RH: CD19 regulates B cell maturation, proliferation, and positive selection in the FDC zone of murine splenic germinal centers. Immunity 2005, 22:749–761. [DOI] [PubMed] [Google Scholar]
- 38.Kasturi SP, Skountzou I, Albrecht RA, Koutsonanos D, Hua T, Nakaya HI, Ravindran R, Stewart S, Alam M, Kwissa M, et al. : Programming the magnitude and persistence of antibody responses with innate immunity. Nature 2011, 470:543–547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Tam HH, Melo MB, Kang M, Pelet JM, Ruda VM, Foley MH, Hu JK, Kumari S, Crampton J, Baldeon AD, et al. : Sustained antigen availability during germinal center initiation enhances antibody responses to vaccination. Proc Natl Acad Sci USA 2016, 113:E6639–E6648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Adachi Y, Onodera T, Yamada Y, Daio R, Tsuiji M, Inoue T, Kobayashi K, Kurosaki T, Ato M, Takahashi Y: Distinct germinal center selection at local sites shapes memory B cell response to viral escape. J Exp Med 2015, 212:1709–1723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.••. de Carvalho RVH, Ersching J, Barbulescu A, Hobbs A, Castro TBR, Mesin L, Jacobsen JT, Phillips BK, Hoffmann H-H, Parsa R, et al. : Clonal replacement sustains long-lived germinal centers primed by respiratory viruses. Cell 2023, 186:131–146 e13. This paper demonstrates that GCs can persist in mice long after an initial influenza infection is cleared. These persistent GCs can be invaded by naïve clones which may not be flu specific, but late founder clones can be exported with high levels of SHM.
- 42.••. Yewdell WT, Smolkin RM, Belcheva KT, Mendoza A, Michaels AJ, Cols M, Angeletti D, Yewdell JW, Chaudhuri J: Temporal dynamics of persistent germinal centers and memory B cell differentiation following respiratory virus infection. Cell Rep 2021, 37:109961. This study shows that respiratory viral infection in mice can produce GCs that last for up to 5 months. These long-lived GCs can continue to produce antiviral MBCs.
- 43.Rothaeusler K, Baumgarth N: B-cell fate decisions following influenza virus infection. Eur J Immunol 2010, 40:366–377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.•. Fallet B, Hao Y, Florova M, Cornille K, de Los Aires AV, Girelli Zubani G, Ertuna YI, Greiff V, Menzel U, Hammad K, et al. : Chronic viral infection promotes efficient germinal center B cell responses. Cell Rep 2020, 30:1013–1026 e7. This study shows that chronic LCMV infection in mice generates sustained GC responses.
- 45.•. Kräutler NJ, Yermanos A, Pedrioli A, Welten SPM, Lorgé D, Greczmiel U, Bartsch I, Scheuermann J, Kiefer JD, Eyer K, et al. : Quantitative and qualitative analysis of humoral immunity reveals continued and personalized evolution in chronic viral infection. Cell Rep 2020, 30:997–1012 e6. This paper demonstrates that chronic LCMV infection stimulates longer-lived GCs in mice than acute LCMV infection.
- 46.••. Lee JH, Sutton HJ, Cottrell CA, Phung I, Ozorowski G, Sewall LM, Nedellec R, Nakao C, Silva M, Richey ST, et al. : Long-primed germinal centres with enduring affinity maturation and clonal migration. Nature 2022, 609:998–1004. In this study, the authors show that long-lived GCs can be stimulated in NHPs that last up to 6 months. A slow-delivery immunization strategy promotes GC persistence, resulting in MBCs with greater levels of SHM.
- 47.Cirelli KM, Carnathan DG, Nogal B, Martin JT, Rodriguez OL, Upadhyay AA, Enemuo CA, Gebru EH, Choe Y, Viviano F, et al. : Slow delivery immunization enhances HIV neutralizing antibody and germinal center responses via modulation of immunodominance. Cell 2019, 177:1153–1171 e28.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Matsuda K, Huang J, Zhou T, Sheng Z, Kang BH, Ishida E, Griesman T, Stuccio S, Bolkhovitinov L, Wohlbold TJ, et al. : Prolonged evolution of the memory B cell response induced by a replicating adenovirus-influenza H5 vaccine. Sci Immunol 2019, 4:eaau2710. [DOI] [PubMed] [Google Scholar]
- 49.Wec AZ, Haslwanter D, Abdiche YN, Shehata L, Pedreño-Lopez N, Moyer CL, Bornholdt ZA, Lilov A, Nett JH, Jangra RK, et al. : Longitudinal dynamics of the human B cell response to the yellow fever 17D vaccine. Proc Natl Acad Sci USA 2020, 117:6675–6685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Andrews SF, Chambers MJ, Schramm CA, Plyler J, Raab JE, Kanekiyo M, Gillespie RA, Ransier A, Darko S, Hu J, et al. : Activation dynamics and immunoglobulin evolution of pre-existing and newly generated human memory B cell responses to influenza hemagglutinin. Immunity 2019, 51:398–410 e5.. [DOI] [PubMed] [Google Scholar]
- 51.Davis CW, Jackson KJL, McElroy AK, Halfmann P, Huang J, Chennareddy C, Piper AE, Leung Y, Albariño CG, Crozier I, et al. : Longitudinal analysis of the human B cell response to Ebola virus infection. Cell 2019, 177:1566–1582 e17.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.•. Sakharkar M, Rappazzo CG, Wieland-Alter WF, Hsieh C-L, Wrapp D, Esterman ES, Kaku CI, Wec AZ, Geoghegan JC, McLellan JS, et al. : Prolonged evolution of the human B cell response to SARS-CoV-2 infection. Sci Immunol 2021, 6:eabg6916. This study demonstrates prolonged affinity maturation of antibodies to SARS-CoV-2 spike protein in humans.
- 53.•. Sokal A, Chappert P, Barba-Spaeth G, Roeser A, Fourati S, Azzaoui I, Vandenberghe A, Fernandez I, Meola A, Bouvier-Alias M, et al. : Maturation and persistence of the anti-SARS-CoV-2 memory B cell response. Cell 2021, 184:1201–1213 e14. This study demonstrates ongoing SHM in SARS-CoV-2-specific B cell clones up to 6 months after infection.
- 54.Gaebler C, Wang Z, Lorenzi JCC, Muecksch F, Finkin S, Tokuyama M, Cho A, Jankovic M, Schaefer-Babajew D, Oliveira TY, et al. : Evolution of antibody immunity to SARS-CoV-2. Nature 2021, 591:639–644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Turner JS, Zhou JQ, Han J, Schmitz AJ, Rizk AA, Alsoussi WB, Lei T, Amor M, McIntire KM, Meade P, et al. : Human germinal centres engage memory and naive B cells after influenza vaccination. Nature 2020, 586:127–132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.••. Turner JS, O’Halloran JA, Kalaidina E, Kim W, Schmitz AJ, Zhou JQ, Lei T, Thapa M, Chen RE, Case JB, et al. : SARS-CoV-2 mRNA vaccines induce persistent human germinal centre responses. Nature 2021, 596:109–113. This study shows persistent GC responses in draining lymph nodes in humans after SARS-CoV-2 mRNA vaccination.
- 57.Kim W, Zhou JQ, Horvath SC, Schmitz AJ, Sturtz AJ, Lei T, Liu Z, Kalaidina E, Thapa M, Alsoussi WB, et al. : Germinal centre-driven maturation of B cell response to mRNA vaccination. Nature 2022, 604:141–145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Mudd PA, Minervina AA, Pogorelyy MV, Turner JS, Kim W, Kalaidina E, Petersen J, Schmitz AJ, Lei T, Haile A, et al. : SARS-CoV-2 mRNA vaccination elicits a robust and persistent T follicular helper cell response in humans. Cell 2022, 185:603–613 e15.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Pape KA, Catron DM, Itano AA, Jenkins MK: The humoral immune response is initiated in lymph nodes by B cells that acquire soluble antigen directly in the follicles. Immunity 2007, 26:491–502. [DOI] [PubMed] [Google Scholar]
- 60.Moon JJ, Suh H, Li AV, Ockenhouse CF, Yadava A, Irvine DJ: Enhancing humoral responses to a malaria antigen with nanoparticle vaccines that expand Tfh cells and promote germinal center induction. Proc Natl Acad Sci 2012, 109:1080–1085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Lindsay KE, Bhosle SM, Zurla C, Beyersdorf J, Rogers KA, Vanover D, Xiao P, Araínga M, Shirreff LM, Pitard B, et al. : Visualization of early events in mRNA vaccine delivery in nonhuman primates via PET-CT and near-infrared imaging. Nat Biomed Eng 2019, 3:371–380. [DOI] [PubMed] [Google Scholar]
- 62.Tew JG, Mandel TE: Prolonged antigen half-life in the lymphoid follicles of specifically immunized mice. Immunology 1979, 37:69–76. [PMC free article] [PubMed] [Google Scholar]
- 63.Phan TG, Grigorova I, Okada T, Cyster JG: Subcapsular encounter and complement-dependent transport of immune complexes by lymph node B cells. Nat Immunol 2007, 8:992–1000. [DOI] [PubMed] [Google Scholar]
- 64.Phan TG, Green JA, Gray EE, Xu Y, Cyster JG: Immune complex relay by subcapsular sinus macrophages and noncognate B cells drives antibody affinity maturation. Nat Immunol 2009, 10:786–793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Fleire SJ, Goldman JP, Carrasco YR, Weber M, Bray D, Batista FD: B cell ligand discrimination through a spreading and contraction response. Science 2006, 312:738–741. [DOI] [PubMed] [Google Scholar]
- 66.Natkanski E, Lee W-Y, Mistry B, Casal A, Molloy JE, Tolar P: B cells use mechanical energy to discriminate antigen affinities. Science 2013, 340:1587–1590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Hauser AE, Junt T, Mempel TR, Sneddon MW, Kleinstein SH, Henrickson SE, von Andrian UH, Shlomchik MJ, Haberman AM: Definition of germinal-center B cell migration in vivo reveals predominant intrazonal circulation patterns. Immunity 2007, 26:655–667. [DOI] [PubMed] [Google Scholar]
- 68.Spillane KM, Tolar P: B cell antigen extraction is regulated by physical properties of antigen-presenting cells. J Cell Biol 2016, 216:217–230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Smith BA, Gartner S, Liu Y, Perelson AS, Stilianakis NI, Keele BF, Kerkering TM, Ferreira-Gonzalez A, Szakal AK, Tew JG, et al. : Persistence of infectious HIV on follicular dendritic cells. J Immunol 2001, 166:690–696. [DOI] [PubMed] [Google Scholar]
- 70.Keşmir C, De, Boer RJ: A mathematical model on germinal center kinetics and termination. J Immunol 1999, 163:2463–2469. [PubMed] [Google Scholar]
- 71.Bachmann MF, Odermatt B, Hengartner H, Zinkernagel RM: Induction of long-lived germinal centers associated with persisting antigen after viral infection. J Exp Med 1996, 183:2259–2269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Baumjohann D, Preite S, Reboldi A, Ronchi F, Ansel KM, Lanzavecchia A, Sallusto F: Persistent antigen and germinal center B cells sustain T follicular helper cell responses and phenotype. Immunity 2013, 38:596–605. [DOI] [PubMed] [Google Scholar]
- 73.Fahey LM, Wilson EB, Elsaesser H, Fistonich CD, McGavern DB, Brooks DG: Viral persistence redirects CD4 T cell differentiation toward T follicular helper cells. J Exp Med 2011, 208:987–999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Deenick EK, Chan A, Ma CS, Gatto D, Schwartzberg PL, Brink R, Tangye SG: Follicular helper T cell differentiation requires continuous antigen presentation that is independent of unique B cell signaling. Immunity 2010, 33:241–253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.•. Röltgen K, Nielsen SCA, Silva O, Younes SF, Zaslavsky M, Costales C, Yang F, Wirz OF, Solis D, Hoh RA, et al. : Immune imprinting, breadth of variant recognition, and germinal center response in human SARS-CoV-2 infection and vaccination. Cell 2022, 185:1025–1040 e14. This study demonstrates the presence of SARS-CoV-2 spike protein in human lymph node GCs up to 60 days postsecond vaccine dose.
- 76.Garg AK, Desikan R, Dixit NM: Preferential presentation of high-affinity immune complexes in germinal centers can explain how passive immunization improves the humoral response. Cell Rep 2019, 29:3946–3957 e5.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Wang S, Mata-Fink J, Kriegsman B, Hanson M, Irvine DJ, Eisen HN, Burton DR, Wittrup KD, Kardar M, Chakraborty AK: Manipulating the selection forces during affinity maturation to generate cross-reactive HIV antibodies. Cell 2015, 160:785–797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.•. Garg AK, Mitra T, Schips M, Bandyopadhyay A, Meyer-Hermann M: Amount of antigen, T follicular helper cells and quality of seeder cells shape the diversity of germinal center B cells. bioRxiv, 2022, doi:〈 10.1101/2022.10.26.513835〉. In this preprint, the authors show that antigen availability on follicular dendritic cells can influence germinal center B cell diversity. In this preprint, the authors show that antigen availability on FDCs can influence Gc B cell diversity.
- 79.Weinstein JS, Herman EI, Lainez B, Licona-Limón P, Esplugues E, Flavell R, Craft J: TFH cells progressively differentiate to regulate the germinal center response. Nat Immunol 2016, 17:1197–1205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Akkaya M, Traba J, Roesler AS, Miozzo P, Akkaya B, Theall BP, Sohn H, Pena M, Smelkinson M, Kabat J, et al. : Second signals rescue B cells from activation-induced mitochondrial dysfunction and death. Nat Immunol 2018, 19:871–884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Arnold CN, Campbell DJ, Lipp M, Butcher EC: The germinal center response is impaired in the absence of T cell-expressed CXCR5. Eur J Immunol 2007, 37:100–109. [DOI] [PubMed] [Google Scholar]
- 82.Ballesteros-Tato A, León B, Graf BA, Moquin A, Adams PS, Lund FE, Randall TD: Interleukin-2 inhibits germinal center formation by limiting T follicular helper cell differentiation. Immunity 2012, 36:847–856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Ryg-Cornejo V, Ioannidis LJ, Ly A, Chiu CY, Tellier J, Hill DL, Preston SP, Pellegrini M, Yu D, Nutt SL, et al. : Severe malaria infections impair germinal center responses by inhibiting T follicular helper cell differentiation. Cell Rep 2016, 14:68–81. [DOI] [PubMed] [Google Scholar]
- 84.de Vinuesa CG, Cook MC, Ball J, Drew M, Sunners Y, Cascalho M, Wabl M, Klaus GGB, MacLennan ICM: Germinal centers without T cells. J Exp Med 2000, 191:485–494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.•. Long Z, Phillips B, Radtke D, Meyer-Hermann M, Bannard O: Competition for refueling rather than cyclic reentry initiation evident in germinal centers. Sci Immunol 2022. 7:eabm0775. In this paper, the authors show that T cell help is not necessary for initiation of cyclic reentry in GC B cell LZ/DZ cycling. Instead T cell help may ‘refuel’ B cells to better promote DZ proliferation and survival. This mechanism could explain how long-lived GCs could support both low and high affinity clones in a diversified response.
- 86.Gitlin AD, Shulman Z, Nussenzweig MC: Clonal selection in the germinal centre by regulated proliferation and hypermutation. Nature 2014, 509:637–640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Bolduc A, Long E, Stapler D, Cascalho M, Tsubata T, Koni PA, Shimoda M: Constitutive CD40L expression on B cells prematurely terminates germinal center response and leads to augmented plasma cell production in T cell areas. J Immunol 2010, 185:220–230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Jain RW, Parham KA, Tesfagiorgis Y, Craig HC, Romanchik E, Kerfoot SM, Autoreactive, Low-Affinity T: Cells preferentially drive differentiation of short-lived memory B cells at the expense of germinal center maintenance. Cell Rep 2018, 25:3342–3355 e5.. [DOI] [PubMed] [Google Scholar]
- 89.Linterman MA, Pierson W, Lee SK, Kallies A, Kawamoto S, Rayner TF, Srivastava M, Divekar DP, Beaton L, Hogan JJ, et al. : Foxp3+ follicular regulatory T cells control the germinal center response. Nat Med 2011, 17:975–982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Jacobsen JT, Hu WR, Castro TB, Solem S, Galante A, Lin Z, Allon SJ, Mesin L, Bilate AM, Schiepers A, et al. : Expression of Foxp3 by T follicular helper cells in end-stage germinal centers. Science 2021, 373:eabe5146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Benet ZL, Marthi M, Ke F, Wu R, Turner JS, Gabayre JB, Ivanitskiy MI, Sethi SS, Grigorova IL: CCL3 promotes germinal center B cells sampling by follicular regulatory T cells in murine lymph nodes. Front Immunol 2018, 9:2044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Calado DP, Sasaki Y, Godinho SA, Pellerin A, Köchert K, Sleckman BP, de Alborán IM, Janz M, Rodig S, Rajewsky K: The cell-cycle regulator c-Myc is essential for the formation and maintenance of germinal centers. Nat Immunol 2012, 13:1092–1100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Dominguez-Sola D, Victora GD, Ying CY, Phan RT, Saito M, Nussenzweig MC, Dalla-Favera R: The proto-oncogene MYC is required for selection in the germinal center and cyclic reentry. Nat Immunol 2012, 13:1083–1091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Ramezani-Rad P, Chen C, Zhu Z, Rickert RC: Cyclin D3 governs clonal expansion of dark zone germinal center B cells. Cell Rep 2020, 33:108403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Finkin S, Hartweger H, Oliveira TY, Kara EE, Nussenzweig MC: Protein amounts of the MYC transcription factor determine germinal center B cell division capacity. Immunity 2019, 51:324–336 e5.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Luo W, Weisel F, Shlomchik MJ: B cell receptor and CD40 signaling are rewired for synergistic induction of the c-Myc transcription factor in germinal center B cells. Immunity 2018, 48:313–326 e5.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Pae J, Ersching J, Castro TBR, Schips M, Mesin L, Allon SJ, Ordovas-Montanes J, Mlynarczyk C, Melnick A, Efeyan A, et al. : Cyclin D3 drives inertial cell cycling in dark zone germinal center B cells. J Exp Med 2020, 218:e20201699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Amanna IJ, Carlson NE, Slifka MK: Duration of humoral immunity to common viral and vaccine antigens. N Engl J Med 2007, 357:1903–1915. [DOI] [PubMed] [Google Scholar]
- 99.Weisel FJ, Zuccarino-Catania GV, Chikina M, Shlomchik MJ: A temporal switch in the germinal center determines differential output of memory B and plasma cells. Immunity 2016, 44:116–130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Cirelli KM, Crotty S: Germinal center enhancement by extended antigen availability. Curr Opin Immunol 2017, 47:64–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Pauthner M, Havenar-Daughton C, Sok D, Nkolola JP, Bastidas R, Boopathy AV, Carnathan DG, Chandrashekar A, Cirelli KM, Cottrell Ca, et al. : Elicitation of robust Tier 2 neutralizing antibody responses in nonhuman primates by HIV envelope trimer immunization using optimized approaches. Immunity 2017, 46:1073–1088 e6.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Ehrenhofer C, Opdebeeck JP: The effects of continuous and intermittent delivery of antigens of Boophilus microplus on the development of murine antibodies. Vet Parasitol 1995, 59:263–273. [DOI] [PubMed] [Google Scholar]
- 103.Higaki M, Azechi Y, Takase T, Igarashi R, Nagahara S, Sano A, Fujioka K, Nakagawa N, Aizawa C, Mizushima Y: Collagen minipellet as a controlled release delivery system for tetanus and diphtheria toxoid. Vaccine 2001, 19:3091–3096. [DOI] [PubMed] [Google Scholar]
- 104.Kemp JM, Kajihara M, Nagahara S, Sano A, Brandon M, Lofthouse S: Continuous antigen delivery from controlled release implants induces significant and anamnestic immune responses. Vaccine 2002, 20:1089–1098. [DOI] [PubMed] [Google Scholar]
- 105.Kuraoka M, Yeh C-H, Bajic G, Kotaki R, Song S, Windsor I, Harrison SC, Kelsoe G: Recall of B cell memory depends on relative locations of prime and boost immunization. Sci Immunol 2022, 7:eabn5311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Reboldi A, Cyster JG: Peyer’s patches: organizing B-cell responses at the intestinal frontier. Immunol Rev 2016, 271:230–245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Bunker JJ, Bendelac A: IgA responses to microbiota. Immunity 2018, 49:211–224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Weinstein PD, Cebra JJ: The preference for switching to IgA expression by Peyer’s patch germinal center B cells is likely due to the intrinsic influence of their microenvironment. J Immunol 1991, 147:4126–4135. [PubMed] [Google Scholar]
- 109.Lécuyer E, Rakotobe S, Lengliné-Garnier H, Lebreton C, Picard M, Juste C, Fritzen R, Eberl G, McCoy KD, Macpherson AJ, et al. : Segmented filamentous bacterium uses secondary and tertiary lymphoid tissues to induce Gut IgA and specific T helper 17 cell responses. Immunity 2014, 40:608–620. [DOI] [PubMed] [Google Scholar]
- 110.Nowosad CR, Mesin L, Castro TBR, Wichmann C, Donaldson GP, Araki T, Schiepers A, Lockhart AAK, Bilate AM, Mucida D, et al. : Tunable dynamics of B cell selection in gut germinal centres. Nature 2020, 588:321–326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Sautès-Fridman C, Petitprez F, Calderaro J, Fridman WH: Tertiary lymphoid structures in the era of cancer immunotherapy. Nat Rev Cancer 2019, 19:307–325. [DOI] [PubMed] [Google Scholar]
- 112.Pitzalis C, Jones GW, Bombardieri M, Jones SA: Ectopic lymphoid-like structures in infection, cancer and autoimmunity. Nat Rev Immunol 2014, 14:447–462. [DOI] [PubMed] [Google Scholar]
- 113.J Gunderson A, Rajamanickam V, Bui C, Bernard B, Pucilowska J, Ballesteros-Merino C, Schmidt M, McCarty K, Philips M, Piening B, et al. : Germinal center reactions in tertiary lymphoid structures associate with neoantigen burden, humoral immunity and long-term survivorship in pancreatic cancer. Oncoimmunology 2021, 10:1900635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Siliņa K, Soltermann A, Attar FM, Casanova R, Uckeley ZM, Thut H, Wandres M, Isajevs S, Cheng P, Curioni-Fontecedro A, et al. : Germinal centers determine the prognostic relevance of tertiary lymphoid structures and are impaired by corticosteroids in lung squamous cell carcinoma. Cancer Res 2018, 78:1308–1320. [DOI] [PubMed] [Google Scholar]
- 115.Posch F, Silina K, Leibl S, Mündlein A, Moch H, Siebehüner A, Samaras P, Riedl J, Stotz M, Szkandera J, et al. : Maturation of tertiary lymphoid structures and recurrence of stage II and III colorectal cancer. Oncoimmunology 2018, 7:e1378844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Helmink BA, Reddy SM, Gao J, Zhang S, Basar R, Thakur R, Yizhak K, Sade-Feldman M, Blando J, Han G, et al. : B cells and tertiary lymphoid structures promote immunotherapy response. Nature 2020, 577:549–555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Gu-Trantien C, Loi S, Garaud S, Equeter C, Libin M, de Wind A, Ravoet M, Le Buanec H, Sibille C, Manfouo-Foutsop G, et al. : CD4+ follicular helper T cell infiltration predicts breast cancer survival. J Clin Invest 2013, 123:2873–2892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Kuraoka M, Schmidt AG, Nojima T, Feng F, Watanabe A, Kitamura D, Harrison SC, Kepler TB, Kelsoe G: Complex antigens drive permissive clonal selection in germinal centers. Immunity 2016, 44:542–552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.••. Tas JMJ, Koo J-H, Lin Y-C, Xie Z, Steichen JM, Jackson AM, Hauser BM, Wang X, Cottrell CA, Torres JL, et al. : Antibodies from primary humoral responses modulate the recruitment of naive B cells during secondary responses. Immunity 2022, 55:1856–1871 e6. This study demonstrates in mice how antibody titers and affinity from primary responses can modulate GC recruitment of naïve B cells during secondary responses.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
No data were used for the research described in the article.