Abstract
Purpose
The VERSIFY phase 3 trial in patients with Crohn’s disease (CD) treated with vedolizumab was the first to include a substudy that used a standardized magnetic resonance enterography (MRE) protocol to assess features of transmural inflammation (bowel edema and wall thickness) and extramural disease activity (enlarged lymph nodes).
Patients and Methods
Patients received intravenous vedolizumab (300 mg) at weeks 0 (baseline), 2, and 6, and then every 8 weeks for 26 or 52 weeks. Post hoc analyses included a subpopulation with a Magnetic Resonance Index of Activity score of ≥7 in at least one bowel segment at baseline and at least one postbaseline MRE assessment. Changes in transmural inflammation, including intramural bowel edema and wall thickness, were evaluated. Patient-level and segment-level analyses were performed.
Results
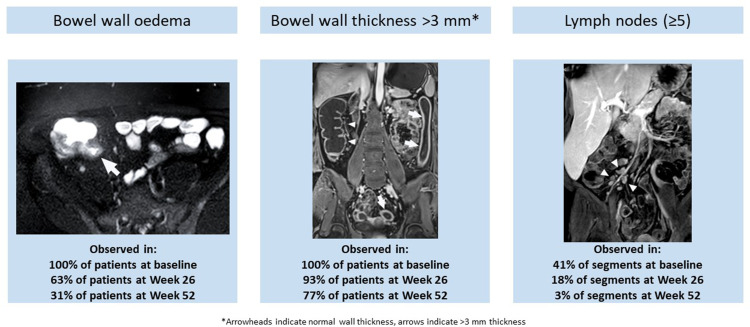
MRE images were evaluated in 27 patients with 83 evaluable bowel segments at baseline and week 26, and 13 patients with 38 evaluable segments at baseline, week 26, and week 52. At baseline, all patients had bowel wall edema and wall thickness of >3 mm in at least one bowel segment. The proportion of patients with edema decreased at weeks 26 (17/27 [63.0%]) and 52 (4/13 [30.8%]) and the proportion with bowel wall thickness of >3 mm decreased at weeks 26 (25/27 [92.6%]) and 52 (10/13 [76.9%]).
Conclusion
In patients with CD treated with vedolizumab for 26 and 52 weeks, the number of patients, and bowel segments, with MRE-detected transmural inflammation was reduced. These results highlight the impact of vedolizumab on components of transmural inflammation in CD and demonstrate that using MRE in CD multicenter clinical trials is feasible.
Trial Registration
ClinicalTrials.gov NCT02425111, April 23, 2015, http://www.clinicaltrials.gov NCT02425111; EU Clinical Trials Register EudraCT 2014–003509-13, https://www.clinicaltrialsregister.eu.
Keywords: vedolizumab, magnetic resonance enterography, Crohn’s disease, clinical trials, imaging
Graphical Abstract
Introduction
Crohn’s disease (CD) is a chronic transmural inflammatory condition of the gastrointestinal tract that causes structural bowel damage and patient morbidity. Monitoring of disease activity and improvements in response to treatment is important for effective disease management.1 Evolving guidance now encourages the inclusion of objective measures of inflammation aside from symptomatic relief in therapeutic strategies to prevent structural bowel damage and subsequent disability. The Selecting Therapeutic Targets in Inflammatory Bowel Disease (STRIDE II) consensus advocates a composite treatment target of symptomatic and endoscopic remission, with the latter defined as resolution of ulceration at ileocolonoscopy or of inflammatory changes on cross-sectional imaging in patients not adequately assessed with ileocolonoscopy.2
Ileocolonoscopy with biopsies is used to evaluate disease extent and activity. However, this approach may provide an incomplete evaluation of the colon and/or terminal ileum owing to the severity of lesions or technical reasons, and its assessment is restricted to the mucosa.3–5 Cross-sectional imaging of the bowel with magnetic resonance enterography (MRE) is a promising alternative to endoscopy—not only to supplement ileocolonoscopy in assessing disease activity beyond the mucosa and extramural disease features and complications in CD, but also for future drug development programs, especially in small bowel involvement in CD.3,6
Although currently there are no formal targets using MRE, changes in transmural inflammation are considered as an additional assessment to detect therapy-related changes.2 Transmural response had demonstrated high correlation between endoscopic changes and changes observed at cross-sectional imaging.7 Transmural healing has been associated with sustained clinical remission, reduced risk of therapeutic escalation, decreased risk of surgery, and preventing progression of bowel damage.8–12
Different MRE indices currently exist to evaluate the activity of CD, but only the Magnetic Resonance Index of Activity (MaRIA), the London index, the Clermont index, and the simplified MaRIA have been formally derived and partially validated.13–17 Among these indices, the one with the most extensively evaluated operational characteristics is MaRIA,18,19 which is a composite of mucosal and bowel wall features, including bowel edema, wall thickening, presence of ulcers, and relative contrast enhancement. MaRIA has been shown to correlate with two validated endoscopic indices of mucosal disease activity; namely, the CD Endoscopic Index of Severity and the Simple Endoscopic Score for CD (SES-CD).9,19,20 The MaRIA score has also been shown to accurately measure the therapeutic response and assess endoscopic mucosal healing in CD.7,9 A segmental MaRIA score of ≥7 in an ileocolonic bowel segment was shown to be highly sensitive, specific, and predictive of active CD,19 while the total segment values (global MaRIA) correlated with the global CD Endoscopic Index of Severity.7,19 Ulcer healing, defined as a segmental MaRIA score of <11 in small bowel segments as assessed by MRE, is predictive of endoscopic ulcer healing and was associated with lower rates of clinical and serological relapse, as well as disease-related hospitalization and surgery.9
In the VERSIFY trial, an open-label phase 3b trial (NCT02425111; EudraCT 2014–003509-13), vedolizumab treatment demonstrated endoscopic, histologic, and radiologic healing in patients with CD.21 The primary endpoint of the study was endoscopic remission assessed by SES-CD of ≤4 at week 26. An exploration of the radiologic response and remission in VERSIFY was based on reductions of the ileocolonic MaRIA score. Radiologic remission was defined as a MaRIA score of <7 in all bowel segments in patients with a baseline MaRIA score of ≥7 in at least one bowel segment, and was observed in 21.9% and 38.1% of patients treated with vedolizumab for 26 and 52 weeks, respectively. That was the first phase 3 clinical trial of CD using MRE as an additional measure of therapeutic efficacy. Although widely used in clinical practice, there may be concerns about the feasibility of implementing a standardized MRE protocol in large multicentric studies. Feasibility data is a critical step toward enabling the recommendation of this technique for use in future clinical trials.
Longitudinal assessment of the MRE features associated with transmural CD can contribute to the understanding of the transmural disease healing processes in CD and the impact of vedolizumab on components of transmural inflammation. It can also potentially help with identifying early markers of therapeutic response. Although the primary publication of the VERSIFY study reported data for radiologic remission at week 26 according to MaRIA score, a deeper exploration of the phenotypes of CD in this study population is an important adjunct for additional learning. Previous reports of MRE assessments have typically been based on ileal, colorectal, and ileocolonic segments, whereas we performed an exploratory analysis of MRE-detected transmural features of active CD in a subset of patients enrolled in the VERSIFY trial, including data on small bowel segments proximal to the terminal ileum. Specifically, we examined the impact of vedolizumab treatment (26 weeks and 52 weeks) on transmural features of bowel edema and bowel wall thickness, and extramural features of enlarged regional lymph nodes in afflicted bowel wall segments of patients with moderately to severely active CD. In addition to MaRIA score findings, this analysis of the kinetics of changes in key components of the MaRIA score (edema and wall thickness) and perienteric changes may help to better understand transmural changes in response to vedolizumab treatment in the VERSIFY study. Specifically, we investigated the technical feasibility of using the standardized MRE protocol across multiple sites.
Materials and Methods
Study Design and Patient Population
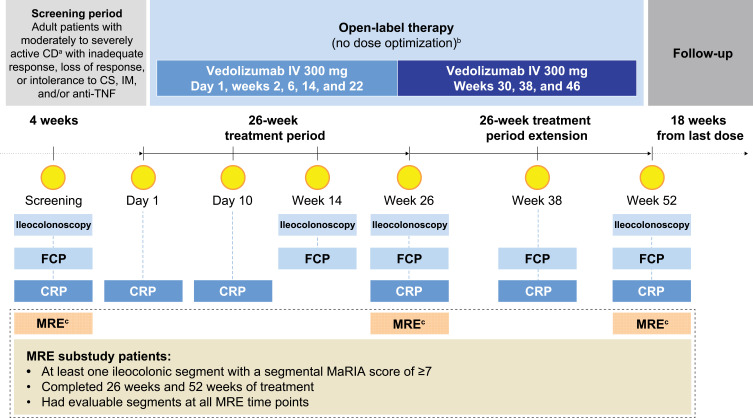
The VERSIFY trial was an open-label, phase 3b, single-group study of vedolizumab conducted from March 2015 to December 2017 in 42 sites in the United States, Canada, and Europe (NCT02425111; EudraCT 2014–003509-13). The study was conducted in accordance with the ethical standards of the institutional and/or national research committee(s) (Listed in Supplementary Material 1) and with the 1964 Helsinki Declaration and its later amendments. Written informed consent was obtained from all study participants. Enrolled patients were aged 18 to 80 years and diagnosed with moderately to severely active CD for ≥3 months, defined as a baseline CD Activity Index score of 220–450 and SES-CD of ≥7, and any ileocolonoscopy-confirmed ulcer, including aphthae, in any bowel segment. Patients received intravenous vedolizumab 300 mg on day 1, weeks 2 and 6, and then every 8 weeks for 26 weeks (original protocol, December 2014) to 52 weeks (postprotocol amendment, April 2016), with no option for dose optimization.21 This exploratory analysis included subsets of patients with CD from selected sites for MRE in the VERSIFY trial that had received expert-to-site technologist training to ensure MRE capture using standardized protocol. Patients had evaluable MRE assessments at baseline and week 26 (MREw26 subset), or baseline, week 26, and week 52 (MREw52 subset), and a baseline MaRIA score of ≥7 in at least one bowel segment (Figure 1).
Figure 1.
Study design of the VERSIFY MRE substudy. aPatients who had a diagnosis of CD for ≥3 months and have active disease defined as a baseline CD Activity Index score of 220–450 and SES-CD of ≥7 with any ulcer (including aphthae) in any bowel segment including the ileum and/or colon documented by centrally read ileocolonoscopy. bNo option at week 10 to increase dose frequency to every 4 weeks based on clinical response (per recommended use in clinical practice). cMRE was used in a subset of 37 patients from the VERSIFY study who had baseline MRE assessments.
Abbreviations: CRP, C-reactive protein; CS, corticosteroid; FCP, fecal calprotectin; IM, immunosuppressant.
Study Assessments
Magnetic Resonance Enterography
MRE was performed at screening, week 26, and week 52. Standard MRE protocol included ingestion of 1500 mL (or a minimum of 800 mL in cases where patients had limited tolerance) of oral, nonabsorbable, contrast solution (mannitol-based solution at 2.5–5%, VoLumen, sorbitol-based solution at 2.5–5%, or an iso-osmotic polyethylene glycol and electrolyte solution such as NuLYTELY and GoLYTELY) administered 45 minutes before starting imaging. MRE examinations were performed using one designated magnetic resonance imaging scanner (1.5 or 3.0 Tesla) per site throughout the study to reduce variability across scanner models. Routine scanning protocol was based on the standard recommendation from the European Society of Gastrointestinal and Abdominal Radiology.22 The sequences included are intended to detect features associated with inflammatory activity in the bowel and CD-associated complications. Scanning sequences included axial and coronal T2 weighted with and without fat saturation, coronal T1 with fat saturation pre- and postgadolinium injection, and axial T1 postgadolinium injection (Table 1). The total time of image acquisition was approximately 30 minutes considering the inherent variability that depended on the site’s model. In order to ensure homogenization of the scanning protocol, a technical manual with a detailed MRE protocol was provided to each substudy site. Each site received expert-to-site technologist training, either face-to-face or via a conference call. Radiologist(s) and the technicians involved in imaging study participants were required to attend the training session. Sites were qualified for study participation after submitting a test scan from a healthy volunteer.
Table 1.
Standardized Magnetic Resonance Enterography Protocol Used in the Study
| Plane | Slice Thickness (mm) | Breath Hold | Fat Saturation | Matrix (mm) | FOV (mm) | |
|---|---|---|---|---|---|---|
| Antiperistaltic agent (10 mg hyoscine metilbromide, or 0.5 mg of glucagon intravenously) | ||||||
| Balanced GRE | Axial | 5 | No | No | 256×256 | 380×256 |
| Coronal | 5 | No | No | 256×256 | 380×305 | |
| T2-w single shot fast spin echo | Axial | 5 | No | No | 256×256 | 380×305 |
| Axial | 5 | No | Yes | 256×256 | 380×305 | |
| Coronal | 5 | No | No | 256×256 | 450×450 | |
| DWI | Axial | 5 | Yes | No | ||
| Antiperistaltic agent (10 mg hyoscine metilbromide, or 0.5 mg of glucagon intravenously) | ||||||
| Fast 3D T1-w, pre Gd | Coronal | <2.5 | Yes | Yes | 320×224 | 440×380 |
| Gadolinium-chelate contrast agent intravenous injection | ||||||
| Fast 3D T1-w, post Gd | Coronala | <2.5 | Yes | Yes | 320×224 | 440×380 |
| Axialb | <2.5 | Yes | Yes | 320×190 | 370×312 | |
Notes: a70 seconds and 120 seconds after contrast injection. b180 seconds after contrast injection.
Abbreviations: 3D, three-dimensional; DWI, diffusion-weighted imaging; FOV, field of view; Gd, gadolinium; GRE, gradient echo; T1-w, T1 weighted; T2-w, T2 weighted.
Images were read centrally by a radiologist experienced in the scoring conventions and blinded to time point assessment and endoscopic and clinical data.
The intestinal tract was divided into six ileocolonic segments (colorectal segments, rectum and sigmoid; descending, transverse, and ascending colon; and terminal ileum, including the last 15 cm from the ileocecal valve or ileocolonic anastomosis). Additionally, the small bowel proximal to the terminal ileum was divided into the proximal ileum and jejunum. The segmental MaRIA scores for each segment were calculated by a central reader with ≥10 years of experience in bowel imaging using the following formula, as previously defined:4,20
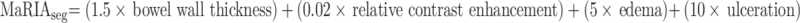 |
Exploratory Analysis Endpoints
Changes in the presence of features of transmural disease in CD from baseline, including bowel wall thickness, bowel edema, and presence of ulcerations, were assessed using MRE (Figure 2). Changes in the presence of extramural disease features of CD were also evaluated where possible, including stricturing, fistula, and mesenteric fat stranding. Owing to the mechanism of action of vedolizumab, which reduces inflammation by blocking recruitment of T cells, the presence of associated gut enlarged lymph nodes was also assessed.
Figure 2.
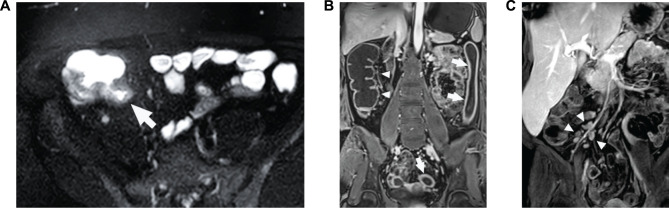
MRE-detected features of active CD disease: (A) bowel wall edema (arrow), (B) bowel wall thickness, and (C) enlarged lymph nodes. (A) Axial magnetic resonance enterography image of bowel edema (arrow) in a patient with CD. (B) Coronal image of increased colon wall thickness (anatomic left, arrows) and normal colon wall thickness (anatomic right, arrowheads) in a patient with CD. (C) Volume rendering of the right lower quadrant showing enlarged lymph nodes (arrowheads) in association with increased wall thickness in the terminal ileum in a patient with CD.
Abbreviation: CD, Crohn’s disease.
Feasibility of MRE Protocol in Multicentric Trials and Image Quality
To evaluate the feasibility of a multicentric trial using MRE in CD, we specifically evaluated the adherence of the protocol (number of sequences not sent for central reading) and number of intestinal segments assessable according to central reader at baseline and week 26 MRE. Exam adequacy was measured as presence of critical sequences (Supplementary Table 1), which included the minimum set of sequences that allow for calculation of the MaRIA score submitted for central reading, correct order of acquisition (ie, not acquiring T2 after gadolinium contrast injection), coverage of full anatomy of the abdomen and the pelvis allowing assessing the whole intestine segments, and the incidence of imaging artifacts.
The incidence of imaging artifacts was also determined, including breath or peristaltic motion, low signal-to-noise ratio, signal drop-out, and feces or gas artifacts. Based on the presence of artifacts, sequences were assessed for quality using a 5-point scale23 (Supplementary Table 2) where 5 is excellent quality and 1 uninterpretable. Imaging was considered successful if all quality scores were ≥3.
Statistical Analyses
This MRE post hoc analysis was performed for patients of the full analyses set of the study (patients who received at least one dose of intravenous vedolizumab) who had a baseline MaRIA score of ≥7 in at least one bowel segment and had follow-up MRE assessments: the week 26 population (MREw26) comprises patients with MRE assessments at baseline and week 26; the week 52 population (MREw52) comprises patients with MRE assessments at baseline, week 26, and week 52. Analyses were conducted using either patient-level or segment-level data. The segment-level analyses included all bowel segments with a baseline MaRIA score of ≥7 in patients who completed 26 or 52 weeks of treatment and had at least one evaluable segment at the relevant time points.
Descriptive statistics were used to summarize patient baseline characteristics. For all continuous variables, descriptive statistics by study visit and mean (standard deviation [SD]) or median (range [minimum, maximum]) changes over time were generated. The MRE ulceration status at any visit was determined by the presence or absence of ulcers across all bowel segments evaluated at that visit.
The McNemar test was used to perform image quality comparisons between paired baseline and follow-up assessments.
Data Accessibility Statement
The datasets—including the redacted study protocol, redacted statistical analysis plan, and individual participants’ data supporting the results reported in this article—will be available 3 months from initial request to researchers who provide a methodologically sound proposal. The data will be provided after their de-identification, in compliance with applicable privacy laws, data protection, and requirements for consent and anonymization. Additional information can be found at: https://vivli.org/ourmember/takeda/
Results
Study Population and VERSIFY Trial MRE Results
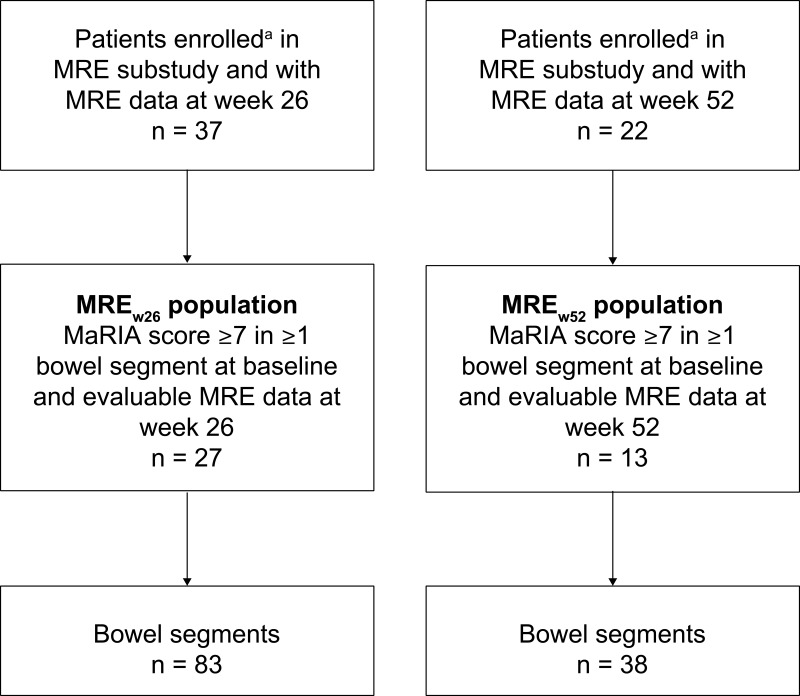
Of the 101 patients in the main VERSIFY study, 37 and 22 patients enrolled into the MRE substudy extensions up to week 26 and week 52. Of these, 27 and 13 patients, respectively, had a baseline MaRIA score of ≥7 in at least one bowel segment and had evaluable follow-up MRE imaging data at week 26 (MREw26 population) and weeks 26 and 52 (MREw52 population), and were included in this post hoc analysis (Figure 3). Of these, 9 of 27 and 3 of 13 in the MREw26 and MREw52 populations, respectively, had at least one small bowel segment with a baseline MaRIA score of ≥7, and 11 of 27 (MREw26) and 10 of 13 (MREw52) had a terminal ileum segment with a MaRIA score of ≥7 at baseline. The 27 patients in the MREw26 population had a total of 83 evaluable bowel segments, and the 13 patients in the MREw52 population had 38 evaluable bowel segments.
Figure 3.
Patient disposition in the VERSIFY MRE substudy. aConsented to 26 of 52 weeks of follow-up and MRE substudy.
Abbreviations: MaRIA, Magnetic Resonance Index of Activity; MRE, magnetic resonance enterography; MREw26, evaluable magnetic resonance enterography assessments at baseline and week 26; MREw52, evaluable magnetic resonance enterography assessments at baseline, week 26, and week 52.
Patients in the MREw26 and MREw52 populations had a mean age of 39.8 and 44.2 years, respectively, and had long-standing disease, with a mean duration (SD) of 12 (9.67) years in the week 26 population and 10.8 (7.29) years in the week 52 population. Prior treatment failure with an antitumor necrosis factor-alpha (anti-TNFα) occurred in 37.0% (10/27) of the patients in the week 26 population and 23.1% (3/13) in the week 52 population. Patients had baseline mean (SD) SES-CD of 15.7 (7.65) and 16.5 (7.82) and CD Activity Index scores of 300.7 (60.71) and 296.6 (78.58) in the week 26 and week 52 populations, respectively (Table 2).
Table 2.
Baseline Patient Demographics and Disease Characteristics of Patients with at Least One Bowel Segment Having a Magnetic Resonance Index of Activity Score of ≥7 at Baselinea
| Characteristics | MREw26 Population (N = 27) | MREw52 Population (N = 13) |
|---|---|---|
| Mean (SD) age (years) | 39.8 (14.3) | 44.2 (15.4) |
| Female, n (%) | 12 (44.4) | 7 (53.8) |
| Mean (SD) weight (kg) | 73.4 (13.3) | 75.6 (15.4) |
| Mean (SD) CD duration (years) | 12.0 (9.67) | 10.8 (7.29) |
| SES-CD | ||
| Mean (SD) | 15.7 (7.65) | 16.5 (7.82) |
| Median (range) | 14 (4–34) | 14 (7–34) |
| n (%) | ||
| SES-CD ≥7 to ≤15 | 14 (51.9) | 7 (53.8) |
| SES-CD >15 | 12 (44.4) | 6 (46.2) |
| CDAI | ||
| Mean (SD) | 300.7 (60.71) | 296.6 (78.58) |
| Median (range) | 290.0 (193–439) | 276.0 (193–439) |
| Smoker, n (%) | 8 (29.6) | 5 (38.5) |
| Prior anti-TNF status, n (%) | ||
| Anti-TNF naïve | 17 (63.0) | 10 (76.9) |
| Anti-TNF failure | 10 (37.0) | 3 (23.1) |
| CRP (mg/L) | ||
| Mean (SD) | 10.9 (16.81) | 8.3 (10.65) |
| Median (range) | 6.4 (0.3–83.4) | 3.1 (0.5–39.3) |
| FCPb (µg/g) | ||
| Mean (SD) | 1617.4 (1332.14) | 1368.3 (1084.95) |
| Median (range) | 1353.0 (44–5464) | 1290.5 (67–3526) |
| Elevated CRP >10 mg/L, n (%) | 10 (37.0) | 4 (30.8) |
| Elevated FCP >500 µg/g, n (%) | 19 (76.0) | 9 (7.0) |
Notes: aPatients with disease detectable by MRE at screening who completed 26 and 52 weeks of treatment and had evaluable bowel segments at all MRE time points were included in this analysis. bFor FCP, N = 25 for MREw26 population and N = 12 for MREw52 population.
Abbreviations: CD, Crohn’s disease; CDAI, Crohn’s Disease Activity Index; CRP, C-reactive protein; FCP, fecal calprotectin; MRE, magnetic resonance enterography; MREw26, evaluable magnetic resonance enterography assessments at baseline and week 26; MREw52, evaluable magnetic resonance enterography assessments at baseline, week 26, and week 52; SD, standard deviation; SES-CD, Simple Endoscopic Score for Crohn’s Disease; TNF, tumor necrosis factor.
MRE Features of Active CD
Segment-Level Analyses
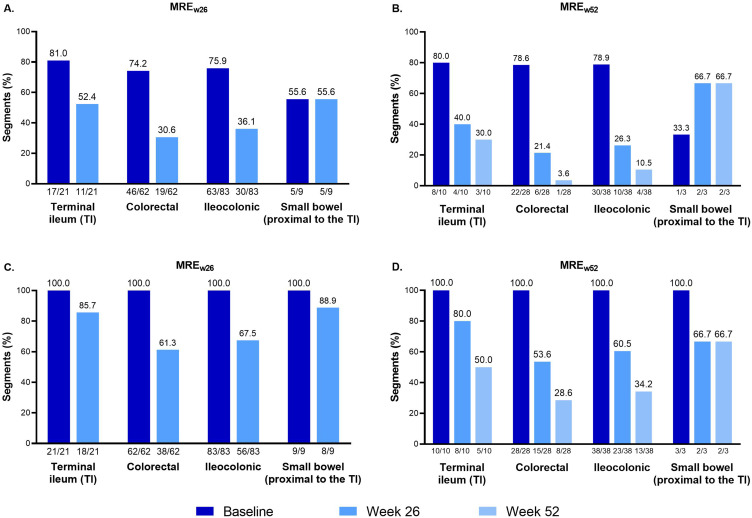
The majority of ileocolonic segments evaluated had bowel edema (MREw26 63/83 [75.9%]; MREw52 30/38 [78.9%]) and all had bowel wall thickness of >3 mm (MREw26 83/83 [100%]; MREw52 38/38 [100%]) at baseline. In the MREw26 population, the proportion of segments with bowel edema decreased to 30 of 83 (36.1%) by week 26. This improvement was greater in colorectal segments (19/62 [30.6%]) than in the terminal ileum (11/21 [52.4%]) (Figure 4A). A similar trend was observed in this population regarding bowel wall thickness, with the proportion decreasing to 56 of 83 (67.5%) at week 26, and with proportions of 38 of 62 (61.3%) and 18 of 21 (85.7%) in colorectal and terminal ileum segments, respectively (Figure 4C).
Figure 4.
Segment-level analyses of magnetic resonance enterography–detected (A and B) bowel edema and (C and D) bowel wall thickness of >3 mm at baseline, week 26, and week 52. The segment-level analysis included bowel segments from patients with a baseline Magnetic Resonance Index of Activity score of ≥7 in at least one bowel segment who completed 26 and 52 weeks of treatment with evaluable segments.
Abbreviations: MREw26, evaluable magnetic resonance enterography assessments at baseline and week 26; MREw52, evaluable magnetic resonance enterography assessments at baseline, week 26, and week 52.
In the MREw52 population, the proportion of segments with bowel edema decreased from 30 of 38 (78.9%) at baseline to 10 of 38 (26.3%) at week 26 and 4 of 38 (10.5%) at week 52. The proportion of segments with bowel wall thickening decreased from 38 of 38 (100%) at baseline to 23 of 38 (60.5%) at week 26 and 13 of 38 (34.2%) at week 52 (Figure 4B and D). Changes were greater in the colorectal segments compared with the terminal ileum and small bowel segments.
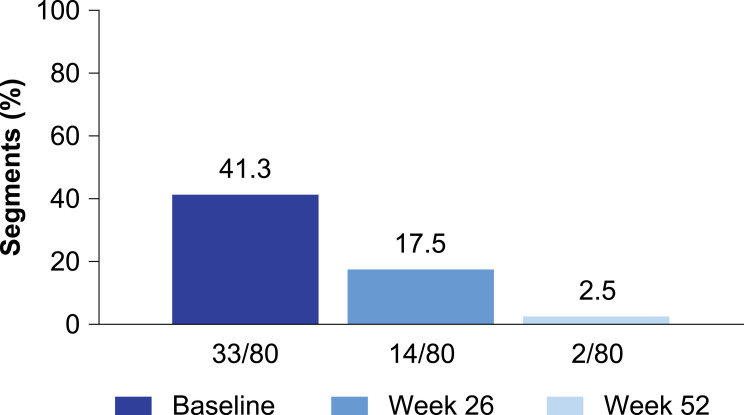
In the MREw52 population, the presence of enlarged gut-associated lymph nodes was assessed in the ileocecal mesentery (80 segments). At baseline, 33 of 80 (41.3%) of segments showed clusters of five or more enlarged lymph nodes. After 26 and 52 weeks, only 14 of 80 (17.5%) and 2 of 80 (2.5%) patients, respectively, still showed as having five or more enlarged lymph nodes (Figure 5).
Figure 5.
Proportion of ileocolonic segments with five or more enlarged lymph nodes. Lymph nodes were quantified in the following ileocolonic segments: rectum, sigmoid colon, descending colon, transverse colon, and terminal ileum/ascending colon. The terminal ileum and ascending colon were evaluated as one segment. Segment population: ileocolonic segments from VERSIFY magnetic resonance enterography patients with evaluable segments at baseline, week 26, and week 52. All segments had Magnetic Resonance Index of Activity score of ≥7 and five or more enlarged lymph nodes at baseline.
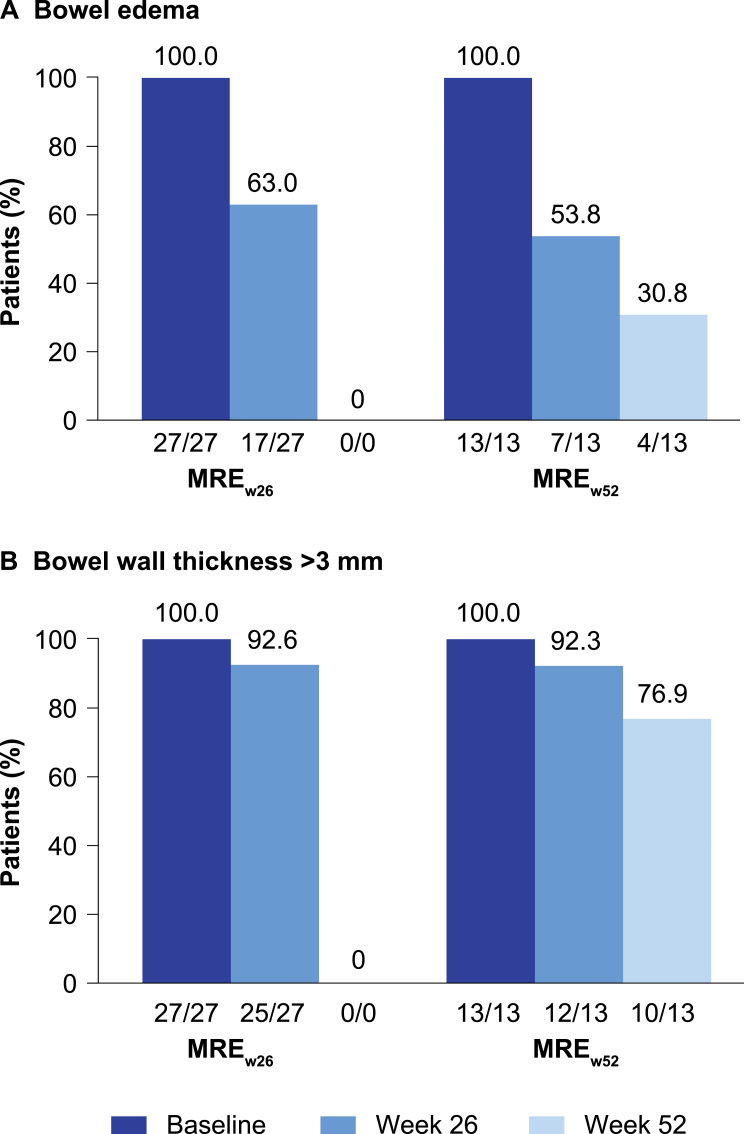
Patient-Level Analyses
All patients evaluated (MREw26, n = 27; MREw52, n = 13) had bowel edema and bowel wall thickness of >3 mm in at least one bowel segment at baseline, as expected based on MaRIA score threshold of 7 for inclusion. At week 26, the proportion of patients with bowel edema in the MREw26 population decreased from baseline to 17 of 27 (63.0%) (Figure 6A). Almost no decrease was noted for the proportion of patients with bowel wall thickening (25/27 [92.6%]) (Figure 6B). In the 13 patients observed until week 52, the proportion of patients with bowel edema and wall thickening decreased from baseline to 7 of 13 (53.8%) and 12 of 13 (92.3%), respectively, at week 26 and decreased further to 4 of 13 (30.8%) and 10 of 13 (76.9%), respectively, at week 52 (Figure 6A and B).
Figure 6.
Patient-level analyses of magnetic resonance enterography–detected (A) bowel edema and (B) bowel wall thickness of >3 mm at baseline, week 26, and week 52. The patient-level analysis included patients with at least one bowel segment with Magnetic Resonance Index of Activity score of ≥7 at screening who completed 26 and 52 weeks of treatment with evaluable segments.
Abbreviations: MREw26, evaluable magnetic resonance enterography assessments at baseline and week 26; MREw52, evaluable magnetic resonance enterography assessments at baseline, week 26, and week 52.
Other MRE Features of Active CD
Other disease features—including the presence of ulcers, strictures, and mesenteric fat stranding—were also observed in a small number of patients with CD at baseline. However, owing to limited data availability, formal analyses of these features were not performed.
Technical Feasibility of MRE Protocol in Clinical Trial
MRE images were reviewed for all 37 patients at baseline (302 sequences total) and for the 30 patients with 26 weeks of follow-up (255 sequences total). Overall, 89% of patients at baseline and 83% at follow-up had MRE sequences suitable for assessing disease activity (ie, had all critical sequences, correct sequence order, and covered full anatomy).
In patients evaluated at both time points (n = 30), there was no significant difference between baseline and follow-up in the proportion of patients with inadequate examinations, analyzed according to the following criteria: missing/incomplete sequences, missing critical sequences, adequate acquisition order, presence of full anatomy on critical sequences, and complete readability on all segments (Table 3). No critical patient sequences were deemed uninterpretable (quality score of 1 or 2) at either time point. At both baseline and follow-up, 98% of patient sequences were interpretable (quality score of ≥3).
Table 3.
Comparisons of Patients (n = 30) with Inadequate Examinations at Follow-Up versus Baseline
| Variable | p value |
|---|---|
| Sequences missing or incomplete | 0.51 |
| Critical sequences missing | 0.13 |
| Adequate acquisition order | 1.00 |
| Full anatomy present on critical sequences | 1.00 |
| All segments readable | 0.73 |
Image Quality
Overall, most MRE sequences (98% at baseline and 99% at follow-up) were considered successful in terms of presence of artifacts that decreased image quality, with quality scores of ≥3 (Table 4). At baseline and follow-up, artifacts were observed in 152/302 sequences (37 patients) and 132/255 sequences (30 patients), respectively (Table 5).
Table 4.
Comparison of Magnetic Resonance Enterography Sequence Image Quality at Baseline and Follow-Up
| Quality Assessment Score | Baseline (% Sequences) | Week 26 (% Sequences) |
|---|---|---|
| 1: Interpretable | 0.7 | 0 |
| 2: Moderate to severe artifacts, markedly diminished confidence (poor quality) | 1.3 | 1.6 |
| 3: Moderate artifacts, mild to moderate decrease in reader confidence (fair quality) | 11.6 | 12.2 |
| 4: Minimum artifacts, no effect on confidence (good quality) | 46.0 | 47.1 |
| 5: Excellent quality | 40.1 | 39.2 |
| Missing sequences | 0.3 | 0 |
Table 5.
Magnetic Resonance Enterography Image Artifacts
| Artifacts | Baseline (% Sequences) | Week 26 (% Sequences) |
|---|---|---|
| Any artifact (including peristaltic motion) | 50.3 | 51.8 |
| Low signal to noise | 14.6 | 15.3 |
| Segmental drop signal | 15.2 | 18.8 |
| Breath motion artifact | 12.9 | 14.5 |
| Feces or gas artifact | 6.3 | 8.6 |
Notes: Total sequences at baseline=302 in 37 patients and total sequences at Week 26=255 in 30 patients.
Discussion
VERSIFY was the first prospective inflammatory bowel disease study to use MRE to assess vedolizumab treatment efficacy in transmural healing in patients with moderately to severely active CD. It was the first multicenter clinical trial that utilized MRE to assess the totality of the disease burden on the gut mucosa, mesentery, and lymph nodes in a small but curated dataset from patients with CD followed longitudinally. This exploratory analysis demonstrated the ability of vedolizumab therapy to effect changes in MRE-detected transmural inflammation in patients with CD who were maintained throughout the treatment course.
Both the proportion of patients and the proportion of bowel segments with transmural inflammation decreased with vedolizumab treatment, with edema being more responsive than wall thickening, in line with previous findings. For example, in a meta-analysis involving adult and pediatric patients with CD, bowel wall enhancement, wall T2 hyperintensity (bowel edema), and the presence of mucosal lesions (including ulcers) were the most consistently useful features to assess bowel wall inflammation.24 The specificity estimate for bowel edema was >90% in the patient analysis and >95% in the bowel segment analysis, which was higher compared with that of bowel wall thickening (0–98%).24 Similarly, bowel edema was shown to be one of the most responsive lesions in segments that achieved endoscopic remission and mucosal healing after treatment, in agreement with our current findings.7,25 Similar observations have been reported in longitudinal studies exploring the radiological response measured by MRE in response to TNF inhibitors.11,26 These studies also show that wall thickness can be less responsive to treatment than other MRE features associated with inflammation.
A parallel decrease in the proportion of ileocolonic segments with enlarged lymph nodes and in segments with a MaRIA score of ≥7 was also observed. In the meta-analysis, the presence of enlarged regional lymph nodes was specific for inflammation at the bowel segment level.24 An increased number of lymph nodes in the mesentery can be a helpful clinical sign to carefully assess bowel segments for involvement with CD. Changes in lymph nodes are of particular interest in response to vedolizumab treatment to investigate any link between lymphocyte activation/inactivation at lymph nodes and detection by MRE.
In both the week 26 and week 52 populations, we observed that when measured by MRE, the response to vedolizumab was lower in the small bowel than in colorectal segments. This observation is aligned with that recently described in a cohort of 58 patients with CD treated with TNF inhibitors and monitored using MRE.27 In that study, the rates of ulcer healing measured by MaRIA score were lower in small bowel segments than in colonic segments, and the location of the disease in the small bowel (opposite to the colon) showed an independent negative predictive value for healing of severe inflammatory lesions. Overall, these observations suggest that the rate of transmural response of severe inflammatory lesions in CD when the disease is located in the small bowel is independent of the mechanism of action of the therapeutic intervention.
MRE has several advantages as a diagnostic and monitoring tool for CD: it lacks ionizing radiation; achieves excellent soft-tissue characterization; allows concomitant evaluation of the small bowel and the colon with less variation owing to operator skills or higher accuracy for disease extension, which is critical in the small bowel,28 compared with ultrasound; enables transmural evaluation of the bowel and detection of extramural CD complications; allows central reading without losing intestinal sections; and has better patient acceptance compared with colonoscopy.20,29
Achieving radiological remission may improve prognosis and, in the future, may be a helpful therapeutic endpoint for CD. There is greatly expanded interest in the use of MRE for assessing therapeutic efficacy in CD, especially for the small bowel. Objective MRE-based disease activity scores strongly correlate with endoscopic mucosal inflammation in the colon and terminal ileum;7,17,20 however, other MRE findings, such as perianal disease, stenosis, and fistulae, and activity beyond the scope of ileoscopy, are better predictors of patient outcomes than endoscopic assessments.5,9,12 Although these results suggest that MRE may be superior to endoscopy for predicting long-term outcomes, the use of MRE in clinical trials is limited by a lack of established MRE treatment targets based on clinically relevant therapeutic outcomes. The results of the current study may help to better understand the longitudinal radiological changes after vedolizumab treatment and further development of radiological endpoints for future trials.
A recent study of patients with CD treated with anti-TNF inhibitors or autologous hematopoietic stem-cell transplantation showed residual evidence of transmural disease even after achieving endoscopic remission. In these patients, who received treatment for a year, luminal strictures, wall thickening, creeping fat, and intestinal wall deposits persisted in afflicted bowel segments of patients in endoscopic remission and were considered signs of established damage.25 Bowel wall thickness >5.9 mm and prior refractory disease were identified as predictors of persistent bowel wall thickening; persistent MRE-detected strictures were associated with earlier recurrence.25 Although some imaging features were found in only a small number of patients in the study, detection of these features highlights the potential of MRE in informing about disease prognosis. Another recent study observed that transmural healing was associated with slower progression of bowel damage and reduced risk of major outcomes compared with endoscopic mucosal healing.30 The prognostic implications of these findings and the development of treat-to-target strategies for inflammatory bowel disease highlight the value of a noninvasive diagnostic modality such as MRE in the management of CD, particularly in close monitoring, which may aid in early disease control and alter the natural course of CD.31
This study reports the feasibility of implementing MRE in large multicentric clinical trials in CD. The majority (>80%) of patients had MRE sequences suitable for assessing disease activity. Overall, MRE data were of very good quality, with limited scan-related artifacts that could substantially diminish reader confidence for image interpretation. Although approximately half of all MRE sequences collected at baseline and follow-up contained artifacts, most had minimal to no effect on image quality. The results of this analysis are consistent with previous analyses demonstrating that disease activity can be assessed in clinical trials using MRE.28,32
This study has some limitations, as described in the previous publication.21 VERSIFY was an open-label study with no comparator or reference treatment arm. A full long-term evaluation was only conducted on a subset of patients who enrolled after the protocol amendment; therefore, the sample may not be representative of the full study population. Another consideration is that this analysis was post hoc and exploratory, and included only patients with MRE data at the time points of week 26 and week 52 (MREw52 population only) and who had MRE performed at baseline and these time points. Patients who dropped out of the study before reaching these time points were not included in the analysis. In addition, only a small number of patients presented with radiologic ulcers, strictures, fistula and/or fluid collections, and creeping fat on MRE, precluding analyses in terms of improvement with vedolizumab treatment. Larger prospective studies are warranted to better define the association of these findings with clinical outcomes and the role of transmural healing with vedolizumab in the natural course of CD.
The study showed strength in its prospective study design, with predefined radiologic endpoints that were read centrally for consistent evaluation. In addition, it demonstrated the feasibility of conducting a phase 3 trial on evaluation of the effect of vedolizumab treatment on CD using MRE. This aligns with the recent joint recommendation from the European Crohn’s and Colitis Organisation and the European Society of Gastrointestinal and Abdominal Radiology on the use of MRE-based indexes to assess disease activity and treatment response in clinical trials.18
Overall, this exploratory analysis contributes to the understanding of transmural disease processes in CD and the impact of vedolizumab on the components of inflammation in CD and potential impact on disease progression. Vedolizumab’s efficacy and safety profile makes it a promising first-line biologic therapeutic option following loss of response to conventional therapy in patients with moderately to severely active CD. MRE is feasible to be implemented in large clinical trials in CD and can be used in CD as an objective, minimally invasive procedure to complement ileocolonoscopy.
Acknowledgments
We thank the patients who participated in the VERSIFY trial, their caregivers, and the trial investigators and members of the VERSIFY trial team. We also thank Maria Kudela, formerly of Takeda, for statistical support. Medical writing support was provided by Paul Hassan, PhD, of Excel Medical Affairs, and funded by Takeda.
Funding Statement
This work was supported by Takeda.
Data Sharing Statement
The datasets—including the redacted study protocol, redacted statistical analysis plan, and individual participants’ data supporting the results reported in this article—will be available 3 months from initial request to researchers who provide a methodologically sound proposal. The data will be provided after their de-identification, in compliance with applicable privacy laws, data protection, and requirements for consent and anonymization. See here for more information: https://vivli.org/ourmember/takeda/
Ethics Approval and Informed Consent
This study was conducted in accordance with the ethical standards of the institutional and/or national research committee(s) and with the 1964 Helsinki Declaration and its later amendments. Written informed consent was obtained from all study participants.
Author Contributions
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; took part in drafting, revising or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Disclosure
J.R. reports consultancy fees from Alimentiv, Boehringer Ingelheim, Janssen, Lumen, Origo Biopharma, Parexel, Takeda, and TiGenix; grant/research support from AbbVie and Genentech; and speaker/honoraria fees from AbbVie, Gilead, Janssen, MSD, and Takeda. J.-F.C. reports research grants from AbbVie, Janssen, and Takeda; payment for lectures from AbbVie, Allergan, Amgen, Ferring, Shire, and Takeda; consulting fees from AbbVie, Amgen, Anaptys Bio, Arena, BMS, Boehringer Ingelheim, Bristol Myers Squibb, Celgene, Celltrion, Eli Lilly, Ferring, Galmed, Genentech, GlaxoSmithKline, Janssen, Kaleido, Imedex, Immunic, Iterative Scopes, Landos, Merck, Microba, Novartis, Otsuka Pharmaceutical, PBM Capital, Pfizer, Protagonist Therapeutics, Sanofi, Takeda, TiGenix, and Vifor; and holds stock options in Intestinal Biotech Development. B.B. reports adviser/speaker fees from AbbVie, Alimentiv, Ferring, Iterative Health, Janssen, Novartis, Merck, Pfizer, and Takeda; adviser for Allergan, Amgen, AMT, BMS, Celgene, Genentech, Gilead, Merck, Microbiome Insights, Pendopharm, Protagonist, and Robarts Clinical Trials; research support for AbbVie, Alvine, Amgen, Boehringer Ingelheim, Viatris, BMS, Celgene, Eli Lilly, Genentech, GSK, Janssen, Merck, and Qu Biologic; and holds Qu Biologics stock options. S.A., J.S., and D.L. are employees of Takeda and hold Takeda stock or stock options. P.C. was an employee of Takeda at the time this research was conducted. S.D. reports lecture fees from AbbVie, Alimentiv, Allergan, Amgen, Applied Molecular Transport, AstraZeneca, Athos Therapeutics, Biogen, Boehringer Ingelheim, Bristol Myers Squibb, Celgene, Celltrion, Dr Falk Pharma, Eli Lilly, Enthera, Ferring, Gilead, Hospira, Inotrem, Janssen, Johnson & Johnson, Merck, Morphic, MSD, Takeda, Mundipharma, Mylan, Pfizer, Roche, Sandoz, Sublimity Therapeutics, Teladoc Health, TiGenix, UCB, Vial, and Vifor; and consultancy fees from AbbVie, Allergan, Biogen, Boehringer Ingelheim, Celgene, Celltrion, Ferring, Hospira, Johnson & Johnson, Merck, MSD, Mundipharma, Pfizer, Sandoz, Takeda, TiGenix, UCB, and Vifor.
References
- 1.Torres J, Mehandru S, Colombel JF, Peyrin-Biroulet L. Crohn’s disease. Lancet. 2017;389(10080):1741–1755. doi: 10.1016/S0140-6736(16)31711-1 [DOI] [PubMed] [Google Scholar]
- 2.Turner D, Ricciuto A, Lewis A, et al. STRIDE-II: an update on the selecting therapeutic targets in inflammatory bowel disease (STRIDE) initiative of the International Organization for the Study of IBD (IOIBD): determining therapeutic goals for treat-to-target strategies in IBD. Gastroenterology. 2021;160(5):1570–1583. doi: 10.1053/j.gastro.2020.12.031 [DOI] [PubMed] [Google Scholar]
- 3.Maaser C, Sturm A, Vavricka SR, et al. ECCO-ESGAR Guideline for Diagnostic Assessment in IBD Part 1: initial diagnosis, monitoring of known IBD, detection of complications. J Crohns Colitis. 2019;13(2):144–164. doi: 10.1093/ecco-jcc/jjy113 [DOI] [PubMed] [Google Scholar]
- 4.Panés J, Bouzas R, Chaparro M, et al. Systematic review: the use of ultrasonography, computed tomography and magnetic resonance imaging for the diagnosis, assessment of activity and abdominal complications of Crohn’s disease. Aliment Pharmacol Ther. 2011;34(2):125–145. doi: 10.1111/j.1365-2036.2011.04710.x [DOI] [PubMed] [Google Scholar]
- 5.Jauregui-Amezaga A, Rimola J, Ordás I, et al. Value of endoscopy and MRI for predicting intestinal surgery in patients with Crohn’s disease in the era of biologics. Gut. 2015;64(9):1397–1402. doi: 10.1136/gutjnl-2014-308101 [DOI] [PubMed] [Google Scholar]
- 6.Bruining DH, Zimmermann EM, Loftus EV, Sandborn WJ, Sauer CG, Strong SA. Consensus recommendations for evaluation, interpretation, and utilization of computed tomography and magnetic resonance enterography in patients with small bowel Crohn’s disease. Radiology. 2018;286(3):776–799. doi: 10.1148/radiol.2018171737 [DOI] [PubMed] [Google Scholar]
- 7.Ordás I, Rimola J, Rodríguez S, et al. Accuracy of magnetic resonance enterography in assessing response to therapy and mucosal healing in patients with Crohn’s disease. Gastroenterology. 2014;146(2):374–382.e371. doi: 10.1053/j.gastro.2013.10.055 [DOI] [PubMed] [Google Scholar]
- 8.Fernandes SR, Rodrigues RV, Bernardo S, et al. Transmural healing is associated with improved long-term outcomes of patients with Crohn’s disease. Inflamm Bowel Dis. 2017;23(8):1403–1409. doi: 10.1097/mib.0000000000001143 [DOI] [PubMed] [Google Scholar]
- 9.Takenaka K, Ohtsuka K, Kitazume Y, et al. Utility of magnetic resonance enterography for small bowel endoscopic healing in patients with Crohn’s disease. Am J Gastroenterol. 2018;113(2):283–294. doi: 10.1038/ajg.2017.464 [DOI] [PubMed] [Google Scholar]
- 10.Buisson A, Hordonneau C, Goutorbe F, et al. Bowel wall healing assessed using magnetic resonance imaging predicts sustained clinical remission and decreased risk of surgery in Crohn’s disease. J Gastroenterol. 2019;54(4):312–320. doi: 10.1007/s00535-018-1505-8 [DOI] [PubMed] [Google Scholar]
- 11.Messadeg L, Hordonneau C, Bouguen G, et al. Early transmural response assessed using magnetic resonance imaging could predict sustained clinical remission and prevent bowel damage in patients with Crohn’s disease treated with anti-tumour necrosis factor therapy. J Crohns Colitis. 2020;14(11):1524–1534. doi: 10.1093/ecco-jcc/jjaa098 [DOI] [PubMed] [Google Scholar]
- 12.Geyl S, Guillo L, Laurent V, D’Amico F, Danese S, Peyrin-Biroulet L. Transmural healing as a therapeutic goal in Crohn’s disease: a systematic review. Lancet Gastroenterol Hepatol. 2021;6(8):659–667. doi: 10.1016/S2468-1253(21)00096-0 [DOI] [PubMed] [Google Scholar]
- 13.Buisson A, Joubert A, Montoriol PF, et al. Diffusion-weighted magnetic resonance imaging for detecting and assessing ileal inflammation in Crohn’s disease. Aliment Pharmacol Ther. 2013;37(5):537–545. doi: 10.1111/apt.12201 [DOI] [PubMed] [Google Scholar]
- 14.Sailer J, Peloschek P, Reinisch W, Vogelsang H, Turetschek K, Schima W. Anastomotic recurrence of Crohn’s disease after ileocolic resection: comparison of MR enteroclysis with endoscopy. Eur Radiol. 2008;18(11):2512–2521. doi: 10.1007/s00330-008-1034-6 [DOI] [PubMed] [Google Scholar]
- 15.Steward MJ, Punwani S, Proctor I, et al. Non-perforating small bowel Crohn’s disease assessed by MRI enterography: derivation and histopathological validation of an MR-based activity index. Eur J Radiol. 2012;81(9):2080–2088. doi: 10.1016/j.ejrad.2011.07.013 [DOI] [PubMed] [Google Scholar]
- 16.Van Assche G, Vanbeckevoort D, Bielen D, et al. Magnetic resonance imaging of the effects of infliximab on perianal fistulizing Crohn’s disease. Am J Gastroenterol. 2003;98(2):332–339. doi: 10.1111/j.1572-0241.2003.07241.x [DOI] [PubMed] [Google Scholar]
- 17.Ordas I, Rimola J, Alfaro I, et al. Development and validation of a simplified magnetic resonance index of activity for Crohn’s disease. Gastroenterology. 2019;157(2):432–439 e431. doi: 10.1053/j.gastro.2019.03.051 [DOI] [PubMed] [Google Scholar]
- 18.Sturm A, Maaser C, Calabrese E, et al. ECCO-ESGAR Guideline for Diagnostic Assessment in IBD Part 2: IBD scores and general principles and technical aspects. J Crohns Colitis. 2019;13(3):273–284. doi: 10.1093/ecco-jcc/jjy114 [DOI] [PubMed] [Google Scholar]
- 19.Rimola J, Ordás I, Rodriguez S, et al. Magnetic resonance imaging for evaluation of Crohn’s disease: validation of parameters of severity and quantitative index of activity. Inflamm Bowel Dis. 2011;17(8):1759–1768. doi: 10.1002/ibd.21551 [DOI] [PubMed] [Google Scholar]
- 20.Rimola J, Rodriguez S, García-Bosch O, et al. Magnetic resonance for assessment of disease activity and severity in ileocolonic Crohn’s disease. Gut. 2009;58(8):1113–1120. doi: 10.1136/gut.2008.167957 [DOI] [PubMed] [Google Scholar]
- 21.Danese S, Sandborn WJ, Colombel JF, et al. Endoscopic, radiologic, and histologic healing with vedolizumab in patients with active Crohn’s disease. Gastroenterology. 2019;157(4):1007–1018. doi: 10.1053/j.gastro.2019.06.038 [DOI] [PubMed] [Google Scholar]
- 22.Taylor SA, Avni F, Cronin CG, et al. The first joint ESGAR/ ESPR consensus statement on the technical performance of cross-sectional small bowel and colonic imaging. Eur Radiol. 2017;27(6):2570–2582. doi: 10.1007/s00330-016-4615-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Siddiki HA, Fidler JL, Fletcher JG, et al. Prospective comparison of state-of-The-art MR enterography and CT enterography in small-bowel Crohn’s disease. AJR Am J Roentgenol. 2009;193(1):113–121. doi: 10.2214/AJR.08.2027 [DOI] [PubMed] [Google Scholar]
- 24.Church PC, Turner D, Feldman BM, et al. Systematic review with meta-analysis: magnetic resonance enterography signs for the detection of inflammation and intestinal damage in Crohn’s disease. Aliment Pharmacol Ther. 2015;41(2):153–166. doi: 10.1111/apt.13024 [DOI] [PubMed] [Google Scholar]
- 25.Rimola J, Alfaro I, Fernández-Clotet A, et al. Persistent damage on magnetic resonance enterography in patients with Crohn’s disease in endoscopic remission. Aliment Pharmacol Ther. 2018;48(11–12):1232–1241. doi: 10.1111/apt.15013 [DOI] [PubMed] [Google Scholar]
- 26.Capozzi N, Ordas I, Fernandez-Clotet A, et al. Validation of the simplified magnetic resonance index of activity [sMARIA] without gadolinium-enhanced sequences for Crohn’s disease. J Crohns Colitis. 2020;14(8):1074–1081. doi: 10.1093/ecco-jcc/jjaa030 [DOI] [PubMed] [Google Scholar]
- 27.Rimola J, Fernandez-Clotet A, Capozzi N, et al. Pre-treatment magnetic resonance enterography findings predict the response to TNF-alpha inhibitors in Crohn’s disease. Aliment Pharmacol Ther. 2020;52(10):1563–1573. doi: 10.1111/apt.16069 [DOI] [PubMed] [Google Scholar]
- 28.Taylor SA, Mallett S, Bhatnagar G, et al. Diagnostic accuracy of magnetic resonance enterography and small bowel ultrasound for the extent and activity of newly diagnosed and relapsed Crohn’s disease (METRIC): a multicentre trial. Lancet Gastroenterol Hepatol. 2018;3(8):548–558. doi: 10.1016/S2468-1253(18)30161-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Miles A, Bhatnagar G, Halligan S, et al. Magnetic resonance enterography, small bowel ultrasound and colonoscopy to diagnose and stage Crohn’s disease: patient acceptability and perceived burden. Eur Radiol. 2019;29(3):1083–1093. doi: 10.1007/s00330-018-5661-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lafeuille P, Hordonneau C, Vignette J, et al. Transmural healing and MRI healing are associated with lower risk of bowel damage progression than endoscopic mucosal healing in Crohn’s disease. Aliment Pharmacol Ther. 2021;53(5):577–586. doi: 10.1111/apt.16232 [DOI] [PubMed] [Google Scholar]
- 31.Chateau T, Peyrin-Biroulet L. Evolving therapeutic goals in Crohn’s disease management. United Eur Gastroenterol J. 2020;8(2):133–139. doi: 10.1177/2050640619887316 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Coimbra AJ, Rimola J, O’Byrne S, et al. Magnetic resonance enterography is feasible and reliable in multicenter clinical trials in patients with Crohn’s disease, and may help select subjects with active inflammation. Aliment Pharmacol Ther. 2016;43(1):61–72. doi: 10.1111/apt.13453 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets—including the redacted study protocol, redacted statistical analysis plan, and individual participants’ data supporting the results reported in this article—will be available 3 months from initial request to researchers who provide a methodologically sound proposal. The data will be provided after their de-identification, in compliance with applicable privacy laws, data protection, and requirements for consent and anonymization. Additional information can be found at: https://vivli.org/ourmember/takeda/