Take Home Message
First-generation neodymium and diode lasers are obsolete in modern practice.
Holmium:yttrium-aluminum-garnet (YAG) and thulium:YAG lasers provide excellent ablation and hemostasis of upper tract urothelial tumors, with excellent survival rates.
The new thulium fiber laser shows similar safety and efficacy to holmium:YAG and thulium:YAG lasers.
Keywords: Upper urinary tract urothelial carcinoma, Nephron-sparing surgery, Endoscopic treatment, Laser, Survival
Abstract
Context
The occurrence of upper urinary tract urothelial carcinoma (UTUC) is uncommon and is usually identified at an advanced and multifocal stage. Currently, there is growing interest in utilizing endoscopic laser ablation (ELA).
Objective
To evaluate the survival rates and perioperative complications of ELA.
Evidence acquisition
We performed a literature search through PubMed, Web of Science, and Scopus. The analysis included observational studies that examined the oncological outcomes of patients with UTUC treated with ELA.
Evidence synthesis
Neodymium and diode lasers are no longer used due to their high complication rates. Holmium:yttrium-aluminum-garnet (YAG) and thulium:YAG lasers provided excellent tumor ablation and hemostasis in both the collecting system and the ureter. These lasers offer good disease-free and cancer-specific survival, especially for low-grade tumors.
Conclusions
Advancements in laser technology and ablation techniques, and understanding of UTUC tumor biology hold significant promise in improving the use of conservative UTUC treatment, with excellent safety and good oncological outcomes for low-grade diseases.
Patient summary
With the advancement of technology, the conservative approach utilizing endoscopic laser ablation for upper tract urothelial tumors has been proved to be both safe and effective, showcasing promising survival rates.
1. Introduction
Upper tract urothelial carcinoma (UTUC) is a relatively uncommon tumor, including only 5–10% of all urothelial carcinomas. Typically, UTUC is diagnosed with invasive features [1]. According to the current European Association of Urology guidelines, the standard treatment for localized high-risk disease is radical nephroureterectomy (RNU) with bladder cuff excision or distal ureterectomy that may be associated with similar oncological outcomes to RNU but with lower morbidity [2]. Indeed, RNU can result in postoperative renal function impairment, leading to a potential decrease in the estimated glomerular filtration rate by 20–25% and an associated risk of developing chronic kidney disease, which can negatively impact overall survival (OS) [3].
In a meta-analysis comparing segmental ureterectomy (SU) and RNU, the postoperative estimated glomerular filtration rate was higher in the SU group, with similar results in 5-yr cancer-specific survival (CSS) and metastasis-free survival. However, the 5-yr recurrence-free survival was lower in the SU group [4]. Therefore, nephron-sparing surgery (NSS) is the preferred approach to preserve renal parenchyma and optimize functional outcomes, specifically in patients with small, localized, low-grade UTUC, and the treatment of choice in those with solitary kidneys, pre-existing chronic kidney disease, or multiple comorbidities [2].
Endoscopic tumor laser ablation is an NSS approach, wherein laser energy helps vaporize or coagulate UTUC percutaneously or ureteroscopically. This targeted approach offers several advantages over traditional surgical methods, including minimal damage to surrounding healthy tissue, reduced bleeding, and shorter hospital stays. In low-stage UTUC, endoscopic laser ablation has demonstrated noninferior oncological outcomes compared with radical surgery while avoiding the morbidities associated with the latter [5].
Technology has significantly contributed to improving endoscopic techniques, particularly in enhancing the accuracy of tumor identification, characterization, and ablation [6].
Despite the promising results in preserving renal function, endoscopic treatment of UTUC remains challenging because the presence of high-grade disease during the initial ureteroscopy significantly impacts prognosis, regardless of the treatment method [7], [8].
In this study, we aimed to perform a scoping review to establish how and which lasers can be used effectively as an NSS approach for endoscopic laser ablation and in which patients this has the most benefit vis-à-vis RNU.
2. Evidence acquisition
2.1. Literature search
The principal focus of this review was to assess prognostic factors and oncological outcomes of endoscopic laser ablation for UTUCs. Literature search was performed through EMBASE, PubMed, and Scopus on April 14, 2023. The following terms and Boolean operators were used: (Conservative treatment OR endoscopic treatment OR nephron-sparing surgery OR laser surgery) AND (Upper Urinary Tract OR collecting system OR pelvis OR ureter) AND (Urothelium cancer OR Urothelial Carcinoma OR UTUC).
2.2. Selection criteria
Only studies reporting endoscopic laser ablation on UTUC were considered. The patient, intervention, comparison, outcome, and study type (PICOS) model was used to frame and respond to the clinical question; P: adult patients with UTUC; I: endoscopic laser ablation; C: comparison with patients treated with RNU or none; O: OS, CSS, bladder recurrence rate, perioperative complications, and postoperative renal function; and S: retrospective, prospective, and randomized studies.
We only accepted studies published in English. Preclinical and pediatric studies were excluded. Additionally, we excluded reviews, letters to the editor, case reports, and meeting abstracts.
2.3. Study screening and selection
Two independent authors screened all the retrieved papers using the Covidence Systematic Review Management (Veritas Health Innovation, Melbourne, Australia). A third author was consulted to resolve the discrepancies. The full text of the papers that were deemed relevant to the purpose of the review was selected after the initial screening process. This review was registered on https://osf.io/registries/ (number osf.io/rc6fw).
3. Evidence synthesis
3.1. Literature screening
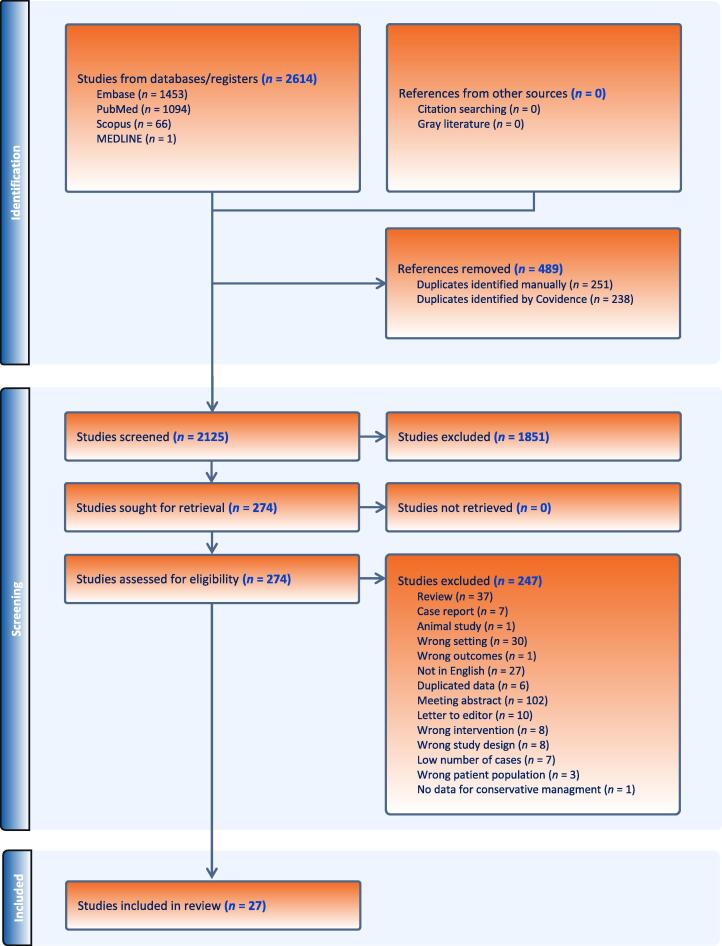
Initially, 2614 papers were identified through the literature search. After removing 489 duplicates, 2125 papers were screened based on their title and abstract. Among these papers, 1851 were further excluded. The remaining 274 full-text papers were evaluated for relevance, and 247 were subsequently excluded. Ultimately, 27 papers were deemed suitable and included in this review [9], [10], [11], [12], [13], [14], [15], [16], [17], [18], [19], [20], [21], [22], [23], [24], [25], [26], [27], [28], [29], [30], [31], [32], [33], [34], [35]. Figure 1 illustrates the flowchart of the literature search process.
Fig. 1.
PRISMA 2009 flow diagram. PRISMA = Preferred Reporting Items for Systematic Reviews and Meta-analyses.
3.2. Study characteristics
Table 1 shows the study characteristics. Most of the studies were retrospective [9], [10], [11], [12], [13], [14], [16], [17], [18], [19], [22], [23], [24], [25], [26], [27], [28], [29], [30], [31], [32], [33], [35], [36], while four were prospective [15], [20], [21], [34]. There were four comparative studies [16], [19], [22], [32], and the other ones were single series [9], [10], [11], [12], [13], [14], [15], [17], [18], [20], [21], [23], [24], [25], [26], [27], [28], [29], [30], [31], [33], [34], [35]. In six studies, the combination between electroresection and laser ablation was applied [9], [10], [11], [12], [26], [27]. Three articles employed neodymium laser [9], [10], [11], [12], [13], [14], one diode laser [14], six holmium laser [15], [16], [17], [18], [19], [20], four thulium laser [21], [22], [23], [24], six the combination of neodymium and holmium laser [25], [26], [27], [28], [29], [30], [31], [32], and three the combination of thulium and holmium laser [33], [34], [35].
Table 1.
Studies on endoscopic laser ablation (ELA) in patients with upper urinary tract urothelial carcinoma (UTUC)
| Author (year) | Country | Study design | Type of technique | Cases (n) | Age (yr) | Grade (HG–G3), n (%) | Postoperative complications, n (%) | Mean follow-up (mo) | Overall survival (%) | Cancer–specific survival (%) | Bladder recurrence, n (%) | Conclusion |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Niţă (2012) [9] | Romania | Retrospective | Nd:YAG or resection | 65 | 67 | 15 (71.4) | – | 60 | – | 5 yr: 52.3% | 20 (30.7) | The most important prognostic factors for UTUC progression are tumor location, size, and grade |
| Martínez-Piñeiro (1996) [10] | Spain | Retrospective | Nd:YAG or resection | 42 | 62.2 | 10 | UUT perforation: 6 (14) Colon perforation: 1 (2.3) Ureteral stricture:7 (16.7) |
31 | – | – | 10 (23.8) | ELA for superficial UTUC is a safe procedure, with low complication rates and moderate recurrence rates |
| Blute (1989) [11] | USA | Retrospective | Nd:YAG or resection | 21 | 65 | 3 (14) | Fever: 2 (9.5) UUT perforation: 3 (14) |
33 | – | – | 4 (19) | Conservative endourological techniques can safely be employed to manage specific cases of UTUC |
| Elliott (1996) [12] | USA | Retrospective | Nd:YAG or electrocautery | 21 | 69 | 6 (29) | UUT perforation: 2 (9.5) Fever: 4 (19) Ureteral stricture: 6 (28.6) |
72 | 5 yr: 66% | – | 44 (43.2) | UTUC can be managed by ELA in selected cases |
| Jabbour (2000) [13] | France | Retrospective | Nd:YAG laser | 61 | – | 0 (0) | – | 48 | – | 4 yr: 95% | – | The low sensitivity and specificity of urine cytology and radiography warrant close and vigilant long-term endoscopic follow-up, especially for stage T1 tumors |
| Jimie (2022) [14] | UK | Retrospective | Diode laser | 30 | 76 | 9 (30) | Hematuria: 1 (3.3) AKI: 1 (3.3) AUR: 1(3.3) Vomiting: 2 (6.7) |
30 | – | – | – | Diode laser proved to be a safe and effective approach for managing UTUC in patients who are not suitable candidates for RNU |
| Matsuoka (2003) [15] | Japan | Prospective | Ho:YAG | 30 | – | 0 (0) | Ureteral stricture: 1 (3.3) | 20 | – | 3 yr with elective indication: 95% 3 yr with imperative indication: 57% |
5 (17) | Ho:YAG laser can be a useful method in limited cases identified in specific treatments groups combined with a strict follow-up |
| Rouprêet (2006) [16] | France | Retrospective | Ho:YAG | 27 URS 16 PEA |
68 | 8 (29.6) URS 5 (31.2) PEA |
URS: 2 perforation requiring urinary stent placement and 1 case of bleeding requiring endoscopic surgery PEA: 0 |
57.5 median | – | 5 yr URS: 80.7% 5 yr PEA: 80% |
5 (12) | Conservative surgery can be recommended for LG or superficial UTUC, determining similar CSS and BR to RNU |
| Painter (2008) [17] | UK | Retrospective | Ho:YAG and/or Nd:YAG | Elective group: 19 Relative group: 16 Palliative group:10 |
65 | Elective group: 0 (0) Relative group: 12 (75) Palliative group: 8 (80) |
Ureteral stricture: 2 (4.4) | 24 | 2 yr elective group: 90% Relative group: 62.5% Palliative group: 70% |
2 yr elective group: 90% | – | ELA is a valid option in elective cases. Even in imperative indications, endoscopic treatment is a safe and feasible approach |
| Cornu (2010) [18] | France | Retrospective | Ho:YAG | 35 | 67 | 6 (17.1) | Sepsis: 2 (5.7) AKI: 1 (2.8) |
30 | 3 yr: 100% | 3 yr: 100% | 23 (65) | ELA can be advocated in selected cases of UTUC as an alternative to RNU |
| Hoffman (2014) [19] | Israel | Retrospective | Ho:YAG | 25 | 64 | 0(0) | – | 26 | 4 yr: 70% | 5 yr: 100% | 11 (44) | EA for LG UTUC guarantees similar CSS to RNU, despite a higher rate of BR |
| Villa (2018) [20] | Italy | Prospective | Ho:YAG | 112 | 69.7 | 13 (14.1) | – | 52.4 | – | 2 yr: 77% | 70 (76.1) | ELA is a valid option in selected cases of UTUC. Tumor size >1 cm and multifocality do not contraindicate the procedure |
| Musi (2018) [21] | Italy | Prospective | Thu:YAG | 42 | 68 | 4 (9) | CD I: 16 (38) CD II: 15 (35.7) CD III: 1 (2.4) |
60 | – | – | – | EA with thulium laser is a safe and effective technique for UTUC treatment. It guarantees optimal lesion vaporization and fine hemostatic control without any major complication |
| Wen (2018) [22] | China | Retrospective | Thu:YAG | 32 | 69.3 | 5 (16) | 0 (0) | – | – | – | 7 (35) | Thulium laser group is associated with higher renal function preservation, but a higher rate of local recurrence |
| Bozzini (2021) [23] | Italy | Retrospective | Thu:YAG | 47 | 69.2 | 29 (37.2) | Hematuria: 12 (15.3) Infections: 9 (11.5) |
11.7 | – | – | 9 (17) | For a short term, thulium laser ablation of UTUC is safe and feasible, especially in low-grade UTUC |
| Proietti (2022) [24] | Italy | Retrospective | TFL | 28 | 73 | 8 (28.6) | CD I–II: 3 (10.7) CD IIIB: 1 (3.6) |
12 | – | 1 yr: 76.5% | – | TFL guarantees that optimal tumor ablation and fine hemostatic control were achieved without major complications in a short-term follow-up |
| Johnson (2005) [25] | USA | Retrospective | Nd:YAG and/or Ho:YAG laser | 35 | – | 14 (22) | Infundibular strictures: 2 (6) Ureteral stricture:1 (3) |
32 | – | – | – | ELA is recommendable for patients with LG UTUC, owing to the low tumor progression risk and low morbidity |
| Sowter (2007) [26] | UK | Retrospective | Nd:YAG and/or Ho:YAG laser or cautery | 37 | 65 | 4 (9.8) | – | 41.6 | – | – | 12 (34.3) | ELA is a is a safe and effective approach in LG UTUC or imperative cases |
| Suh (2003) [27] | USA | Retrospective | Nd:YAG and/or Ho:YAG laser or resection | 58 | 70.7 | 20 (33) | Hematuria: 4 (6.9) Flank pain leading to admission: 3 (5.2) Atrial arrhythmia: 2 (3.4) Ureteral stricture :2 (3.4) |
21.0 | – | – | – | Endoscopic treatment is associated with a high risk of local recurrence and retreatment. Patients with low-grade, solitary, or less bulky diseases have higher recurrence-free survival |
| Chen (2000) [28] | USA | Retrospective | Nd:YAG and/or Ho:YAG laser | 23 | 65 | 1 (4.4) | Ureteral strictures: 2 (8.8) | 35 | – | – | 7 (30) | ELA of small, low-grade UTUC can be a safe alternative treatment to RNU in patients with healthy contralateral kidneys |
| Boorjian (2004) [29] | USA | Retrospective | Nd:YAG and/or Ho:YAG laser | 38 | 70.9 | 2 (5) | – | 37.2 | – | 3 yr: 66% | – | Selective cytology examination may play a significant role in the decision-making process for patients with UTUC |
| Scotland (2020) [30] | USA | Retrospective | Nd:YAG and/or Ho:YAG laser | 168 | 70 | 28 (16.7) | Sepsis: 2 (1.2) UTI: 4 (2.4) Ureteral stricture: 1 (0.6) |
66 | 5 yr: 80.9% | 5 yr: 92.6% | – | ELA can guarantee satisfactory oncological outcomes for UTUC, especially low-grade ones, while sparing renal function |
| Shvero (2020) [31] | Israel | Retrospective | Nd:YAG and/or Ho:YAG laser | 59 | 70 | – | CD I: 12 (20.3) CD II: 7 (11.8) CD IIIa: 1 (1.6) CD IIIb: 4 (6.7) |
22 | – | 2 yr: 100% | 27 (45.7) | ELA for large, multifocal, low-grade UTUC guarantees short-term oncological outcomes, with a low comorbidity rate |
| Shen (2022) [32] | Taiwan | Retrospective | Nd:YAG and/or Ho:YAG laser | 23 | 66.0 | – | – | 33.6 | 5 yr: 94.5% | – | – | In Ta-T1 UTUC, ELA offers similar oncological outcomes to RNU. However, for high-grade tumors, strict surveillance is needed |
| Defidio (2019) [33] | Italy | Retrospective | Thu-Ho:YAG Duo laser |
178 | 70.8 | 60 (33.7) | CD I: 17 (9.6) | 28.7 | – | – | – | ELA with the thulium-holmium:YAG Duo laser has been proved to be a safe and effective treatment option, demonstrating long-term oncological radicality and minimal morbidity |
| Sanguedolce (2021) [34] | Italy | Prospective | Ho:YAG or Thu:YAG | 47 | 75 | 8 (17) | CD I: 1 (2.1) CD II: 4(8.5) |
24 | – | – | 11 (23.4) | ELA is a feasible procedure guaranteeing renal function preservation. Tumor size seems to be associated with BR, while the number of recurrences seems to be associated with UTUC progression |
| Proietti (2021) [35] | Italy | Retrospective | Ho:YAG and/or Thu:YAG | 29 with 137 procedures | 69 | 18 (62) | CD III: 3 (2.2) CD IV: 1 (0.7) |
24 | 2 yr: 96% | 2 yr: 31.3% | 9 (31) | ELA in patients with imperative indications is a feasible alternative to RNU |
AKI = acute kidney injury; AUR = acute urinary retention; BR = bladder recurrence; CD = Clavien-Dindo; CSS = cancer-specific survival; EA = endoscopic ablation; HG = high grade; Ho = holmium; LG = low grade; ND = neodymium; PEA = percutaneous endoscopic ablation; RNU = radical nephroureterectomy; TFL = thulium fiber laser; Thu = thulium; URS = ureteroscopy; UTI = urinary tract infection; UUT = upper urinary tract; YAG = yttrium-aluminum-garnet.
3.3. Discussion
3.3.1. Use of neodymium and diode lasers
Neodymium:yttrium-aluminum-garnet (Nd:YAG) laser emits light in the near-infrared range (ie, 1.064 nm). Hemoglobin is its selected target. This allows simultaneous coagulation and ablation on target tissues. However, Nd:YAG laser has the drawback of higher tissue penetration of 5–6 mm with a high degree of scatter, causing large tissue volume to be coagulated if used as a free beam [36]. These effects are potentially harmful for the upper urinary tract. Indeed, its application for UTUC treatment has led to a high incidence of ureteric stricture in the past [37]. With the rapid development of new laser systems during the past decades, this type of laser source has been used more frequently in combination with other laser energies. Thus, the use of Nd:YAG laser as the only laser system for the conservative surgical management of UTUC has been evaluated by only a few studies [9], [10], [11], [12], [13].
Elliott et al. [12] retrospectively analyzed 44 UTUC cases treated by different endoscopic approaches and reported outcomes of 18 patients who underwent treatment with Nd:YAG laser with a power setting of 20–45 W through a flexible or rigid ureteroscope, and a pulse setting of 2 s. For this subgroup, a recurrence rate of 39% was observed, with 11% of patients experiencing two or more recurrences.
Similarly, Martínez-Piñeiro et al. [10], in their retrospective case series of 19 cases, found a recurrence rate of 42.1%. They also reported a poorer prognosis for patients with ureteral tumors than those with renal pelvis [14]. This was attributed to ureteric location, concerns about inadvertent perforation, and poor visibility during endoscopic laser ablation. However, it is noteworthy that the latter study considered endoscopic surgery before 1995; their findings may not be applicable nowadays in light of technological advancements since then.
Generally, studies reporting on Nd:YAG laser as the only laser energy for the conservative surgical management of UTUC observed more frequent recurrences (39–42%) than those with other laser systems [11], [13]. Moreover, those studies employed different energies (ie, Nd:YAG laser, electrical fulguration, or electrical resection), and most data were presented for the overall population, making a correct analysis of the efficacy of Nd:YAG laser difficult.
Diode laser refers to the laser generated by a diode source that emits at 810 or 980 nm wavelength and can work in a pulsed or continuous mode. Thanks to its photothermal effect, a diode laser can be applied by an excision technique or by ablation/vaporization [38]. Despite its potential benefit in soft tissue ablation, diode laser has not been used extensively in UTUC conservative management.
Jimie et al. [14] showed that management of UTUC with diode laser was safe and effective, providing symptom control in patients unfit for radical treatment. In their series, the authors reported its successful use in 63.3% of grade 2 tumors and 30% in grade 3 tumors, with an overall rate of 16.7% Clavien grade ≤2 complications, with no patient needing a blood transfusion.
3.3.2. Use of holmium:YAG laser
One of the most widely utilized lasers in urology is holmium:yttrium-aluminum-garnet (Ho:YAG) laser. This laser is particularly valuable in the treatment of UTUC due to its high absorption in water, resulting in a reduced penetration depth of 0.4–0.7 mm. The pulsed nature of the laser, coupled with its significant peak power, leads to substantial vapor generation upon firing and provides effective coagulation properties [39].
Matsuoka et al. [15] reported the oncological outcomes of 30 UTUC patients, with a follow-up period of 36 mo. In the imperative indication group, the tumor-free and recurrence rates after the initial treatment were 57% and 86%, respectively. In contrast, the elective indication group demonstrated more favorable results with rates of 95% and 20%, respectively.
In a retrospective review involving 97 patients from two centers, Rouprêt et al. [16] detailed their experience of the use of Ho:YAG laser with a 200-μm fiber. They started the procedure with a low-energy (0.6 J) and low-frequency (6 Hz) setting. The 5-yr disease-specific survival rates after RNU and conservative laser treatment were comparable (84% vs 80.7%, p = 0.89), as were the 5-yr tumor-free survival rates (75.3% vs 71.5%, p = 0.78). Based on these findings, the authors concluded that endoscopic laser ablation can be recommended as an alternative to RNU for low-grade UTUC.
Painter et al. [17] conducted a study involving 45 patients who underwent endoscopic laser ablation. The authors divided treatments as follows: (1) elective indication: patients with normal contralateral kidneys, normal global renal function, no major comorbidity, and favorable tumor characteristics (smaller lesions, all grade ≤2); (2) relative indication: patients with less favorable tumor characteristics or moderate chronic renal impairment, or who refused radical surgery; and (3) imperative treatment: patients with a solitary kidney or deemed unfit for RNU. Among the 19 patients in the elective treatment group, none showed evidence of disease progression during a median surveillance period of 24 mo. In contrast, 75% of 16 patients in the relative indication group required RNU after 24 mo from surgery. Among these patients, half had been diagnosed with T2 disease or a more advanced stage based on the final pathological evaluation. Out of the ten patients who received treatment solely for palliative purposes, seven were alive at the last follow-up, although some of them have experienced frequent disease recurrences. Regarding postoperative complications, only two out of 45 patients developed ureteral strictures, likely as a result of mitomycin C instillation.
In another case series conducted by Cornu et al. [18], a total of 35 patients underwent endoscopic laser ablation using Ho:YAG laser. Three patients experienced postoperative complications, with two developing severe sepsis and the remaining patient suffering from an acute renal injury requiring dialysis. Local recurrence was observed in 21 patients (60%), leading to RNU in four cases due to tumor progression. Given the risk of recurrence, the authors recommended a comprehensive and rigorous follow-up plan, including regular ureteroscopies every 3 mo for 2 yr.
Hoffman et al. [19] also employed Ho:YAG laser for the treatment of 25 patients with low-grade UTUC. During the 26-mo follow-up period, bladder recurrence was observed in 11 cases, with four patients experiencing multiple recurrences. The authors inferred that disease-related mortality following a nephron-sparing endoscopic approach or RNU for low-grade upper tract transitional cell carcinoma is excellent. However, the former was associated with a relatively high rate of ureteral and bladder recurrence, and therefore, they advocated for a stringent follow-up protocol.
In a prospective study by Villa et al. [20], 112 patients underwent flexible ureteroscopy with Ho:YAG laser photoablation. After an 18-mo follow-up period, 21 patients (22.8%) required RNU due to significant local recurrence. The progression-free survival (PFS) rates at 12 and 24 mo after ureteroscopy were 86% and 77%, respectively. Based on these findings, the authors concluded that endoscopic laser ablation can be a suitable procedure for managing low-grade UTUC, providing a rigorous follow-up. They demonstrated a significant difference in disease PFS at 1 and 2 yr following surgery based on tumor grade.
In summary, the use of Ho:YAG laser for the conservative management of UTUC is both feasible and safe. However, it is crucial to emphasize the importance of a tailored and personalized therapeutic approach and strict follow-up to effectively prevent recurrences and achieve improved outcomes.
3.3.3. Use of thulium:YAG laser and thulium fiber laser
Thulium:yttrium-aluminum-garnet (thulium:YAG) laser operates at 1940–2013 nm wavelength, in a continuous wave mode, and water is its target chromophore. Thulium:YAG laser has an optical penetration of only 0.2 mm, which allows high energy density, leading to smooth incision and rapid tissue vaporization. This translates into a smaller zone of thermal damage in the remaining tissue [40]. These characteristics make thulium:YAG laser an appealing energy source for endoscopic UTUC treatment.
Musi et al. [21] reported their experience of 42 UTUC cases conservatively treated by thulium:YAG ablation. In this retrospective case series, endoscopic surgery was performed by Revolix 200 W or Quanta System Cyber 150 W laser fibers of 272 and 365 μm (for flexible and semirigid ureteroscope, respectively), and ablation energy of 10–20 W. No major perioperative complications occurred (only Clavien grade I and II complications were observed, with a rate of 38% and 47.6%, respectively). Of the cases, 12% underwent a second procedure for residual disease persistence due to nonadequate vaporization, but of note, all lesions were larger >15 mm. Concerning the oncological outcomes, 19% of cases had a clinical recurrence, with median estimated recurrence-free survival of 44 mo. Moreover, 9.5% of patients underwent RNU, while no progression or upstaging was observed at a median follow-up of 26.3 mo.
Similarly, Wen et al. [22] presented the outcomes of 32 patients with UTUC managed conservatively by thulium:YAG laser ablation by a rigid or flexible ureteroscope using the Vela XL 1.9 μm thulium laser system at an energy setting of 30–50 W. All patients were managed successfully, with no major perioperative complications, but four cases of ureteral strictures were recorded 3 mo after surgery. Comparing their data with 107 RNU cases, the authors found that the laser group had a lower loss of renal function (postoperative creatinine level of 89 ± 7.5 vs 123 ± 9.4 μmol/l, p < 0.01), shorter length of hospital stay (3.6 ± 1.9 vs 8.6 ± 2.4 d, p < 0.01), but a higher rate of local recurrence (21.9% vs 7.8%, p < 0.01), which led authors to suggest thulium:YAG laser ablation as an acceptable treatment for selected cases under an intensive surveillance program.
Bozzini et al. [23] retrospectively evaluated thulium:YAG laser for ureteroscopic UTUC ablation in a multicenter study including 78 cases. A 272 μm laser fiber was used, at 15–30 and 15 W for the ablation of pelvic and ureteric lesions, respectively. Regarding postoperative complications, there were just Clavien I and II complications (15.3% and 11.5%, respectively), and no strictures or perforations were observed during the follow-up. After endoscopic treatment, 39.8% of patients underwent RNU (mainly for high-grade disease), whereas the remaining patients were on follow-up, and 19.2% experienced disease recurrence after a mean of 11.7 mo. Moreover, seven cases developed low-grade bladder cancer.
The thulium fiber laser (TFL) is the latest laser energy introduced in urological clinical practice. Launched in 2018, TFL is gaining popularity due to its versatility. TFL emits at 1.940 nm wavelength in both continuous and pulsed mode. TFL wavelength is near the absorption peak of water and has an optical penetration depth of only 0.077 mm [41]. After traveling the distance of its optical penetration depth, the TFL energy pulse reduces to 1.7% only, and this in conjunction with its high water absorption ensures a high energy delivery to the target tissue with a very thin layer of carbonization, followed by larger layers of cellular vacuolization and thermal-coagulation zone, providing adequate hemostasis in highly vascular tissue such as in UTUC [42]. These characteristics are a good compromise between the excellent hemostasis of thulium:YAG laser and the tissue cutting/ablation of Ho:YAG laser with an acceptable degree of carbonization.
Proietti et al. [24] reported their experience with TFL ablation for endoscopic conservative treatment of UTUC. In this case series of 28 patients, the ablation was performed with the SuperPulsed mode, consisting of 1 J, 10 Hz, short pulse using a 200 μm laser fiber. Overall, 95 endoscopic procedures were performed, with no intraoperative complications. Only one IIIb grade complication during the follow-up period occurred, and no ureteric stricture was observed. The recurrence rate was 21.7% at 6 mo and 17.7% at 12-mo follow-up.
3.3.4. Combination of Ho:YAG and Nd:YAG lasers
Using a combination of Nd:YAG and Ho:YAG lasers was adopted in the 1980s for the endoscopic treatment of UTUC. Nd:YAG laser provides a coagulative and ablative effect on the tumor due to its long pulse and high energy. On the contrary, Ho:YAG laser, set at 0.6–1.0 J and 5–10 Hz with a short pulse, can resect the tumor from the ureteral wall, reducing the risk of perforation and strictures [25]. In the initial case series, the utilization of either one of the two lasers or their combination during ureteroscopy involved electroresection until there was no visible tumor [26], [27]. The majority of patients (95%) achieved complete macroscopic eradication of tumor tissue, although the recurrence rate was high and ranged from 74% to 88%. However, renal function preservation was observed in >70% of cases.
The combined use of these lasers was first presented in 2000 by Chen and Bagley [28] in a series of 23 patients. This combined treatment approach involved performing laser coagulation of the bulk of the tumor using Nd:YAG laser, followed by resection/ablation with Ho:YAG laser. Although the overall recurrence rate was high, 15 out of 23 patients (65%) were tumor free in the ipsilateral upper urinary tract, with a mean follow-up of 17 mo. Only four patients required RNU, resulting in a 100% OS rate. Three patients needed endoscopic dilatation of ureteral strictures (Clavien grade IIIb).
In a series by Boorjian et al. [29] of 38 patients with UTUC, the preoperative urinary cytology role in predicting tumor grade and stage was highlighted. The authors indicated that among patients who underwent endoscopic ablation for UTUC and had positive cytology, 94.1% experienced at least one recurrence, while only 47.1% of patients with initially negative selective cytology demonstrated recurrences during their follow-up. Importantly, tumor recurrence in patients with positive urinary cytology was unrelated to the pathological grade.
Despite the high percentage of recurrence rate, a low progression rate independent of tumor grade at presentation has been observed. In one of the studies with 5 yr of follow-up, Scotland et al. [30] reported a 75% PFS rate, even though only 30% of the 168 included patients were recurrence free. RNU was performed in 50 patients (19.4%), ensuring a CSS rate of 92.6%.
Similarly, Shvero et al. [31] reported a PFS rate of 93.2% despite 74.1% of the patients experiencing at least one recurrence, with a median time to recurrence of 6.5 mo after surgery. Most of those recurrences were small and were managed endoscopically with a stringent follow-up. They observed a higher incidence of local recurrence among patients with collecting system tumors, which can be attributed to the continuous circulation of irrigation fluid within the pelvis during the procedure and the subsequent risk of tumor seeding. Moreover, they did not find any association between multifocality or tumor size and the time to tumor progression.
Shen et al. [32] conducted a comparative study including 65 UTUC patients with similar oncological baseline characteristics, which were managed either endoscopically (23) or with RNU (42). At 5-yr follow-up, the OS was similar between the groups (94.5% vs 94.6%), with a PFS rate of 58.6% in the endoscopic group compared with 55.8% in the RNU group (p = 0.083), indicating the oncological safety of this treatment. However, patients treated with RNU experienced a significant loss of estimated glomerular filtration rate. According to their findings, high-grade tumors were found to have an association solely with recurrence-free survival, while they did not impact OS, PFS, and CSS. Additionally, tumor multiplicity was identified as the only independent factor for bladder recurrence.
Regarding surgical complications, all studies reported some cases of ureteral stricture, which were resolved endoscopically and were related to the ablative effect of Nd:YAG laser. The overall complication rate was below 10%, primarily consisting of Clavien I events such as transient hematuria, urinary tract infections, and stent-related symptoms.
3.3.5. Combination of Ho:YAG and thulium:YAG lasers
The use of the combination of Ho:YAG and thulium:YAG lasers brings in the advantages of both lasers in UTUC conservative treatment, combining the specific advantages of each system.
The efficacy of ablation thulium:YAG laser can be enhanced using Ho:YAG laser to eliminate the necrotic tissue layer that covers the deep layers of the lesion. When used in contact with the neoplastic tissue, thulium:YAG laser produces necrotic coagulated tissues that can be eliminated by Ho:YAG laser, providing a better view of the tumor base, enabling clearer ablation with thulium:YAG laser, and reducing bleeding and operation time.
The principal report on this combined treatment approach is a study by Defidio et al. [33], which included a retrospective multicentric cohort of 178 UTUC cases treated with this laser combination at 10–15 W power with a 270-μm laser fiber (Revolix Duo; Lisa Laser, Katlenburg-Lindau, Germany). Surgery was standardized with urine wash cytology specimens, and multiple biopsies (tumor and base), followed by thulium:YAG laser coagulation of tumor and base were sequentially performed at 10–15 W power. Coagulated necrotic tissue was dislodged with Ho:YAG laser at the same power settings. The study reported no major complications, with only 10% of patients experiencing self-limiting hematuria (Clavien I). At the last follow-up, 69.3% were recurrence free, 21.7% continued conservative treatment for low-risk recurrence, and 8.9% underwent RNU due to progression.
Another retrospective single-series study by Sanguedolce et al. [34] reported the outcomes of the duo laser combination in 47 UTUC patients (61.7% high grade and 38.3% low grade), with a median follow-up of 24 mo. The authors highlighted the limitations of using the two laser sources alone (disruptive effect of Ho:YAG laser and charring effect of thulium:YAG laser), opting for the combined device (Revolix Duo; Lisa Laser) due to its physical advantages. Complications were reported in five patients, including one case of self-limiting hematuria (Clavien I), three cases of urinary tract infections, and one case of major bleeding requiring blood transfusion (Clavien II). One patient developed severe ureteral stenosis 7 mo after treatment, necessitating ureteral end-to-end anastomosis. UTUC recurrence was reported in 13 patients (28.3%), while bladder recurrence occurred in 11 (23.4%). Nine patients (19%) experienced progression and underwent RNU.
Proietti et al. [35] reported a series of 29 patients treated with either Ho:YAG or thulium:YAG laser ablation for UTUC management in “imperative” cases, such as advanced age, multiple comorbidities, solitary kidney, and bilateral UTUC. The median follow-up was 23 mo, and the study reported a recurrence rate of 61.1% (18 patients), including cases of high-grade tumors. The OS was 96.4%, with a mean recurrence-free survival rate of 31.7%. The authors noted a low likelihood of complications, although three Clavien III and one Clavien IV complications occurred during the study period.
3.3.6. Take-home messages and guidelines
The present scoping review infers the following messages about the use of lasers in endoscopic ablation of UTUC:
-
1.
Nd:YAG laser is associated with a disadvantages of a deeper tissue penetration and a significant degree of scatter, resulting in the coagulation of a large tissue volume when used as a free beam. Its use has previously been linked to a high occurrence of ureteric stricture. As a consequence, Nd:YAG laser is nowadays not used for endoscopic UTUC ablation, being replaced by new lasers.
-
2.
Thanks to its high affinity for water, leading to a reduced penetration depth of 0.4–0.7 mm and effective coagulation properties, Ho:YAG laser is an excellent energy source for UTUC endoscopic laser ablation. Our review showed that studies using Ho:YAG laser demonstrated excellent ablative effects with a good safety profile, particularly with a negligible rate of ureteral stricture and acceptable rate of recurrence. Being available in most centers due to its use in stone and prostate surgery, Ho:YAG laser can be suggested as the laser of choice for practicing urologists approaching UTUC endoscopic ablation.
-
3.
Thulium:YAG laser is an attractive energy source for endoscopic UTUC treatment due to its optical penetration depth of just 0.2 mm, which allows for a high energy density and, as a consequence, in smooth incisions and rapid tissue vaporization. Compared with Ho:YAG laser, thulium:YAG laser showed a low rate of high-grade complications and good oncological outcomes in selected cases.
-
4.
Currently, the combination of Ho:YAG and thulium:YAG lasers is probably the best approach for endoscopic UTUC treatment because the effectiveness of thulium:YAG laser ablation can be improved by incorporating Ho:YAG laser to remove the necrotic tissue layer that overlays the deeper regions of the lesion. This results in a clearer view of the tumor base, enabling more precise ablation with thulium:YAG laser, and subsequently, reducing both bleeding and the duration of the procedure.
-
5.
TFL ensure an efficient delivery of high energy to the target tissue with a very thin layer of carbonization and thermal coagulation. These features offer a balance between the excellent hemostasis provided by thulium:YAG laser and the tissue cutting/ablation capabilities of Ho:YAG laser. TFL could be the laser of choice for UTUC ablation, but further studies are needed to confirm its role.
The good safety profile of lasers and oncological outcomes shown in our scoping review should be read in light of the current guidelines [2], which suggest the use of conservative treatment of UTUC in the following cases:
-
1.
Primary treatment as elective treatment in low-risk disease: unifocal disease, tumor size <2 cm, negative for high-grade cytology, low-grade tumor on ureteroscopy biopsy, and no invasive aspect on computed tomography (strong rating)
-
2.
Treatment with imperative indications on a case-by-case basis for high-risk disease such as solitary kidney, bilateral UTUC, chronic kidney disease, or any other comorbidity compromising the use of RNU
-
3.
An early single adjuvant intracavitary upper tract instillation of mitomycin C in patients with low-grade UTUC might reduce the risk of local recurrence
-
4.
Follow-up should be stringent with urine cytology and ureteroscopy, but there is no suggested schedule
4. Conclusions
Our scoping review provides insight into all studies reporting the use of different laser sources in endoscopic laser ablation of UTUC. Studies reporting results with Ho:YAG and thulium:YAG lasers demonstrate that both lasers provide excellent results in terms of safety and oncological outcomes in well-selected UTUC patients with a very low rate of ureteral strictures and bladder recurrence. Prospective comparative studies are required to evaluate the efficiency of endoscopic laser ablation for UTUC.
Author contributions: Carlo Giulioni had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Study concept and design: Giulioni.
Acquisition of data: Giulioni, Castellani, Brocca, Tramanzoli, Pirola.
Analysis and interpretation of data: Giulioni, Castellani.
Drafting of the manuscript: Giulioni, Castellani, Stramucci, Mantovan, Perpepaj, Cicconofri, Gauhar, Pirola, Maggi.
Critical revision of the manuscript for important intellectual content: Galosi.
Statistical analysis: Giulioni.
Obtaining funding: None.
Administrative, technical, or material support: None.
Supervision: Galosi.
Other: None.
Financial disclosures: Carlo Giulioni certifies that all conflicts of interest, including specific financial interests and relationships and affiliations relevant to the subject matter or materials discussed in the manuscript (eg, employment/affiliation, grants or funding, consultancies, honoraria, stock ownership or options, expert testimony, royalties, or patents filed, received, or pending), are the following: None.
Funding/Support and role of the sponsor: None.
Associate Editor: Silvia Proietti
References
- 1.Siegel R.L., Miller K.D., Fuchs H.E., Jemal A. Cancer statistics, 2021 [published correction appears in CA Cancer J Clin. 2021 Jul; 71(4):359] CA Cancer J Clin. 2021;71:7–33. [Google Scholar]
- 2.Rouprêt M., Seisen T., Birtle A.J., et al. European Association of Urology guidelines on upper urinary tract urothelial carcinoma: 2023 update. Eur Urol. 2023;84:49–64. doi: 10.1016/j.eururo.2023.03.013. [DOI] [PubMed] [Google Scholar]
- 3.Ham W.S., Park J.S., Jang W.S., Kim J. Nephron-sparing approaches in upper tract urothelial carcinoma: current and future strategies. Biomedicines. 2022;10:2223. doi: 10.3390/biomedicines10092223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Veccia A., Antonelli A., Checcucci E., et al. Segmental ureterectomy for upper tract urothelial carcinoma: a systematic review and meta-analysis of comparative studies. Clin Genitourin Cancer. 2020;18:e10–e20. doi: 10.1016/j.clgc.2019.10.015. [DOI] [PubMed] [Google Scholar]
- 5.Yakoubi R., Colin P., Seisen T., et al. Radical nephroureterectomy versus endoscopic procedures for the treatment of localised upper tract urothelial carcinoma: a meta-analysis and a systematic review of current evidence from comparative studies. Eur J Surg Oncol. 2014;40:1629–1634. doi: 10.1016/j.ejso.2014.06.007. [DOI] [PubMed] [Google Scholar]
- 6.Audenet F., Traxer O., Yates D.R., Cussenot O., Rouprêt M. Potential role of photodynamic techniques combined with new generation flexible ureterorenoscopes and molecular markers for the management of urothelial carcinoma of the upper urinary tract. BJU Int. 2012;109:608–614. doi: 10.1111/j.1464-410X.2011.10363.x. [DOI] [PubMed] [Google Scholar]
- 7.Grasso M., Fishman A.I., Cohen J., Alexander B. Ureteroscopic and extirpative treatment of upper urinary tract urothelial carcinoma: a 15-year comprehensive review of 160 consecutive patients. BJU Int. 2012;110:1618–1626. doi: 10.1111/j.1464-410X.2012.11066.x. [DOI] [PubMed] [Google Scholar]
- 8.Krambeck A.E., Thompson R.H., Lohse C.M., et al. Endoscopic management of upper tract urothelial carcinoma in patients with a history of bladder urothelial carcinoma. J Urol. 2007;177:1721–1726. doi: 10.1016/j.juro.2007.01.006. [DOI] [PubMed] [Google Scholar]
- 9.Niţă G., Georgescu D., Mulţescu R., et al. Prognostic factors in laser treatment of upper urinary tract urothelial tumours. J Med Life. 2012;5:33–38. [PMC free article] [PubMed] [Google Scholar]
- 10.Martínez-Piñeiro J.A., García Matres M.J., Martínez-Piñeiro L. Endourological treatment of upper tract urothelial carcinomas: analysis of a series of 59 tumors. J Urol. 1996;156(2 Pt 1):377–385. doi: 10.1097/00005392-199608000-00011. [DOI] [PubMed] [Google Scholar]
- 11.Blute M.L., Segura J.W., Patterson D.E., Benson R.C., Jr, Zincke H. Impact of endourology on diagnosis and management of upper urinary tract urothelial cancer. J Urol. 1989;141:1298–1301. doi: 10.1016/s0022-5347(17)41286-9. [DOI] [PubMed] [Google Scholar]
- 12.Elliott D.S., Blute M.L., Patterson D.E., Bergstralh E.J., Segura J.W. Long-term follow-up of endoscopically treated upper urinary tract transitional cell carcinoma. Urology. 1996;47:819–825. doi: 10.1016/S0090-4295(96)00043-X. [DOI] [PubMed] [Google Scholar]
- 13.Jabbour M.E., Desgrandchamps F., Cazin S., Teillac P., Le Duc A., Smith A.D. Percutaneous management of grade II upper urinary tract transitional cell carcinoma: the long-term outcome. J Urol. 2000;163:1105–1107. quiz 1295. [PubMed] [Google Scholar]
- 14.Jimie J., Kwok A., Housami F., Elfar M., Hussein B. Diode laser for the management of upper tract urothelial cancer (UTUC) – case series. J Endolum Endourol. 2022;5:e14–e21. [Google Scholar]
- 15.Matsuoka K., Iida S., Tomiyasu K., Inoue M., Noda S. Transurethral endoscopic treatment of upper urinary tract tumors using a holmium:YAG laser. Lasers Surg Med. 2003;32:336–340. doi: 10.1002/lsm.10184. [DOI] [PubMed] [Google Scholar]
- 16.Rouprêt M., Hupertan V., Traxer O., et al. Comparison of open nephroureterectomy and ureteroscopic and percutaneous management of upper urinary tract transitional cell carcinoma. Urology. 2006;67:1181–1187. doi: 10.1016/j.urology.2005.12.034. [DOI] [PubMed] [Google Scholar]
- 17.Painter D.J., Denton K., Timoney A.G., Keeley F.X. Ureteroscopic management of upper-tract urothelial cancer: an exciting nephron-sparing option or an unacceptable risk? J. Endourol. 2008;22:1237–1239. doi: 10.1089/end.2008.0187. [DOI] [PubMed] [Google Scholar]
- 18.Cornu J.N., Rouprêt M., Carpentier X., et al. Oncologic control obtained after exclusive flexible ureteroscopic management of upper urinary tract urothelial cell carcinoma. World J Urol. 2010;28:151–156. doi: 10.1007/s00345-009-0494-x. [DOI] [PubMed] [Google Scholar]
- 19.Hoffman A., Yossepowitch O., Erlich Y., Holland R., Lifshitz D. Oncologic results of nephron sparing endoscopic approach for upper tract low grade transitional cell carcinoma in comparison to nephroureterectomy—a case control study. BMC Urol. 2014;14:97. doi: 10.1186/1471-2490-14-97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Villa L., Haddad M., Capitanio U., et al. Which patients with upper tract urothelial carcinoma can be safely treated with flexible ureteroscopy with Holmium:YAG laser photoablation? Long-term results from a high volume institution. J Urol. 2018;199:66–73. doi: 10.1016/j.juro.2017.07.088. [DOI] [PubMed] [Google Scholar]
- 21.Musi G., Mistretta F.A., Marenghi C., et al. Thulium laser treatment of upper urinary tract carcinoma: a multi-institutional analysis of surgical and oncological outcomes. J Endourol. 2018;32:257–263. doi: 10.1089/end.2017.0915. [DOI] [PubMed] [Google Scholar]
- 22.Wen J., Ji Z.G., Li H.Z. Treatment of upper tract urothelial carcinoma with ureteroscopy and thulium laser: a retrospective single center study. BMC Cancer. 2018;18:196. doi: 10.1186/s12885-018-4118-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bozzini G., Gastaldi C., Besana U., et al. Thulium-laser retrograde intra renal ablation of upper urinary tract transitional cell carcinoma: an ESUT study. Minerva Urol Nephrol. 2021;73:114–121. doi: 10.23736/S2724-6051.20.03689-9. [DOI] [PubMed] [Google Scholar]
- 24.Proietti S., Johnston T., Pupulin M., et al. Effectiveness and safety of thulium fiber laser in the conservative management of patients with upper tract urothelial carcinoma. Eur Urol Open Sci. 2022;46:99–104. doi: 10.1016/j.euros.2022.10.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Johnson G.B., Fraiman M., Grasso M. Broadening experience with the retrograde endoscopic management of upper urinary tract urothelial malignancies. BJU Int. 2005;95(Suppl 2):110–113. doi: 10.1111/j.1464-410x.2005.05210.x. [DOI] [PubMed] [Google Scholar]
- 26.Sowter S.J., Ilie C.P., Efthimiou I., Tolley D.A. Endourologic management of patients with upper-tract transitional-cell carcinoma: long-term follow-up in a single center. J Endourol. 2007;21:1005–1009. doi: 10.1089/end.2006.9922. [DOI] [PubMed] [Google Scholar]
- 27.Suh R.S., Faerber G.J., Wolf J.S., Jr. Predictive factors for applicability and success with endoscopic treatment of upper tract urothelial carcinoma. J Urol. 2003;170(6 Pt 1):2209–2216. doi: 10.1097/01.ju.0000097327.20188.c1. [DOI] [PubMed] [Google Scholar]
- 28.Chen G.L., Bagley D.H. Ureteroscopic management of upper tract transitional cell carcinoma in patients with normal contralateral kidneys. J Urol. 2000;164:1173–1176. [PubMed] [Google Scholar]
- 29.Boorjian S., Ng C., Munver R., et al. Abnormal selective cytology results predict recurrence of upper-tract transitional-cell carcinoma treated with ureteroscopic laser ablation. J Endourol. 2004;18:912–916. doi: 10.1089/end.2004.18.912. [DOI] [PubMed] [Google Scholar]
- 30.Scotland K.B., Hubbard L., Cason D., et al. Long term outcomes of ureteroscopic management of upper tract urothelial carcinoma. Urol Oncol. 2020;38:850.e17–850.e26. doi: 10.1016/j.urolonc.2020.06.027. [DOI] [PubMed] [Google Scholar]
- 31.Shvero A., Zilberman D.E., Dotan Z.A., et al. Endoscopic management of upper tract urothelial carcinoma-tips and tricks. Transl Androl Urol. 2020;9:1815–1820. doi: 10.21037/tau.2020.01.07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Shen C.Y., Jou Y.C., Kan W.C., Tzai T.S., Tsai Y.S. Outcome of non-muscle invasive upper tract urothelial carcinoma receiving endoscopic ablation: an inverse probability of treatment weighting analysis. J Clin Med. 2022;11:1307. doi: 10.3390/jcm11051307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Defidio L., Antonucci M., De Dominicis M., Fuchs G., Patel A. Thulium-Holmium:YAG duo laser in conservative upper tract urothelial cancer treatment: 13 years’ experience from a tertiary national referral center. J Endourol. 2019;33:902–908. doi: 10.1089/end.2019.0308. [DOI] [PubMed] [Google Scholar]
- 34.Sanguedolce F., Fontana M., Turco M., et al. Endoscopic management of upper urinary tract urothelial carcinoma: oncologic outcomes and prognostic factors in a contemporary cohort. J Endourol. 2021;35:1593–1600. doi: 10.1089/end.2021.0133. [DOI] [PubMed] [Google Scholar]
- 35.Proietti S., Marchioni M., Eisner B.H., et al. Conservative treatment of upper urinary tract carcinoma in patients with imperative indications. Minerva Urol Nephrol. 2021;73:245–252. doi: 10.23736/S2724-6051.20.03710-8. [DOI] [PubMed] [Google Scholar]
- 36.Garden J.M., Geronemus R.G. Dermatologic laser surgery. J Dermatol Surg Oncol. 1990;16:156–168. doi: 10.1111/j.1524-4725.1990.tb00035.x. [DOI] [PubMed] [Google Scholar]
- 37.Schmeller N.T., Hofstetter A.G. Laser treatment of ureteral tumors. J Urol. 1989;141:840–843. doi: 10.1016/s0022-5347(17)41027-5. [DOI] [PubMed] [Google Scholar]
- 38.Azma E., Safavi N. Diode laser application in soft tissue oral surgery. J Lasers Med Sci. 2013;4:206–211. [PMC free article] [PubMed] [Google Scholar]
- 39.Taratkin M., Kovalenko A., Laukhtina E., et al. Ex vivo study of Ho: YAG and thulium fiber lasers for soft tissue surgery: which laser for which case? Lasers Med Sci. 2020;37:149–154. doi: 10.1007/s10103-020-03189-7. [DOI] [PubMed] [Google Scholar]
- 40.Castellani D., Pirola G.M., Pacchetti A., Saredi G., Dellabella M. State of the art of thulium laser enucleation and vapoenucleation of the prostate: a systematic review. Urology. 2020;136:19–34. doi: 10.1016/j.urology.2019.10.022. [DOI] [PubMed] [Google Scholar]
- 41.Kronenberg P., Traxer O. The laser of the future: reality and expectations about the new thulium fiber laser—a systematic review. Transl Androl Urol. 2019;8(Suppl 4):S398–S417. doi: 10.21037/tau.2019.08.01. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Fried N.M., Murray K.E. High-power thulium fiber laser ablation of urinary tissues at 1.94 micron. J Endourol. 2005;19:25–31. doi: 10.1089/end.2005.19.25. [DOI] [PubMed] [Google Scholar]