Abstract
Background/purpose
Clinically, dentists are suggested to immerse autopolymerizing interim fixed restorations in hot water during fabrication. However, this suggestion, without including the best temperature, mostly comes from clinical experience instead of scientific evidence. This in vitro study evaluated the effect of water temperature on the cytotoxicity of interim partial fixed dental prostheses (FDPs) and examined its correlation with residual MMA.
Materials and methods
Tempron was chosen as the autopolymerizing polymethyl methacrylate material. Tempron was mixed and then soaked in water at different temperatures, except control group (Controlair) was not being soaking in water. The specimens were incubated with conditioned medium. The concentration of residual MMA was determined by liquid chromatography-tandem mass spectrometry (LC-MS-MS). The cell viability of human gingival fibroblasts (HGFs) was evaluated by MTT assay.
Results
The 60 °C and 80 °C groups exhibited significantly higher cell viabilities than those of the other groups (P < 0.05) at 48 and 72 h. The concentration of residual MMA was highly correlated with this outcome: the higher the concentration of residual MMA detected in the eluates, the poorer the cell viability was; the longer the incubation time was, the stronger the correlation was between the concentration of residual MMA and the cell viability.
Conclusion
Autopolymerizing PMMA interim FDPs that are polymerized in water up to at least 60 °C could reduce cell toxicity. Higher water temperature could certainly decrease the amount of residual MMA, which is closely correlated with the outcome of cell viability.
Keywords: Provisional fixed restorations, Temporary fixed restorations, Residual monomers, Water immersion, Biocompatibility
Introduction
Interim fixed dental prostheses (FDPs) play several important roles in prosthodontic treatment.1 The most commonly used material in interim FDPs has been autopolymerizing polymethyl methacrylate (PMMA) since 1940.2,3 Autopolymerizing PMMA resins are relatively cheap and can be easily fabricated or modified.4, 5, 6, 7 However, there are still many disadvantages when using autopolymerizing PMMA resins to make interim FDPs, such as polymerization shrinkage, high polymerization exotherm during setting, poor physical properties and biological harms.1,2,5, 6, 7, 8, 9, 10 Nevertheless, one must emphasize that autopolymerizing acrylic resin has a higher level of residual monomer, which may cause many biocompatible problems.1,5,11, 12, 13, 14, 15 However, research over the past few years has mostly focused on the material properties of interim FDPs for physical and clinical applications but has not mentioned their cytotoxic effects.4
The cytotoxic effects of acrylic resin materials are mostly believed to be caused by resin monomers in current investigations.15,16 Methyl methacrylate (MMA) is the resin monomer of autopolymerizing PMMA materials. MMA may cause local irritation of tissues, inflammation or allergic reaction to both patients and operators.12, 13, 14, 15, 16, 17, 18, 19 According to the manufacturers' recommendation, dentists are suggested to immerse autopolymerizing interim FDPs in hot water during fabrication clinically, which can reduce the amount of cytotoxic residual MMA and improve the conversion rate during polymerization.1,3,18 However, this suggestion, without including the best temperature for autopolymerizing interim FDPs, mostly comes from clinical experience instead of scientific evidence.5
The purpose of this in vitro study was to evaluate the effect of water temperature on the cytotoxicity of interim FDPs and to examine its correlation with residual MMA. The null hypotheses were that interim FDPs immersed in different water temperatures during fabrication would not influence the cytotoxicity and the concentration of residual MMA.
Materials and methods
This in vitro study stimulated the direct technique to fabricate interim FDPs clinically by using autopolymerizing PMMA resin to explore whether immersion in different water temperatures before complete polymerization would influence the cytotoxic effect and the amount of residual MMA.
Specimen preparation
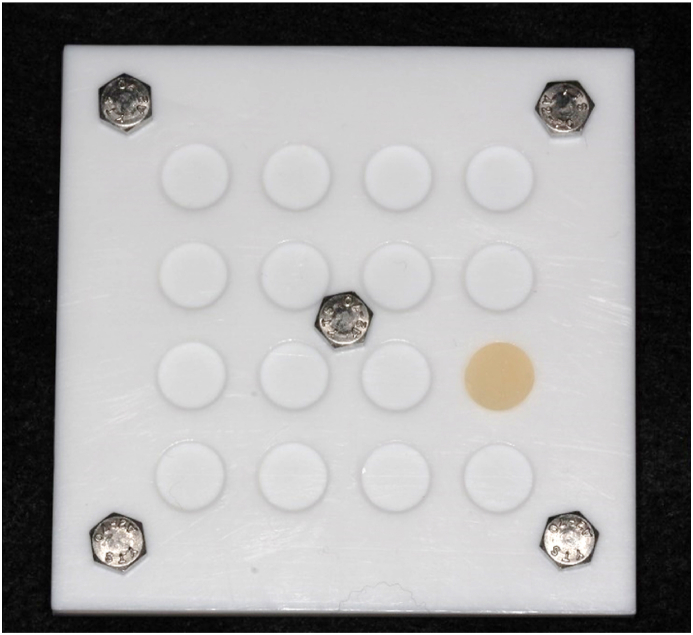
Tempron (GC Corp., Tokyo, Japan) was chosen as the autopolymerizing PMMA material since it was the most common brand used in Taiwan. Specimens were made according to the powder-to-liquid ratio recommended by the manufacturer20: the first graduation of the liquid pipette of Tempron liquid was taken into the mixing jar; Tempron powder was slowly added up to the first graduation on the powder measure and mixed for 20–30 s at room temperature (approximately 24 °C). Then, pour the mixed creamy paste into the Teflon mold for 3 min to form Φ = 10 mm and h = 1 mm for each specimen (Fig. 1). Next, each specimen was carefully removed before it was fully set. The total operating time was within 5 min.
Figure 1.
Specimens were made in the Teflon mold to form Φ = 10 mm and h = 1 mm in shape.
In our experiment, all specimens were placed in water at different temperatures except for Controlair. In the Controlair group, specimens were set at room temperature without immersion in water. The water temperatures we used in this research were room temperature, 4 °C, 30 °C, 40 °C, 50 °C, 60 °C and 80 °C. Sterile conical centrifuge tubes containing 10 mL sterile double-distilled water were placed in a water bath to reach each experimental temperature except for the 4 °C group, which was prepared in the refrigerator before making the specimens. Once the specimens reached the initial polymerizing stage (approximately 5 min as mentioned above), they were put into sterile conical centrifuge tubes for another 5 min to be fully set in each experimental group. In addition, in the Controlair group, after 5 min of making the specimens, the specimens were set in empty sterile centrifuge tubes at room temperature for another 5 min to be fully set similarly.
Sample eluate preparation
For eluate collection, specimens set under the same conditions were taken out and transferred into a new sterile centrifuge tube containing 5 mL Dulbecco's modified Eagle medium (DMEM) (Sigma-Aldrich, St. Louis, MO, USA) culture medium with 1% penicillin-streptomycin. The ratio of the surface area of the specimens to the volume of culture medium was 3.01 cm2/mL, conforming to International Standards Organization (ISO) 10,993–12.21 The eluates were conducted for 4 days at 37 °C under an atmosphere of 5% CO2 and 95% air. After 4 days, the eluates were filtered through a sterile syringe filter (0.20 μm), and 10% fetal bovine serum (FBS) (Gibco, New York, NY, USA) was added immediately before quantifying the amount of residual MMA and cytotoxicity testing.
Cytotoxic assay
Cytotoxicity tests were performed by using the 3-(4,5-dimethyl-2-thiazolyl)-2,5-diphenyl-2H-tetrazorium bromide (MTT) (Sigma-Aldrich) assay according to ISO 10993–5.22 Human gingival fibroblasts (HGFs) purchased from Lifeline Cell Technology (Frederick, MD, USA) were plated at 1 × 103 cells/well in DMEM with 1% penicillin-streptomycin and 10% fetal bovine serum (FBS) in a 96-well dish and incubated at 37 °C in 5% CO2 and 100% relative humidity. After 24 h of incubation, the culture medium was removed from each well, and 100 μL of 60% diluted eluate was added for another 24 h, 48 h and 72 h since the cytotoxic result showed no difference between groups by adding 100% eluate (data not shown). Culture medium with 10% FBS was used as the negative control (100% cell viability), whereas 0.02% sodium dodecyl sulfate (SDS) was used as the positive control. To measure cell viability (n = 4) after the treatment, cells in each well were incubated with 10 μL of MTT dissolved in phosphate buffered saline (PBS) solution at a concentration of 5 mg/mL at 37 °C. After incubation for 2 h, the medium was removed, and 100 μL dimethyl sulfoxide (DMSO) was added to each well to dissolve the intracellularly stored MTT formazan. The absorbance at 595 nm was spectrophotometrically measured.
Chromatography and mass spectrometry conditions
Liquid chromatography-tandem mass spectrometry (LC-MS-MS) was used to determine the amount of residual MMA in eluates. A stock solution of MMA (Merck KGaA, Darmstadt, Germany) was serially diluted with methanol to obtain calibration standard stock solutions of gradient concentrations. For all detection and quantification of analytes, a Waters ACQUITY UPLC system (Waters Corporation, Milford, MA, USA) coupled with Finnigan TSQ Quantum Ultra triple-quadrupole MS (Thermo Electron, San Jose, CA, USA) in combination with Xcalibur software (Thermo-Finnigan, Bellefonte, PA, USA) was used. The LC–MS–MS system was equipped with an electrospray ion source (ESI) and was run in positive mode. The injection volume was 10 μL on an ACQUITY UPLC BEH C18 Column (130 Å, 1.7 μm, 2.1 mm × 50 mm, Waters Corporation, Milford, MA, USA) equipped with a filter (Waters Acquity UPLC™ BEH C18 column, 1.7 μm, 2.1 mm × 5 mm) in front of the column.
The flow rate was 200 μL/min, and the column temperature was 40 °C. The solvents were A: 0.1% acetic acid in water and B: 0.1% acetic acid in acetonitrile. Solvent programming was 0.0–0.2 min, 30% B; 2.5 min, 80% B; 3.0–5.0 min, 30% B. MS–MS interphase settings were as follows: spray voltage, 4000 V; sheath gas (N2) pressure, 28 psi; auxiliary gas (N2) pressure, 10psi; capillary temperature, 350 °C; collision gas (Ar) pressure, 1.0 mTorr. Information about the precursor/product ions in positive mode, retention time of the analytes separated on the LC column, individual collision energies, and individual tube lenses are listed in Table 1.
Table 1.
Information about the precursor/product ions in positive mode, retention time of the analytes separated on the LC column, individual collision energies, and individual tube lenses when testing the amount of residual MMA monomers.
| Analyte | Precursor/product ions (m/z) | Retention time (min) | Collision energy (V) | Tube lens (V) |
|---|---|---|---|---|
| MMA | 101/73 | 1.83 | 23 | 42 |
Note: MMA, methyl methacrylate; m/z, mass-to-charge ratio; V, volt.
Statistical analysis
Data were statistically analyzed using IBM SPSS Statistics v20 (SPSS Inc., Chicago, IL, USA). One-way ANOVA followed by Bonferroni's post hoc comparisons tests or Dunnett's T3 was used to evaluate the values of cell viabilities between different groups (α = 0.05). The correlation between the amount of residual MMA in eluates and the values of cell viabilities were analyzed with Spearman's rank correlation coefficient test at a significance level of P ≤ 0.05.
Results
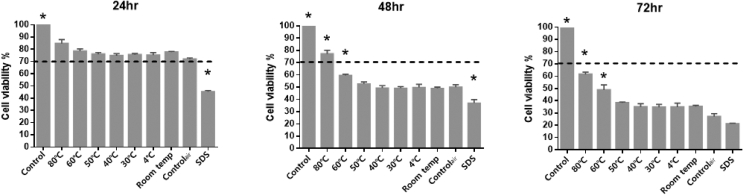
Cytotoxic effect of the eluates, which were extracted from Tempron specimens polymerized at different water temperatures, on HGFs was shown in Fig. 2 by MTT assay at 24 h, 48 h and 72 h. According to ISO 10993-5 guidelines for biological evaluation of biomedical devices, the cell viability rate lower than 70% is considered to be cytotoxic. After culturing for 24 h, no cytotoxic effects were found in any of the tested groups, and the 80 °C group showed the highest cell viability rate of 84.7%. However, there was no significant difference between all tested groups (P > 0.05). After culturing for 48 h, the cell viability rates were lower than 70% in all tested groups except the 80 °C group, which showed a 77.3% cell viability rate. Moreover, the 80 °C and 60 °C groups showed significantly higher cell viability rates than the other groups (P < 0.05). After culturing for 72 h, although the cell viability rates were lower than 70% in all tested groups, the 80 °C and 60 °C groups still showed higher cell viability rates and revealed statistically significant differences among the groups (P < 0.05). In addition, strong cytotoxicity was found in specimens set at water temperatures below 50 °C, and no significant difference was found among the groups (P > 0.05). In summary, the 60 °C and 80 °C groups showed better cell viabilities, and the 80 °C group exhibited the best cell viability at each time point.
Figure 2.
The cell viability of HGF by MTT assay at 24, 48, and 72 h. The dotted line indicates 70% cell viability (cytotoxic indicator line according to ISO 10993–5). Control group: culture medium; 4 °C, 30 °C, 40 °C, 50 °C, 60 °C, 80 °C and Room temp groups: cultured with eluate from specimens were set at indicated temperature in water; Controlair group: cultured with eluate from specimens were set at room temperature without immersion in water; SDS group: culture medium with 0.02% sodium dodecyl sulfate. ∗ Significantly different from all the other groups (P < 0.05) as determined by one-way ANOVA.
The concentration of residual MMA in each tested eluate was detected by LC-MS-MS and is presented in Table 2. The Controlair group in which the specimen was set at room temperature without immersion in water showed the highest concentration of residual MMA (108 ppm). Meanwhile, the concentration of residual MMA in the group in which the specimen was set in water at room temperature was approximately half that of the Controlair group (51 ppm). The concentration of residual MMA in the 4 °C group was 66.4 ppm, which was the highest among all water-immersing groups. The concentration of residual MMA in the 60 °C group was 26.7 ppm, which was approximately half of the concentration of the 30 °C group (53.1 ppm). In addition, the lowest concentration of residual MMA was found in the 80 °C group (8.6 ppm). According to these findings, we can conclude that the water immersion treatment can surely decrease the amount of residual MMA. Nevertheless, in all the groups in which specimens were placed in water, we observed that the lower the water temperature was, the higher the concentration of residual MMA would be.
Table 2.
The concentration of residual methyl methacrylate (MMA) in all the eluate conditions according to liquid chromatography-tandem mass spectrometry (LC-MS-MS).
| Eluate conditions | Controlair | Room temp | 4 °C | 30 °C | 40 °C | 50 °C | 60 °C | 80 °C |
|---|---|---|---|---|---|---|---|---|
| The concentration of residual MMA (ppm) | 108.0 | 51.0 | 66.4 | 53.1 | 49.2 | 38.1 | 26.7 | 8.6 |
Note: MMA, methyl methacrylate. Controlair group: eluate from specimens were set at room temperature without immersion in water; 4 °C, 30 °C, 40 °C, 50 °C, 60 °C, 80 °C and Room temp groups: eluate from specimens were set at indicated temperature in water.
The correlation between the concentration of residual MMA and the cell viability at different water temperatures was shown in Table 3. After culturing for 24 and 48 h, significant negative correlations were found between the concentration of residual MMA and cell viability, with Spearman's rho values of −0.714 and −0.75, respectively. After culturing for 72 h, highly significant negative correlations were found between the concentration of residual MMA and cell viability, as Spearman's rho reached −0.929 (P < 0.01). In other words, the data indicated that the higher the concentration of residual MMA detected in the eluates, the poorer the cell viability was presented; the longer the incubation time was, the stronger the correlation we could find between the concentration of residual MMA and the cell viability.
Table 3.
Correlation between the concentration of residual MMA and the cell viability (%) at different water temperatures.
| Spearman's rank correlation coefficient |
|||
|---|---|---|---|
| 24 h Cell viability | 48 h Cell viability | 72 h Cell viability | |
| MMA monomer (ppm) | −0.714✝ | −0.750✝ | −0.929✱✱ |
Note: MMA, methyl methacrylate.
✝P value < 0.1.
✱P value < 0.05.
✱✱P value < 0.01.
Discussion
In this study, as the cell viability rates varied in different temperature groups in the cytotoxicity test and the concentration of residual MMA differed in each tested eluate detected by LC-MS-MS, the null hypothesis was rejected.
The effect of water temperature on the cytotoxicity of interim FDPs has never been mentioned before. Few studies have discussed the effect of polymerization temperature on the physicomechanical properties or marginal accuracy of interim FDPs. Ogawa et al.23 demonstrated that polymerizing interim FDPs at higher water temperatures could greatly enhance their mechanical properties. The 60 °C and 80 °C groups produced two times greater strength than the Controlair group.23 In addition, Chhabra et al. found the highest transverse strength in the 60 °C group.24 A recent study also claimed that if interim FDPs require additional strength, they should not be placed in cold water during polymerization.5 However, it is worth mentioning that when speaking of marginal accuracy, most studies25, 26, 27 have preferred a water temperature of approximately 20–30 °C. To date, our study is the first to discuss the biocompatibility of interim FDPs polymerized at different water temperatures. In our study, we found that the 60 °C and 80 °C groups showed better cell viabilities, and the 80 °C group revealed the best cell viability at each time point. As we know from the above, the interim FDPs polymerized in 60 °C and 80 °C water could have better strength and lower toxicity but poorer marginal fit. Thus, this result is also consistent with previous studies showing that the material of interim FDPs is currently never perfect.1,9
In this study, we found that a higher water temperature could certainly decrease the amount of residual MMA, which is closely correlated with the outcome of cell viability. The levels of residual MMA were strongly affected by the degree of conversion. Heat was certainly a good way to improve the conversion rate.1,7,11,13,28, 29, 30 However, it was recommended not to heat near 100 °C because monomers may be vaporized, with subsequent formation of interim FDPs porosity.1 Due to this, the highest temperature we used in this study was 80 °C. In addition, immersion in water diminished the negative effect of oxygen and removed residual monomers after polymerization.5,11,28 Similarly, in this study, the concentration of residual MMA in the group in which the specimen was placed in water at room temperature was approximately half that of the Controlair group (51 ppm). According to this finding, we can conclude that the water immersion treatment can surely decrease the amount of residual MMA. Currently, the most recent studies believe that residual monomers are the primary issue in the biocompatibility problem of acrylic resin.11,12,15, 16, 17, 18 In this study, we observed that the higher the concentration of the residual MMA was, the lower the cell viability was presented. Thus, we could say that the relation of residual MMA and cell toxicity was strong. Decreasing residual MMA could benefit the safety of interim FDPs.
A limitation of the current study is that the in vitro study may not perfectly reflect the real metabolism in humans. Previous studies once mentioned that MMA could be rapidly hydrolyzed by enzymes in blood serum.13,18 On the other hand, another study reported that residual monomers could be detected for up to several years after use.12,29 In our studies, we could not completely know if the cytotoxicity lasted for the entire time that the toxicity continued to come from the residual MMA after using interim FDPs for several months. Nevertheless, we only used one kind of autopolymerizing acrylic resin. Studies have shown that MMA may affect the lipid bilayer in biological membranes.18 Therefore, different monomers may have diverse cytotoxic effects due to the different lipophilicities.16 Additionally, we should still keep in mind potentially cytotoxic factors other than incomplete monomer conversion or leaching residual components. Further research is needed to investigate the above ambiguities.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgments
The authors gratefully acknowledge the support of research grant from the Ministry of Science and Technology of Taiwan (MOST 111-2314-B-037-044-MY2).
Contributor Information
Je-Kang Du, Email: dujekang@gmail.com.
Yan-Hsiung Wang, Email: yhwang@kmu.edu.tw.
References
- 1.Rosenstiel S.F., Land M.F., Fujimoto J. fifth ed. Mosby Elserver; 2015. Contemporary Fixed Prosthodontics; pp. 401–423. [Google Scholar]
- 2.Burns D.R., Beck D.A., Nelson S.K. A review of selected dental literature on contemporary provisional fixed prosthodontic treatment: report of the committee on research in fixed prosthodontics of the academy of fixed prosthodontics. J Prosthet Dent. 2003;90:474–497. doi: 10.1016/s0022-3913(03)00259-2. [DOI] [PubMed] [Google Scholar]
- 3.Ranganath L.M., Shet R.G., Rajesh A.G., Abraham S. The effect of fiber reinforcement on the dimensional changes of poly methyl methacrylate resin after processing and after immersion in water: an in vitro study. J Contemp Dent Pract. 2011;12:305–317. doi: 10.5005/jp-journals-10024-1051. [DOI] [PubMed] [Google Scholar]
- 4.Ye R.R., Shen Q.P., Qiao G.Y. Cytotoxicity of three kinds of temporary crown and bridge materials on mouse fibroblasts in vitro. Shang Hai Kou Qiang Yi Xue. 2008;17:308–312. [PubMed] [Google Scholar]
- 5.Morita K., Tsuka H., Kato K., Tsuga K. Effect of polymerization temperature on the properties of autopolymerizing resin. J Prosthet Dent. 2017;119:840–844. doi: 10.1016/j.prosdent.2017.07.022. [DOI] [PubMed] [Google Scholar]
- 6.Campaner M., Takamiya A.S., Bitencourt S.B., et al. Cytotoxicity and inflammatory response of different types of provisional restorative materials. Arch Oral Biol. 2020;111 doi: 10.1016/j.archoralbio.2019.104643. [DOI] [PubMed] [Google Scholar]
- 7.Raszewski Z. Influence of polymerization method on the cytotoxicity of three different denture base acrylic resins polymerized in different methods. Saudi J Biol Sci. 2020;27:2612–2616. doi: 10.1016/j.sjbs.2020.05.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Strassler H.E. Fixed prosthodontics provisional materials: making the right selection. Comp Cont Educ Dent. 2013;34:22–24. [PubMed] [Google Scholar]
- 9.Strassler H.E., Lowe R.A. Chairside resin-based provisional restorative materials for fixed prosthodontics. Comp Cont Educ Dent. 2011;32:10–12. 14 passim. [PubMed] [Google Scholar]
- 10.Astudillo-Rubio D., Delgado-Gaete A., Bellot-Arcis C., Montiel-Company J.M., Pascual-Moscardo A., Almerich-Silla J.M. Mechanical properties of provisional dental materials: a systematic review and meta-analysis. PLoS One. 2018;13 doi: 10.1371/journal.pone.0193162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lee S.Y., Lai Y.L., Hsu T.S. Influence of polymerization conditions on monomer elution and microhardness of autopolymerized polymethyl methacrylate resin. Eur J Oral Sci. 2002;110:179–183. doi: 10.1034/j.1600-0722.2002.11232.x. [DOI] [PubMed] [Google Scholar]
- 12.Kedjarune U., Charoenworaluk N., Koontongkaew S. Release of methyl methacrylate from heat-cured and autopolymerized resins: cytotoxicity testing related to residual monomer. Aust Dent J. 1999;44:25–30. doi: 10.1111/j.1834-7819.1999.tb00532.x. [DOI] [PubMed] [Google Scholar]
- 13.Gautam R., Singh R.D., Sharma V.P., Siddhartha R., Chand P., Kumar R. Biocompatibility of polymethylmethacrylate resins used in dentistry. J Biomed Mater Res B Appl Biomater. 2012;100:1444–1450. doi: 10.1002/jbm.b.32673. [DOI] [PubMed] [Google Scholar]
- 14.Fletcher A.M., Purnaveja S., Amin W.M., Ritchie G.M., Moradians S., Dodd A.W. The level of residual monomer in self-curing denture-base materials. J Dent Res. 1983;62:118–120. doi: 10.1177/00220345830620020601. [DOI] [PubMed] [Google Scholar]
- 15.Keul C., Seidl J., Guth J.F., Liebermann A. Impact of fabrication procedures on residual monomer elution of conventional polymethyl methacrylate (PMMA)-a measurement approach by UV/Vis spectrophotometry. Clin Oral Invest. 2020;24:4519–4530. doi: 10.1007/s00784-020-03317-1. [DOI] [PubMed] [Google Scholar]
- 16.Lee J.H., Jun S.K., Moon H.J., Lee H.H. Cytotoxicity and proinflammatory cytokine expression induced by interim resin materials in primary cultured human dental pulp cells. J Prosthet Dent. 2017;118:524–534. doi: 10.1016/j.prosdent.2016.11.014. [DOI] [PubMed] [Google Scholar]
- 17.Issa Y., Watts D.C., Brunton P.A., et al. Resin composite monomers alter MTT and LDH activity of human gingival fibroblasts in vitro. Dent Mater. 2004;20:12–20. doi: 10.1016/s0109-5641(03)00053-8. [DOI] [PubMed] [Google Scholar]
- 18.Leggat P.A., Kedjarune U. Toxicity of methyl methacrylate in dentistry. Int Dent J. 2003;53:126–131. doi: 10.1111/j.1875-595x.2003.tb00736.x. [DOI] [PubMed] [Google Scholar]
- 19.Frazer R.Q., Byron R.T., Osborne P.B., et al. PMMA: an essential material in medicine and dentistry. J Long Term Eff Med Implants. 2005;15:629–639. doi: 10.1615/jlongtermeffmedimplants.v15.i6.60. [DOI] [PubMed] [Google Scholar]
- 20.The instruction of Tempron: https://europe.gc.dental/sites/europe.gc.dental/files/products/downloads/tempron/ifu/IFU_Tempron_W.pdf.
- 21.ISO . International Organization for Standardization; Basel, Switzerland: 2012. ISO 10993-12 Biological Evaluation of Medical Devices-Part 12: Sample Preparation and Reference Materials. [Google Scholar]
- 22.ISO . International Organization for Standardization; Basel, Switzerland: 2009. ISO 10993-5 Biological Evaluation of Medical Devices-Part 5: Tests for in Vitro Cytotoxicity. [Google Scholar]
- 23.Ogawa T., Tanaka M., Koyano K. Effect of water temperature during polymerization on strength of autopolymerizing resin. J Prosthet Dent. 2000;84:222–224. doi: 10.1067/mpr.2000.108574. [DOI] [PubMed] [Google Scholar]
- 24.Chhabra A., Rudraprasad I.V., Nandeeshwar D.B., et al. A comparative study to determine strength of autopolymerizing acrylic resin and autopolymerizing composite resin influenced by temperature during polymerization: an in Vitro study. Indian J Dent Res. 2017;28:442–449. doi: 10.4103/ijdr.IJDR_564_10. [DOI] [PubMed] [Google Scholar]
- 25.Ogawa T., Aizawa S., Tanaka M., et al. Effect of water temperature on the fit of provisional crown margins during polymerization. J Prosthet Dent. 1999;82:658–661. doi: 10.1016/s0022-3913(99)70006-5. [DOI] [PubMed] [Google Scholar]
- 26.Dhillon N., Kumar M., D'Souza D. Effect of water temperature and duration of immersion on the marginal accuracy of provisional crowns. Med J Armed Forces India. 2011;67:237–240. doi: 10.1016/S0377-1237(11)60049-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ramkumar V., Sangeetha A., Kumar V. Effect of water temperature on the fit of provisional crown margins during polymerization: an in vitro study. J Pharm BioAllied Sci. 2012;4:S376–S383. doi: 10.4103/0975-7406.100288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Tsuchiya H., Hoshino Y., Tajima K., et al. Leaching and cytotoxicity of formaldehyde and methyl methacrylate from acrylic resin denture base materials. J. Prosthet Dent. 1994;71:618–624. doi: 10.1016/0022-3913(94)90448-0. [DOI] [PubMed] [Google Scholar]
- 29.Bural C., Aktas E., Deniz G., et al. Effect of post-polymerization heat-treatments on degree of conversion, leaching residual MMA and in vitro cytotoxicity of autopolymerizing acrylic repair resin. Dent Mater. 2011;27:1135–1143. doi: 10.1016/j.dental.2011.08.007. [DOI] [PubMed] [Google Scholar]
- 30.Campanha N.H., Pavarina A.C., Giampaolo E.T., et al. Cytotoxicity of hard chairside reline resins: effect of microwave irradiation and water bath postpolymerization treatments. Int J Prosthodont (IJP) 2006;19:195–201. [PubMed] [Google Scholar]