Abstract
Background
Platelet concentrate (PC) transfusions are crucial in prevention and treatment of bleeding in infection, surgery, leukemia, and thrombocytopenia patients. Although the technology for platelet preparation and storage has evolved over the decades, there are still challenges in the demand for platelets in blood banks because the platelet shelf life is limited to 5 days due to bacterial contamination and platelet storage lesions (PSLs) at 20–24°C under constant horizontal agitation. In addition, the relations between some adverse effects of platelet transfusions and PSLs have also been considered. Therefore, understanding the mechanisms of PSLs is conducive to obtaining high quality platelets and facilitating safe and effective platelet transfusions.
Objective
This review summarizes developments in mechanistic research of PSLs and their relationship with clinical practice, providing insights for future research.
Methods
Authors conducted a search on PubMed and Web of Science using the professional terms “PSL” and “platelet transfusion.” The obtained literature was then roughly categorized based on their research content. Similar studies were grouped into the same sections, and further searches were conducted based on the keywords of each section.
Results
Different studies have explored PSLs from various perspectives, including changes in platelet morphology, surface molecules, biological response modifiers (BMRs), metabolism, and proteins and RNA, in an attempt to monitor PSLs and identify intervention targets that could alleviate PSLs. Moreover, novel platelet storage conditions, including platelet additive solutions (PAS) and reconsidered cold storage methods, are explored. There are two approaches to obtaining high‐quality platelets. One approach simulates the in vivo environment to maintain platelet activity, while the other keeps platelets at a low activity level in vitro under low temperatures.
Conclusion
Understanding PSLs helps us identify good intervention targets and assess the therapeutic effects of different PSLs stages for different patients.
Keywords: biological response modifiers, cold storage, platelet additive solutions, platelet storage lesions, platelet transfusion
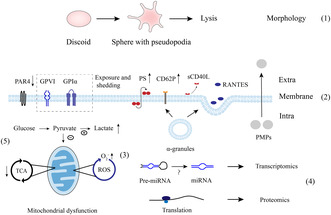
Some major biological response modifiers (BMRs) and the potential mechanisms of platelet storage lesions (PSLs). (1) During storage, platelet morphology transforms from discoid to sphere with pseudopodia and lysis at extended storage. (2) Changes in molecules on the surface are reduced G‐protein‐coupled protease‐activated receptors (PAR4), exposure and shedding of CD42b (GPI bα) and GPVI, and inverse phosphatidylserine (PS), which are different from classical platelet activation. In parallel, α‐granules release CD62P to the membrane and soluble CD40 ligand (sCD40L) and regulated on normal activation T cells expressed and secreted (RANTES) to the extracellular space. Platelet microparticles (PMPs) are also produced by platelets. (3) These BMRs including GPI bα, GPVI, PS and PMPs may be associated with reactive oxygen species (ROS) as demonstrated by elevated O2‐ level. microRNAs (miRNAs) that may be derived from precursor miRNAs (pre‐miRNAs) and proteins that are synthesized in the existing process of translation (4) can be detected. (5) The proportion of glucose that can access the tricarboxylic acid cycle (TCA) decreases contributes to the accumulated lactate due to mitochondrial dysfunction.
1. INTRODUCTION
In the 1950s, platelet transfusions were found to save the lives of leukemia patients by reducing bleeding. 1 In the 1970s, platelets preserved at 22°C under constant agitation and oxygen exchange were found to have excellent functions. 2 To date, the preparation and storage of platelets have been developed for more than 60 years, and the procedures from platelet storage to transfusion have become relatively mature. Critical variables of platelet storage in the blood bank are temperature (20–24°C), oxygen and continuous agitation. However, the platelet shelf life changed from 3–5 days to 7 days and was finally limited to 5 days because of bacterial contamination and impaired platelet function during storage. 3 Long‐term platelet storage is closely followed by unsatisfactory platelet recovery and survival after transfusion. Although some social interventions have been tried, 4 discarded platelets still exist due to their short shelf life. This will not only lead to a waste of social resources but also a window period of inadequate platelet supply in hospitals. Another problem is the shortage of PCs in some countries due to a lack of blood donors. The production of platelets in vitro may be another viable option. However, even if we are able to replace donor‐derived platelets with those produced in vitro, we cannot overlook the issue of platelet storage. This is because in situations where timely transfusion of in vitro‐produced platelets is not possible, such as in remote areas, challenges in platelet storage may still arise.
It is well known that platelet transfusions are life‐saving, but some adverse effects have also been noted. Common transfusion‐associated adverse reactions (TAARs) are allergic reactions, febrile nonhemolytic transfusion reaction (FNHTR) and transfusion‐related acute lung injury (TRALI). A recent randomized controlled trial (RCT) by Anna Curley 5 showed that platelet transfusion in neonates at a low threshold (25,000 per cubic millimeter) is associated with lower infant bleeding and mortality than that at a high threshold (50,000 per cubic millimeter), suggesting that platelet transfusions may cause harm in neonates independently of the disease process. Some retrospective studies have also suggested that platelet transfusion may be associated with the prognosis of patients. There are various factors contributing to TARRs. Expect for the impact of plasma and leukocytes, transfused stored platelets can individually cause these side effects. 6 In addition to their classical hemostatic function, it has also been reported that platelets have immunological and proinflammatory effects by interacting with leukocytes. 7 , 8 In the process of collection, preparation and storage of platelets, due to shear stress and some in vitro stimulation, the morphology, metabolism and function of platelets will gradually change, resulting in a decrease in the quality of platelets. Biological response modifiers (BMRs), such as platelet microparticles (PMPs), microRNA, soluble CD40 ligand (sCD40L) and regulated on activation normal T cells expressed and secreted (RANTES), are released by platelets during storage, which are not only reflections of platelet function but also relevant to clinical practice as their immunological roles. Meanwhile, clinical platelet transfusions will face the challenge, platelet immune and inflammation function.
Although there have been a variety of mature detection techniques in vitro, including platelet aggregometry, electron microscopy, flow cytometry, and omics research, to monitor the changes in the function, morphology, activation, and metabolism of platelets during storage, the biological mechanisms of platelet storage lesions (PSLs) are still not clear, which makes it difficult to further extend the storage time of platelets and to obtain high‐quality platelets.
Recent studies have evaluated novel platelet storage conditions, 9 platelet additive solutions (PAS) and cold storage methods. And the reduction of reactive oxygen species (ROS) is able to alleviate PSLs. 10 , 11 Cryopreserved and cold storage conditions are reconsidered to lengthen the storage time of platelets and reduce bacterial contamination, despite the deleterious effects and reduced recoveries of refrigerated platelets as demonstrated by Murphy S 12 and other studies. Additionally, the characteristics of platelets at 4°C that are more capable of coagulating has received increasing attention, which may be more suitable for patients with acute bleeding. 13 Different types of PAS have been shown to reduce the incidence of allergic reactions, FNHTR and TRALI and may also be helpful to mitigate PSLs for extended storage time. While there are lower corrected count increments (CCI) of platelets stored in PAS‐E than those stored in plasma but with no significant difference. 14
This review will discuss current research progresses on PSLs and efforts undertaken to mitigate PSLs. Considering the role of platelets beyond coagulation, this paper will also review recent findings of BMRs during storage and their potential connections with clinical practice.
2. PHYSIOLOGY OF NORMAL PLATELETS
Platelets are small, discoid, anucleate cells with a diameter of 2–3 μm. Two‐thirds of platelets are present in the peripheral blood after shedding from megakaryocytes, which plays major roles in blood clot formation and maintains the integrity of the vessel wall, while the rest are stored in the spleen and liver. Recent studies have shown that the lung may also be an important organ for platelet production. 15 The lifespan of platelets is limited to 7–14 days because of shear forces and the absence of a nucleus. With the aging of platelets, the size decreases, and surface molecules are exposed, such as glycoproteins and phosphatidylserine (PS). Finally, senescent platelets are eliminated by macrophages. When bleeding, the resting platelets are activated immediately, and then the shape of the platelet changes from smooth disk‐shaped structures to irregular spheres with pseudopodia. Activated platelets bind to the subendothelial matrix by the surface GPIb/IX/V complex and circulating Von Willebrand factor (vWF) for adhesion and combine with collagen by GPIIb/IIIa for aggregation. In parallel, activated platelets further contribute to platelet activation by releasing substances stored in dense granules, α‐granules, and newly synthesized thromboxane A2 (TXA2). In addition, a variety of coagulation factors can also be adsorbed by activated platelets to promote secondary hemostasis. After performing their normal physiological functions, platelets in vivo are cleared. Quach 16 has discussed common mechanisms of in vivo clearance of activated and senescent platelets, such as platelet apoptosis signaling pathways (Bax and Bak), mitochondrial dysfunction, GPIbα activation, and PS externalization. Inhibiting these clearance processes may potentially enhance the functionality of stored platelets after transfusion.
3. PREPARATION AND STORAGE OF PC IN THE BLOOD BANK
Platelet concentrates (PC) are isolated by centrifugation. There are three types of PC (Figure 1): buffy coat (BC) PC, platelet‐rich plasma (PRP) PC and apheresis PC. 17 , 18 Apheresis platelets are separated by automated instruments, and then the remaining blood components are transfused back into the donors where all steps are performed with no manual processing. Another benefit of apheresis PC is that platelets from one donor are relatively sufficient for one receptor, which avoids platelet mixing and reduces the incidence of immune reactions. BC‐derived PC and PRP‐derived PC are isolated from whole blood donation by hard spin and soft spin, respectively. However, platelets produced by the PRP method tend to aggregate during the preparation process. 18 During the preparation of platelets, other techniques are applied for different aims. Pathogen reduction technologies (PRTs) reduce microbial contamination to secure platelet transfusions by chemical or physical (ultraviolet light illumination) methods but may lead to platelet activation, 19 ROS generation, PS exposure and apoptotic feature. 20 , 21 Even gamma irradiation as the known safe method also showed to increase ROS generation and pro‐apoptotic function in platelets albeit it does not increase the storage dependent levels of an overt apoptosis. 21 Leukoreduction by filtration can effectively decrease cytokine concentrations. 22 PAS as storage media have protective effects on platelets. 14 Shear stress and artificial materials are considered as the first step in the development of PSLs. 17 Some research has conducted experiments to investigate the relationship between shear stress and PSLs. In Hosseini study, 23 lower GPVI shedding/expression of platelets in manually mixed PCs (MM‐PCs) than continuously agitated PCs (CAG‐PCs) during 4 days storage, which indicated shear stress may contribute to GPVI shedding. Additionally, shear stress may activate Ca2+ channels. 24 In Wang review, 25 the shear stress threshold of platelets in vitro is lower because the time that platelets experienced is longer and persistent than in vivo.
FIGURE 1.
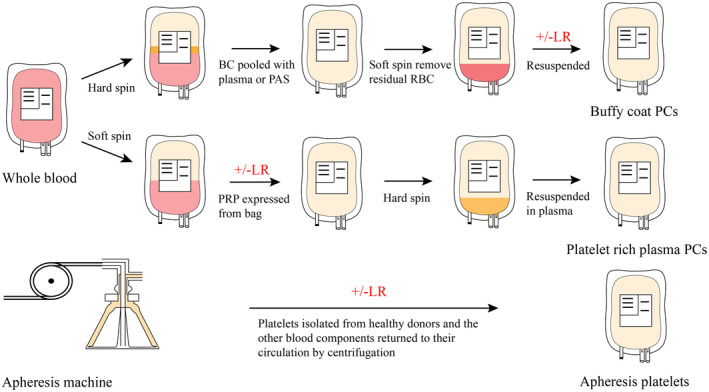
Platelet concentrate (PC) preparation methods. There are three schemes of platelet preparation established, buffy‐coat (BC) PC, platelet‐rich plasma (PRP) PC and apheresis PC production (top, middle and bottom). 17 , 18 BC is first separated from whole blood by hard spin and then pooled with plasma or platelet additive solutions (PAS), where remanent red blood cells are removed under soft centrifugation. On the contrary, PRP is isolated by soft spin. Subsequently, platelets are segregated via hard centrifugation. As for apheresis PC, automated blood cell separator is utilized to collect platelets and transfuse back the other blood components into donors. BC and apheresis platelets can be resuspended in PAS and leukoreduction (LR) technique can be applied.
Different storage conditions have been explored such as vertical agitation, continuous agitation 26 and manually mixed. 23 It is an established practice that platelets are stored at 20–24°C under constant horizontal agitation in n‐Butyryl tri‐n‐hexyl citrate (BTHC) or tri‐(ethylhexyl)‐trimellitate (TEHTM) plasticized polyvinyl chloride (PVC) bags. 27 Temperature, agitation, pH (6.4–7.4), fuel availability and respiratory capacity are requisite for the maintenance of platelet function.
4. PSLS AT 20–24°C UNDER CONSTANT HORIZONTAL AGITATION
Methods for monitoring the function of stored platelets in vitro are changes in platelet morphology, surface molecules, platelet metabolism and BMRs, whereas it is a challenge to accurately predict platelet function after transfusion by these tests. One reason is that the mechanisms of platelet lesions are not well understood and that most of these monitoring methods are used to detect platelet activation and apoptosis. The following text will summarize changes observed during platelet storage in recent studies and present them through Figure 2.
FIGURE 2.
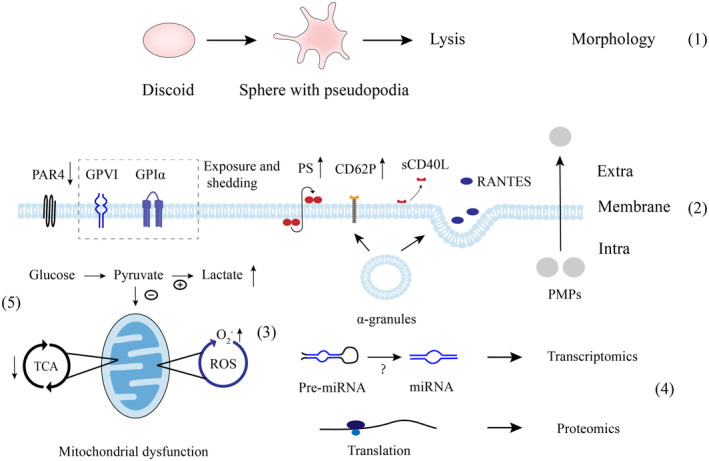
Some major biological response modifiers (BMRs) and the potential mechanisms of platelet storage lesions (PSLs). (1) During storage, platelet morphology transforms from discoid to sphere with pseudopodia and lysis at extended storage. 17 , 18 , 27 (2) Changes in molecules on the surface are reduced G‐protein‐coupled protease‐activated receptors (PAR4), 38 exposure and shedding of CD42b (GPI bα) 29 and GPVI, 30 and inverse phosphatidylserine (PS), 106 which are different from classical platelet activation. In parallel, α‐granules release CD62P 30 to the membrane and soluble CD40 ligand (sCD40L) 66 and regulated on normal activation T cells expressed and secreted (RANTES) 67 to the extracellular space. Platelet microparticles (PMPs) 72 are also produced by platelets. (3) These BMRs including GPI bα, GPVI, PS and PMPs may be associated with reactive oxygen species (ROS) as demonstrated by elevated O2‐ level. 34 microRNAs (miRNAs) 50 , 59 that may be derived from precursor miRNAs (pre‐miRNAs) and proteins that are synthesized in the existing process of translation (4) can be detected. (5) The proportion of glucose that can access the tricarboxylic acid cycle (TCA) decreases contributes to the accumulated lactate due to mitochondrial dysfunction. 32
4.1. Changes in platelet morphology
Apart from the first stimuli during the platelet preparation process, PSLs mainly occur in storage. The normal morphology of platelets is the basis for their normal function. The change in platelet morphology from discoid to spheres leads to the loss of platelet function and low recovery in vivo. Increased mean platelet volume (MPV) and platelet distribution width (PDW) may indicate platelet activation during the early stage of storage. While in the extended stage of storage, over 5 days, decreased MPV may be the result of aging and cytolysis. 28 The extent of shape change (ESC) and hypotonic shock response test (HSR) are both assessed by light‐scattering techniques. ESC is the degree of morphological alteration that platelets response to agonists. HSR reflects the structural integrity and stability of platelets in hypotonic media. 3 Platelet morphological score (PMS) assigns different types of platelet morphology (balloons = 0, dendrites = 1, spheres = 2 and disks = 4). 29 Swirling assessment is a simple and useful macroscopic method to evaluate PC quality and is associated with PH and CCI (no swirling = 0, poor = 1, good = 2, excellent = 3). 23 Higher HSR, ESC, swirling and PMS scores indicate better platelet function. Nonetheless, these parameters that reflect changes in platelet morphology are inconsistent across studies.
4.2. Platelet activation and surface molecules modification
Platelets contain some well‐known receptors as markers of platelet activation, which can be detected by flow cytometry. Shedding of CD42b (GPIbα) may be associated with decreased surface exposure and attenuate platelet response to ristocetin, 30 and it may cause decreased capability of platelet adhesion at high shear or premature platelet clearance. In parallel, inhibiting CD42b shedding improves posttransfusion platelet recovery and hemostatic function. 31 GPVI, as a more important collagen receptor, is also likely to be shed in storage 30 and related with functional deficit of platelets including lowering responses to collagen‐induced aggregation as well as gradual loss of platelet adhesion to collagen. 10 CD62P (P‐selectin), transported from α‐granules to the membrane in activated platelets, is significantly increased during storage. 29 , 30 The expression of GPIbα (significantly on day 3) and GPVI (significantly on day 5) both came down always with the storage time on stored platelets surface, as indicated by the mean fluorescence intensity (MFI). Furthermore, these proteins could be detected in the supernatant. In contrast, the levels of CD62P and PS showed a continuous increase throughout the storage period, with a significant difference observed on day 3. 30 , 32 There are three processes of PS exposure, including activation, apoptosis and an unknown way. 33 During storage, it was also reported that platelets mitochondrial dysfunction was associated with PS exposure. 32 , 34 In addition to its coagulation function, PS exerts immune effects 35 and provides an “eat me” signal, but its inflammatory roles in blood components are unclear. 36 But, it is reported that higher PS‐exposed platelets are more suitable for high‐risk patients to stop bleeding. 37 A decrease in G‐protein‐coupled protease‐activated receptors 1 and 4 (PAR1, PAR4), bound with thrombin, is related to a reduction in platelet aggregation potency. 38 It has been reported that tissue factor (TF), as the main activator of blood coagulation, would increase on stored platelets, but the connections with platelet transfusions need further study. 39 Beyond activation, the occurrence of ROS and 34 mitochondrial dysfunction 32 may also be important factors affecting platelet function because these alterations, such as surface markers (GPVI and PS), are not as classical as platelet activation. Some studies hypothesized ROS and mitochondrial dysfunction may be associated with platelet apoptosis. 40 However, in Pleines study, the BAK/BAX depletion of murine platelets was able to lengthen platelet life span in vivo but had no protective effects on platelet function in vitro, which indicated the loss of granular contents was more important to PSLs than apoptosis during platelet storage. 41 The relationship among PSLs, GPVI shedding, mitochondrial dysfunction and PS exposure needs further study.
4.3. Metabolism of platelets
Similar to other living cells, platelets still undergo adenosine triphosphate (ATP) consumption during storage to ensure their function, which is mainly from the tricarboxylic acid cycle (TCA). The process of ATP metabolism is the consumption of oxygen and glucose to generate ATP as well as water and carbon dioxide, but in the case of abnormal glycolysis and mitochondrial function, 32 lactate accumulates and the pH drops as a result of inadequate clearance of metabolites. Therefore, glucose, pH and lactate levels are also used as markers to evaluate the function of platelets during storage. 17 The level of lactate dehydrogenase (LDH), a key enzyme in glycolysis, is also increased when platelets are fragmented. Metabolomics studies show that the metabolic changes in platelets during storage are not linear but suddenly change on the fourth and seventh days. 42 During short‐term storage (0–3 days), without hypoxia, 40% glucose is consumed through the glycolytic pathway, and the levels of lactate and malate increase while the pH decreases. During intermediate storage (4–6 days), lactate production continues, but TCA become active. In addition, changes in platelet lipid metabolism include decreased cholesterol and increased sphingomyelin (SM) and ceramide. 43 Moreover, changes in fatty acid metabolism are associated with platelet recoveries after transfusion. 44 Although several other metabolites have been identified in the Paglia G 45 and Zimring JC 44 studies and Paglia G compares the metabolism of platelets from different preparation methods after storage, the reasons for the metabolic changes in platelets during storage are not clear. There are still few studies on the relationship between platelet metabolism during prolonged storage of platelets and platelet recoveries after transfusion.
4.4. Alterations in proteins and RNA
By platelet proteome analysis, the levels of 21 proteins significantly changed of which 12 decreased, including vWF (significantly decreased on day 5), platelet factor 4 (PF4, significantly decreased on day 13) in platelet α‐granules and some non‐α‐granule proteins (histone H2A, significantly decreased on day 2), while proteins in dense granules and lysosomes were not lost, which indicates a gradual release of α‐granules. 46 In the supernatant of PC, 25 proteins exhibited a significant increase, and 42 proteins exhibited a significant decrease on day 5. Proteins released by platelets were associated with exosomes, two clusters of which were related to hemostasis and RNA binding, respectively. 47 During in vitro storage, there is a close relationship between the changes in platelet RNA and protein levels because the processes of protein translation still exist. From mRNA to proteins, microRNAs (miRNAs) are important regulators after gene expression, which implies another interesting way to monitor PSLs. 48 Yan Y 49 discussed the role of miRNA during platelet storage, and both increased and decreased levels of miRNA have been reported. The increased miRNA levels may be from precursor miRNAs (pre‐miRNAs) or miRNA cleavage because there is no transcription in stored platelets. It was reported that miR‐127, miR‐191 and miR‐320a were highly expressed and that miR‐127 and miR‐320a were biomarkers of PSLs. 50 , 51 In a study by Norouzi M, miR‐326 and miR‐145 were positively correlated with glucose concentrations and can be potential biomarkers to reflect platelet function. 52 Other miRNAs were associated with platelet apoptosis. Mir‐326 and mir‐let‐7b, which interacted with Bcl‐xL and Bak, had high expression levels during storage, 53 , 54 and mir‐103b downregulating integrin beta 3 (ITGB3) promoted platelet apoptosis. 55 Overexpression of miR‐570‐3p in platelets resulted in reduced levels of mitochondrial ATP synthase subunit g (ATP5L) mRNA and concomitant ATP loss. 56 miR‐181a 57 and miR‐320c 58 reduce platelet activation by Ras‐related protein (RAP1) downregulation. Apart from miRNA, some other RNAs, such as mRNA, long noncoding RNA (lncRNA) and circular RNA (circRNA), also showed interesting level changes during platelet preservation by whole transcriptome analysis. 59 Recent studies have suggested the possibility of RNA, especially miRNA, to predict PSLs, and the relationship between miRNAs and platelet apoptosis, platelet metabolism, and mitochondrial disorders has also been reported. Besides, the biological mechanisms may be complex because of changes in a variety of miRNAs as reported in different studies. When identifying miRNAs with bioinformatics tools, we need to be aware that the technique may have limitations. One limitation is the updating of precursor and mature microRNA sequence databases. A recent study, 60 based on the latest version of miRbase, comprehensively summarizes the diverse repertoire of miRNAs that are identified in platelets stored in blood banks for 6 days. These findings are further validated using quantitative real‐time polymerase chain reaction (qPCR). Explaining the changes of miRNAs during platelet storage is equally important. Obtaining the key causal relationships from numerous clues can help us understand these changes and discover potential intervention targets.
4.5. Major BMRs released by platelets
There are some well‐studied BMRs in PC supernatant, including PMPs and proteins in α‐granules (Table 1). Contents in platelet α‐granules, including sCD40L, RANTES, PF4, β‐thromboglobulin (β‐TG) and transforming growth factor (TGF)‐β1, which have vital functions in hemostasis, are released into the platelet supernatant during storage. 17 , 61 These accumulated soluble mediators can inhibit the immune response of dendritic cells to lipopolysaccharide (LPS) in vitro, 62 suggesting a nonnegligible immune function of platelets. As a transmembrane receptor, CD40L plays an important role in innate and adaptive immunity, mainly from activated T cells and platelets. 63 During the storage process, CD40L is cleaved into soluble CD40L (sCD40L). In vitro, sCD40L activates B cells and neutrophils, and also plays a role in allergic reactions, FNHTR, and TRALI. 64 , 65 sCD40L along with dysfunctional vascular tissue in recipients may result in adverse reactions. 66 RANTES, also known as chemokine ligand 5 (CCL5), migrates eosinophils 67 and may serve as a new measure of platelet secretory function during storage. 68 Higher levels in PC supernatant are also associated with allergic reactions and FNHTR but not alone. 67 , 69 PF4 and β‐TG have long been known to be in platelet α‐granules, and their release correlates with platelet activation, as their concentrations also increase during storage. 61 , 70 Additional cytokines derived from leukocytes in PC supernatants, such as interleukin (IL)‐6, IL‐8, IL‐2 and tumor necrosis factor (TNF‐α), can be diminished by leukoreduction, especially pre‐storage leukoreduced (pre‐LR) in contrast to post‐storage leukoreduced (post‐LR), which significantly reduce the prevalence of TAARs. 22 However, during the process of leukoreduction by the filter, platelets under extra shear stress may be activated, which is considered by the accumulated RANTES. 71 In this case, downgrading major BMRs from PC appears to be particularly important.
TABLE 1.
Major BMRs released by platelets at 22°C storage and their relations with clinical practice.
| BMRs | Location in platelets | Function | Changes in BMRs and their potential relationship with clinical practice |
|---|---|---|---|
| sCD40L (CD154) 6 , 63 , 64 , 65 , 66 , 90 , 93 | α‐ Granule |
|
|
| RANTES (CCL5) 6 , 67 , 68 , 69 , 86 , 90 , 93 | α‐ Granule |
|
|
| Phosphatidylserine (PS) 16 , 35 , 36 , 37 , 106 | Platelet plasma membrane |
|
|
| Platelet Microparticles (PMPs) 72 , 76 , 79 , 80 , 81 , 90 , 93 |
|
|
|
| microRNA 50 , 51 , 52 , 53 , 54 , 55 , 56 , 57 , 58 |
|
Another important type of BMRs is PMPs. Microparticles (MPs) in circulating blood are platelet‐derived MPs (PMPs, Annexin‐V+ /CD41a+), red blood cell‐derived MPs (RMPs, CD235+), leukocyte‐derived MPs (LMPs, CD45+), monocyte‐derived MPs (MMPs, CD14+) and endothelial cell‐derived MPs (EMPs, CD144+). Although different types of MPs can be detected in PCs, 72 , 73 we primarily focus on PMPs because the concentration of other cells in PCs is minimal. PMPs, small vesicles with a diameter of 0.1–1 microns, are primarily thought to be a way for platelets to exert coagulation function through membrane or intrinsic mediators because PMPs can trigger monocytic cell aggregation and release of procoagulant tissue factor‐expressing MPs. 74 However, in later studies, the characteristics of PMPs extensively involved in immunity and inflammation are considered since they more easily cross tissue barriers than platelets. 75 PMPs account for a major proportion of MPs both in stored PC and blood. 72 The elevated PMP levels in the preparation and storage process are mainly generated by resting platelets, a fraction of which produced by activated platelets (CD62P+) also significantly increased during storge (day 3). 72 , 73 Unlike cytokines, the PMP concentrations have no significant changes after LR. 72 , 76 PS externalization on PMPs surface staining with annexin‐V is associated with desialylation, which promotes phagocytosis by HepG2 cells. 77 Some evidence has also suggested that PMPs from PC have potential for immune function due to proteins. HLA molecule expression highlights the contribution of PMPs to alloimmunization mechanisms. 78 Proteins from plasma (C1q, C3 fragments and factor H) can also be acquired by PMPs, apart from those in platelets. 79 In vitro experiments, PMPs from PCs are able to interact with various cells, such as reducing the responsiveness of macrophages and dendritic cells to LPS, 79 and stimulating the differentiation of B lymphocytes. 80 Furthermore, sCD40L carried by PMPs induce polymorphonuclears (PMN) respiration burst, which may be related to TRALI. 81 However, the interpretations are inadequate.
5. PAS, COLD AND FROZEN PLATELETS–RECONSIDERED CONDITIONS
There are some formulations of PAS that consist of various constituents (acetate, potassium, magnesium, phosphate, bicarbonate or additional glucose) to alternate plasma and provide buffering, fuel sources and cations. 9 In the latest review, PAS are found to be effective strategies for reducing the incidence of allergic reactions, FNHTR and TRALI, as well as alleviating platelet lesions after PRT procedures. Additionally, there are other interesting and noteworthy parameters, such as prolonged storage time in PAS‐F (Isoplate) and reliable 24 h CCI in PAS‐E (PAS‐5, PAS IIIM, SSP+). 14 Actually, studies that investigate CCI after transfusion of platelet stored in different PAS are not sufficient. In an observational study, 82 it was observed that PCs stored in PAS‐C showed a significant 16% decrease in 24 h CCI compared to platelets stored in plasma. On the other hand, platelets stored in PAS‐E exhibited a nonsignificant 2% decrease in 24 h CCI compared to platelets stored in plasma. In a randomized study, 83 20 h CCI after transfusion of platelets in PAS‐B were significantly lower than in plasma (11.5 ± 8.0 vs. 9.5 ± 8.0). In vitro, platelets incubated in PAS‐B have metabolic improvement. 84 Platelets prepared in PAS‐C (Intersol) are remarkably activated (CD62P+, PS+ staining by annexin‐V and sCD40L) compared with those in PAS‐E and plasma, while the use of PAS contributes to complement (C3b and C4b) diminution, which may be associated with FNHTR. 85 BMRs (RANTES, sCD40L, β‐TG and PF4) released by platelets stored in both PAS and plasma accumulate, while PAS‐C supplemented with magnesium and potassium can reverse the increase and platelet activation. 70 , 86 Platelets resuspended in PAS‐E are also preserved satisfactorily in comparison to those resuspended in PAS‐B. 87 , 88 In parallel, the use of PAS‐E and PAS‐G (M‐Sol) maintained platelet metabolic activity, as evidenced by PH and lactate. 89 PAS containing glucose, magnesium and potassium also exhibit attractive advantages in other experiments. 90 , 91 Increased PMP levels in PAS tend to bind to vWF, 92 and washing by another PAS, bicarbonate Ringer's solution supplemented with acid‐citrate‐dextrose Formula A (BRS‐A) used in Japan, can reduce PMPs, sCD40L and RANTES levels. 93 Substitution of plasma with PAS may alleviate transfusion reactions by reducing some of the inflammatory substances in plasma, but this approach needs to be considered carefully in patients with coagulation factor deficiency. Meanwhile, reliable CCI and platelet function should also be guaranteed. In addition, PAS had some help in alleviating PSLs in some in vitro studies, which was due to its inclusion of magnesium ions, potassium ions and glucose.
Platelet storage under cold conditions was abandoned in the 1970s due to unsatisfactory recoveries and survival after transfusion but remains attractive because of its long preservation time. Indeed, recent research has reconsidered cold storage platelets (CSP) for their procoagulant characteristics that may produce a rapid hemostatic response in bleeding patients. 13 Compared to 22°C, the irreversible discoid‐to‐sphere shape, inhibition of platelet metabolism, mitochondrial dysfunction, GPIbα clustering and desialylation for clearance and elevated BMRs in cold storage conditions have been reported. 13 , 25 , 94 , 95 Although the CD62P expression of cryopreserved platelets (CP) were lower than PCs stored in room temperature, the aggregation rate of CP decreased. And the pathways “SNARE interactions in vesicular transport” and “Vasopressin‐regulated water reabsorption” were affected by cold storage in Wang study. 96 However, the profiles of CSP demonstrate superior capabilities to form stiffer and stronger clots binding with blood coagulation factor XIII. 97 Although the recoveries of CSP in vivo are lower than those at 22°C, CSP in the extended storage time up to 20 days still maintain the hemostatic response. 98 Recent RCT supports this standpoint. During complex cardiothoracic surgery, chest drain output of patients who accept platelets at 22°C or CSP, as well as platelet counts, number of blood components, occurrence of thromboembolic events, length of stay in intensive care and mortality, are comparable. 99 Moreover, CSP with PAS‐C can ensure platelet function and metabolism, reduce platelet activation and improve platelet survival. 100 In CP with DMSO, GPIbα shedding and enhanced PS exposure may be associated with decreased adhesion and increased coagulation function respectively. 101 PMP levels in CP are 10–15 folds higher than those at room temperature, which potentially promotes their coagulation. 102 Recent RCTs also show that in patients with acute leukemia, 103 thrombocytopenia 104 and high‐risk cardiothoracic surgery, 105 CSP transfusions are safe and have hemostatic effects. However, the side effects of the hypercoagulable characteristic should be taken into account. Although the superiority of hemostatic function of CSP has been reported in vitro, the advanced evidence suggesting the characteristic in vivo is insufficient especially abundant studies for the same clinical scenario, which highlights the urgency of excellent RCTs to clarify posttransfusion function of the reconsidered and interesting product in vivo.
6. WHAT ARE WE WORKING FOR?
Whether the platelets are stored at 22°C under agitation or cold conditions, the ultimate goal is high‐quality platelets for safe and effective transfusions. Basically, there are two approaches. One is to preserve platelets at 22°C under agitation to ensure that they are always in a normal state of function and maintain the viability of platelets during storage. This approach may be analogous to finding the right conditions, such as sufficient nutrients and oxygen, appropriate temperature and even consistent agitation, for platelets to subsist as they circulate in vivo. However, TAARs and limited platelet shelf life emerge because of PSLs and bacterial contamination beyond plasma or other blood cell factors. To understand the complex biological mechanisms of PSLs other than activation or apoptosis, we conduct mechanistic studies, including monitoring changes in platelet morphology, metabolic alterations, surface markers modification, transcriptomic research, major BMRs released by platelets, and the mechanism of platelet clearance in vivo. It is well known that platelets are small cells without nuclei that are relatively fragile compared to nucleated cells and that platelets survive in the body for approximately 2 weeks. In this case, platelet senility is inescapable, although PAS may be helpful in some way.
Another approach is cold storage at 4°C and cryopreservation at −80°C, which is similar to in vitro cell preservation, in which the function of platelets is maintained at a low or even zero level in vitro before they are resuscitated and transfused into patients. Indeed, bacterial contamination is reduced and storage time is extended under these conditions, but the tremendous stimulus of low temperature will lead to irreversible lesions in platelets, which are fragile cells, thus affecting the quality and recoveries of platelets. Cold storage conditions have been reconsidered in recent studies, as the higher hemostasis function may be appropriate for some acute bleeding patients, and growth factors in expired PC can be reused. However, more experimental data are needed to evaluate its safety, especially in patients with hypercoagulable disorders.
In this review, we discuss clinical issues and research progress related to platelet transfusion. As platelets are essential for hemostasis in the body, their transfusion is crucial for preventing bleeding and saving the lives of critically ill patients. However, clinical platelet transfusion encounters two significant challenges. Firstly, due to PSLs, platelets can only be stored for up to 5 days, limiting their shelf life. Additionally, transfusion of platelets may lead to adverse reactions. Therefore, extensive research is focused on understanding the mechanisms behind PSLs to monitor platelet function and ensure the availability of high‐quality platelets for transfusion. Accordingly, different studies conduct numerous experiments at temperatures of 22°C or under cold conditions, both with and without PAS, to maintain platelets in an ideal quiescent state where there are no PSLs.
Novel and specific negative regulatory mechanisms may help us move closer to this goal of temporarily switching platelets “off” and then “on,” without causing damages. As reported in Hosseini study, ROS and mitochondrial dysfunction play an important role in PSLs, which is associated with GPVI. Therefore, reducing the oxidative–reductive state during platelet storage is beneficial for platelet viability, while preventing stored dependent receptor or MPs shedding events. Based on current research, another approach to effectively use stored platelets is to provide platelets with different levels of PSLs for different patients. As discussed above, significant changes that we monitor during platelet storage mainly happen on day 3 such as CD62P, PS, metabolism molecules, BMRs, proteins and RNA. It is possible that different stages of PSLs have different therapeutic effects on different patients. Maybe, before that, PSLs should be uniformly quantified.
FUNDING INFORMATION
This work was supported by National Natural Science Foundation of China (Program No. 82072352).
CONFLICT OF INTEREST STATEMENT
The authors declare that they have no competing interests.
Liu C, Su Y, Guo W, Ma X, Qiao R. The platelet storage lesion, what are we working for? J Clin Lab Anal. 2024;38:e24994. doi: 10.1002/jcla.24994
DATA AVAILABILITY STATEMENT
Data sharing not applicable to this article as no datasets were generated or analyzed during the current study.
REFERENCES
- 1. Freireich EJ. Origins of platelet transfusion therapy. Transfus Med Rev. 2011;25(3):252‐256. [DOI] [PubMed] [Google Scholar]
- 2. Murphy S, Gardner FH. Platelet storage at 22 degrees C: role of gas transport across plastic containers in maintenance of viability. Blood. 1975;46(2):209‐218. [PubMed] [Google Scholar]
- 3. Shrivastava M. The platelet storage lesion. Transfus Apher Sci. 2009;41(2):105‐113. [DOI] [PubMed] [Google Scholar]
- 4. Lee HJ, Oh SH, Jo SY, Kim IS. Platelet inventory management program: development and practical experience. Ann Lab Med. 2021;41(1):95‐100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Curley A, Stanworth SJ, Willoughby K, et al. Randomized trial of platelet‐transfusion thresholds in neonates. N Engl J Med. 2019;380(3):242‐251. [DOI] [PubMed] [Google Scholar]
- 6. Cognasse F, Hally K, Fauteux‐Daniel S, et al. Effects and side effects of platelet transfusion. Hamostaseologie. 2021;41(2):128‐135. [DOI] [PubMed] [Google Scholar]
- 7. Ghasemzadeh M, Hosseini E. Platelet‐leukocyte crosstalk: linking proinflammatory responses to procoagulant state. Thromb Res. 2013;131(3):191‐197. [DOI] [PubMed] [Google Scholar]
- 8. Okubo K, Kurosawa M, Kamiya M, et al. Macrophage extracellular trap formation promoted by platelet activation is a key mediator of rhabdomyolysis‐induced acute kidney injury. Nat Med. 2018;24(2):232‐238. [DOI] [PubMed] [Google Scholar]
- 9. Capocelli KE, Dumont LJ. Novel platelet storage conditions: additive solutions, gas, and cold. Curr Opin Hematol. 2014;21(6):491‐496. [DOI] [PubMed] [Google Scholar]
- 10. Hosseini E, Solouki A, Roudsari ZO, Kargar F, Ghasemzadeh M. Reducing state attenuates ectodomain shedding of GPVI while restoring adhesion capacities of stored platelets: evidence addressing the controversy around the effects of redox condition on thrombosis. J Thromb Thrombolysis. 2020;50(1):123‐134. [DOI] [PubMed] [Google Scholar]
- 11. Hosseini E, Ghasemzadeh M, Atashibarg M, et al. ROS scavenger, N‐acetyl‐l‐cysteine and NOX specific inhibitor, VAS2870 reduce platelets apoptosis while enhancing their viability during storage. Transfusion. 2019;59(4):1333‐1343. [DOI] [PubMed] [Google Scholar]
- 12. Murphy S, Gardner FH. Effect of storage temperature on maintenance of platelet viability: deleterious effect of refrigerated storage. N Engl J Med. 1969;280:1094‐1098. [DOI] [PubMed] [Google Scholar]
- 13. Jimenez‐Marco T, Castrillo A, Hierro‐Riu F, Vicente V, Rivera J. Frozen and cold‐stored platelets: reconsidered platelet products. Platelets. 2022;33(1):27‐34. [DOI] [PubMed] [Google Scholar]
- 14. van der Meer PF, de Korte D. Platelet additive solutions: a review of the latest developments and their clinical implications. Transfus Med Hemother. 2018;45(2):98‐102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Lefrancais E, Ortiz‐Munoz G, Caudrillier A, et al. The lung is a site of platelet biogenesis and a reservoir for haematopoietic progenitors. Nature. 2017;544(7648):105‐109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Quach ME, Chen W, Li R. Mechanisms of platelet clearance and translation to improve platelet storage. Blood. 2018;31(14):1512‐1521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Ng MSY, Tung JP, Fraser JF. Platelet storage lesions: what more do we know now? Transfus Med Rev. 2018;32:144‐154. [DOI] [PubMed] [Google Scholar]
- 18. Devine DV, Serrano K. The platelet storage lesion. Clin Lab Med. 2010;30(2):475‐487. [DOI] [PubMed] [Google Scholar]
- 19. Magron A, Laugier J, Provost P, Boilard E. Pathogen reduction technologies: the pros and cons for platelet transfusion. Platelets. 2018;29(1):2‐8. [DOI] [PubMed] [Google Scholar]
- 20. Hosseini E, Kianinodeh F, Ghasemzadeh M. Irradiation of platelets in transfusion medicine: risk and benefit judgments. Platelets. 2022;33(5):666‐678. [DOI] [PubMed] [Google Scholar]
- 21. Hosseini E, Nodeh FK, Ghasemzadeh M. Gamma irradiation induces a pro‐apoptotic state in longer stored platelets, without progressing to an overt apoptosis by day 7 of storage. Apoptosis. 2023;28(7):1141‐1153. [DOI] [PubMed] [Google Scholar]
- 22. Chang CC, Lee TC, Su MJ, et al. Transfusion‐associated adverse reactions (TAARs) and cytokine accumulations in the stored blood components: the impact of prestorage versus poststorage leukoreduction. Oncotarget. 2017;9(4):4385‐4394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Hosseini E, Solouki A, Haghshenas M, Ghasemzadeh M, Schoenwaelder SM. Agitation‐dependent biomechanical forces modulate GPVI receptor expression and platelet adhesion capacity during storage. Thromb J. 2022;20(1):3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Ilkan Z, Wright JR, Goodall AH, Gibbins JM, Jones CI, Mahaut‐Smith MP. Evidence for shear‐mediated Ca2+ entry through mechanosensitive cation channels in human platelets and a megakaryocytic cell line. J Biol Chem. 2017;292(22):9204‐9217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Wang S, Liu Q, Cheng L, Wang L, Xu F, Yao C. Targeting biophysical cues to address platelet storage lesions. Acta Biomater. 2022;151:118‐133. [DOI] [PubMed] [Google Scholar]
- 26. Torres R, Tormey CA, Stack G. Fluid motion and shear forces in platelet storage bags with different modes of agitation. Vox Sang. 2016;111(2):209‐212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. van der Meer PF, de Korte D. Platelet preservation: agitation and containers. Transfus Apher Sci. 2011;44(3):297‐304. [DOI] [PubMed] [Google Scholar]
- 28. Fijnheer R, Pietersz RN, de Korte D, Roos D. Monitoring of platelet morphology during storage of platelet concentrates. Transfusion. 1989;29(1):36‐40. [DOI] [PubMed] [Google Scholar]
- 29. Vucetic D, Ilic V, Vojvodic D, et al. Flow cytometry analysis of platelet populations: usefulness for monitoringthe storage lesion in pooled buffy‐coat platelet concentrates. Blood Transfus. 2018;16(1):83‐92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Hosseini E, Ghasemzadeh M, Nassaji F, Jamaat ZP. GPVI modulation during platelet activation and storage: its expression levels and ectodomain shedding compared to markers of platelet storage lesion. Platelets. 2017;28(5):498‐508. [DOI] [PubMed] [Google Scholar]
- 31. Chen W, Liang X, Syed AK, et al. Inhibiting GPIbalpha shedding preserves post‐transfusion recovery and hemostatic function of platelets after prolonged storage. Arterioscler Thromb Vasc Biol. 2016;36(9):1821‐1828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Perales Villarroel JP, Figueredo R, Guan Y, et al. Increased platelet storage time is associated with mitochondrial dysfunction and impaired platelet function. J Surg Res. 2013;184(1):422‐429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Nagata S, Sakuragi T, Segawa K. Flippase and scramblase for phosphatidylserine exposure. Curr Opin Immunol. 2020;62:31‐38. [DOI] [PubMed] [Google Scholar]
- 34. Ghasemzadeh M, Hosseini E, Roudsari ZO, Zadkhak P. Intraplatelet reactive oxygen species (ROS) correlate with the shedding of adhesive receptors, microvesiculation and platelet adhesion to collagen during storage: does endogenous ROS generation downregulate platelet adhesive function? Thromb Res. 2018;163:153‐161. [DOI] [PubMed] [Google Scholar]
- 35. Wang J, Yu C, Zhuang J, et al. The role of phosphatidylserine on the membrane in immunity and blood coagulation. Biomark Res. 2022;10(1):4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Saas P, Angelot F, Bardiaux L, Seilles E, Garnache‐Ottou F, Perruche S. Phosphatidylserine‐expressing cell by‐products in transfusion: a pro‐inflammatory or an anti‐inflammatory effect? Transfus Clin Biol. 2012;19(3):90‐97. [DOI] [PubMed] [Google Scholar]
- 37. Noulsri E, Lerdwana S. Quantitation of phosphatidylserine‐exposing platelets and platelet‐derived microparticles in platelet products: a new strategy to improve efficacy of platelet transfusion. Med Hypotheses. 2020;145:110306. [DOI] [PubMed] [Google Scholar]
- 38. Schlagenhauf A, Kozma N, Leschnik B, Wagner T, Muntean W. Thrombin receptor levels in platelet concentrates during storage and their impact on platelet functionality. Transfusion. 2012;52(6):1253‐1259. [DOI] [PubMed] [Google Scholar]
- 39. Vignoli A, Giaccherini C, Marchetti M, et al. Tissue factor expression on platelet surface during preparation and storage of platelet concentrates. Transfus Med Hemother. 2013;40(2):126‐132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Bakry R, Sayed D, Galal H, Shaker S. Platelet function, activation and apoptosis during and after apheresis. Ther Apher Dial. 2010;14(5):457‐464. [DOI] [PubMed] [Google Scholar]
- 41. Pleines I, Lebois M, Gangatirkar P, et al. Intrinsic apoptosis circumvents the functional decline of circulating platelets but does not cause the storage lesion. Blood. 2018;132(2):197‐209. [DOI] [PubMed] [Google Scholar]
- 42. Paglia G, Sigurjonsson OE, Rolfsson O, et al. Comprehensive metabolomic study of platelets reveals the expression of discrete metabolic phenotypes during storage. Transfusion. 2014;54(11):2911‐2923. [DOI] [PubMed] [Google Scholar]
- 43. Green SM, Padula MP, Marks DC, Johnson L. The lipid composition of platelets and the impact of storage: an overview. Transfus Med Rev. 2020;34(2):108‐116. [DOI] [PubMed] [Google Scholar]
- 44. Zimring JC, Slichter S, Odem‐Davis K, et al. Metabolites in stored platelets associated with platelet recoveries and survivals. Transfusion. 2016;56(8):1974‐1983. [DOI] [PubMed] [Google Scholar]
- 45. Paglia G, Sigurjonsson OE, Rolfsson O, et al. Metabolomic analysis of platelets during storage: a comparison between apheresis‐ and buffy coat‐derived platelet concentrates. Transfusion. 2015;55(2):301‐313. [DOI] [PubMed] [Google Scholar]
- 46. Rijkers M, van den Eshof BL, van der Meer PF, et al. Monitoring storage induced changes in the platelet proteome employing label free quantitative mass spectrometry. Sci Rep. 2017;7(1):11045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Kamhieh‐Milz J, Mustafa SA, Sterzer V, et al. Secretome profiling of apheresis platelet supernatants during routine storage via antibody‐based microarray. J Proteomics. 2017;150:74‐85. [DOI] [PubMed] [Google Scholar]
- 48. Dahiya N, Sarachana T, Vu L, et al. Platelet MicroRNAs: an overview. Transfus Med Rev. 2015;29(4):215‐219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Yan Y, Zhang J, Zhang Q, Chen Y, Zhu X, Xia R. The role of microRNAs in platelet biology during storage. Transfus Apher Sci. 2017;56(2):147‐150. [DOI] [PubMed] [Google Scholar]
- 50. Pontes TB, Moreira‐Nunes Cde F, Maues JH, et al. The miRNA profile of platelets stored in a blood Bank and its relation to cellular damage from storage. PLoS One. 2015;10(6):e0129399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Vieira PCM, Maues J, Lamarao LM, et al. MicroRNA 320a and membrane antigens as tools to evaluate the pathophysiology of platelets stored in blood banks. Curr Issues Mol Biol. 2022;44(5):1838‐1850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Norouzi M, Mesbah‐Namin SA, Deyhim MR. Analysis of changes in the expression pattern of miR‐326 and miR‐145 during storage of platelet concentrate in blood bank condition and its relationship with some markers of platelet quality. J Thromb Thrombolysis. 2021;52(4):1036‐1042. [DOI] [PubMed] [Google Scholar]
- 53. Elgendy W, Swelem R, Aboudiba N, Elwafa RA. Role of MicroRNA‐326 and its target genes Bcl‐xL and Bak as potential markers in platelet storage lesion in blood banks. Indian J Hematol Blood Transfus. 2022;38(4):731‐738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Yan Y, Xie R, Zhang Q, Zhu X, Han J, Xia R. Bcl‐xL/Bak interaction and regulation by miRNA let‐7b in the intrinsic apoptotic pathway of stored platelets. Platelets. 2019;30(1):75‐80. [DOI] [PubMed] [Google Scholar]
- 55. Dahiya N, Atreya C. MiRNA‐103b downregulates ITGB3 and mediates apoptosis in ex vivo stored human platelets. Microrna. 2021;10(2):123‐129. [DOI] [PubMed] [Google Scholar]
- 56. Dahiya N, Sarachana T, Kulkarni S, et al. miR‐570 interacts with mitochondrial ATPase subunit g (ATP5L) encoding mRNA in stored platelets. Platelets. 2017;28(1):74‐81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Dahiya N, Atreya CD. MiR‐181a reduces platelet activation via the inhibition of endogenous RAP1B. Microrna. 2020;9(3):240‐246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Dahiya N, Atreya CD. RAP1 downregulation by miR‐320c reduces platelet activation in ex‐vivo storage. Microrna. 2019;8(1):36‐42. [DOI] [PubMed] [Google Scholar]
- 59. Heililahong H, Jin P, Lei H, et al. Whole transcriptome analysis of platelet concentrates during storage. Blood Transfus. 2023;21(2):146‐156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Maues J, Moreira‐Nunes CFA, Burbano RMR. Computational identification and characterization of new microRNAs in human platelets stored in a blood bank. Biomolecules. 2020;10(8):1173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Wadhwa M, Seghatchian MJ, Dilger P, et al. Cytokines in WBC‐reduced apheresis PCs during storage: a comparison of two WBC‐reduction methods. Transfusion. 2000;40(9):1118‐1126. [DOI] [PubMed] [Google Scholar]
- 62. Perros AJ, Christensen AM, Flower RL, Dean MM. Soluble mediators in platelet concentrates modulate dendritic cell inflammatory responses in an experimental model of transfusion. J Interferon Cytokine Res. 2015;35(10):821‐830. [DOI] [PubMed] [Google Scholar]
- 63. Aloui C, Prigent A, Sut C, et al. The signaling role of CD40 ligand in platelet biology and in platelet component transfusion. Int J Mol Sci. 2014;15(12):22342‐22364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Cognasse F, Sut C, Fromont E, Laradi S, Hamzeh‐Cognasse H, Garraud O. Platelet soluble CD40‐ligand level is associated with transfusion adverse reactions in a mixed threshold‐and‐hit model. Blood. 2017;130(11):1380‐1383. [DOI] [PubMed] [Google Scholar]
- 65. Khan SY, Kelher MR, Heal JM, et al. Soluble CD40 ligand accumulates in stored blood components, primes neutrophils through CD40, and is a potential cofactor in the development of transfusion‐related acute lung injury. Blood. 2006;108(7):2455‐2462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Sahler J, Spinelli S, Phipps R, Blumberg N. CD40 ligand (CD154) involvement in platelet transfusion reactions. Transfus Clin Biol. 2012;19(3):98‐103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Wakamoto S, Fujihara M, Kuzuma K, et al. Biologic activity of RANTES in apheresis PLT concentrates and its involvement in nonhemolytic transfusion reactions. Transfusion. 2003;43(8):1038‐1046. [DOI] [PubMed] [Google Scholar]
- 68. Tormey CA, Stack G. Use of a cytokine‐release assay to demonstrate loss of platelet secretory capacity during blood bank processing and storage. Arch Pathol Lab Med. 2014;138(11):1481‐1487. [DOI] [PubMed] [Google Scholar]
- 69. Savage WJ, Savage JH, Tobian AA, et al. Allergic agonists in apheresis platelet products are associated with allergic transfusion reactions. Transfusion. 2012;52(3):575‐581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Shanwell A, Falker C, Gulliksson H. Storage of platelets in additive solutions: the effects of magnesium and potassium on the release of RANTES, beta‐thromboglobulin, platelet factor 4 and interleukin‐7, during storage. Vox Sang. 2003;85(3):206‐212. [DOI] [PubMed] [Google Scholar]
- 71. Seghatchian J. Platelet storage lesion: an update on the impact of various leukoreduction processes on the biological response modifiers. Transfus Apher Sci. 2006;34(1):125‐130. [DOI] [PubMed] [Google Scholar]
- 72. Gao M, Zhang B, Zhang Y, et al. The effects of apheresis, storage time, and leukofiltration on microparticle formation in apheresis platelet products. Transfusion. 2018;58(10):2388‐2394. [DOI] [PubMed] [Google Scholar]
- 73. Rank A, Nieuwland R, Liebhardt S, et al. Apheresis platelet concentrates contain platelet‐derived and endothelial cell‐derived microparticles. Vox Sang. 2011;100(2):179‐186. [DOI] [PubMed] [Google Scholar]
- 74. Lin HC, Chang HW, Hsiao SH, Chou ML, Seghatchian J, Burnouf T. Platelet‐derived microparticles trigger THP‐1 monocytic cell aggregation and release of pro‐coagulant tissue factor‐expressing microparticles in vitro. Transfus Apher Sci. 2015;53(2):246‐252. [DOI] [PubMed] [Google Scholar]
- 75. Puhm F, Boilard E, Machlus KR. Platelet extracellular vesicles: beyond the blood. Arterioscler Thromb Vasc Biol. 2021;41(1):87‐96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Nollet KE, Saito S, Ono T, Ngoma A, Ohto H. Microparticle formation in apheresis platelets is not affected by three leukoreduction filters. Transfusion. 2013;53(10):2293‐2298. [DOI] [PubMed] [Google Scholar]
- 77. van der Wal DE, Rey Gomez LM, Hueneburg T, Linnane C, Marks DC. Changes in glycans on platelet microparticles released during storage of apheresis platelets are associated with phosphatidylserine externalization and phagocytosis. Transfusion. 2022;62(6):1289‐1301. [DOI] [PubMed] [Google Scholar]
- 78. Pannetier L, Tamagne M, Bocquet T, Pirenne F, Ansart‐Pirenne H, Vingert B. HLA molecule expression on the surface of cells and microparticles in platelet concentrates. Transfusion. 2021;61(4):1023‐1028. [DOI] [PubMed] [Google Scholar]
- 79. Sadallah S, Eken C, Martin PJ, Schifferli JA. Microparticles (ectosomes) shed by stored human platelets downregulate macrophages and modify the development of dendritic cells. J Immunol. 2011;186(11):6543‐6552. [DOI] [PubMed] [Google Scholar]
- 80. Yari F, Motefaker M, Nikougoftar M, Khayati Z. Interaction of platelet‐derived microparticles with a human B‐lymphoblast cell line: a clue for the immunologic function of the microparticles. Transfus Med Hemother. 2018;45(1):55‐61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Xie RF, Hu P, Li W, et al. The effect of platelet‐derived microparticles in stored apheresis platelet concentrates on polymorphonuclear leucocyte respiratory burst. Vox Sang. 2014;106(3):234‐241. [DOI] [PubMed] [Google Scholar]
- 82. Drawz SM, Marschner S, Yañez M, et al. Observational study of corrected count increments after transfusion of platelets treated with riboflavin pathogen reduction technology in additive solutions. Transfusion. 2015;55(7):1745‐1751. [DOI] [PubMed] [Google Scholar]
- 83. de Wildt‐Eggen J, Nauta S, Schrijver JG, van Marwijk Kooy M, Bins M, van Prooijen HC. Reactions and platelet increments after transfusion of platelet concentrates in plasma or an additive solution: a prospective, randomized study. Transfusion. 2000;40(4):398‐403. [DOI] [PubMed] [Google Scholar]
- 84. Johannsson F, Guethmundsson S, Paglia G, et al. Systems analysis of metabolism in platelet concentrates during storage in platelet additive solution. Biochem J. 2018;475(13):2225‐2240. [DOI] [PubMed] [Google Scholar]
- 85. de Wit YES, Vlaar R, Gouwerok E, et al. Platelet concentrates in platelet additive solutions generate less complement activation products during storage than platelets stored in plasma. Blood Transfus. 2022;21:157‐167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Cardigan R, Sutherland J, Wadhwa M, Dilger P, Thorpe R. The influence of platelet additive solutions on cytokine levels and complement activation in platelet concentrates during storage. Vox Sang. 2003;84(1):28‐35. [DOI] [PubMed] [Google Scholar]
- 87. Cid J, Magnano L, Molina P, et al. Automated preparation of whole blood‐derived platelets suspended in two different platelet additive solutions and stored for 7 days. Transfusion. 2014;54(2):426‐433. [DOI] [PubMed] [Google Scholar]
- 88. Valkonen S, Mallas B, Impola U, et al. Assessment of time‐dependent platelet activation using extracellular vesicles, CD62P exposure, and soluble glycoprotein V content of platelet concentrates with two different platelet additive solutions. Transfus Med Hemother. 2019;46(4):267‐275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Bashir S, Kemsley K, Min K, Swann ID, Cardigan R. Platelet storage in more than 90% additive solution containing glucose and bicarbonate has the potential to increase shelf life. Transfusion. 2018;58(12):2959‐2968. [DOI] [PubMed] [Google Scholar]
- 90. Nogawa M, Naito Y, Chatani M, et al. Parallel comparison of apheresis‐collected platelet concentrates stored in four different additive solutions. Vox Sang. 2013;105(4):305‐312. [DOI] [PubMed] [Google Scholar]
- 91. Morrison A, McMillan L, Radwanski K, Blatchford O, Min K, Petrik J. Storage of apheresis platelet concentrates after manual replacement of >95% of plasma with PAS 5. Vox Sang. 2014;107(3):247‐253. [DOI] [PubMed] [Google Scholar]
- 92. Yari F, Azadpour S, Shiri R. Platelet storage media change the expression characteristics of the platelet‐derived microparticles. Indian J Hematol Blood Transfus. 2014;30(3):169‐174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93. Oikawa S, Minegishi M, Endo K, et al. Impact of the platelet washing process on in vitro platelet properties, and the levels of soluble CD40 ligand and platelet‐derived microparticles in the storage media. Transfusion. 2019;59(3):1080‐1089. [DOI] [PubMed] [Google Scholar]
- 94. Johnson L, Tan S, Wood B, Davis A, Marks DC. Refrigeration and cryopreservation of platelets differentially affect platelet metabolism and function: a comparison with conventional platelet storage conditions. Transfusion. 2016;56(7):1807‐1818. [DOI] [PubMed] [Google Scholar]
- 95. Hoffmeister KM, Felbinger TW, Falet H, et al. The clearance mechanism of chilled blood platelets. Cell. 2003;122:1‐20. [DOI] [PubMed] [Google Scholar]
- 96. Wang S, Jiang T, Fan Y, Zhao S. A proteomic approach reveals the variation in human platelet protein composition after storage at different temperatures. Platelets. 2019;30(3):403‐412. [DOI] [PubMed] [Google Scholar]
- 97. Nair PM, Pandya SG, Dallo SF, et al. Platelets stored at 4 degrees C contribute to superior clot properties compared to current standard‐of‐care through fibrin‐crosslinking. Br J Haematol. 2017;178(1):119‐129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98. Stolla M, Bailey SL, Fang L, et al. Effects of storage time prolongation on in vivo and in vitro characteristics of 4 degrees C‐stored platelets. Transfusion. 2020;60(3):613‐621. [DOI] [PubMed] [Google Scholar]
- 99. Strandenes G, Sivertsen J, Bjerkvig CK, et al. A pilot trial of platelets stored cold versus at room temperature for complex cardiothoracic surgery. Anesthesiology. 2020;133(6):1173‐1183. [DOI] [PubMed] [Google Scholar]
- 100. Bailey SL, Fang LY, Fitzpatrick L, Byrne D, Pellham E, Stolla M. In vitro and in vivo effects of short‐term cold storage of platelets in PAS‐C. Haematologica. 2022;107(4):988‐990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101. Six KR, Delabie W, Devreese KMJ, et al. Comparison between manufacturing sites shows differential adhesion, activation, and GPIbalpha expression of cryopreserved platelets. Transfusion. 2018;58(11):2645‐2656. [DOI] [PubMed] [Google Scholar]
- 102. Tegegn TZ, De Paoli SH, Orecna M, et al. Characterization of procoagulant extracellular vesicles and platelet membrane disintegration in DMSO‐cryopreserved platelets. J Extracell Vesicles. 2016;5:30422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103. Napolitano M, Mancuso S, Raso S, et al. Buffy coat‐derived platelets cryopreserved using a new method: results from a pivotal clinical trial on thrombocytopenic patients with acute leukaemia. Transfus Apher Sci. 2019;58(6):102666. [DOI] [PubMed] [Google Scholar]
- 104. Slichter SJ, Dumont LJ, Cancelas JA, et al. Safety and efficacy of cryopreserved platelets in bleeding patients with thrombocytopenia. Transfusion. 2018;58(9):2129‐2138. [DOI] [PubMed] [Google Scholar]
- 105. Reade MC, Marks DC, Bellomo R, et al. A randomized, controlled pilot clinical trial of cryopreserved platelets for perioperative surgical bleeding: the CLIP‐I trial (Editorial, p. 2759). Transfusion. 2019;59(9):2794‐2804. [DOI] [PubMed] [Google Scholar]
- 106. Dasgupta SK, Argaiz ER, Mercado JE, et al. Platelet senescence and phosphatidylserine exposure. Transfusion. 2010;50(10):2167‐2175. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data sharing not applicable to this article as no datasets were generated or analyzed during the current study.


