Abstract
Extracellular Yersinia disables the immune system of its host by injecting effector Yop proteins into host cells. We show that a Yersinia enterocolitica nonpolar lcrG mutant is severely impaired in the translocation of YopE, YopH, YopM, YpkA/YopO, and YopP into eukaryotic cells. LcrG is thus required for efficient internalization of all the known Yop effectors.
The capacity of Yersinia species (Yersinia pestis, Y. pseudotuberculosis, and Y. enterocolitica) to resist the immune systems of their hosts depends on the Yop virulon, which is encoded by the 70-kb pYV plasmid. This virulon allows extracellular bacteria adhering to the surfaces of eukaryotic cells to inject bacterial proteins into the cytosol in order to disable these cells (9). Translocation of the intracellular effectors (YopE, YopH, YpkA/YopO, YopM, and YopP) across the eukaryotic cell membrane requires at least two other secreted proteins, namely, YopB and YopD (5, 12–14, 19, 22, 28, 33–35). The Yop proteins are secreted outside the bacterial cell by a contact (type III) secretion apparatus called Ysc (1, 2, 4, 10, 16, 18, 23, 24, 37). The translocators YopB and YopD are encoded by a large operon that also encodes LcrV and LcrG (3, 20, 26). LcrG is a 96-amino-acid (11-kDa) protein that appears to be involved in the control of Yop release (31). In addition, LcrG has been shown to bind LcrV, a protein required for the secretion of YopB and YopD (21, 29). Yop secretion occurs only when bacteria are in contact with eukaryotic cells or deprived of Ca2+ ions. A nonpolar lcrG mutant of Y. pestis is Ca2+ blind, secreting large amounts of Yops in the absence as well as in the presence of eukaryotic cells or Ca2+ ions, like the yopN mutants of Y. pseudotuberculosis and Y. enterocolitica and the tyeA mutant of Y. enterocolitica (5, 11, 14, 28, 31). In spite of their deregulated phenotype, yopN45 mutant bacteria (i.e., bacteria in which the yopN gene is interrupted after codon 45) can efficiently deliver Yop effectors into the cytosol of eukaryotic cells (5). YopN is thus thought to act at the level of Yop release as the stop valve of the secretion apparatus. TyeA is required for the translocation of a subset of Yop effectors (14). We have recently shown that LcrG can bind to HeLa cells via heparan sulfate proteoglycans and that addition of exogenous heparin can interfere with the translocation of Yops into HeLa cells (7). We inferred that LcrG could have an important role to play in translocation and that interaction with heparan sulfate could affect the activity of LcrG. In this work, we present evidence that LcrG is indeed essential for efficient translocation to occur.
Construction and characterization of an lcrG mutant.
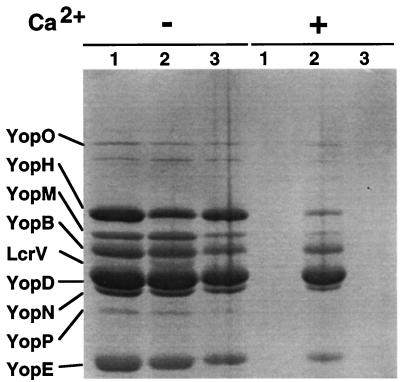
To investigate the role of LcrG in the secretion of Yops and their subsequent translocation into eukaryotic cells, we constructed an lcrG nonpolar mutant. First, we inactivated the chromosomal gene encoding β-lactamase A of Y. enterocolitica E40(pYV40) (34) with the mutator plasmid pKNG105 (15) to produce strain MRS40(pYV40). Next, 147 bp (bp 22 to 169) of lcrG were deleted from pMRS22 (Table 1) by site-directed mutagenesis (17) with oligonucleotide MIPA310 (5′-AGTCTTCCCATTTTGATAAGCTAGCGGAGCGCGAG-3′), which is identical to nucleotides 5 to 21 and nucleotides 170 to 187 of lcrG but which changes Pro58 to Leu. The mutated allele of lcrG, called lcrGΔ8–57, was verified by sequencing, cloned in a suicide vector, and introduced into MRS40(pYV40) to create strain MRS40(pMRS4043) (Table 1). The lcrG mutant strain was tested for Ca2+ dependency and in vitro Yop secretion (2, 8). The mutant was unable to grow at 37°C in the presence or absence of Ca2+ (data not shown) and as such was defined as growth thermosensitive. The Y. enterocolitica lcrG mutant secreted all the Yops in the presence and absence of Ca2+ (Fig. 1) and was thus Ca2+ blind, as was previously described for Y. pestis (31). The translocators YopB and YopD, whose genes are situated downstream of LcrG, are efficiently secreted, demonstrating the nonpolarity of the lcrG mutation. Yop secretion was prevented by Ca2+ ions after the introduction of plasmid pMSK23, containing lcrG alone transcribed from the yopE promoter, into MRS40(pMRS4043) (Table 1; Fig. 1). This confirmed the nonpolarity of the lcrG mutation.
TABLE 1.
Plasmids used in this work
| Plasmid | Genotype and/or description | Reference or origin |
|---|---|---|
| pAB409 | pYV40 yopHΔ1–352 yopE21 yopOΔ65–558 yopP23 yopM23 yopBΔ89–217 | 6, 19, 30, 36 |
| pABL403 | pYV40 yopHΔ1–352 yopE21 yopOΔ65–558 yopP23 yopM23 | 6, 19, 36 |
| pAB6 | pTM100 PyopM yopM100-cyaA′+; encodes YopM100-Cya | 5 |
| pCD10 | pTM100 PsycE yopO143-cyaA′+; encodes YopO143-Cya | 14 |
| pIM41 | pYV40 yopN45 | 5 |
| pMRS20 | pBluescriptII SK+ + PCR-amplified fragment (using MIPA271 [CCGGAATTCACTTTCATACCAAGAGCTGA] and MIPA64 [ATGTCGACCTGTCGTCTCTTGTTG]) of pYV227 (8) cloned in EcoRI and SalI sites; contains lcrRGV | This work |
| pMRS22 | pBluescriptII SK+ + HindIII-XmnI fragment of pMRS20; contains lcrR′ lcrG lcrV′ | This work |
| pMRS42 | pMRS22 lcrGΔ8–57; contains lcrR′ lcrGΔ8–57 lcrV′ | This work |
| pMRS43 | pKNG101 (15) + XbaI-SalI fragment of pMRS42; encoding lcrGΔ8–57 | This work |
| pMRS99 | pYV40 yopN45 lcrGΔ8–57 | 5; this work |
| pMRS4043 | pYV40 lcrGΔ8–57 | This work |
| pMS3 | pACYC184 + oriTRK2 + yopE sycE | 32 |
| pMS111 | pTM100 sycE+, PyopE yopE130-cyaA′+; encodes SycE and YopE130-Cya | 33 |
| pMSK3 | pTM100 PyopE yopP99-cyaA′+; encodes YopP99-Cya | 35 |
| pMSK23 | XbaI-HindIII deletion of pMRS72 (29); pBC19R; PyopE lcrG | This work |
| pMSK48 | pYV40 yopHΔ1–352 yopE21 yopOΔ65–558 yopP23 yopM23 lcrGΔ8–57 | 6, 19, 36; this work |
| pMSL41 | pYV40 yscNΔ169–177 (secretion mutant) | 34, 37 |
| pMSLH99 | pTM100 PyopH yopH99-cyaA′+; encodes YopH99-Cya | 34 |
| pPW401 | pYV40 yopBΔ89–217 (translocation mutant) | 5, 30 |
FIG. 1.
The lcrG mutant strain MRS40(pMRS4043) is Ca2+ blind. Yop secretion by the Y. enterocolitica wild-type strain MRS40(pYV40) (lane 1), the lcrG mutant strain MRS40(pMRS4043) (lane 2), and the complemented strain MRS40(pMRS4043)(pMSK23) (lane 3) in the absence (−) and in the presence (+) of Ca2+ was analyzed. Bacteria were grown in brain heart infusion-oxalate or brain heart infusion-Ca2+, and Yop secretion was induced for 4 h at 37°C. Purified Yops were subjected to sodium dodecyl sulfate-polyacrylamide gel electrophoresis and stained with Coomassie blue.
LcrG is involved in the translocation of the YopE cytotoxin.
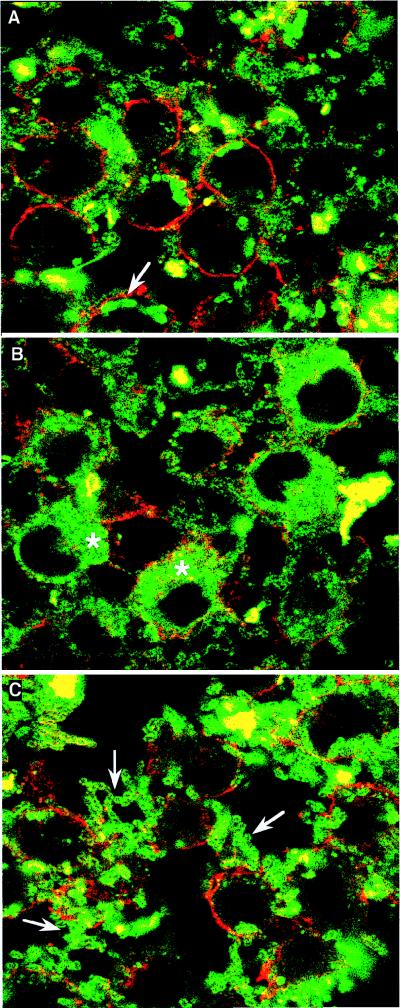
Wild-type Y. enterocolitica induces a cytotoxic response on HeLa cells that is characterized by the rounding up and detachment of the target cells due to the disruption of actin microfilaments (25, 27). After 2 h of infection, the lcrG mutant bacteria were unable to induce this cytotoxicity (data not shown). This observation suggested that the lcrG mutant was impaired in its ability to internalize YopE, the major cytotoxin, inside HeLa cells. To investigate this further, we introduced plasmid pMS111, encoding YopE130-Cya (i.e., a hybrid protein made of 130 residues of YopE fused to Cya), into wild-type Y. enterocolitica, a yscN secretion mutant, the lcrG mutant, a yopN mutant, and a yopB translocation mutant (Table 1). Cultured PU5-1.8 macrophages were infected with each of these strains in the presence of cytochalasin D. We monitored both the release of hybrid adenylate cyclase into the culture medium and the accumulation of cyclic AMP (cAMP) inside the eukaryotic cells. In good agreement with the Ca2+ blind phenotype, the lcrG and yopN mutant bacteria secreted much more YopE130-Cya into the culture medium than the wild-type strain (5) (Table 2). Hence, the lcrG mutant strain was able to efficiently secrete Yops in the presence of eukaryotic cells, but this Yop secretion was deregulated and probably independent of eukaryotic cell contact. Unlike the yopN mutant bacteria but like the yopB mutant bacteria, the lcrG mutant bacteria were unable to induce high levels of cAMP accumulation in the cytosol of PU5-1.8 macrophages (Table 2). LcrG was thus involved in the delivery of YopE into eukaryotic cells. Introduction of lcrG on plasmid pMSK23 into the lcrG mutant strain resulted in the recovery of the translocation ability of YopE, thus showing that the translocation phenotype was due solely to the defect in the lcrG gene (Table 2). To visualize directly the internalization of YopE inside eukaryotic cells, macrophages infected with wild-type and mutant lcrG isogenic Y. enterocolitica overproducing YopE from plasmid pMS3 (32) were subjected to immunostaining and examined by confocal microscopy. YopE appeared dispersed in the cytosol of macrophages infected with the wild-type bacteria but not in the cytosol of cells infected with the mutant lcrG bacteria (Fig. 2). Taken together, these results led us to conclude that LcrG is essential for the efficient translocation of YopE across the eukaryotic cell membrane.
TABLE 2.
Role of LcrG in the translocation of YopE-Cya, YopH-Cya, and YopM-Cya into PU5-1.8 macrophages
| Hybrid protein | Plasmid(s) | Characteristic(s) | cAMP concna | AC activity in RPMI mediumb |
|---|---|---|---|---|
| YopE130-Cya | pYV40 | Wild type | 5.7 ± 2.5 | 12.6 ± 7.5 |
| pMSL41 | yscN | 0.36 ± 0.14 | 0.30 | |
| pIM41 | yopN45 | 9.2 ± 2.5 | 125 | |
| pPW401 | yopBΔ89–217 | 0.09 ± 0.03 | 12.3 | |
| pMRS4043 | lcrGΔ8–57 | 0.24 ± 0.21 | 69.2 ± 15.8 | |
| pMRS99 | yopN45, lcrGΔ8–57 | 0.09 ± 0.02 | ND | |
| pMRS4043, pMSK23 | lcrGΔ8–57, lcrG+ | 9.6 | ND | |
| pYV40, pMSK23 | Wild type, lcrG+ | 8.5 | ND | |
| YopH99-Cya | pYV40 | Wild type | 2.0 ± 0.8 | 2.3 |
| pPW401 | yopBΔ89–217 | 0.27 ± 0.14 | 2.8 | |
| pMRS4043 | lcrGΔ8–57 | 0.17 ± 0.06 | 9.4 | |
| YopM100-Cya | pYV40 | Wild type | 1.2 ± 0.7 | 2.7 |
| pPW401 | yopBΔ89–217 | 0.03 ± 0.03 | 7.0 | |
| pMRS4043 | lcrGΔ8–57 | 0.38 ± 0.24 | 10.6 |
Data are means of two experiments (in nanomoles per milligram of protein) or three experiments (in nanomoles per milligram of protein ± standard deviation), each carried out in duplicate. Cells were infected with bacteria for 2 h in the presence of cytochalasin D.
Adenylate cyclase (AC) units per milliliter of supernatant from RPMI medium of macrophages. ND, not determined.
FIG. 2.
Delivery of YopE into macrophages. PU5-1.8 macrophages grown on coverslips were infected for 2 h with Y. enterocolitica MRS40(pPW401)(pMS3), a yopB mutant bacterium overproducing YopE (A); MRS40(pYV40)(pMS3), a wild-type bacterium overproducing YopE (B); or MRS40(pMRS4043)(pMS3), an lcrG mutant bacterium also overproducing YopE (C). The asterisk indicates YopE inside the cytosol of the macrophages. The arrows indicate bacteria. After infection, the cells were fixed, incubated with purified anti-YopE antibodies, stained with fluorescein isothiocyanate-labelled anti-rabbit antiserum, and examined by confocal microscopy. The eukaryotic cell membranes were labelled with wheat germ agglutinin-Texas red. Each panel shows a single optical plane at the level of the nucleus. Note that in panel C, bacteria are heavily stained because of deregulated and depolarized Yop secretion.
We also tested the secretion and translocation phenotypes of a Yersinia lcrG yopN double mutant strain (pMRS99) (Table 1). This strain was Ca2+ blind for Yop secretion like the lcrG and yopN individual mutant strains (data not shown). However, the lcrG yopN mutant strain did not significantly translocate YopE130-Cya into macrophages (Table 2). Thus, the function of LcrG is not solely to control the opening of the Yop secretion pore by YopN to allow Yop release and subsequent translocation. Rather, LcrG is itself independently required for optimal translocation of YopE.
LcrG is involved in the internalization of YopH, YopM, YopO, and YopP.
We then investigated whether translocation of YopH99-Cya and YopM100-Cya was also dependent on LcrG (Table 1). Although the lcrG mutant bacteria secreted more YopH99-Cya and YopM100-Cya into the culture medium than the wild-type bacteria, they did not induce significant accumulation of cAMP in infected macrophages (Table 2). Thus, the efficient internalization of YopH and YopM was also dependent on the presence of LcrG.
We also wanted to look at the translocation of YopO143-Cya and YopP99-Cya into eukaryotic cells (Table 3). Because these Yops are not translocated as efficiently as YopE, YopH, and YopM, this must be studied in a Y. enterocolitica strain lacking the Yop effectors YopE, YopH, YopO, YopP, and YopM (12, 14, 35). Due to the lack of competition for the secretion and translocation apparatuses, the translocation of the Yop-Cya hybrid is optimized. We thus introduced the lcrGΔ8–57 allele into the Yop effector polymutant strain MRS40(pABL403) (Table 1). As can be seen in Table 3, the translocation of YopO143-Cya, YopP99-Cya, and the other Yop-Cya hybrid proteins was greatly reduced in the lcrG mutant strain compared to that in the parental strain. The level of translocation of each of the hybrid Cya proteins by the polymutant lcrG was almost similar to that of the polymutant yopB strain. Thus, LcrG is involved in the translocation of all the known effector Yops.
TABLE 3.
Role of LcrG in the translocation of YopO-Cya and YopP-Cya into PU5-1.8 macrophages
| Hybrid protein | pYV | Genotype | cAMP concna |
|---|---|---|---|
| YopE130-Cya | pABL403 | yopH yopE yopO yopP yopM | 7.8 |
| pAB409 | yopH yopE yopO yopP yopM yopB | 0.35 | |
| pMSK48 | yopH yopE yopO yopP yopM lcrG | 0.58 | |
| YopH99-Cya | pABL403 | yopH yopE yopO yopP yopM | 9.5 |
| pAB409 | yopH yopE yopO yopP yopM yopB | 0.71 | |
| pMSK48 | yopH yopE yopO yopP yopM lcrG | 1.4 | |
| YopM100-Cya | pABL403 | yopH yopE yopO yopP yopM | 12.8 |
| pAB409 | yopH yopE yopO yopP yopM yopB | 0.09 | |
| pMSK48 | yopH yopE yopO yopP yopM lcrG | 0.28 | |
| YopO143-Cya | pABL403 | yopH yopE yopO yopP yopM | 10.7 |
| pAB409 | yopH yopE yopO yopP yopM yopB | 0.03 | |
| pMSK48 | yopH yopE yopO yopP yopM lcrG | 0.25 | |
| YopP99-Cya | pABL403 | yopH yopE yopO yopP yopM | 1.4 |
| pAB409 | yopH yopE yopO yopP yopM yopB | 0.08 | |
| pMSK48 | yopH yopE yopO yopP yopM lcrG | 0.10 |
Data are means of two experiments (in nanomoles per milligram of protein) carried out in duplicate. Cells were infected for 2 h in the presence of cytochalasin D.
Conclusions.
The phenotype of the newly constructed Y. enterocolitica lcrG mutant is unique. Not only is it Ca2+ blind like the yopN and tyeA mutants (5, 11, 14, 28, 31), but it is also a weak Yop translocator like the yopB and yopD mutants (5, 22, 28). This phenotype clearly shows that LcrG is involved in translocation of all the Yop effectors. This is in contrast to the yopN mutant, which translocates all the Yops efficiently, and the tyeA mutant, which is required for the translocation of only a subset of Yop effectors, namely, YopE and YopH (5, 18).
There are several possibilities regarding the role of LcrG in translocation. LcrG could be an essential element of the translocation machinery along with YopB and YopD. It is also possible that LcrG is an element regulating the deployment of the translocation apparatus or the action of the translocation process itself. We have recently shown that LcrG can bind to HeLa cells via heparan sulfate proteoglycans and that heparin can interfere with the translocation of Yops inside HeLa cells (7). Thus, LcrG could be a Yop apparatus ligand whose interaction with heparan sulfate proteoglycans augments its function in the translocation of Yops into eukaryotic cells. We plan to investigate these possibilities in greater detail in our future work.
Acknowledgments
We thank D. Desnoeck, I. Lambermont, and C. Kerbouch for excellent technical assistance.
M.R.S. was a recipient of a Sociéte Générale de Belgique fellowship and A.P.B. was a recipient of a Brenninkmeijer fellowship, both awarded by I.C.P. This work was supported by the Belgian FRSM (3.4595.97), the Direction générale de la Recherche Scientifique-Communauté Française de Belgique (ARC 94/99-172), and the Belgium Federal Office for Scientific, Technical and Cultural affairs (PAI 4/03). Confocal microscopy was funded by credit 9.4531.94F from FRSM (Loterie Nationale).
REFERENCES
- 1.Allaoui A, Woestyn S, Sluiters C, Cornelis G R. YscU, a Yersinia enterocolitica inner membrane protein involved in Yop secretion. J Bacteriol. 1994;176:4534–4542. doi: 10.1128/jb.176.15.4534-4542.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Allaoui A, Scheen R, Lambert de Rouvroit C, Cornelis G R. VirG, a Yersinia enterocolitica lipoprotein involved in Ca2+ dependency, is related to ExsB of Pseudomonas aeruginosa. J Bacteriol. 1995;177:4230–4237. doi: 10.1128/jb.177.15.4230-4237.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bergman T, Håkansson S, Forsberg Å, Norlander L, Macellaro A, Bäckman A, Bölin I, Wolf-Watz H. Analysis of the V antigen lcrGVH-yopBD operon of Yersinia pseudotuberculosis: evidence for a regulatory role of LcrH and LcrV. J Bacteriol. 1991;173:1607–1616. doi: 10.1128/jb.173.5.1607-1616.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bergman T, Erickson K, Galyov E, Persson C, Wolf-Watz H. The lcrB (yscN/U) gene cluster of Yersinia pseudotuberculosis is involved in Yop secretion and shows high homology to the spa gene clusters of Shigella flexneri and Salmonella typhimurium. J Bacteriol. 1994;176:2619–2626. doi: 10.1128/jb.176.9.2619-2626.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Boland A, Sory M-P, Iriarte M, Kerbourch C, Wattiau P, Cornelis G R. Status of YopM and YopN in the Yersinia Yop virulon: YopM of Y. enterocolitica is internalized inside the cytosol of PU5-1.8 macrophages by the YopB, D, N delivery apparatus. EMBO J. 1996;15:5191–5201. [PMC free article] [PubMed] [Google Scholar]
- 6.Boland A, Cornelis G R. Role of YopP in suppression of tumor necrosis factor alpha release by macrophages during Yersinia infection. Infect Immun. 1998;66:1878–1884. doi: 10.1128/iai.66.5.1878-1884.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Boyd A P, Sory M-P, Iriarte M, Cornelis G R. Heparin interferes with translocation of Yop proteins into HeLa cells and binds to LcrG, a regulatory component of the Yersinia Yop apparatus. Mol Microbiol. 1998;27:425–436. doi: 10.1046/j.1365-2958.1998.00691.x. [DOI] [PubMed] [Google Scholar]
- 8.Cornelis G R, Vanooteghem J C, Sluiters C. Transcription of the yop regulon from Y. enterocolitica requires trans acting pYV and chromosomal genes. Microb Pathog. 1987;2:367–379. doi: 10.1016/0882-4010(87)90078-7. [DOI] [PubMed] [Google Scholar]
- 9.Cornelis G R, Wolf-Watz H. The Yersinia Yop virulon: a bacterial system for subverting eukaryotic cells. Mol Microbiol. 1997;23:861–867. doi: 10.1046/j.1365-2958.1997.2731623.x. [DOI] [PubMed] [Google Scholar]
- 10.Fields K A, Plano G V, Straley S C. A low-Ca2+ response (LCR) secretion (ysc) locus lies within the lcrB region of the LCR plasmid in Yersinia pestis. J Bacteriol. 1994;176:569–579. doi: 10.1128/jb.176.3.569-579.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Forsberg A, Viitanen A M, Skurnik M, Wolf-Watz H. The surface-located YopN protein is involved in calcium signal transduction in Yersinia pseudotuberculosis. Mol Microbiol. 1991;5:977–986. doi: 10.1111/j.1365-2958.1991.tb00773.x. [DOI] [PubMed] [Google Scholar]
- 12.Håkansson S, Galyov E E, Rosqvist R, Wolf-Watz H. The Yersinia YpkA Ser/Thr kinase is translocated and subsequently targeted to the inner surface of the HeLa cell plasma membrane. Mol Microbiol. 1996;20:593–603. doi: 10.1046/j.1365-2958.1996.5251051.x. [DOI] [PubMed] [Google Scholar]
- 13.Håkansson S, Schesser K, Persson C, Galyov E E, Rosqvist R, Homble F, Wolf-Watz H. The YopB protein of Yersinia pseudotuberculosis is essential for the translocation of Yop effector proteins across the target cell plasma membrane and displays a contact dependent membrane disrupting activity. EMBO J. 1996;15:5812–5823. [PMC free article] [PubMed] [Google Scholar]
- 14.Iriarte M, Sory M-P, Boland A, Boyd A P, Mills S D, Lambermont I, Cornelis G R. TyeA: a protein involved in control of Yop release and in translocation of Yersinia Yop effectors. EMBO J. 1998;17:1907–1918. doi: 10.1093/emboj/17.7.1907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kaniga K, Delor I, Cornelis G R. A wide-host-range suicide vector for improving reverse genetics in gram-negative bacteria: inactivation of the blaA gene of Yersinia enterocolitica. Gene. 1991;109:137–141. doi: 10.1016/0378-1119(91)90599-7. [DOI] [PubMed] [Google Scholar]
- 16.Koster M, Bitter W, de Cock H, Allaoui A, Cornelis G R, Tommassen J. The outer membrane component, YscC, of the Yop secretion machinery of Yersinia enterocolitica forms a ring-shaped multimeric complex. Mol Microbiol. 1997;26:789–797. doi: 10.1046/j.1365-2958.1997.6141981.x. [DOI] [PubMed] [Google Scholar]
- 17.Kunkel T A, Roberts J D, Zakour R A. Rapid and efficient site-specific mutagenesis without phenotypic selection. Methods Enzymol. 1987;154:367–382. doi: 10.1016/0076-6879(87)54085-x. [DOI] [PubMed] [Google Scholar]
- 18.Michiels T, Vanooteghem J-C, Lambert de Rouvroit C, China B, Gustin A, Boudry P, Cornelis G R. Analysis of virC, an operon involved in the secretion of Yop proteins by Yersinia enterocolitica. J Bacteriol. 1991;173:4994–5009. doi: 10.1128/jb.173.16.4994-5009.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mills S D, Boland A, Sory M-P, van der Smissen P, Kerbouch C, Finlay B B, Cornelis G R. Yersinia enterocolitica induces apoptosis in macrophages by a process requiring functional type III secretion and translocation mechanisms and involving YopP, presumably acting as an effector protein. Proc Natl Acad Sci USA. 1997;94:12638–12643. doi: 10.1073/pnas.94.23.12638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mulder B, Michiels T, Simonet M, Sory M-P, Cornelis G R. Identification of additional virulence determinants on the pYV plasmid of Yersinia enterocolitica W227. Infect Immun. 1989;57:2534–2541. doi: 10.1128/iai.57.8.2534-2541.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Nilles M L, Williams A W, Skrzypek E, Straley S C. Yersinia pestis LcrV forms a stable complex with LcrG and may have a secretion-related regulatory role in the low-Ca2+ response. J Bacteriol. 1997;179:1307–1316. doi: 10.1128/jb.179.4.1307-1316.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Persson C, Nordfelth R, Holmström A, Håkansson S, Rosqvist R, Wolf-Watz H. Cell-surface-bound Yersinia translocate the protein tyrosine phosphatase YopH by a polarized mechanism into the target cell. Mol Microbiol. 1995;18:135–150. doi: 10.1111/j.1365-2958.1995.mmi_18010135.x. [DOI] [PubMed] [Google Scholar]
- 23.Plano G V, Straley S C. Multiple effects of lcrD mutations in Yersinia pestis. J Bacteriol. 1993;175:3536–3545. doi: 10.1128/jb.175.11.3536-3545.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Plano G V, Straley S C. Mutations in yscC, yscD, and yscG prevent high-level expression and secretion of V antigen and Yops in Yersinia pestis. J Bacteriol. 1995;177:3843–3854. doi: 10.1128/jb.177.13.3843-3854.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Portnoy D A, Moseley S L, Falkow S. Characterization of plasmids and plasmid-associated determinants of Yersinia enterocolitica pathogenesis. Infect Immun. 1981;31:775–782. doi: 10.1128/iai.31.2.775-782.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Price S B, Leung K Y, Barve S S, Straley S C. Molecular analysis of lcrGVH, the V antigen operon of Yersinia pestis. J Bacteriol. 1989;171:5646–5653. doi: 10.1128/jb.171.10.5646-5653.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Rosqvist R, Forsberg Å, Wolf-Watz H. Intracellular targeting of the Yersinia YopE cytotoxin in mammalian cells induces actin microfilament disruption. Infect Immun. 1991;59:4562–4569. doi: 10.1128/iai.59.12.4562-4569.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Rosqvist R, Magnusson K E, Wolf-Watz H. Target cell contact triggers expression and polarized transfer of Yersinia YopE cytotoxin into mammalian cells. EMBO J. 1994;13:964–972. doi: 10.1002/j.1460-2075.1994.tb06341.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sarker M R, Neyt C, Stainier I, Cornelis G R. The Yersinia Yop virulon: LcrV is required for extrusion of the translocators YopB and YopD. J Bacteriol. 1998;180:1207–1214. doi: 10.1128/jb.180.5.1207-1214.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Schulte R, Wattiau P, Hartland E L, Robins-Browne R M, Cornelis G R. Differential secretion of interleukin-8 by human epithelial cell lines upon entry of virulent or nonvirulent Yersinia enterocolitica. Infect Immun. 1996;64:2106–2113. doi: 10.1128/iai.64.6.2106-2113.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Skrzypek E, Straley S C. LcrG, a secreted protein involved in negative regulation of the low-calcium response in Yersinia pestis. J Bacteriol. 1993;175:3520–3528. doi: 10.1128/jb.175.11.3520-3528.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sory M-P, Kaniga K, Goldenberg S, Cornelis G R. Expression of the eukaryotic Trypanosoma cruzi CRA gene in Yersinia enterocolitica and induction of an immune response against CRA in mice. Infect Immun. 1992;60:3830–3836. doi: 10.1128/iai.60.9.3830-3836.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sory M-P, Cornelis G R. Translocation of a hybrid YopE-adenylate cyclase from Yersinia enterocolitica into HeLa cells. Mol Microbiol. 1994;14:583–594. doi: 10.1111/j.1365-2958.1994.tb02191.x. [DOI] [PubMed] [Google Scholar]
- 34.Sory M-P, Boland A, Lambermont I, Cornelis G R. Identification of YopE and YopH domains required for secretion and internalization into the cytosol of macrophages, using the cyaA gene fusion approach. Proc Natl Acad Sci USA. 1995;92:11998–12002. doi: 10.1073/pnas.92.26.11998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Sory, M.-P., C. Kerbouch, and G. R. Cornelis. Unpublished results.
- 36.Wattiau P, Cornelis G R. SycE, a chaperone-like protein of Yersinia enterocolitica involved in the secretion of YopE. Mol Microbiol. 1993;8:123–131. doi: 10.1111/j.1365-2958.1993.tb01209.x. [DOI] [PubMed] [Google Scholar]
- 37.Woestyn S, Allaoui A, Wattiau P, Cornelis G R. YscN, the putative energizer of the Yersinia Yop secretion machinery. J Bacteriol. 1994;176:1561–1569. doi: 10.1128/jb.176.6.1561-1569.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]