Abstract
The neck control strategies of early-stage oral squamous cell carcinoma (OSCC) patients with clinical node-negative neck remain uncertain. These patients could be benefit from elective neck dissection (END) alongside primary tumor excision; but current evidence on END versus observation for OSCC of stage I only is not yet analyzed collectively in detail. Herein, this short communication aimed to evaluate the neck control strategies of stage I OSCC, mainly END versus observation. A total of 740 patients with stage I OSCC, comprising 434 underwent END and 306 received observation, were identified from literature. The results showed that stage I OSCC patients would not be benefit from END based on the analysis of neck nodal recurrence and overall survival. An ideal strategy would likely be to avoid neck dissection for stage I OSCC patients with N0 neck. Immune checkpoint therapy is such a potential strategy, which aims at eliciting potent antitumor immune responses within lymph nodes hold promise for treating patients with early-stage OSCC and may prove more efficacious than lymphadenectomy in a variety of scenarios. Consequently, neck dissection for stage I OSCC could be approached with caution, particularly in patients receiving immune checkpoint therapy.
Keywords: Immune checkpoint blockade, Immunotherapy, Lymph node metastasis, Oral cancer, Elective neck dissection
Introduction
Oral squamous cell carcinoma (OSCC) is characterized by a high risk of neck regional lymph node (LN) metastasis, especially the tumor-draining lymph nodes (tdLNs).1 LN metastasis is the most significant prognostic factor for recurrence and survival rate, reducing the survival rate by 50%.2 Although there is a consensus that neck dissection must be considered when apparent LN metastasis is clinically found, the strategies of neck control of early-stage OSCC patients with clinical node-negative (cN0) neck remain uncertain. Increasing evidence indicates that elective neck dissection (END) alongside tumor excision for early-stage OSCC patients with cN0 neck can reduce the risk of neck nodal recurrence and improve overall/disease-free survival compared to observation alone.2 Of note, the previous systematic review and meta-analyses always analyzed stage I (T1N0M0) in combined with stage II (T2N0M0);3, 4, 5 but the evidence on END versus observation for OSCC of stage I only is not yet analyzed collectively in detail.
Immunotherapy that changed the therapeutic scenario in oncology are involved in the interplay between tumor cells and T lymphocytes. Programmed cell death (PD)-1/PD-ligand 1 (L1) axis and cytotoxic T-lymphocyte antigen 4 family represent the targets of immune checkpoint blockade (ICB) therapy.6 For head and neck cancer, the vast majority of the preclinical and clinical studies on immunotherapy were conducted in locally advanced and recurrent/metastatic head and neck squamous cell carcinomas (HNSCC).6, 7, 8 In contrast, few studies on immunotherapy were conducted in early-stage head and neck cancer. Using paired tumor and LN samples (uninvolved [uiLN] and/or metastatic [metLN]) obtained from patients with locally advanced HNSCC, a high-impact study by Rahim et al.9 recently reported that uiLNs play a pivotal role in ICB immunotherapy. Surgical removal of the uiLNs was demonstrated to be disrupt responses to ICB therapy in mouse models and human patients.10
In such a context, the objective of this short communication is to evaluate the neck control strategies of stage I OSCC including END, observation, and immunotherapy, and put forward a perspective that stage I OSCC patients received primary tumor resection without neck dissection benefit from ICB immunotherapy.
Materials and methods
As per the methodology described previously,3, 4, 5 a systematic literature search regarding the studies in English language on END versus observation for OSCC of stage I only from PubMed, Cochrane libraries, and EMBASE databases was conducted on Mar 22, 2023. Medical subject term “stage I or T1N0∗“, “neck dissection”, “immunotherapy” “immune checkpoint”, and “OSCC” and its synonyms in title/abstract were used, according to the search strategy described in Supplementary Table S1. Studies included in this analysis met the following inclusion criteria: (i) physical examination (neck palpation), ultrasonography, and imaging examination (computed tomography, magnetic resonance imaging, or positron emission tomography–computed tomography) confirmed stage I (cT1N0M0); (ii) The diagnosis of OSCC was confirmed by pathologic examination; (iii) These patients did not receive any prior treatment and received surgical excision of the primary tumor with or without END. (iv) The studies had reported the clinical outcomes for both groups and the reported outcome measures included neck nodal recurrence (NNR), disease-free survival (DFS), or overall survival (OS). Studies with insufficient data and animal experiments were excluded. Data search and extraction were undertaken independently by two investigators (C.Y. and W.L.), and any disagreement was resolved in a consensus symposium. Statistical analysis was performed by applying Review manager version 5.4 (Cochrane Collaboration, Oxford, UK), as per the statistical methods described by Cai et al.5
Results
Evaluation of elective neck dissection versus observation for stage I oral squamous cell carcinoma
As presented in Table 1, there were 8 eligible studies which addressed the issue of END versus observation for OSCC of stage I. These were retrospective studies with different follow-up times (range, 1–196 months) from 6 countries. A total of 740 patients with stage I OSCC, comprising 434 treated with END and 306 received observation, were identified. The mean/median age ranged from 52 to 60.7 years, and male patients outnumbered female patients. For primary tumor sites involved by OSCC, 6 studies contained only tongue carcinoma as the most common OSCC. Based on the available data on the outcomes of END versus observation, 7 studies reported the outcome of neck nodal recurrence (NNR), 5 and 3 studies reported the outcome of DFS and OS, respectively. Begg's funnel plot showed that there was no evidence for publication bias in these studies on the outcomes of NNR, DFS, and OS (P > 0.05, Egger's test; Supplementary Fig. S1).
Table 1.
Characteristics of included studies on elective neck dissection (END) versus observation (OBS) for OSCC of stage I.
| Authors, year | Location | Study Design | No. of patients | Age (Mean/median, range, y) | Male/Female | Follow-up (Mean, range, m) | Cancer site | END/OBS (n) | Outcome measure |
|---|---|---|---|---|---|---|---|---|---|
| Davies et al. 201711 | UK | Retrospective | 148 | NA | NA | NA | Oral cavity | 88/60 | NNR, OS |
| Huang et al. 201712 | China | Retrospective | 101 | NA | NA | 4–84 | Tongue | 67/34 | DFS, OS |
| Peng et al. 201413 | USA | Retrospective | 123 | 56, 27-92 | 64/59 | 29, 1-196 | Tongue | 88/35 | NNR, DFS |
| Zhang et al. 201414 | USA | Retrospective | 65 | 60.7, 24-91 | 32/33 | 56.8, 3-148 | Tongue | 36/29 | NNR, DFS |
| Liu et al. 201115 | China | Retrospective | 131 | 52, 21-91 | 79/52 | NA | Tongue | 88/43 | NNR, DFS, OS |
| Ryott, 201116 | Sweden | Retrospective | 74 | NA | NA | 40 | Tongue | 30/44 | NNR |
| An et al. 200817 | Korea | Retrospective | 49 | 56, 26-88 | 35/28 | 59, 12-191 | Tongue | 13/36 | NNR, DFS, OS |
| Dias et al. 200118 | Brazil | Retrospective | 49 | 59, 37-92 | 32/17 | 57, 7-153 | Tongue, mouth floor | 24/25 | NNR, DFS, OS |
DFS, disease-free survival; NA, not available; NNR, neck nodal recurrence; OS, overall survival; OSCC, oral squamous cell carcinomas.
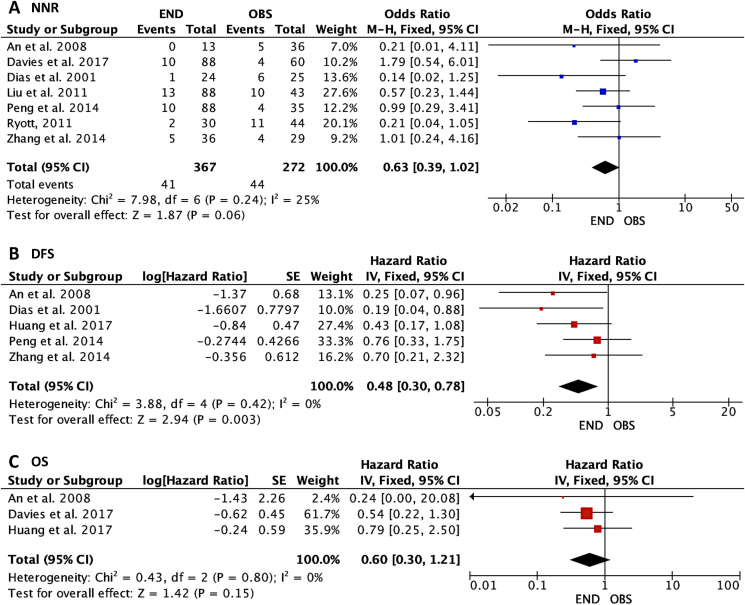
As shown in Fig. 1, 367 patients underwent END and 272 received observation were identified to analyze the outcome of NNR in stage I OSCC. A marginally significant association of NNR with END versus observation was found with the fixed effect (odds ratio, 0.63; 95% confidence interval [CI], 0.39–1.02; P = 0.06), suggested that END could have a lower risk of NNR compared to the observation group. As for DFS, 228 patients underwent END and 159 received observation were identified to analyze this outcome. A significant association of DFS with END versus observation was found with the fixed effect (hazard ratio, 0.48; 95%CI, 0.30–0.78; P = 0.003), suggested that END decreased the risk of DFS compared with the observation group. As for OS, 168 patients underwent END and 130 received observation were identified to analyze this outcome. Lack of association of OS with END versus observation was found with the fixed effect (hazard ratio, 0.60; 95%CI, 0.30–1.21; P = 0.15), suggested that END could not influence OS compared with the observation group.
Figure 1.
Forest plots of the association between elective neck dissection (END) versus observation (OBS) in cT1N0 oral squamous cell carcinoma (OSCC) and (A) neck nodal recurrence (NNR), (B) disease-free survival (DFS), and (C) overall survival (OS).
Perspective on immune checkpoint therapy for stage I oral squamous cell carcinoma
There was lack of eligible study which addressed the issue of ICB immunotherapy for stage I OSCC. Based on the results of a high-impact study by Rahim et al.,9 we put forward a perspective that stage I OSCC patients may benefit from ICB immunotherapy. Rahim et al.9 identified the progenitor exhausted T (Tpex) cells being a population of CD8+ T cells from uiLNs of human HNSCC in modulating anti-tumor in response to ICB immunotherapy. Tpex cells are clonally increased in uiLNs and peripheral blood of HNSCC patients following anti-PD-L1 immunotherapy before surgery, these clone cells expanded within the primary tumor, suggestive of migration from tdLNs and differentiation into intermediate exhausted T cells.9 Thus, the efficacy of ICB immunotherapy is reliant upon activation of tdLN-resident lymphocytes whose subsequent migration to the tumor microenvironment is an essential feature of effective antitumor immunity. Meanwhile, the presence of LN metastases in patients correlated with impaired activation of these T cells within the metLNs and reduced responses to anti-PD-L1 immunotherapy.9 This indicates metLNs may be less responsive to ICB owing to suppression of Tpex cell populations, and metLNs removal might prove less consequential than earlier stages where tdLNs still harbor the potential for activation following ICB immunotherapy. Thus, an ideal strategy would likely be to avoid neck dissection for OSCC patients with N0 neck, particularly in patients receiving ICB immunotherapy.
Discussion
Although early-stage (stage I/II) OSCC patients with clinical N0 neck could be benefit from END alongside primary tumor excision, definitive evidence of its value is lacking due to publication bias and certain limitations mainly being heterogeneity among those studies.3, 4, 5 Moreover, some clinicians and investigators prefer and recommend an observation policy partly because neck dissection adversely affects patients' quality of life and increased postoperative complications and costs. Neck dissection cause damage to neck structures and might be too invasive for patients with N0 neck; after all, over 70% of early-stage OSCC patients eventually remain node negativity.2 A systematic review and meta-analysis reported that 10.5% (95%CI, 8.7–12.7%) of occult metastatic incidence among T1 tumors was significantly lower than 24.5% (95%CI, 22.1–27.0%) for T2 tumors.2 More importantly, a systematic review and meta-analysis reported that patients following pathologically node-negative neck dissection (pN0) staged T1-T2 with regional nodal recurrence was still 7.5% (95%CI, 6.4–8.7%), and concluded that a pathologically negative neck did not guarantee against future recurrence.19 Consistently, the results of this report indicated that stage I OSCC patients would not be benefit from END based on the analysis of neck nodal recurrence and OS.
LNs are key facilitators of adaptive immunity and harbor vast numbers of potentially tumor-reactive lymphocytes. There is increasing evidence that specific subsets of CD8+ T cells must be recruited from the LNs to the tumor to drive therapeutic responses.10 Antitumor immunity and the generation of tumor-specific cytotoxic CD8+ T cells are reliant upon the processes within LNs, which is essential for the efficacy of immunotherapy and targeting ICB to LNs can enhance efficacy. Indeed, studies in mice and humans have shown that Tpex cells provide a continual reservoir of antigen-specific T cells, robustly respond to ICB immunotherapy.10 By targeting anti-PD-L1 to tdLNs, Tpex cell seeding of tumors is increased and the therapeutic response is enhanced. Direct surgical removal of the LNs disrupts responses to ICB in mouse models and human patients.10 On the one hand, the procedure removes a reservoir of potentially metastatic cells within metLNs and their immunosuppressive effects, perhaps preventing locoregional LN recurrence and improving progression-free survival. On the other hand, it also removes the main hubs of immune education and tumor antigen presentation in entire LN basin (both uiLNs and metLNs), thus impeding the capacity of the immune system to generate systemic antitumor immunity and improve overall survival. Future immunotherapies would likely benefit from the inclusion of features to specifically target tdLNs, where are essential sites for priming antitumor T cell responses and they can drive tumor antigen-specific immunity and treat metastatic disease.
Collectively, an ideal strategy would likely be to avoid neck dissection for stage I OSCC patients with N0 neck, since the therapeutic benefit of neck dissection remains uncertain. The strategy that aims at eliciting potent antitumor immune responses within LNs hold promise for treating patients with early-stage OSCC and may prove more efficacious than neck dissection in a variety of scenarios. Consequently, for stage I OSCC, we argue that neck dissection should be approached with caution, particularly in patients receiving ICB immunotherapy. Hence, it is urgent to assess the design of clinical trials to investigate whether early-stage (N0 neck) OSCC patients received primary tumor resection without neck dissection benefit from ICB immunotherapy.
Declaration of competing interest
The authors have no conflicts of interest relevant to this article.
Acknowledgments
This work was supported by National Natural Science Foundation of China (82170952), Two hundred talent project of Shanghai Jiao Tong University School of Medicine, and Innovative Research Team of High-level Local Universities in Shanghai (SHSMU-ZLCX20212401).
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.jds.2023.09.006.
Contributor Information
Linjun Shi, Email: shi-linjun@hotmail.com.
Wei Liu, Email: liuweb@hotmail.com.
Appendix A. Supplementary data
The following are the Supplementary data to this article.
References
- 1.Chamoli A., Gosavi A.S., Shirwadkar U.P., et al. Overview of oral cavity squamous cell carcinoma: risk factors, mechanisms, and diagnostics. Oral Oncol. 2021;121 doi: 10.1016/j.oraloncology.2021.105451. [DOI] [PubMed] [Google Scholar]
- 2.Massey C., Dharmarajan A., Bannuru R.R., Rebeiz E. Management of N0 neck in early oral squamous cell carcinoma: a systematic review and meta-analysis. Laryngoscope. 2019;129:e284–e298. doi: 10.1002/lary.27627. [DOI] [PubMed] [Google Scholar]
- 3.Oh L.J., Phan K., Kim S.W., Low T.H., Gupta R., Clark J.R. Elective neck dissection versus observation for early-stage oral squamous cell carcinoma: systematic review and meta-analysis. Oral Oncol. 2020;105 doi: 10.1016/j.oraloncology.2020.104661. [DOI] [PubMed] [Google Scholar]
- 4.Ibrahim S.A., Ahmed A.N.A., Elsersy H.A., Darahem I.M.H. Elective neck dissection in T1/T2 oral squamous cell carcinoma with N0 neck: essential or not? A systematic review and meta-analysis. Eur Arch Oto-Rhino-Laryngol. 2020;277:1741–1752. doi: 10.1007/s00405-020-05866-3. [DOI] [PubMed] [Google Scholar]
- 5.Cai H., Zhu Y., Wang C., Zhang Y., Hou J. Neck nodal recurrence and survival of clinical T1-2 N0 oral squamous cell carcinoma in comparison of elective neck dissection versus observation: a meta-analysis. Oral Surg Oral Med Oral Pathol Oral Radiol. 2020;129:296–310. doi: 10.1016/j.oooo.2019.10.012. [DOI] [PubMed] [Google Scholar]
- 6.Fasano M., Corte C.M.D., Liello R.D., et al. Immunotherapy for head and neck cancer: present and future. Crit Rev Oncol Hematol. 2022;174 doi: 10.1016/j.critrevonc.2022.103679. [DOI] [PubMed] [Google Scholar]
- 7.Nindra U., Hurwitz J., Forstner D., Chin V., Gallagher R., Liu J. A systematic review of neoadjuvant and definitive immunotherapy in locally advanced head and neck squamous cell carcinoma. Cancer Med. 2023;12:11234–11247. doi: 10.1002/cam4.5815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cai X.J., Zhang H.Y., Zhang J.Y., Li T.J. Bibliometric analysis of immunotherapy for head and neck squamous cell carcinoma. J Dent Sci. 2023;18:872–878. doi: 10.1016/j.jds.2023.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Rahim M.K., Okholm T.L.H., Jones K.B., et al. Dynamic CD8+ T cell responses to cancer immunotherapy in human regional lymph nodes are disrupted in metastatic lymph nodes. Cell. 2023;186:1127. doi: 10.1016/j.cell.2023.02.021. 43.e18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Reticker-Flynn N.E., Engleman E.G. Lymph nodes: at the intersection of cancer treatment and progression. Trends Cell Biol. 2023 doi: 10.1016/j.tcb.2023.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Davies I., Boyes H., Ryba F., et al. Early oral cavity squamous cell carcinoma (pT1): a selective approach to surgical intervention in the neck is justified: a 10-year single centre retrospective review. Br J Oral Maxillofac Surg. 2017;55:e80. [Google Scholar]
- 12.Huang C., Zhuang S.M., Li J.J., et al. Can we identify the patients with clinically T1-2N0 oral tongue squamous cell carcinoma benefiting from neck dissection? Int J Clin Exp Med. 2017;10:4023–4034. [Google Scholar]
- 13.Peng K.A., Chu A.C., Lai C., et al. Is there a role for neck dissection in T1 oral tongue squamous cell carcinoma? The UCLA experience. Am J Otolaryngol. 2014;35:741–746. doi: 10.1016/j.amjoto.2014.06.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zhang T., Lubek J.E., Salama A., Dyalram D., Liu X., Ord R.A. Treatment of cT1 N0 M0 tongue cancer: outcome and prognostic parameters. J Oral Maxillofac Surg. 2014;72:406–414. doi: 10.1016/j.joms.2013.05.028. [DOI] [PubMed] [Google Scholar]
- 15.Liu T.R., Chen F.J., Yang A.K., et al. Elective neck dissection in clinical stage I squamous cell carcinoma of the tongue: does it improve regional control or survival time? Oral Oncol. 2011;47:136–141. doi: 10.1016/j.oraloncology.2010.11.018. [DOI] [PubMed] [Google Scholar]
- 16.Ryott M. Recurrence of T1N0 oral tongue squamous cell carcinoma in Stockholm, Sweden 2000-2009. Conference: ASCO Annual Meeting. J Clin Oncol. 2011;29(15 suppl):5550. [Google Scholar]
- 17.An S.Y., Jung E.J., Lee M., et al. Factors related to regional recurrence in early stage squamous cell carcinoma of the oral tongue. Clin Exp Otorhinolaryngol. 2008;1:166–170. doi: 10.3342/ceo.2008.1.3.166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Dias F.L., Kligerman J., Matos de Sa G., et al. Elective neck dissection versus observation in stage I squamous cell carcinomas of the tongue and floor of the mouth. Otolaryngol Head Neck Surg. 2001;125:23–29. doi: 10.1067/mhn.2001.116188. [DOI] [PubMed] [Google Scholar]
- 19.Chegini S., Schilling C., Walgama E.S., et al. Neck failure following pathologically node-negative neck dissection (pN0) in oral squamous cell carcinoma: a systematic review and meta-analysis. Br J Oral Maxillofac Surg. 2021;59:1157–1165. doi: 10.1016/j.bjoms.2021.04.002. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.