Abstract
Women experiencing intimate partner violence (IPV) experience a heightened prevalence of alcohol use disorder (AUD). Hypothalamic–pituitary–adrenal (HPA)-axis functioning has been associated with increased risk for AUD in other populations, including individuals with posttraumatic stress disorder (PTSD) symptoms. The goal of the present study was to determine whether PTSD symptom severity exacerbates the relationship between HPA-axis functioning and AUD. Participants were 151 community women who had experienced physical or sexual IPV in the past 30 days by their current male partners and used any amount of alcohol or drugs. A two-phase emotion induction protocol was utilized: Neutral mood induction followed by randomly assigned negative, positive, or neutral emotion induction. Saliva cortisol samples were obtained immediately following the neutral mood induction (baseline HPA-axis functioning), 20 min following the individualized emotion induction script (HPA-axis reactivity), and 40 min post the emotionally evocative cue (HPA-axis recovery). Findings revealed that PTSD symptom severity moderated the relations between baseline HPA-axis functioning and HPA-axis recovery and log odds of meeting criteria for AUD. Specifically, baseline HPA-axis functioning was positively associated with log odds of meeting criteria for AUD at high (but not low) PTSD symptom severity, whereas HPA-axis recovery was negatively associated with log odds of meeting criteria for AUD at high (but not low) PTSD symptom severity. Results contribute to our understanding of the biological processes involved in the etiology and maintenance of AUD among women experiencing IPV—specifically the prominent role of PTSD symptom severity.
Keywords: intimate partner violence, alcohol use disorder, posttraumatic stress disorder, HPA axis, cortisol
Intimate partner violence (IPV) is an international public health concern associated with substantial personal and societal costs (Cadilhac et al., 2015). Alcohol use disorder (AUD) is one common correlate of IPV that is of particular clinical significance (Coker et al., 2002). An epidemiological study found a significantly higher prevalence rate of AUD among individuals who had experienced IPV (7.3%) versus those who had not experienced IPV (2.3%; Okuda et al., 2011). Moreover, studies find a clear positive association between alcohol use and IPV among women (Devries et al., 2014). For instance, women who had experienced IPV were twice as likely to report daily alcohol use compared to those with no history of IPV (Carbone-López et al., 2006). Further, women in the general population who experienced past-year IPV had a greater odds of moderate and severe (vs. mild) AUD (La Flair et al., 2012). Speaking to the directionality of this association, Martino et al. (2005) found that women who experienced IPV were at an increased risk for later heavy drinking, whereas earlier heavy drinking did not predict later IPV. Notably, alcohol-related harm among women experiencing IPV is linked to numerous negative outcomes including psychological distress (Sullivan & Holt, 2008), legal problems (Oberleitner et al., 2013), and economic strain (Peterson et al., 2018). The heightened prevalence and significant impacts of alcohol-related harm in women experiencing IPV underscore the importance of research in this area.
Among women experiencing IPV, hypothalamic–pituitary–adrenal (HPA)-axis functioning may be particularly important to assess in relation to AUD. Production of cortisol, which is triggered by stress-induced activation of the HPA-axis, has been studied extensively in relation to AUD (Stephens & Wand, 2012; Wand, 2008). Notably, studies report a correlation between cortisol responsivity and activity within the mesolimbic dopaminergic pathway, which underlies AUD development, maintenance, and exacerbation (Oswald et al., 2005; Stephens & Wand, 2012; Wand et al., 2007). Altered HPA-axis responsivity may be present before alcohol exerts toxic effects on the central nervous system and subsequently contribute to initial vulnerability to AUD (Stephens & Wand, 2012). Studies suggest that cortisol may facilitate neurotransmission of dopaminergic neurons and, consequently, the reward circuity involved in AUD (Saal et al., 2003). Allostatic alterations—or the process by which HPA-axis function attempts to restore to homeostasis thus elevating stress peptide levels—have been posited to, among other things, contribute to the maintenance of AUD by altering brain reward pathways, ultimately resulting in increasing alcohol cravings (Stephens & Wand, 2012). Given the association between HPA-axis functioning and AUD, it is important to investigate this relation among women experiencing IPV.
Past research has identified cortisol levels as a valid biomarker of HPA-axis functioning (Nicolson, 2007), including among women experiencing IPV (Feinberg et al., 2011; Kim et al., 2015; Pinna et al., 2014; Pinto et al., 2016). When faced with acute stressors, the HPA-axis activates to preserve the “fight-or-flight” response, leading to glucocorticoid release, such as cortisol release in the process of allostasis (McEwen & Wingfield, 2003). In turn, this process can cause alterations in HPA-axis functioning, leading to changes in cortisol levels (Guilliams & Edwards, 2010). For instance, some individuals experience a lack of adaptation during chronic stress exposure (e.g., baseline cortisol levels are typically elevated and cortisol response to acute stress is blunted), resulting in excessive cortisol exposure following each stressful event. After repetitive activation, the system exhibits allostatic injury, marked by elevated basal cortisol levels and an inability to mount an acute response (McEwen & Gianaros, 2010; Stephens & Wand, 2012). Other studies suggest that chronic exposure to stressors may lead to periods of elevated cortisol levels that are not reduced appropriately by negative feedback inhibition (i.e., HPA-axis recovery), creating further HPA-axis abnormalities (Guilliams & Edwards, 2010). These shifts in cortisol levels can be observed in the laboratory across three phases: (a) a baseline HPA-axis functioning phase, which reflects unstimulated, nonstressed HPA activity; (b) an HPAaxis reactivity phase in which cortisol increases from baseline (i.e., preemotional stimuli) levels following the onset of emotional stimuli; and (c) an HPA-axis recovery phase in which cortisol levels return to baseline levels following the offset of the emotional stimuli (McEwen, 1998).
Importantly, existing literature underscores the potential role of posttraumatic stress disorder (PTSD) in attenuating HPA-axis functioning (Inslicht et al., 2006; Johnson et al., 2008; Pinna et al., 2014). PTSD is a severe psychiatric condition characterized by the development and persistence of intrusions, avoidance, alterations in mood and cognitions, and arousal and reactivity following exposure to a traumatic event (American Psychiatric Association [APA], 2013). PTSD is widespread among women experiencing IPV (Spencer et al., 2019), and the combination of IPV and PTSD among women is linked to deleterious outcomes, including increased alcohol use (Sullivan et al., 2016). Yehuda and LeDoux (2007) hypothesized that individuals with PTSD fail to achieve recovery and restitution of physiological homeostasis after exposure to trauma, yet, the literature is mixed regarding the specific nature of HPA-axis dysfunction in PTSD. For example, a systematic review found that several studies reported a positive association between PTSD and cortisol levels, while others either found no association or a negative association (Speer et al., 2019). In contrast, research consistently supports PTSD and AUD occur concomitantly (Debell et al., 2014). Indeed, Szabo et al. (2020) concluded that high levels of PTSD symptom severity, coupled with stress, dysregulate cortisol and increase risk of AUD, supporting a bidirectional impact, such that alcohol is used as a coping mechanism in response to distress and related HPA-axis dysfunction, but can also dysregulate HPA-axis functioning and cortisol levels itself. Given these findings, and that cortisol can serve as an objective index of stress reactivity—therefore functioning as a more rigorous tool for assessing the role of HPA-axis functioning—it is clinically relevant to further investigate the unique role of PTSD symptom severity in the relation between HPA-axis functioning and AUD.
Addressing a critical gap in the literature, the goal of the present study was to explicate whether PTSD symptom severity influences the strength of the relationship between HPA-axis functioning (i.e., baseline, reactivity, and recovery cortisol responding) and log odds of meeting criteria for AUD among a community sample of women experiencing IPV. We hypothesized that higher levels of PTSD symptom severity would strengthen the association between HPAaxis functioningandlogodds ofmeetingcriteriaforAUD,such thata positive association between HPA-axis functioning and AUD will be stronger for those with higher PTSD symptoms. We chose to include both women with and without a PTSD diagnosis in the present sample because most trauma-exposed people experience some PTSD symptoms, though they may not rise to the threshold of PTSD diagnosis (Zlotnick et al., 2002). Among women experiencing IPV, the experience of PTSD symptoms is related to significant impairmentin functioning, even when they don’t meet full diagnostic criteria for PTSD (Hellmuth et al., 2014). As such, it is important to include individuals who are experiencing subthreshold PTSD symptoms to fully capture the population of trauma-exposed women experiencing IPV.
Method
Study Overview
Data were collected as part of a larger study examining the proximal role and temporal ordering of emotion dysregulation in the relations between PTSD symptoms and substance use and Human Immunodeficiency Virus (HIV)/sexual risk. All procedures were reviewed and approved by the [redacted] Institutional Review Board. The larger study entailed (a) a baseline session, (b) an experimental session, (c) 30 days of experience sampling using interactive voice technology, and (d) a follow-up session. The present study extracted data from the baseline and experimental sessions. To limit participant burden, these sessions were conducted on separate days. Individual interviews were conducted in-person by bachelor’s- or masters-level clinical psychology doctoral students in private offices to protect participants’ safety and confidentiality. During the baseline session, participants completed a structured diagnostic assessment, answered self-report questionnaires, and received a standardized protocol for developing individualized emotion inductions. In the experimental session, participants underwent a two-phase emotion induction protocol. The first phase involved a neutral mood induction. The second phase was the presentation of a randomly assigned emotion induction (negative, positive, or neutral) delivered via headphones. Participants provided saliva samples at three points: Following the neutral mood induction, at 20 min postindividualized emotion induction, and at 40 min postindividualized emotion induction.
Participants
Recruitment materials were posted in community establishments throughout Providence County, Rhode Island such as grocery stores, laundromats, and shops; selected state offices such as the Office of Housing and Community Development; and waiting rooms, bathrooms, and exam rooms of urban-area primary care clinics; as well as in website postings (e.g., Craigslist). Eligibility was determined through a phone screen. Participants were women who had experienced physical or sexual victimization in the past 30 days by their current male partners and used any amount of drugs or alcohol during that time. Additional inclusion criteria were: (a) age 18 or older, (b) fluent in the English language, and (c) current involvement in a relationship of at least 6 months’ duration with current contact at least twice a week. Exclusion criteria were (a) current mania/psychosis [assessed in the baseline session with the Structured Clinical Interview (SCID-5) for Diagnostic and Statistical Manual of Mental Disorders, Fifth Edition (DSM-5); First & Williams, 2016], (b) current impairment in cognitive functioning (assessed in the baseline session using the Mini-Mental Status Exam and requiring a score >24; Folstein et al., 1975), (c) self-reported current pregnancy, (d) colorblindness, (e) cardiovascular disease, and (f) residenceinashelter or grouphome.The final sampleincluded151women who participated in the baseline and experimental sessions (see Procedures); demographic characteristics are summarized in Table 1.
Table 1.
Sample Demographic and Descriptive Characteristics
| Demographic and descriptive characteristics | M (SD) | Range | n (%) |
|---|---|---|---|
|
| |||
| Age | 40.81 (11.64) | 19–65 | |
| Racial/ethnic background | |||
| Black or African American | 46 (30.5%) | ||
| White | 65 (43.0%) | ||
| American Indian/Alaska Native | 12 (7.9%) | ||
| Hispanic or Latina | 17 (11.3%) | ||
| Not listed | 9 (6.0%) | ||
| Prefer not to respond | 2 (1.3%) | ||
| Years of education completed | 12.39 (2.04) | 6–18 | |
| Employment | |||
| Full time (35+ hr per week) | 8 (5.3%) | ||
| Part time (less than 35 hr per week or sporadic employment) | 17 (11.3%) | ||
| Unemployed | 115 (76.2%) | ||
| Prefer not to respond | 11 (7.3%) | ||
| Monthly household income | $1278.03 ($1594.52) | $0–$10416.67 | |
| Relationship status | |||
| Married | 12 (7.9%) | ||
| Unmarried | 113 (74.8%) | ||
| Separated or divorced | 13 (8.6%) | ||
| Prefer not to respond | 13 (8.6%) | ||
| Relationship length (in years) | 6.08 (5.88) | 0.5–30 | |
| Days with partner per week | 5.80 (1.89) | 0–7 | |
| Current AUD diagnosis | 81 (53.6%) | ||
| Current PTSD diagnosis | 67 (39.0%) | ||
| Lifetime PTSD diagnosis | 103 (59.9%) | ||
| PTSD symptom severity | 34.15 (21.79) | 0–80 | |
| Indices of HPA-axis functioning | |||
| Baseline HPA-axis functioning (ug/dL) | 0.21 (0.40) | 0–4.32 | |
| HPA-axis reactivity (ug/dL) | –0.02 (0.16) | −1.06–1.28 | |
| HPA-axis recovery (ug/dL) | 0.06 (0.99) | −1.19–11.76 | |
| Covariates | |||
| Number of lifetime traumatic events | 6.04 (5.34) | 0–17 | |
| Time since last cigarette (in minutes) | 234.89 (532.92) | 10–4,320 | |
| Current menstruation | 18 (11.9%) | ||
Note. AUD = alcohol use disorder; PTSD = posttraumatic stress disorder; HPA = hypothalamic–pituitary–adrenal.
Measures
Diagnostic Measure
A computerized version of the SCID-5 was administered to establish AUD and PTSD diagnoses (First & Williams, 2016). The SCID-5, a gold standard semi-structured assessment instrument for psychiatric disorders, has been found to yield valid and reliable current and lifetime diagnoses across a variety of common psychiatric disorders, including AUD and PTSD. A recent assessment of interrater reliability of the SCID-5 found evidence of moderate to excellent reliability across major diagnostic categories, including kappas of .84 and .80 for AUD and PTSD, respectively (Osório et al., 2019). SCID-5 interviews were conducted by clinical psychology doctoral students trained to reliability with the principal investigator, a licensed clinical psychologist in the state of Rhode Island. All data were reviewed by the principal investigator. In the case of ambiguous responses, data were discussed by the principal investigator and interviewer until a consensus was reached.
Self-Report Measures
Traumatic Exposure.
The Life Events Checklist for DSM-5 (LEC-5; Weathers, Blake, et al., 2013) is a 17-item self-report measure designed to assess lifetime exposure to traumatic events. The LEC-5 assesses exposure to 16 traumatic events, with a final item assessing for any other stressful event not captured in the first 16 items. For each event, the respondent is asked to indicate if: (a) it happened to them, (b) they witnessed it, (c) they learned about it, (d) they experienced it as part of their job, (e) they are not sure if they experienced it, or (f) they did not experience it. Any of the first four response options indicated a positive Criterion A traumatic event endorsement (APA, 2013). The LEC has shown convergent validity with measures assessing traumatic exposure and psychopathology known to relate to traumatic exposure (Weathers, Blake, et al., 2013). In the present study, the LEC-5 was used to identify an index trauma for subsequent assessment of PTSD symptoms on the PTSD Checklist for DSM-5 (PCL-5) as well as a covariate (i.e., number of lifetime traumatic events), given evidence for the influence of trauma exposure in HPA-axis functioning (de Kloet et al., 2007; Klaassens et al., 2009).
Posttraumatic Stress Disorder Symptom Severity.
The PCL-5 (Weathers, Litz, et al., 2013) is a 20-item self-report measure that assesses past-month PTSD symptoms consistent with DSM-5 criteria (APA, 2013). Participants completed the PCL-5 in response to the most distressing traumatic event endorsed on the Life Events Checklist for the DSM-5. Each item was rated using a 5-point Likert-type scale (0 = not at all, 4 = extremely). Possible scores range from 0 to 80, with higher values indicating increased severity of PTSD symptoms, and with a recommended cut-off score of 31 or higher to identify probable PTSD diagnosis (Blevins et al., 2015; Bovin et al., 2016). The PCL-5 has excellent psychometric properties (Blevins et al., 2015; Bovin et al., 2016; Wortmann et al., 2016). Cronbach’s α was .99 in the current sample.
Menstrual Cycle.
Female participants were asked whether they were currently menstruating (yes/no). This variable was included as a covariate in the present study given evidence for an impact of menstrual cycle phase on cortisol (Kirschbaum et al., 1999).
Biological Measure
Salivary cortisol samples were obtained at three timepoints during the experimental session: (a) immediately following neutral mood induction (i.e., baseline HPA-axis functioning), (b) 20 min following the individualized emotion induction script (i.e., HPA-axis reactivity; given evidence that cortisol levels peak approximately 20 min after presentation of an emotionally evocative cue (see Dickerson & Kemeny, 2004; Nicolson, 2007), and (c) 40 min post emotionally evocative cue (i.e., HPA-axis recovery). Saliva samples were collected by instructing participants to pool saliva in their mouth, then transfer the saliva into a centrifuge tube with a Salivette. Approximately 0.5 mL of saliva was collected, then sealed, and stored in a freezer. All samples were assayed in duplicate for salivary cortisol offsite using a highly sensitive enzyme immunoassay. The test used 25 μL of saliva per determination, has a lower limit of sensitivity of 0.003 μg/dL, standard curve range from 0.012 μg/dL to 3.0 μg/dL, an average intra-assay coefficient of variation (CV) of 3.8%, and an average inter-assay CV of 5.1%. Following recommended guidelines (see Nicolson, 2007 for guidelines in assessing cortisol response to acute stimuli in the laboratory) data on the time of day of cortisol collection and time since last use of nicotine were collected and included as covariates in the study analyses.
Procedures
Baseline Session
Participants provided informed consent, followed by an interview using a computerized version of the SCID-5 and completed a battery of self-report measures that included the LEC-5 and the PCL-5. Prior to the baseline session, participants were randomly assigned to one of three emotion induction conditions (negative, positive, or neutral). For participants in the negative and positive emotion induction conditions, a standardized protocol for developing individualized emotion induction scripts was followed. Participants were asked to recall a recent or vivid event during which they became “very angry” (negative condition) or “very excited” (positive condition) that did not involve substances or trauma. This portion of the session was audio recorded so that the interviewer could subsequently create a script using the participant’s own language. Participants were asked to picture the situation in their mind and try to remember as vividly as possible what the event entailed and their feelings at the time. Participants were then asked to describe the incident in as much detail as possible. The interviewer probed for key aspects of the event (e.g., time and place of the event, as well as emotions, thoughts, and bodily sensations experienced during the event).
Prior to the experimental session, a personalized script consisting of a series of autobiographical statements, appraisals, and emotional responses generated from the interview was recorded onto an audiotape. This script was approximately 1 min in length and the narrator wasconsistentacrossallscripts(theprincipal investigator). All scripts were presented in a female voice with a neutral tone (to reduce reactivity giventhatthe sample is characterized by experiences of IPV with a male partner). The method for generating these individualized emotion induction scripts was based on procedures originally developed by Lang and colleagues (see Lang & Cuthbert, 1984; Levin et al., 1982). The script is designed to maximize emotional responses by depicting the events in a salient, emotion-focused form in second person, present tense. This procedure reliably induces emotional responses in trauma-exposed samples (Lang et al., 1983; Orr et al., 1993; Pitman et al., 1987; Tull et al., 2011, 2019).
Neutral scripts were also developed for this study. Consistent with Keane et al. (1998), the neutral script was standardized and consistent across participants. It provided a description of activities involved in getting up in the morning (e.g., brushing teeth, getting dressed). The neutral script was also approximately 1 min in length and similarly consisted of descriptions of morning events, as well as thoughts and feelings that a person may experience in response.
At the end of the baseline session, participants were instructed to abstain from alcohol and illicit drugs for a period of at least 4 days prior to the experimental session. This criterion reduces the risk for intoxication and acute withdrawal (see Coffey et al., 2006, 2011). Participants were compensated with $40 for completing the baseline session.
Experimental Session
At the start of the experimental session (approximately 4–7 days after the baseline session), participants’ compliance with substance use restrictions was assessed. Specifically, a urine drug screen (iCup by Alere Toxicology Services) was administered at the start of the experimental session to test for metabolites of Tetrahydrocannabinol (THC), cocaine, opiates, amphetamines, benzodiazepines, methamphetamine, oxycodone, propoxyphene, barbiturates, and 3,4-methylenedioxy-methamphetamine (MDMA). To assess recent alcohol intoxication, expired air samples were analyzed (Alco-sensor IV, Intoximeters Inc., St. Louis, MO). Participants who tested positive for illicit drugs or who had a blood alcohol level >.01 were rescheduled. Due to the long half-life of THC metabolites, participants who tested positive for THC and reported marijuana use in the past 30 days, but not past 4 days, were allowed to participate in the experimental session. Participants were also asked to report whether they were currently menstruating. Following this, we induced a neutral mood by displaying colors, one after another, on a screen in front of the participants for 5 min. This procedure, called the “vanilla baseline procedure,” has been found to produce a more neutral mood (e.g., less anxiety) compared with an absence of activities (i.e., having the participant sit still and do nothing for 5 min; Jennings et al., 1992). Participants provided the first salivary cortisol sample after neutral mood induction and then listened to the 1-min individualized emotion induction script developed during the baseline session. Once the tape was finished, participants were instructed to close their eyes and imagine vividly the event taking place in real-time for 1 min. Participants provided additional saliva samples 20- and 40-min postindividualized emotion induction. Participants were compensated $25 for completing the experimental session and were provided with a list of community resources. This study was not preregistered. Code and materials are available upon request to the corresponding author.
Analytic Strategy
To address the question of whether HPA-axis functioning [i.e., baseline, reactivity (reflected by a change score calculated by subtracting baseline cortisol values from cortisol values assessed 20 min postemotion induction), and recovery (reflected by a change score calculated by subtracting cortisol values assessed 20 min postemotion induction from cortisol values assessed 40 min postemotion induction) cortisol responding], PTSD symptom severity, and their interactions are associated with log odds of membership in the AUD group (defined as meeting criteria for mild, moderate, or severe AUD), three moderation analyses were conducted using the PROCESS SPSS macro (Model 1) as recommended by Hayes (2018). The PROCESS procedures use ordinary least squares regression and bootstrapping methodology, which confers more statistical power than do standard approaches to statistical inference and does not rely on distributional assumptions. Bootstrapping was done with 5,000 random samples generated from the observed covariance matrix to estimate bias-corrected 95% confidence intervals (CIs) and significance values. For interactions found to be significant, following the methods described by Aiken et al. (1991), we plotted regression slopes of differences in log odds of membership in the AUD group and conducted follow-up analyses to examine whether the slopes of the regression lines differed significantly from zero. All models included total number of lifetime traumatic events, time of day at which saliva data was collected, time since last cigarette, and current menstruation as covariates.
While the full sample was used for analyses examining the effect of baseline HPA-axis functioning, the sample was restricted only to those participants randomized to positive and negative emotion induction conditions for models examining HPA-axis reactivity and recovery. No significant differences were detected between the positive and negative emotion induction condition with respect to any of the three HPA-axis functioning indices; thus, to improve statistical power, these groups were combined. Sensitivity analyses revealed that we were adequately powered to detect medium effects d = .56, which corresponds to r = .27 and R2 = .07; our effects were above this threshold.
Results
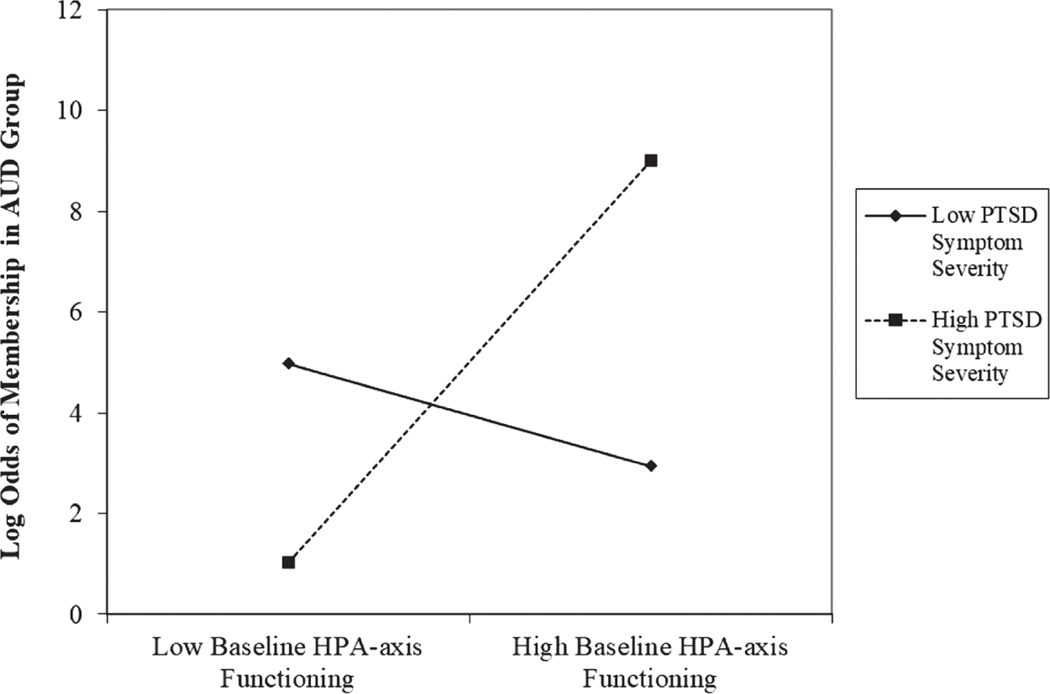
Moderation analyses testing the hypothesis that higher levels of PTSD symptom severity would strengthen the association between HPA-axis functioning and AUD, controlling for time of day of saliva sample collection, time since last cigarette use, number of lifetime traumatic experiences, and current menstruation, are summarized in Table 2. In the model examining baseline HPA-axis functioning, no significant main effects were detected for either baseline HPA-axis functioning, b = 3.71, SE = 2.30, z = 1.62, p = .11, 95% CI [–.79, 8.21], or PTSD symptom severity, b = .02, SE = .01, z = 1.68, p = .09, 95% CI [–.004, .05], predicting log odds of meeting criteria for AUD. However, the interaction of baseline HPA-axis functioning and PTSD symptom severity was significantly associated with the log odds of meeting criteria for AUD, b = .29, SE = .14, z = 2.04, p = .04, 95% CI [.01, .56]. As is summarized in Figure 1, analysis of simple slopes revealed that baseline HPAaxis functioning was significantly positively associated with log odds of meeting criteria for AUD at high, b = 10.10, SE = 4.48, z = 2.26, p = .02, 95% CI [1.32, 18.87], but not low, b = −2.67, SE = 3.18, z = −0.84, p = .40, 95% CI [–8.90, 3.55], levels of PTSD symptom severity.
Table 2.
Summary of Moderation Analyses Predicting Membership in the AUD (vs. no AUD) Group
| Variable of interest | b | SE | z | p | 95% CI |
|---|---|---|---|---|---|
|
| |||||
| Model 1: Baseline HPA-axis functioning (n = 93) | |||||
| Intercept | 1.49 | .74 | 2.02 | .04 | [.04, 2.94] |
| Baseline HPA-axis functioning | 3.71 | 2.30 | 1.62 | .11 | [–.79, 8.21] |
| PTSD symptom severity | .02 | .01 | 1.68 | .09 | [–.004, .05] |
| Baseline HPA-axis functioning × PTSD symptom severity | .29 | .14 | 2.04 | .04 | [.01, .56] |
| Number of lifetime traumatic events | −.02 | .05 | −0.34 | .74 | [−.11, .08] |
| Time of day | −.62 | .37 | −1.68 | .09 | [−1.35, .11] |
| Time since last cigarette | .001 | .001 | 1.16 | .24 | [.00, .002] |
| Current menstruation | .06 | .79 | 0.07 | .94 | [–1.49, 1.60] |
| Model 2: HPA-axis Reactivity (n = 56) | |||||
| Intercept | 1.42 | 1.02 | 1.39 | .17 | [−.58, 3.43] |
| HPA-axis reactivity | −.43 | 5.71 | −0.08 | .94 | [−11.62, 10.76] |
| PTSD symptom severity | .01 | .01 | 0.45 | .66 | [−.02, .03] |
| HPA-axis reactivity × PTSD symptom severity | −.01 | .33 | −0.04 | .97 | [−.65, .62] |
| Number of lifetime traumatic events | −.01 | .07 | −0.17 | .86 | [−.14, .12] |
| Time of day | −.59 | .43 | −1.38 | .17 | [−1.42, .25] |
| Time since last cigarette | .00 | .001 | −0.17 | .87 | [−.002, .002] |
| Current menstruation | .59 | .94 | 0.62 | .53 | [–1.25, 2.43] |
| Model 3: HPA-axis recovery (n = 55) | |||||
| Intercept | 3.00 | 1.41 | 2.12 | .03 | [.23, 5.76] |
| HPA-axis recovery | −13.20 | 10.52 | −1.26 | .21 | [−33.82, 7.42] |
| PTSD symptom severity | .01 | .02 | 0.59 | .55 | [−.02, .04] |
| HPA-axis recovery × PTSD symptom severity | −1.64 | .73 | −2.25 | .02 | [−3.07, −.21] |
| Number of lifetime traumatic events | −.06 | .07 | −0.82 | .41 | [−.21, .08] |
| Time of day | −1.22 | .56 | −2.17 | .03 | [−2.32, −.12] |
| Time since last cigarette | .00 | .001 | 0.05 | .96 | [−.002, .002] |
| Current menstruation | −.89 | 1.17 | −0.76 | .45 | [−3.99, 1.40] |
Note. Bolded typeface indicates significance at the level p < .05. AUD = alcohol use disorder; HPA = hypothalamic–pituitary–adrenal; PTSD = posttraumatic stress disorder.
Figure 1.
Baseline HPA-Axis Functioning by PTSD Symptom Severity Interaction for Log Odds of Membership in the AUD Group
Note. HPA = hypothalamic–pituitary–adrenal; PTSD = posttraumatic stress disorder; AUD = alcohol use disorder.
In the model examining HPA-axis reactivity, no significant main effects were detected for either HPA-axis reactivity, b = −.43, SE = 5.71, z = −0.08, p = .94, 95% CI [–11.62, 10.76], or PTSD symptom severity, b = .01, SE = .01, z = 0.45, p = .66, 95% CI [–.02, .03], predicting log odds of meeting criteria for AUD. Additionally, the interaction of HPA-axis reactivity and PTSD symptom severity was not significantly associated with log odds of meeting criteria for AUD, b = −.01, SE = .33, z = −0.04, p = .97, 95% CI [–.65, .62].
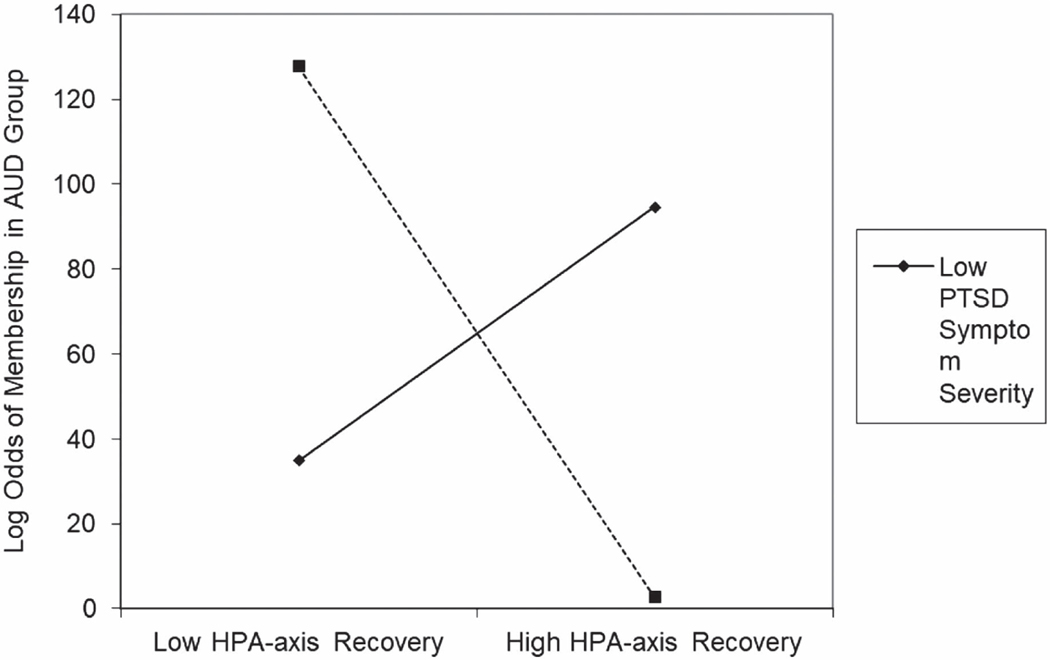
Finally, in the model examining HPA-axis recovery, no significant main effects were detected for either HPA-axis recovery, b = −13.20, SE = 10.52, z = −1.26, p = .21, 95% CI [–33.82, 7.42], or PTSD symptom severity, b = .01, SE = .02, z = 0.59, p = .55, 95% CI [–.02, .04]. However, the interaction of HPA-axis reactivity and PTSD symptom severity was significantly associated with the log odds of meeting criteria for AUD, b =−1.64, SE = .73, z = −2.25, p = .02, 95% CI [–3.07, −.21]. As is summarized in Figure 2, analysis of simple slopes revealed that HPA-axis recovery was significantly negatively associated with log odds of meeting criteria for AUD at high, b = −51.18, SE = 20.88, z = −2.45, p = .01, 95% CI [–92.11, −10.26], but not low, b = 24.78, SE = 18.81, z = 1.32, p = .19, 95% CI [–12.09, 61.65], levels of PTSD symptom severity.
Figure 2.
HPA-Axis Recovery by PTSD Symptom Severity Interaction for Log Odds of Membership in the AUD Group
Note. HPA = hypothalamic–pituitary–adrenal; PTSD = posttraumatic stress disorder; AUD = alcohol use disorder.
Exploratory Analyses
Given recent calls to explore biological stress responsivity within racial and ethnic subgroups, we conducted exploratory analyses to examine potential differences in HPA-axis functioning, PTSD (symptom severity and diagnosis), and AUD. We found that there were no significant between-group differences with respect to basal cortisol, F(5, 139) = 0.36, p = .88, cortisol reactivity, F(5, 139) = 1.72, p = .14, cortisol recovery, F(5, 137) = 0.20, p = .96, or PTSD symptom severity, F(5, 136) = 1.18, p = .32, nor were there differences in the proportion of participants who met criteria for PTSD, χ2(5) = 7.08, p = .22, or AUD, χ2(5) = 3.50, p = .62, across racial/ethnic groups. We also examined correlations between our variables of interest by racial/ethnic group and largely did not find evidence for significant relations (rs from −.25 to .23, ps from .15 to .995), except between basal cortisol and PTSD symptom severity for Black participants (r = .35, p = .03).
Discussion
The purpose of the present study was to elucidate the combined role of PTSD symptomology and HPA axis function on risk for AUD among women experiencing IPV. Consistent with study hypotheses, baseline HPA-axis functioning was positively associated, and HPA-axis recovery negatively associated, with log odds of meeting criteria for AUD at high—but not low—levels of PTSD symptom severity. These findings advance research on the relation between HPA-axis functioning and AUD by highlighting the important influence of PTSD symptom severity in these associations.
Overall, our results extend previous findings concerning the association of HPA-axis functioning with AUD (Lee et al., 2018; Oswald et al., 2005; Stephens & Wand, 2012; Wand, 2008; Wand et al., 2007) and PTSD (Speer et al., 2019; Szabo et al., 2020) separately, underscoring the moderating role of PTSD symptom severity in the relationships between both baseline HPA-axis functioning and HPA-axis recovery and AUD. Specifically, our findings suggest that severity of PTSD symptoms exacerbates the link between HPA-axis functioning and AUD among women experiencing IPV. This may suggest an important role of the mesolimbic dopamine reward pathway, which is involved in stress response and addiction (Cleck & Blendy, 2008; Sinha et al., 2005).
Of note, we found no significant main effect for HPA-axis reactivity predicting log odds of meeting criteria for AUD. Given evidence that allostatic injury can cause the HPA-axis to become less sensitive, marked by elevated basal levels and an inability to mount an acute response, following subsequent stressful episodes, it is possible that tests of HPA-axis reactivity among individuals experiencing chronic trauma, such as women experiencing IPV, do not capture HPA-axis functionality in the relation to AUD due to blunted cortisol activity (Guilliams & Edwards, 2010; McEwen & Gianaros, 2010; Sinha et al., 2009; Stephens & Wand, 2012). In fact, our finding that mean reactivity reflected a negligible decrease from baseline is consistent with previous work regarding blunted cortisol reactivity in AUD (see Table 1). This aligns with previous literature that finds significant blunted-to-absent cortisol reactivity among people with AUD (Adinoff et al., 2005) including after exposure to a psychological stressor (Lovallo et al., 2000). Indeed, blunted cortisol reactivity response has been found when using personalized stressful imagery among individuals with AUD (Sinha et al., 2009) along with suppressed cortisol in trauma-exposed veterans diagnosed with PTSD relative to controls (de Kloet et al., 2007). Decreased HPA-axis reactivity has been shown to be associated with increased maladaptive behaviors including alcohol use among those who have experienced significant life stressors (Kim, 2017; Walton et al., 2018). Furthermore, our result, which found that for participants with high PTSD symptom severity, HPA-axis recovery was negatively associated with greater odds of AUD, also suggests that blunted cortisol reactivity is a manifestation of allostatic load among IPV-exposed women who are exposed to chronic stressors.
Our significant findings among HPA-axis at baseline and recovery, but not reactivity, may be understood through the drinking to cope self-medication model (Khantzian, 1997). Individuals with PTSD may misuse alcohol to self-medicate a negative affective state, including anxiogenic and hyperarousal symptoms associated with the disorder (Gilpin & Weiner, 2017; Hawn et al., 2020). Indeed, alcohol has been identified as the most commonly used substance among individuals with PTSD (Shorter et al., 2015). One study found a longitudinal association between IPV, alcohol-related harm, and drinking to cope, such that drinking to cope mediates the relationship between IPV and both alcohol-related harm and consumption (Øverup et al., 2015). Research suggests that IPV-exposed women use alcohol to alleviate their PTSD symptoms, such that elevated PTSD symptom severity is related to greater likelihood of drinking and amount of alcohol consumed (Sullivan et al., 2020). While individuals without severe PTSD may experience a normal decrease in negative affect after experiencing an emotionally evocative cue (e.g., HPA-axis recovery), it is possible that those with severe PTSD symptoms do not. Thus, perhaps severe PTSD symptoms are influencing the relation between HPA-axis and AUD at baseline and recovery, rather than at reactivity, when individuals are attempting to manage and diminish the effects of a prolonged affective state. Alternatively, perhaps this unexpected finding is due to additional barriers related to understanding HPA-axis reactivity (i.e., blunted reactivity); such that HPA-axis reactivity may be altered in ways that can persist into adulthood for individuals who have experienced early-life trauma (Dunlop & Wong, 2019).
Results of the present study help explain the influence of PTSD symptom severity on the strength of the relationships between HPA-axis functioning (i.e., baseline, reactivity, and recovery cortisol responding) and AUD among a community sample of women experiencing IPV. However, our study should be interpreted in the context of its limitations. First, our study only measured cortisol at three timepoints: Baseline, reactivity (i.e., 20-min postemotion induction), and recovery (i.e., 20-min postreactivity sample). This measure of cortisol reactivity should be noted as a limitation given that it is possible it may not fully capture timing and peak cortisol reactivity of the cortisol response curve. Future research may consider a repeated 10-min saliva sampling to better capture cortisol responsivity as has been done in other research (Gozansky et al., 2005). Second, we included current menstruation as a covariate in analyses given evidence suggesting that hormonal fluctuations regarding the menstrual cycle phase can influence HPA-axis functioning (Montero-López et al., 2018). However, we were limited in our assessment of menstrual cycle and menstrual phase; future work should include more thorough assessment to further elucidate the role of menstrual cycle-related cortisol fluctuations in relation to AUD. Lastly, our study assessed PTSD among a sample of community women experiencing IPV, thus our results may not generalize to other trauma-exposed samples, such as those identified by other index traumas or in different settings (e.g., treatment-seeking clinical settings); other clinical populations including people who have depression given than HPA-Axis may be blunted in depressed patients (Saxbe, 2008), which commonly co-occurs with both PTSD (Smith et al., 2016) and AUD (Grant et al., 2015); and among men for whom HPA-axis reactivity has been found to be stronger than among women (Saxbe, 2008). Relatedly, given that women suffer disproportionately from IPV (Caldwell et al., 2012), and the unique presentation of IPV across sexual minority populations, the current sample focused on women-identified individuals in heterosexual relationships. Future research should investigate the influence of PTSD on the relationship between HPA axis and AUD in different settings, among men, within specific racial and ethnic subgroups (Price et al., 2021), and across gender and sexual minorities.
Despite these limitations, the findings of the present study advance our understanding of the associations among HPA-axis functioning, PTSD symptom severity, and AUD among women experiencing IPV. Specifically, we found that baseline HPA-axis functioning was significantly positively associated with log odds of meeting criteria for AUD at high (but not low) PTSD symptom severity, whereas HPA-axis recovery was significantly negatively associated with log odds of meeting criteria for AUD at high (but not low) PTSD symptom severity. Our findings contribute a novel understanding of the biological processes involved in the development of AUD among women experiencing IPV—specifically the prominent role of PTSD symptom severity.
Public Health Significance.
This study highlights how PTSD symptom severity moderates the relationship between HPA-axis functioning and odds of meeting criteria for alcohol use disorder (AUD). Results contribute to our understanding of the biological processes involved in the etiology and maintenance of AUD among women experiencing IPV—specifically the prominent role of PTSD symptom severity.
Acknowledgments
This research was supported by National Institute on Drug Abuse Grant K23 DA039327, awarded to Nicole H. Weiss. Nicole H. Weiss also acknowledges the support from the Center for Biomedical Research and Excellence (COBRE) on Opioids and Overdose funded by the National Institute on General Medical Sciences (P20 GM125507). Work on this article by Silvi C. Goldstein was supported by National Institute on Alcohol Abuse and Alcoholism Grant 1F31AA029274-01A1.
Silvi C. Goldstein played lead role in writing of original draft and equal role in conceptualization. Melissa R. Schick played lead role in formal analysis and methodology and lead role in writing of original draft. Lisa Weyandt played equal role in writing of review and editing. Tami P. Sullivan played equal role in writing of review and editing. Hans Saint-Eloi Cadely played equal role in writing of review and editing. Nicole H. Weiss played lead role in funding acquisition and writing of review and editing and equal role in conceptualization.
The authors would like to acknowledge and thank the study participants for their role in this research, for without them this study would not have been possible.
Footnotes
All authors contributed in a significant way to the manuscript, and all authors have read and approved the final manuscript.
This study was not preregistered. Code and materials are available upon request to the corresponding author.
The authors have no known conflict of interest to disclose.
References
- Adinoff B, Krebaum SR, Chandler PA, Ye W, Brown MB, & Williams MJ. (2005). Dissection of hypothalamic-pituitary-adrenal axis pathology in 1-month-abstinent alcohol-dependent men, part 1: Adrenocortical and pituitary glucocorticoid responsiveness. Alcoholism: Clinical and Experimental Research, 29(4), 517–527. 10.1097/01.ALC.0000158940.05529.0A [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aiken LS, West SG, & Reno RR. (1991). Multiple regression: Testing and interpreting interactions. Sage Publications. [Google Scholar]
- American Psychiatric Association (APA). (2013). Diagnostic and statistical manual of mental disorders (DSM-5®). [DOI] [PubMed] [Google Scholar]
- Blevins CA, Weathers FW, Davis MT, Witte TK, & Domino JL. (2015). The posttraumatic stress disorder checklist for DSM-5 (PCL-5): Development and initial psychometric evaluation. Journal of Traumatic Stress, 28(6), 489–498. 10.1002/jts.22059 [DOI] [PubMed] [Google Scholar]
- Bovin MJ, Marx BP, Weathers FW, Gallagher MW, Rodriguez P, Schnurr PP, & Keane TM. (2016). Psychometric properties of the ptsd checklist for diagnostic and statistical manual of mental disorders–fifth edition (PCL-5) in veterans. Psychological Assessment, 28, 1379–1391. 10.1037/pas0000254 [DOI] [PubMed] [Google Scholar]
- Cadilhac DA, Sheppard L, Cumming TB, Thayabaranathan T, Pearce DC, Carter R, & Magnus A. (2015). The health and economic benefits of reducing intimate partner violence: An Australian example. BMC Public Health, 15(1), Article 625. 10.1186/s12889-015-1931-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caldwell JE, Swan SC, & Woodbrown VD. (2012). Gender differences in intimate partner violence outcomes. Psychology of Violence, 2(1), 42–57. 10.1037/a0026296 [DOI] [Google Scholar]
- Carbone-López K, Kruttschnitt C, & Macmillan R. (2006). Patterns of intimate partner violence and their associations with physical health, psychological distress, and substance use. Public Health Reports, 121(4), 382–392. 10.1177/003335490612100406 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cleck JN, & Blendy JA. (2008). Making a bad thing worse: Adverse effects of stress on drug addiction. The Journal of Clinical Investigation, 118(2), 454–461. 10.1172/JCI33946 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coffey SF, Schumacher JA, Baschnagel JS, Hawk LW, & Holloman G. (2011). Impulsivity and risk-taking in borderline personality disorder with and without substance use disorders. Personality Disorders, 2(2), 128–141. 10.1037/a0020574 [DOI] [PubMed] [Google Scholar]
- Coffey SF, Stasiewicz PR, Hughes PM, & Brimo ML. (2006). Trauma-focused imaginal exposure for individuals with comorbid posttraumatic stress disorder and alcohol dependence: Revealing mechanisms of alcohol craving in a cue reactivity paradigm. Psychology of Addictive Behaviors, 20(4), 425–435. 10.1037/0893-164X.20.4.425 [DOI] [PubMed] [Google Scholar]
- Coker AL, Davis KE, Arias I, Desai S, Sanderson M, Brandt HM, & Smith PH. (2002). Physical and mental health effects of intimate partner violence for men and women. American Journal of Preventive Medicine, 23(4), 260–268. 10.1016/S0749-3797(02)00514-7 [DOI] [PubMed] [Google Scholar]
- de Kloet CS, Vermetten E, Heijnen CJ, Geuze E, Lentjes EG, & Westenberg HG. (2007). Enhanced cortisol suppression in response to dexamethasone administration in traumatized veterans with and without posttraumatic stress disorder. Psychoneuroendocrinology, 32(3), 215–226. 10.1016/j.psyneuen.2006.12.009 [DOI] [PubMed] [Google Scholar]
- Debell F, Fear NT,Head M, Batt-Rawden S,Greenberg N,Wessely S, & Goodwin L. (2014). A systematic review of the comorbidity between PTSDand alcohol misuse.SocialPsychiatryandPsychiatric Epidemiology, 49(9), 1401–1425. 10.1007/s00127-014-0855-7 [DOI] [PubMed] [Google Scholar]
- Devries KM, Child JC, Bacchus LJ, Mak J, Falder G, Graham K, Watts C, & Heise L. (2014). Intimate partner violence victimization and alcohol consumption in women: A systematic review and meta-analysis. Addiction, 109(3), 379–391. 10.1111/add.12393 [DOI] [PubMed] [Google Scholar]
- Dickerson SS, & Kemeny ME. (2004). Acute stressors and cortisol responses: A theoretical integration and synthesis of laboratory research. Psychological Bulletin, 130(3), 355–391. 10.1037/0033-2909.130.3.355 [DOI] [PubMed] [Google Scholar]
- Dunlop BW, & Wong A. (2019). The hypothalamic-pituitary-adrenal axis in PTSD: Pathophysiology and treatment interventions. Progress in Neuro-Psychopharmacology and Biological Psychiatry, 89, 361–379. 10.1016/j.pnpbp.2018.10.010 [DOI] [PubMed] [Google Scholar]
- Feinberg ME, Jones DE, Granger DA, & Bontempo D. (2011). Relation of intimate partner violence to salivary cortisol among couples expecting a first child. Aggressive Behavior, 37(6), 492–502. 10.1002/ab.20406 [DOI] [PMC free article] [PubMed] [Google Scholar]
- First MB, & Williams JBW. (2016). SCID-5-CV: Structured clinical interview for DSM-5 disorders: Clinician version. American Psychiatric Association Publishing. [Google Scholar]
- Folstein MF, Folstein SE, & McHugh PR. (1975). “Mini-mental state.” A practical method for grading the cognitive state of patients for the clinician. Journal of Psychiatric Research, 12, 189–198. 10.1016/0022-3956(75)90026-6 [DOI] [PubMed] [Google Scholar]
- Gilpin NW, & Weiner JL. (2017). Neurobiology of comorbid posttraumatic stress disorder and alcohol-use disorder. Genes Brain and Behavior, 16(1), 15–43. 10.1111/gbb.12349 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gozansky WS, Lynn JS, Laudenslager ML, & Kohrt WM. (2005). Salivary cortisol determined by enzyme immunoassay is preferable to serum total cortisol for assessment of dynamic hypothalamic—pituitary—adrenal axis activity. Horumon To Rinsho, 63(3), 336–341. 10.1111/j.1365-2265.2005.02349.x [DOI] [PubMed] [Google Scholar]
- Grant BF, Goldstein RB, Saha TD, Chou SP, Jung J, Zhang H, Pickering RP, Ruan WJ, Smith SM, Huang B, & Hasin DS. (2015). Epidemiology of DSM-5 alcohol use disorder: Results from the national epidemiologic survey on alcohol and related conditions III. JAMA Psychiatry, 72(8), 757–766. 10.1001/jamapsychiatry.2015.0584 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guilliams TG, & Edwards L. (2010). Chronic stress and the HPA axis. The Standard, 9(2), 1–12. https://scholar.google.com/scholar_lookup?journal=The+standard&title=Chronic+stress+and+the+HPA+axis.&author=TG+ Guilliams&author=L+Edwards&volume=9&publication_year=2010& pages=1-12& [Google Scholar]
- Hawn SE, Cusack SE, & Amstadter AB. (2020). A systematic review of the self-medication hypothesis in the context of posttraumatic stress disorder and comorbid problematic alcohol use. Journal of Traumatic Stress, 33(5), 699–708. 10.1002/jts.22521 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayes AF. (2018). Introduction to mediation, moderation and conditional process analysis: A regression-based approach (2nd ed.). The Guilford Press. [Google Scholar]
- Hellmuth JC, Jaquier V, Swan SC, & Sullivan TP. (2014). Elucidating posttraumatic stress symptom profiles and their correlates among women experiencing bidirectional intimate partner violence. Journal of Clinical Psychology, 70(10), 1008–1021. 10.1002/jclp.22100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inslicht SS, Marmar CR, Neylan TC, Metzler TJ, Hart SL, Otte C, McCaslin SE, Larkin GL, Hyman KB, & Baum A. (2006). Increased cortisol in women with intimate partner violence-related posttraumatic stress disorder. Psychoneuroendocrinology, 31(7), 825–838. 10.1016/j.psyneuen.2006.03.007 [DOI] [PubMed] [Google Scholar]
- Intoximeters, Inc. Alco-sensor IV. St Louis, MO. https://www.intox.com/product/alco-sensor-iv/ [Google Scholar]
- Jennings JR, Kamarck T, Stewart C, Eddy M, & Johnson P. (1992). Alternate cardiovascular baseline assessment techniques: Vanilla or resting baseline. Psychophysiology, 29(6), 742–750. 10.1111/j.1469-8986.1992.tb02052.x [DOI] [PubMed] [Google Scholar]
- Johnson DM, Delahanty DL, & Pinna K. (2008). The cortisol awakening response as a function of PTSD severity and abuse chronicity in sheltered battered women. Journal of Anxiety Disorders, 22(5), 793–800. 10.1016/j.janxdis.2007.08.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keane TM, Kolb LC, Kaloupek DG, Orr SP, Blanchard EB, Thomas RG, Hsieh FY, & Lavori PW. (1998). Utility of psychophysiological measurement in the diagnosis of posttraumatic stress disorder: Results from a department of Veterans affairs cooperative study. Journal of Consulting and Clinical Psychology, 66(6), 914–923. 10.1037/0022-006X.66.6.914 [DOI] [PubMed] [Google Scholar]
- Khantzian EJ. (1997). The self-medication hypothesis of substance use disorders: A reconsideration and recent applications. Harvard Review of Psychiatry, 4(5), 231–244. 10.3109/10673229709030550 [DOI] [PubMed] [Google Scholar]
- Kim HK, Tiberio SS, Capaldi DM, Shortt JW, Squires EC, & Snodgrass JJ. (2015). Intimate partner violence and diurnal cortisol patterns in couples. Psychoneuroendocrinology, 51, 35–46. 10.1016/j.psyneuen.2014.09.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim YH. (2017). Associations of adverse childhood experiences with depression and alcohol abuse among Korean college students. Child Abuse and Neglect: The International Journal, 67, 338–348. 10.1016/j.chiabu.2017.03.009 [DOI] [PubMed] [Google Scholar]
- Kirschbaum C, Kudielka BM, Gaab J, Schommer NC, & Hellhammer DH. (1999). Impact of gender, menstrual cycle phase, and oral contraceptives on the activity of the hypothalamus-pituitary-adrenal axis. Psychosomatic Medicine, 61(2), 154–162. 10.1097/00006842-199903000-00006 [DOI] [PubMed] [Google Scholar]
- Klaassens ER, van Noorden MS, Giltay EJ, van Pelt J, van Veen T, & Zitman FG. (2009). Effects of childhood trauma on HPA-axis reactivity in women free of lifetime psychopathology. Progress in Neuro-Psychopharmacology & Biological Psychiatry, 33(5), 889–894. 10.1016/j.pnpbp.2009.04.011 [DOI] [PubMed] [Google Scholar]
- La Flair LN, Bradshaw CP, Storr CL, Green KM, Alvanzo AA, & Crum RM. (2012). Intimate partner violence and patterns of alcohol abuse and dependence criteria among women: A latent class analysis. Journal of Studies on Alcohol and Drugs, 73(3), 351–360. 10.15288/jsad.2012.73.351 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lang PJ, & Cuthbert BN. (1984). Affective information processing and the assessment of anxiety. Journal of Behavioral Assessment, 6(4), 369–395. 10.1007/BF01321326 [DOI] [PubMed] [Google Scholar]
- Lang PJ, Levin DN, Miller GA, & Kozak MJ(1983).Fear behavior, fear imagery, and the psychophysiology of emotion: The problem of affective response integration. Journal of Abnormal Psychology, 92(3), 276–306. 10.1037/0021-843X.92.3.276 [DOI] [PubMed] [Google Scholar]
- Lee RS, Oswald LM, & Wand GS. (2018). Early life stress as a predictor of co-occurring alcohol use disorder and post-traumatic stress disorder. Alcohol Research : Current Reviews, 39(2), 147–159. [PMC free article] [PubMed] [Google Scholar]
- Levin D, Cook E, & Lang P. (1982). Fear imagery and fear behaviorpsychophysiological analysis of clients receiving treatment for anxiety disorders. Psychophysiology, 19(5), 571–572. [Google Scholar]
- Lovallo WR, Dickensheets SL, Myers DA, Thomas TL, & Nixon SJ. (2000). Blunted stress cortisol response in abstinent alcoholic and polysubstance-abusing men. Alcoholism: Clinical and Experimental Research, 24(5), 651–658. 10.1111/j.1530-0277.2000.tb02036.x [DOI] [PubMed] [Google Scholar]
- Martino SC, Collins RL, & Ellickson PL. (2005). Cross-lagged relationships betweensubstanceuse and intimate partnerviolence amonga sample of young adult women. Journal of Studies on Alcohol, 66(1), 139–148. 10.15288/jsa.2005.66.139 [DOI] [PubMed] [Google Scholar]
- McEwen BS. (1998). Protective and damaging effects of stress mediators. New England Journal of Medicine, 338(3), 171–179. 10.1056/NEJM199801153380307 [DOI] [PubMed] [Google Scholar]
- McEwen BS, & Gianaros PJ. (2010). Central role of the brain in stress and adaptation: Links to socioeconomic status, health, and disease. Annals of the New York Academy of Sciences, 1186, 190–222. 10.1111/j.1749-6632.2009.05331.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- McEwen BS, & Wingfield JC. (2003). The concept of allostasis in biology and biomedicine. Hormones and Behavior, 43(1), 2–15. 10.1016/S0018-506X(02)00024-7 [DOI] [PubMed] [Google Scholar]
- Montero-López E, Santos-Ruiz A, García-Ríos MC, RodríguezBlázquez M, Rogers HL, & Peralta-Ramírez MI. (2018). The relationship between the menstrual cycle and cortisol secretion: Daily and stress-invoked cortisol patterns. International Journal of Psychophysiology, 131, 67–72. 10.1016/j.ijpsycho.2018.03.021 [DOI] [PubMed] [Google Scholar]
- Nicolson NA. (2007). Measurement of cortisol. In Luecken LJ & Gallo LC (Eds.), Handbook of physiological research methods in health psychology (pp. 37–74). Sage Publications. [Google Scholar]
- Oberleitner LM, Mandel DL, & Easton CJ. (2013). Treatment of co-occurring alcohol dependence and perpetration of intimate partner violence: The role of anger expression. Journal of Substance Abuse Treatment, 45(3), 313–318. 10.1016/j.jsat.2013.03.001 [DOI] [PubMed] [Google Scholar]
- Okuda M, Olfson M, Hasin D, Grant BF, Lin K-H, & Blanco C. (2011). Mental health of victims of intimate partner violence: Results from a national epidemiologic survey. Psychiatric Services, 62(8), 959–962. 10.1176/ps.62.8.pss6208_0959 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orr SP, Pitman RK, Lasko NB, & Herz LR. (1993). Psychophysiological assessment of posttraumatic stress disorder imagery in World War II and Korean combat veterans. Journal of Abnormal Psychology, 102(1), 152–159. 10.1037/0021-843X.102.1.152 [DOI] [PubMed] [Google Scholar]
- Osório FL, Loureiro SR, Hallak JEC, Machado-de-Sousa JP, Ushirohira JM, Baes CVW, Apolinario TD, Donadon MF, Bolsoni LM, Guimarães T, Fracon VS, Silva-Rodrigues APC, Pizeta FA, Souza RM, Sanches RF, dos Santos RG, MartinSantos R, & Crippa JAS. (2019). Clinical validity and intrarater and test-retest reliability of the structured clinical interview for DSM-5—clinician version (SCID-5-CV). Psychiatry and Clinical Neurosciences, 73(12), 754–760. 10.1111/pcn.12931 [DOI] [PubMed] [Google Scholar]
- Oswald LM, Wong DF, McCaul M, Zhou Y, Kuwabara H, Choi L, Brasic J, & Wand GS. (2005). Relationships among ventral striatal dopamine release, cortisol secretion, and subjective responses to amphetamine. Neuropsychopharmacology, 30(4), 821–832. 10.1038/sj.npp.1300667 [DOI] [PubMed] [Google Scholar]
- Øverup CS, DiBello AM, Brunson JA, Acitelli LK, & Neighbors C. (2015). Drowning the pain: Intimate partner violence and drinking to cope prospectively predict problem drinking. Addictive Behaviors, 41, 152–161. 10.1016/j.addbeh.2014.10.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peterson C, Kearns MC, McIntosh WL, Estefan LF, Nicolaidis C, McCollister KE, Gordon A, & Florence C. (2018). Lifetime economic burden of intimate partner violence among US adults. American Journalof Preventive Medicine, 55(4), 433–444. 10.1016/j.amepre.2018.04.049 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pinna KL, Johnson DM, & Delahanty DL. (2014). PTSD, comorbid depression, and the cortisol waking response in victims of intimate partner violence: Preliminary evidence. Anxiety, Stress, and Coping, 27(3), 253–269. 10.1080/10615806.2013.852185 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pinto RJ, Correia-Santos P, Costa-Leite J, Levendosky AA, & Jongenelen I. (2016). Cortisol awakening response among women exposed to intimate partner violence. Psychoneuroendocrinology, 74, 57–64. 10.1016/j.psyneuen.2016.08.024 [DOI] [PubMed] [Google Scholar]
- Pitman RK, Orr SP, Forgue DF, de Jong JB, & Claiborn JM. (1987). Psychophysiologic assessment of posttraumatic stress disorder imagery in Vietnam combat veterans. Archives of General Psychiatry, 44(11), 970–975. 10.1001/archpsyc.1987.01800230050009 [DOI] [PubMed] [Google Scholar]
- Price JL, Bruce MA, & Adinoff B. (2021). Addressing structural racism in psychiatry with steps to improve psychophysiologic research. JAMA Psychiatry, 79(1):70–74. 10.1001/jamapsychiatry.2021.2663 [DOI] [PubMed] [Google Scholar]
- Saal D, Dong Y, Bonci A, & Malenka RC. (2003). Drugs of abuse and stress trigger a commonsynapticadaptationin dopamine neurons.Neuron, 37(4), 577–582. 10.1016/S0896-6273(03)00021-7 [DOI] [PubMed] [Google Scholar]
- Saxbe DE. (2008). A field (researcher’s) guide to cortisol: Tracking HPA axis functioning in everyday life. Health Psychology Review, 2(2), 163–190. 10.1080/17437190802530812 [DOI] [Google Scholar]
- Shorter D, Hsieh J, & Kosten TR. (2015). Pharmacologic management of comorbid post-traumatic stress disorder and addictions. American Journal on Addictions, 24(8), 705–712. 10.1111/ajad.12306 [DOI] [PubMed] [Google Scholar]
- Sinha R,Fox HC,Hong KA, Bergquist K, Bhagwagar Z, & Siedlarz KM. (2009). Enhanced negative emotion and alcohol craving, and altered physiological responses following stress and cue exposure in alcohol dependent individuals. Neuropsychopharmacology, 34(5), 1198–1208. 10.1038/npp.2008.78 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sinha R, Lacadie C, Skudlarski P, Fulbright RK, Rounsaville BJ, Kosten TR, & Wexler BE. (2005). Neural activity associated with stress-induced cocaine craving: A functional magnetic resonance imaging study. Psychopharmacology, 183(2), 171–180. 10.1007/s00213-005-0147-8 [DOI] [PubMed] [Google Scholar]
- Smith SM, Goldstein RB, & Grant BF. (2016). The association between post-traumatic stress disorder and lifetime DSM-5 psychiatric disorders among veterans: Data from the national epidemiologic survey on alcohol and related conditions-III (NESARC-III). Journal of Psychiatric Research, 82, 16–22. 10.1016/j.jpsychires.2016.06.022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Speer KE, Semple S, Naumovski N, D’Cunha NM, & McKune AJ. (2019). HPA axis function and diurnal cortisol in post-traumatic stress disorder: A systematic review. Neurobiology of Stress, 11, Article 100180. 10.1016/j.ynstr.2019.100180 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spencer C, Mallory AB, Cafferky BM, Kimmes JG, Beck AR, & Stith SM. (2019). Mental health factors and intimate partner violence perpetration and victimization: A meta-analysis. Psychology of Violence, 9(1), 1–17. 10.1037/vio0000156 [DOI] [Google Scholar]
- Stephens MAC, & Wand G. (2012). Stress and the HPA axis: Role of glucocorticoids in alcohol dependence. Alcohol Research: Current Reviews, 34(4), 468–483. [PMC free article] [PubMed] [Google Scholar]
- Sullivan TP, Armeli S, Tennen H, Weiss NH, & Hansen NB. (2020). Fluctuations in daily PTSD symptoms are related to proximal alcohol use: A micro-longitudinal study of women victims of intimate partner violence. American Journal of Drug and Alcohol Abuse, 46(1), 98–108. 10.1080/00952990.2019.1624765 [DOI] [PubMed] [Google Scholar]
- Sullivan TP, & Holt LJ. (2008). PTSD symptom clusters are differentially related to substance use among community women exposed to intimate partner violence. Journal of Traumatic Stress, 21(2), 173–180. 10.1002/jts.20318 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sullivan TP, Weiss NH, Flanagan JC, Willie TC, Armeli S, & Tennen H. (2016). PTSD and daily co-occurrence of drug and alcohol use among women experiencing intimate partner violence. Journal of Dual Diagnosis, 12(1), 36–42. 10.1080/15504263.2016.1146516 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szabo YZ, Breeding T, Hejl C, Guleria RS, Nelson SM, & Zambrano-Vazquez L. (2020). Cortisol as a biomarker of alcohol use in combat veterans: A literature review and framework for future research. Journal of Dual Diagnosis, 16(3), 322–335. 10.1080/15504263.2020.1771504 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tull MT, Forbes CN, Weiss NH, & Gratz KL. (2019). An investigation of the effect of trauma script exposure on risk-taking among patients with substance use disorders and posttraumatic stress disorder. Journal of Anxiety Disorders, 62, 77–85. 10.1016/j.janxdis.2019.01.002 [DOI] [PubMed] [Google Scholar]
- Tull MT, McDermott MJ, Gratz KL, Coffey SF, & Lejuez CW. (2011). Cocaine-related attentional bias following trauma cue exposure among cocaine dependent in-patients with and without post-traumatic stress disorder. Addiction, 106(10), 1810–1818. 10.1111/j.1360-0443.2011.03508.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walton JL, Raines AM, Cuccurullo LJ, Vidaurri DN, Villarosa-Hurlocker MC, & Franklin CL. (2018). The relationship between DSM-5 PTSD symptom clusters and alcohol misuse among military veterans. American Journal on Addictions, 27(1), 23–28. 10.1111/ajad.12658 [DOI] [PubMed] [Google Scholar]
- Wand G. (2008). The influence of stress on the transition from drug use to addiction. Alcohol Research & Health, 31(2), 119–136. [PMC free article] [PubMed] [Google Scholar]
- Wand GS, Oswald LM, McCaul ME, Wong DF, Johnson E, Zhou Y, Kuwabara H, & Kumar A. (2007). Association of amphetamineinduced striatal dopamine release and cortisol responses to psychological stress. Neuropsychopharmacology, 32(11), 2310–2320. 10.1038/sj.npp.1301373 [DOI] [PubMed] [Google Scholar]
- Weathers FW, Blake DD, Schnurr PP, Kaloupek DG, Marx BP, & Keane TM. (2013). The life events checklist for DSM-5 (LEC-5). Instrument available from the National Center for PTSD at https://www.ptsd.va.gov/professional/assessment/te-measures/life_events_checklist.asp [Google Scholar]
- Weathers FW, Litz BT, Keane TM, Palmieri PA, Marx BP, & Schnurr PP. (2013). The PTSD checklist for DSM-5 (PCL-5). Instrument available from the National Center for PTSD at https://www.ptsd.va.gov/professional/assessment/adult-sr/ptsd-checklist.asp
- Wortmann JH, Jordan AH, Weathers FW, Resick PA, Dondanville KA, Hall-Clark B, Foa EB, Young-McCaughan S, Yarvis JS, Hembree EA, Mintz J, Peterson AL, & Litz BT. (2016). Psychometric analysis of the PTSD checklist-5 (PCL-5) among treatment-seeking military service members. Psychological Assessment, 28, 1392–1403. 10.1037/pas0000260 [DOI] [PubMed] [Google Scholar]
- Yehuda R, & LeDoux J. (2007). Response variation following trauma: A translational neuroscience approach to understanding PTSD. Neuron, 56(1), 19–32. 10.1016/j.neuron.2007.09.006 [DOI] [PubMed] [Google Scholar]
- Zlotnick C, Franklin CL, & Zimmerman M. (2002). Does “subthreshold” posttraumatic stress disorder have any clinical relevance? Comprehensive Psychiatry, 43(6), 413–419. 10.1053/comp.2002.35900 [DOI] [PubMed] [Google Scholar]