Abstract
OBJECTIVES:
Bloodstream infections (BSIs) acquired in the ICU represent a detrimental yet potentially preventable condition. We determined the prevalence of BSI acquired in the ICU (ICU-onset BSI), pathogen profile, and associated risk factors.
DESIGN:
Retrospective cohort study.
DATA SOURCES:
Eighty-five U.S. hospitals in the Cerner Healthfacts Database.
PATIENT SELECTION:
Adult hospitalizations between January 2009 and December 2015 including a (≥ 3 d) ICU stay.
DATA EXTRACTION AND DATA SYNTHESIS:
Prevalence of ICU-onset BSI (between ICU Day 3 and ICU discharge) and associated pathogen and antibiotic resistance distributions were compared with BSI present on (ICU) admission (ICU-BSIPOA); and BSI present on ICU admission day or Day 2. Cox models identified risk factors for ICU-onset BSI among host, care setting, and treatment-related factors. Among 150,948 ICU patients, 5,600 (3.7%) had ICU-BSIPOA and 1,306 (0.9%) had ICU-onset BSI. Of those with ICU-BSIPOA, 4,359 (77.8%) were admitted to ICU at hospital admission day. Patients with ICU-onset BSI (vs ICU-BSIPOA) displayed higher crude mortality of 37.9% (vs 20.4%) (p < 0.001) and longer median (interquartile range) length of stay of 13 days (8–23 d) (vs 5 d [3–8 d]) (p < 0.001) (considering all ICU stay). Compared with ICU-BSIPOA, ICU-onset BSI displayed more Pseudomonas, Acinetobacter, Enterococcus, Candida, and Coagulase-negative Staphylococcus species, and more methicillin-resistant staphylococci, vancomycin-resistant enterococci, ceftriaxone-resistant Enterobacter, and carbapenem-resistant Enterobacterales and Acinetobacter species, respectively. Being younger, male, Black, Hispanic, having greater comorbidity burden, sepsis, trauma, acute pulmonary or gastrointestinal presentations, and pre-ICU exposure to antibacterial and antifungal agents was associated with greater ICU-onset BSI risk after adjusted analysis. Mixed ICUs (vs medical or surgical ICUs) and urban and small/medium rural hospitals were also associated with greater ICU-onset BSI risk. The associated risk of acquiring ICU-onset BSI manifested with any duration of mechanical ventilation and 7 days after insertion of central venous or arterial catheters.
CONCLUSIONS:
ICU-onset BSI is a serious condition that displays a unique pathogen and resistance profile compared with ICU-BSIPOA. Further scrutiny of modifiable risk factors for ICU-onset BSI may inform control strategies.
Keywords: antibiotic resistance, bacteremia, bloodstream, infection, intensive care unit, risk factors, bacteremia, epidemiology, intensive care unit, nosocomial, prevention, risk factors
Bloodstream infection (BSI) carries a high burden of morbidity, mortality, and healthcare costs (1) and is a common reason for ICU admission. However, BSI that develops as a nosocomial complication of ICU stay (i.e., ICU-onset BSI) is often an avoidable condition and might represent an indicator of ICU care quality (2). Understanding the occurrence rate of and risk factors for ICU-BSI might inform ICU providers’ trigger for initiating antibiotics, prompt behavioral modifications for prevention, and minimize associated morbidity and mortality. Understanding the epidemiology of ICU-onset BSI might also benefit antibiotic stewardship. At any given time, approximately 70% of patients in ICUs worldwide are on antibiotics (3), and this pattern has not decreased over time (4). Differentiating bloodstream pathogens and associated antibiotic resistance phenotypes that are more likely to be encountered with ICU-onset BSI versus BSI present-on-(ICU)-admission (ICU-BSIPOA) might optimize the spectrum of antibiotic therapy prescribed in either setting.
Existing evidence on prevalence of and risk factors for ICU-onset BSI has limited generalizability due to differences in regional microbial epidemiology, case mix, and ICU care practices across reports and over time (5–8). Furthermore, the relatively limited sample size of prior studies (9, 10) has precluded simultaneous assessment of many candidate risk factors or BSI populations beyond a specific BSI type (e.g., central venous catheter-related BSI). We conducted a large database analysis of ICU patients in U.S. hospitals to determine the prevalence, patient and care setting, and treatment-related factors associated with the risk of acquiring ICU-onset BSI.
MATERIALS AND METHODS
Data Source
A retrospective cohort study was conducted using the Cerner Healthfacts Database, a deidentified clinical data repository from U.S. hospitals using Cerner electronic health record (EHR) systems (North Kansas City, MO). It includes EHR-based clinical and administrative data and spans demographic data, diagnoses, procedures, medications, and laboratory and physiologic data elements and has been leveraged in prior studies on critically ill patients (11). Given the deidentified nature of the data, its HIPAA-compliant derivation, exclusive secondary usage, and the nonuse of human subjects, the study was deemed not to require ethics board review by the Office of Human Subjects Research Protections, National Institutes of Health, under the revised Common Rule.
Study Population and Case Definition
Adult inpatients greater than or equal to 20 years old admitted between January 1, 2009, and December 31, 2015, at database hospitals whose encounter included an ICU stay spanning greater than or equal to 3 consecutive days were included (online supplement, section A, http://links.lww.com/CCM/H200). Only the first ICU stay per patient encounter was analyzed. The ICU length of stay (LOS) was considered from admission to discharge. Patients in the ICU with evidence of bacterial or candida bloodstream isolates were dichotomized by time of BSI onset into ICU-onset BSI (BSI recorded on blood cultures drawn between ICU Day 3 and ICU discharge based on Centers for Disease Control and Prevention [CDC] definitions [12]) and ICU-BSIPOA (blood cultures drawn on the day of ICU admission or ICU Day 2 with onset presumed prior to ICU stay). Patients with BSI due to the same species reported in the 14 days prior to their index ICU admission were excluded. Patients who did not have bacteremia during their ICU stay (despite blood sampling when required by ICU care) were classified as non-BSI.
Microbiology
Bacterial species generally considered contaminants in blood culture were excluded. For microbiology data, noncontaminant species were grouped into the following categories: Staphylococcus aureus, coagulase-negative Staphylococcus (CoNS), Streptococci, Enterococci, Enterobacterales (Escherichia coli, Klebsiella, and Enterobacter species), Acinetobacter species, Candida albicans and nonalbicans species, and other. Among CoNS species in blood culture, S. Lugdinensis was always considered a pathogen, whereas non-Lugdinensis CoNS were considered pathogens only if isolated from two consecutive blood cultures drawn from two different vascular sites on the same or on consecutive days. We excluded blood cultures with all species of the following genera presuming them to be contaminants: Aerococcus, Corynebacterium, Bacillus, Diphtheroids, Micrococcus, and Propionibacterium. Common treatment-limiting antibiotic-resistance phenotypes were defined based on CDC criteria and identified based on reported interpretations of resistance. Additional details of this methodology applied to the study database have been reported previously (online supplement, section B, http://links.lww.com/CCM/H200) (14).
Candidate Risk Factors of ICU-Onset BSI
Risk factors for ICU-onset BSI were compared with the non-BSI cohort. Candidate risk factors were selected based on prior evidence of their role as potential risk factors identified in the literature and/or by investigators based on prior knowledge, that is, variables suspected to be associated with ICU stay and development of BSI. Demographic and center-level and care setting-level data were reported for encounters in the database. Other variables were derived from International Classification of Diseases, 9th revision diagnosis and procedure codes and specific EHR orders (14). Comorbidity burden was estimated using the Elixhauser comorbidity index (14–16). Acute presentation groupings were adapted from those previously reported in the Multiparameter Intelligent Monitoring in Intensive Care II database (www.physionet.org), and acute organ failures were aggregated using the Sequential Organ Failure Assessment (SOFA) score (17). Inpatient (pre-ICU) antimicrobial agent use was assessed from pharmacy data (18). Select time-varying candidate risk factors were identified using date-stamped procedure codes and included vascular catheters (central venous catheter, arterial catheter), mechanical ventilation, cardiopulmonary resuscitation, and blood product transfusion (online supplement, section C, http://links.lww.com/CCM/H200). The list of variables, definitions, and codes are presented in eTables 1–7, http://links.lww.com/CCM/H200).
Statistical Analysis
Differences in characteristics between groups were tested using analysis of variance procedures for continuous variables and chi-square tests for categorical variables using the compareGroups package in R 3.5.0 (19, 20). Risk factors for ICU-onset BSI were assessed using an inferential Cox model using the coxph() function in the survival package (21, 22). Candidate baseline and time-varying variables were identified based on prior studies, clinical importance deemed by investigators and availability in the database. Multicollinearity was assessed by comparing z score change; the SOFA score was determined to be colinear with vasopressor use, liver disease, coagulopathy, and being on mechanical ventilator prompting its exclusion from the final model. Overlap in hospital characteristics (e.g., large hospitals tend to be clustered in urban locations) were mitigated by using a combined variable stratified on both bed-capacity and urbanicity. Each time-varying covariate was allowed to have different early and late effects relative to exposure using 7 days post exposure as the cut off, based on prior studies assessing risk (23). This dichotomization allowed for detailing whether the instantaneous risk of ICU-onset BSI increased markedly after a week of being exposed to the time varying factor. The dichotomization of risk periods for the time varying factors was assessed by likelihood ratio tests. Sensitivity analyses were performed: 1) replacing the Elixhauser comorbidity index with individual comorbid conditions (e.g., liver disease, hypertension, coagulopathy, etc.) to evince risk effects by comorbidity type and 2) defining ICU-onset BSI as occurring on or after Day 4 (in lieu of Day 3). All analyses were performed in R 3.5.0 (19).
RESULTS
Among 2,529,158 inpatient encounters at 169 hospitals between 2009 and 2015, 150,948 unique inpatient encounters (6%) with an initial ICU stay spanning greater than or equal to 3 days were identified at 85 hospitals (Fig. 1). Of these, 143,589 patients (95.4%) were nonbacteremic, 5,600 (3.7%) had ICU-BSIPOA, and 1,306 (0.9%) had ICU-onset BSI. Among the cohort, 41.5% of them had at least one blood sampling during their ICU stay, including 38.7% of the non-BSI group (eFig. 1, http://links.lww.com/CCM/H200). The median (interquartile range [IQR]) onset of ICU-onset BSI was 6 days (4–11 d) post ICU admission. The median LOS after ICU-onset BSI was 5 days with an IQR of (2–11 d). ICU-onset BSI was monomicrobial in 88% of cases. Although BSI source was not readily discernible, 71% of these encounters had a site-specific infection diagnosis code. Baseline characteristics are compared between patients with ICU-onset BSI and ICU-BSIPOA and non-BSI ICU patients in Table 1 and eTable 8 (http://links.lww.com/CCM/H200). The median (IQR) SOFA score at ICU admission was 5 (3–8) in both ICU-BSI groups and 3 (1–5) for the non-BSI group. The median (IQR) ICU LOS was longer for the ICU-onset BSI (13 d [8–23 d]) vs ICU-BSIPOA (5 d [3–8 d]) and non-BSI groups (4 d [3–7 d]) (p < 0.001). Crude mortality was 10% in the nonbacteremia group, 20.4% in the ICU-BSIPOA group, and 37.9% in the ICU-onset BSI group (p < 0.001).
Figure 1.
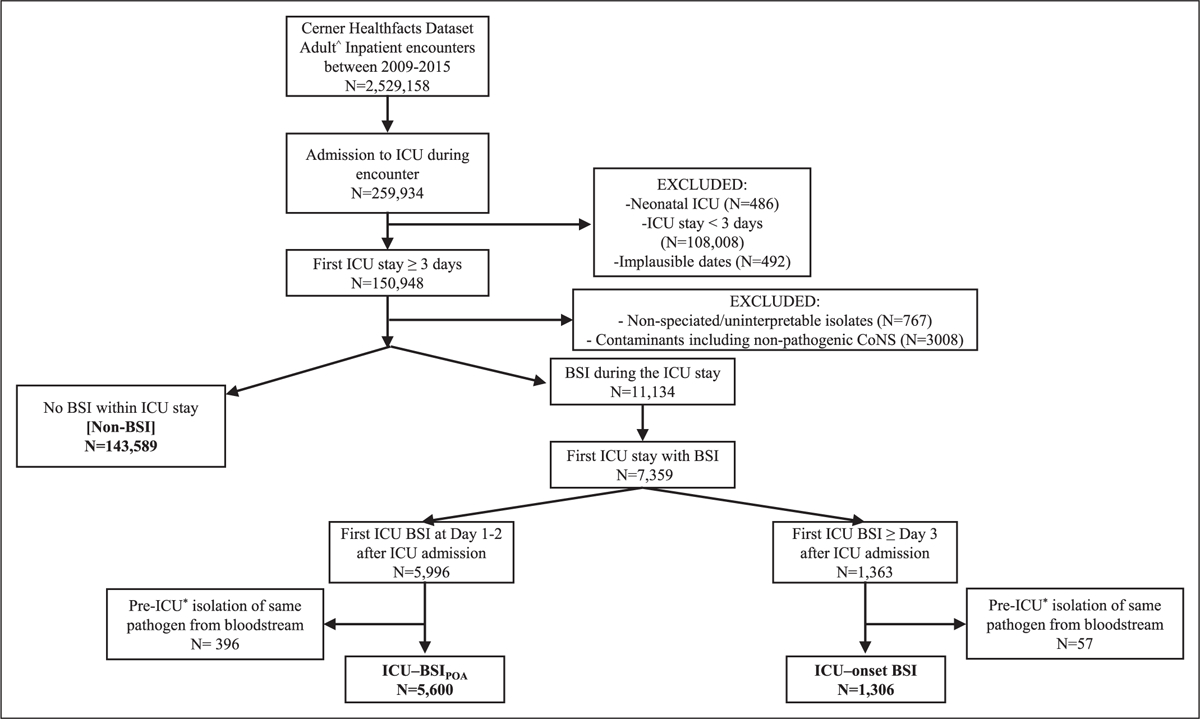
Case selection flowchart. BSI present-on-(ICU)-admission (ICU-BSIPOA) represents BSI with onset on the day of admission to the ICU or the day after. BSI acquired in the ICU (ICU-onset BSI) represents BSIs with onset on the third day of ICU admission or thereafter. ^Greater than or equal to 20 yr old; *limited to inpatient period of up to 14 d preceding index ICU admission. BSI = bloodstream infection.
TABLE 1.
Baseline Characteristics
| Variables | ICU-Related BSI, N = 1,306) | BSI at Days, 1–2 of ICU Stay, N = 5,600 | No BSI During ICU Stay, N = 143,589 |
|---|---|---|---|
| Host characteristics | |||
| Age, median (IQR) | 63 (52–73) | 66 (54–77) | 65 (53–76) |
| Gender female, n (%) | 567 (43.4) | 2,665 (47.6) | 6,7351 (46.9) |
| Race White, n (%) | 820 (62.8) | 3,849 (68.7) | 10,4807 (73.0) |
| Race Black, n (%) | 337 (25.8) | 1,263 (22.6) | 26,430 (18.4) |
| Race Asian, n (%) | 12 (0.92) | 78 (1.4) | 1,731 (1.2) |
| Race Hispanic, n (%) | 26 (2.0) | 64 (1.1) | 1,479 (1.0) |
| Race others, n (%) | 72 (5.5) | 231 (4.1) | 5,084 (3.5) |
| Elixhauser index, median (IQR) | 4.0 (2.0–6.0) | 4.0 (2.0–6.0) | 4.0 (2.0–5.0) |
| Immunocompromised, n (%) | 156 (11.9) | 724 (12.9) | 11,210 (7.8) |
| ICU admission | |||
| Baseline Sepsis Organ Failure Assessment score at ICU admission, median (IQR) | 5.0 (3.0–8.0) | 5.0 (3.0–8.0) | 3.0 (1.0–5.0) |
| Hospital day onset for ICU admission, median (IQR) | 1.0 (1.0–2.0) | 1.0 (1.0–1.0) | 1.0 (1.0–2.0) |
| Sepsis shock at ICU admission, n (%) | 88 (6.7) | 913 (16.3) | 3,917 (2.7) |
| Mechanical ventilation at ICU admission, n (%) | 413 (31.6) | 1,221 (21.8) | 21,843 (15.2) |
| ECMO at ICU admission, n (%) | 1 (0.08) | 3 (0.05) | 52 (0.04) |
| Heart circulatory assist at ICU admission, n (%) | 27 (2.1) | 11 (0.2) | 1,360 (0.95) |
| ECMO and heart circulatory assist at ICU admission, n (%) | 28 (2.1) | 14 (0.25) | 1,395 (0.97) |
| ICU length of stay, median (IQR) | 13.0 (8.0–23.0) | 5.0 (3.0–8.0) | 4.0 (3.0–7.0) |
| Central catheter at ICU admission, n (%) | 19 (1.5) | 44 (0.79) | 1,294 (0.90) |
| Burn, n (%) | 9 (0.69) | 7 (0.12) | 440 (0.31) |
| Trauma, n (%) | 133 (10.2) | 83 (1.5) | 7,333 (5.1) |
| Dialysis required at ICU admission, n (%) | 7 (0.54) | 47 (0.84) | 618 (0.43) |
| Neutropenia at ICU admission, n (%) | 12 (0.92) | 114 (2.0) | 829 (0.58) |
| Parenteral nutrition at ICU admission, n (%) | 10 (0.77) | 29 (0.52) | 359 (0.25) |
| Vasopressor at ICU admission, n (%) | 434 (33.2) | 2,042 (36.5) | 27,418 (19.1) |
BSI = bloodstream infection, ECMO = extracorporeal membrane oxygenation, IQR = interquartile range, Detected at day 3 or later of ICU stay.
Prevalence, Pathogen Profile, and Antibiotic Resistance
Based on cumulative exposure time, ICU-onset BSI prevalence was 1.47 cases per 1,000 patient-ICU-days. On the other hand, the prevalence of ICU-BSIPOA was over four-fold greater at 6.32 cases per 1,000 patient-ICU-days. Overall, the most common organisms causing BSI among ICU patients was S. aureus (Fig. 2A for organism distribution). However, there were notable differences in the species distribution between the two types of ICU-BSI (Fig. 2B): Acinetobacter species, CoNS, and Candida, Bacteroides, and Enterococcus species, respectively, were more often the pathogen involved in ICU-onset BSI (vs ICU-BSIPOA), whereas S. aureus, E. coli, and Streptococcus species were more often the etiology for ICU-BSIPOA (vs ICU-onset BSI). In general, resistance phenotypes tended to be more prevalent across bloodstream pathogens causing ICU-onset BSI (vs ICU-BSIPOA). More specifically, there were more methicillin-resistant S. aureus and CoNS, vancomycin-resistant enterococci, ceftriaxone-resistant Enterobacterales, and carbapenem-resistant E. coli, Klebsiella, and Acinetobacter species among the bloodstream pathogens causing ICU-onset BSI (vs ICU BSIPOA), respectively (Fig. 2C). Seventy-eight percent of patients with ICU-BSIPOA were in fact admitted to the ICU on the day of hospital admission and were found to display similar pathogen distribution compared with the overall ICU-BSIPOA group (eTables 9–11, http://links.lww.com/CCM/H200).
Figure 2.
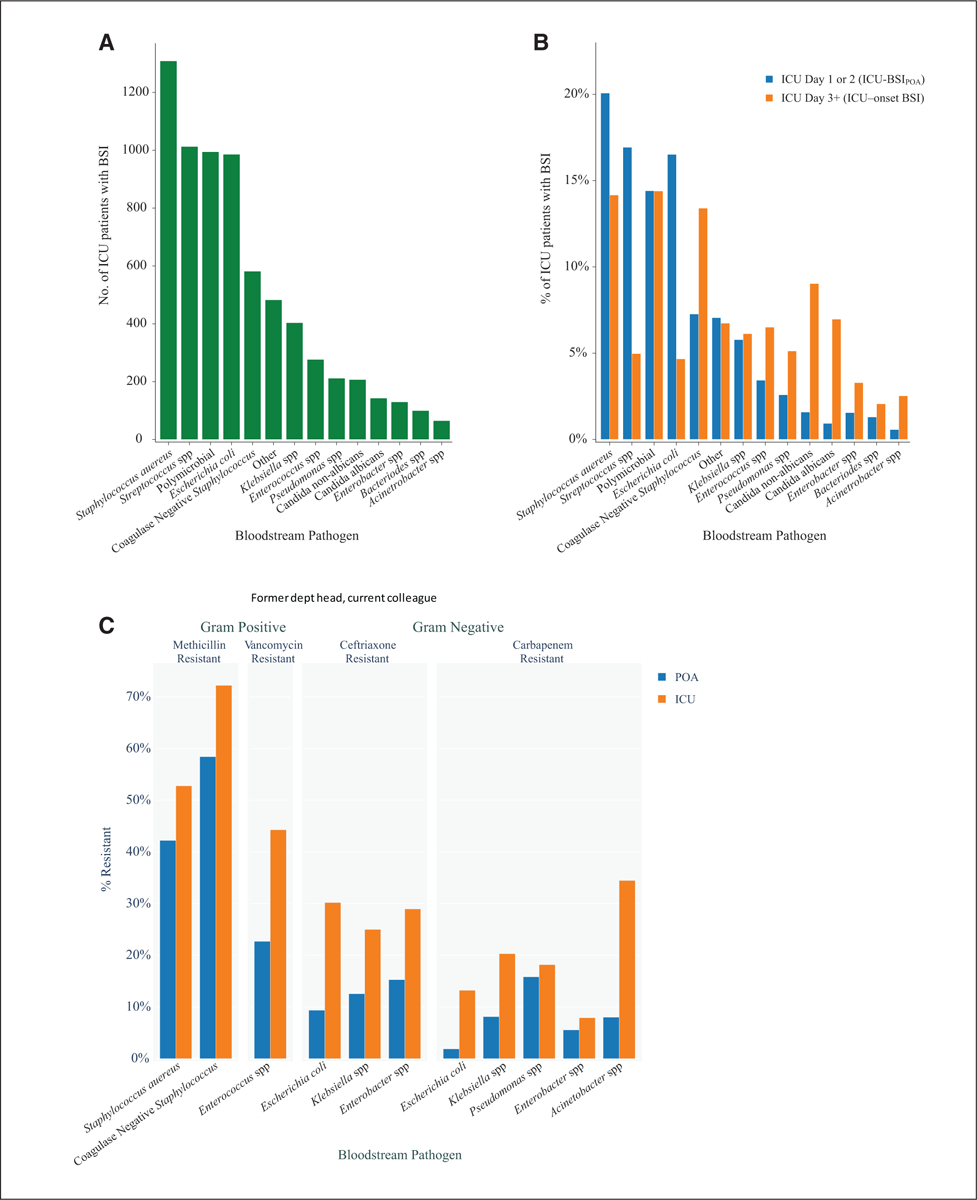
Distribution of bloodstream pathogens among ICU patients. A, The distribution of bloodstream pathogens across all ICU patients with bloodstream infection (BSI) (green bars). B, The percentage distributions of bloodstream pathogens separately for BSI present on (ICU) admission (ICU-BSIPOA) (blue bars) and BSI acquired in the ICU (ICU-onset BSI) (orange bars), respectively, and displayed as paired graphs to enable visual comparisons. C, The distribution of bloodstream pathogens according to resistance profile separately for ICU-BSIPOA (blue bars) and ICU-onset BSI (orange bars), respectively.
Risk Factors for ICU-Onset BSI Compared With Non-BSI
Demographic Factors and Comorbid Conditions.
In the multivariable model, among demographic characteristics, younger age, male sex, and Blacks and Hispanics (vs Whites, respectively) and residing in a healthcare facility were independent risk factors for ICU-onset BSI (Table 2 for hazard ratio estimates and 95% CI). Higher Elixhauser comorbidity index was associated with higher ICU-onset BSI risk, whereas having codes for neutropenia and immunosuppression was not.
TABLE 2.
Risk Factors for Acquiring Bloodstream Infection Acquired in the ICU
| Category | Hazard Ratio (95% CI) |
|---|---|
| Demographics/comorbidities | |
| Age | 0.93 (0.89–0.96)a |
| Female (vs male) | 0.82 (0.73–0.92)a |
| Asian (vs White) | 0.85 (0.48–1.52) |
| Black (vs White) | 1.35 (1.17–1.55)a |
| Hispanic (vs White) | 1.9 (1.26–2.87)a |
| Other race (vs White) | 1.76 (1.37–2.26)a |
| Unknown race (vs White) | 1.08 (0.76–1.53) |
| Elixhauser comorbidity index | 1.03 (1.0–1.06)a |
| Immunocompromised | 0.98 (0.82–1.17) |
| Healthcare facility origin | 1.14 (0.97–1.35) |
| Acute illness diagnoses | |
| Burns | 1.76 (0.78–3.98) |
| Trauma | 1.96 (1.48–2.59)a |
| Cardiac | 0.98 (0.86–1.13) |
| Gastrointestinal | 1.35 (1.2–1.53)a |
| Hematology/oncology | 1.01 (0.89–1.15) |
| Infectious disease/sepsis | 1.46 (1.24–1.71)a |
| Neurologic | 0.96 (0.85–1.09) |
| Other unspecified | 0.95 (0.79–1.15) |
| Pulmonary | 2.09 (1.8–2.43)a |
| Renal/metabolic/toxic | 0.8 (0.66–0.96)a |
| Skin soft tissue/musculoskeletal | 0.96 (0.85–1.08) |
| TBI alone (vs no trauma) | 1.34 (0.92–1.97) |
| TBI with other complication (vs no trauma) | 2.63 (1.74–3.96)a |
| Other procedural complication (vs no trauma) | 1.37 (1.18–1.6)a |
| Center/care setting | |
| Large urban hospital (vs large rural hospital) | 1.9 (1.37–2.62)a |
| Medium rural hospital (vs large rural hospital) | 1.65 (1.14–2.37)a |
| Medium urban hospital (vs large rural hospital) | 2.07 (1.54–2.77)a |
| Small rural hospital (vs large rural hospital) | 1.56 (1.04–2.34)a |
| Small urban hospital (vs large rural hospital) | 2.4 (1.67–3.46)a |
| Cardiac ICU (vs nonspecialty ICU) | 0.91 (0.76–1.09) |
| Medical ICU (vs nonspecialty ICU) | 0.57 (0.45–0.71)a |
| Neurologic ICU (vs nonspecialty ICU) | 0.71 (0.46–1.1) |
| Surgical ICU (vs nonspecialty ICU) | 0.8 (0.66–0.97)a |
| Northeast census region (vs east) | 1.34 (1.1–1.63)a |
| South census region (vs east) | 1.06 (0.89–1.26) |
| West census region (vs east) | 0.86 (0.67–1.1) |
| Academic teaching facility | 1.01 (0.84–1.22) |
| Annual bacteremia volume | 1.0 (1.0–1.0) |
| Treatment related | |
| Pre-ICU antibacterial agent exposure | 2.65 (2.05–3.42)a |
| Pre-ICU antifungal agent exposure | 2.72 (2.37–3.12)a |
| Pre-ICU antiviral agent exposure | 1.0 (0.78–1.27) |
| Dialysis required upon ICU admission | 0.81 (0.38–1.7) |
| Vasopressor administration upon ICU admission | 2.7 (2.38–3.06)a |
| Extracorporeal membrane oxygenation/hearth circulatory assistance required during admission | 2.11 (1.41–3.16)a |
| Parenteral nutrition required during admission | 1.24 (0.64–2.39) |
| Time varying | |
| Mechanical ventilation | 3.76 (2.88–4.91)a |
| CPR >7 d | 1.93 (1.19–3.12)a |
| CPR < 7 d | 1.14 (0.83–1.58) |
| Central catheter > 7 d | 1.69 (1.38–2.07)a |
| Central catheter < 7 d | 1.15 (0.99–1.32) |
| Arterial catheter > 7 d | 1.46 (1.05–2.03)a |
| Arterial catheter < 7 d | 0.97 (0.75–1.24) |
| Blood product transfusion > 7 d | 0.9 (0.69–1.17) |
| Blood product transfusion < 7 d | 0.93 (0.77–1.12) |
CPR = cardiopulmonary rescue, TBI = traumatic brain injury.
p < 0.05.
Point estimates (along with 95% CIs) from the Cox proportional hazards model bearing statistical significance. For time varying covariates, the 7-d cut off represents 7 d from the time of procedure.
Center and Care Setting–Related Risk Factors.
In multivariate analysis, among regions, centers in the Northeast were associated with the highest risk of ICU-onset BSI; among centers stratified by size and urbanicity, large rural centers were associated with the lowest risk; teaching status did not appear to be associated with higher or lower risk of ICU-onset BSI. Compared with ICUs serving a mixed or undifferentiated population, cardiac and neurologic ICUs were each associated with a similar risk. Medical and surgical ICUs were each associated with a lower risk for ICU-onset BSI, respectively. The associated risk of ICU-onset BSI doubled among patients admitted with (vs without) a diagnosis of trauma (with or without brain injury). Admission with (vs without) acute pulmonary and gastrointestinal diagnoses was associated with higher, and admissions for renal, toxic, and metabolic derangements (vs other indications) were associated with lower ICU-onset BSI risk, respectively.
Treatment-Related Risk Factors.
Exposure to antibacterial and antifungal agents while in the hospital in the days preceding the index ICU stay was independently associated with increased ICU-onset BSI risk, whereas exposure to antivirals agents was not. The need for vasopressors and mechanical circulatory support on ICU admission also independently increased the risk of acquiring ICU-onset BSI whereas having the code for parenteral nutrition did not. Among the five variables with date-stamped procedure codes that were examined as time varying factors: mechanical ventilation was associated with ICU-onset BSI risk right from time of intubation, with an increased risk sustained during all mechanical ventilation procedure. On the other hand, ICU-onset BSI risk from central venous and arterial catheters placement and following cardiopulmonary resuscitation event, only manifested in the week following the procedure/event, with a risk starting from week 1 until line removal. Blood product transfusions did not appear to influence ICU-onset BSI risk.
Sensitivity Analyses.
When comorbidities were included individually (in lieu of the Elixhauser index), paralysis and other neurologic disorders, coagulopathy, liver disease, arrhythmias, and weight loss were identified as risk factors for ICU-onset BSI, whereas risk was significantly lower in those with (vs without) HIV/AIDS, diabetes with complications, and hypertension (eTable 12, http://links.lww.com/CCM/H200).
When the period for considering a BSI as ICU-onset was changed from ICU Day 3 onwards to Day 4 onwards, the statistical significance (or lack thereof) of all but one coefficient remained unchanged: Surgical ICU type went from being significantly protective in the primary analysis to not protective in this sensitivity analysis (eTable 13, http://links/lww.com/CCM/H200).
DISCUSSION
This report represents the largest study thus far show-casing the descriptive epidemiology of ICU-onset BSI in U.S. hospitals. In a cohort of over 150,000 critically ill adults at U.S. hospitals between 2009 and 2015, ICU-onset BSI occurred in approximately 1% of ICU encounters (prevalence = 1.47 cases per 1,000 person-ICU days), 41.5% of patients having at least one blood culture drawn during their ICU stay. This estimate is near the lower end of the range reported in prior studies (range = 1.2–6.7% of all ICU admissions) (8–10, 24). Of note, real world data provide more of a “sampled prevalence” which will vary by scenario (i.e., ICU-BSIPOA vs ICU-onset BSI). Our estimate was likely robust to confounding by under sampling given even approximately 39% the nonbacteremic ICU study population had blood cultures drawn. The variation might also be attributed to differences in ICU types, regions, populations, and infection control practices. Although relatively infrequent compared with ICU-BSIPOA, ICU-onset BSI carried a high risk of mortality (crude mortality = 37.9%), and these serious infections might often represent secondary phenomena of healthcare-associated infections, which in turn are often avoidable (such as central-line associated BSI [CLABSI], catheter-associated urinary tract infections, and ventilator-associated-pneumonia) (6). Benchmarking risk-adjusted prevalence of ICU-onset BSI could aid as an indicator of overall infection prevention in an ICU and pose an opportunity to audit and, where applicable, improve existing mitigation practices.
The pathogen and antibiotic resistance distributions in our study resemble previously published estimates from contemporaneous but slightly differing BSI populations in the United States (25, 26). Additionally, our study helps compartmentalize two unique bacteremic patient populations encountered by ICU providers: compared with ICU-BSIPOA, ICU-onset BSI displayed a different distribution of causative pathogens, greater associated resistance burdens, and greater crude mortality and longer ICU stays. This pattern is likely multifactorial: the ICU-BSIPOA group predominantly comprises patients arriving to the hospital with BSI likely enriched for community-onset pathogens. The ICU-onset BSI group is critically ill upon acquisition, which itself increases the likelihood of acquiring a more resistant variety of bloodstream pathogens. Furthermore, some pathogens can coharbor hypervirulence and resistance (27, 28). Critical care providers often need to make instantaneous selections of empiric antibiotic therapy for patients arriving with encephalopathy or with unclear healthcare exposures and microbial and treatment history, and as such, these data might positively influence their treatment decisions.
Prior studies have identified some factors that might heighten the risk of ICU-onset BSI including immunosuppression, prolonged stay, liver disease or gastrointestinal bleeding, surgical admission, trauma, invasive devices, sepsis, and other healthcare-associated infections (5, 6, 29) but focused either on specific types of BSI (e.g., CLABSI [2, 30, 31]) or could only examine a few factors to avoid overfitting models. Our large study sample enabled simultaneous examination of several candidate risk factors at the level of the pathogen, host, care setting, institution as well as treatment and procedural exposures. In addition to sample size, being well-distributed on geography, urbanicity, teaching status, bed capacity, and baseline volume of BSI encounters enhanced generalizability of findings, at least partially, to other U.S. hospitals. Our study identified demographic factors, chronic conditions, and acute presentations that providers could be cognizant of while gauging risk of ICU-onset BSI. Black and Hispanic patients displayed a higher risk for ICU-onset BSI compared with White patients further widening existing healthcare disparities even around healthcare-associated conditions (32, 33). Chronic conditions including neurologic and hepatic disorders, coagulopathy, arrhythmias, weight loss, and acute presentations such as trauma, acute respiratory, and gastrointestinal dysfunction were associated with acquisition of ICU-onset BSI. In this context, weight loss and arrhythmias might be surrogate markers of uncontrolled hypercatabolic disease processes and the associated frailty that necessitates longer stays; the protective effect observed for overdose admissions might in fact indicate an effect of shorter stays and less exposure time. Interestingly, some previously recognized risk factors like parenteral nutrition and immunosuppression were not identified as such in our study (34). However, our model was not designed to identify risk factors for specific ICU-onset BSI types (e.g., CLABSI or Candidemia), for which perhaps total parenteral nutrition might have displayed a different risk profile and administrative data may have precluded a comprehensive capture of immunosuppression.
Our study also confirms prior reports on the heightened risk of BSI upon prior exposure to antibacterial and antifungal agents and might be related to alteration of the microbiome. Our study also identified other potentially modifiable operational and management strategies that warrant further exploration for mitigating acquisition of ICU-onset BSI. Smaller and urban hospitals and undifferentiated ICUs demonstrated higher risk of ICU-onset BSI, which should prompt further investigation to understand the drivers of risk (e.g., training, personnel, equipment, policies etc.) in these specific care settings. Reducing the duration of mechanical ventilation has known advantages, such as lower risk of ventilator-associated pneumonia and lung injury (35). Our study found mechanical ventilation of any duration was associated with a risk of acquiring ICU-onset BSI. Although mechanical ventilation might represent a surrogate for severely ill patients, a prior study found secondary BSI in 20% of patients with ventilator-associated pneumonia (36). As such, our study offers another reason to minimize duration of mechanical ventilation whenever possible. On the other hand, we found that a statistically significant risk of putative CLABSI and arterial catheter-related BSI were only observed a week or later into the ICU stay. A clinical trial conducted between 2006 and 2008 showed a significant increase in risk of arterial catheter infections after 7 days of the catheter being in situ (23). In our study, both arterial catheters and central catheters similarly displayed an associated risk of ICU-onset BSI acquisition that appeared to manifest only beyond 7 days from insertion. Our study complements this trial with more recent data and offers reassurance when retaining arterial catheters during initial resuscitation and monitoring. Nonetheless, providers must continue to encourage removal of vascular catheters as soon as they are deemed nonessential.
Our study has important limitations. Our study relies heavily on administrative data (e.g., diagnosis codes) to identify certain risk factors, and as such our findings must be interpreted in the context of their variable sensitivity and inability in reflecting condition intensity. Notably, identified risk-factors are hypothesis-generating at best, considering the potential for residual confounding. Missingness encountered in our analysis was handled by exclusion, although it was relatively low (2.7% in ICU-onset BSI group). Physiologic perturbations and laboratory values (e.g., WBCs) could not be analyzed and might represent unmeasured risk factors. Patients admitted with trauma tend to be admitted directly to ICUs from the emergency department leaving lesser pre-ICU healthcare exposure time to develop nosocomial BSI. The lack of access to progress notes precluded scrutiny of historical exposures, checklists, and compliance with infection control measures (37). Some BSI cases might have been misclassified, (e.g., delayed diagnosis of ICU-BSIPOA being misclassified as ICU-onset BSI); however, findings persisted in sensitivity analyses. Findings might be less generalizable to current times; our study period ended in 2015, analyses were completed in 2019, but the manuscript was delayed accommodating authors’ needs to prioritize mission critical COVID-19 research. Interestingly, a growth in CLABSIs has been observed during the COVID-19 pandemic (38, 39), and patients with COVID-19 have been reported to display a higher risk of ICU-onset BSI (40, 41). This could be related to the disease itself or the potential breakdown of infection control standards secondary to surging caseloads. As such, future studies on ICU-onset BSI risk should investigate mechanisms and employ causal inferences to substantiate modification of management practices and behaviors in different ICU care settings. Our findings might not be generalizable to other global regions with disparate healthcare systems, policies, training, and staffing patterns, case-mix, and pathogen epidemiology. We encourage similar investigations from other regions and more recent data to enable more global and contemporary perspectives on the topic.
CONCLUSIONS
In conclusion, our study quantifies the burden of ICU-onset BSI in a cohort of 85 U.S. hospitals, describes how these burdens and their associated pathogen and antibiotic resistance distributions might compare between ICU-onset BSI to BSI present on ICU admission, and identifies potential target areas in infection control, triage, policy, and treatment interventions that might may eventually enable us to alter practices and behavior with the goal of minimizing prevalence of these serious, yet often avoidable, infections.
Supplementary Material
ACKNOWLEDGMENTS
We are thankful to David Fram, MA (Commonwealth Informatics Inc., Waltham, MA), for assistance with data curation, Judith Welsh (Clinical Informationist, former NIH Librarian, Office of Research Services, National Institutes of Health) for help with the extensive bibliography research, and Kelly Byrne (Critical Care Medicine Department, National Institutes of Health) for assistance with formatting the article.
Supported, in part, by the intramural research programs of the National Institutes of Health Clinical Center and National Institutes for Allergy and Infectious Diseases, respectively.
Drs. Swihart, Warner, Strich, Follmann, and Kadri received support for article research from the National Institutes of Health. Drs. Swihart, Warner, Strich, Mancera, Follmann, and Kadri disclosed government work. The remaining authors have disclosed that they do not have any potential conflicts of interest.
The opinions expressed in this article are those of the authors and do not represent any position or policy of the National Institutes of Health, the U.S. Department of Health and Human Services, or the U.S. government.
Footnotes
Supplemental digital content is available for this article. Direct URL citations appear in the printed text and are provided in the HTML and PDF versions of this article on the journal’s website (http://journals.lww.com/ccmjournal).
REFERENCES
- 1.Goto M, Al-Hasan MN: Overall burden of bloodstream infection and nosocomial bloodstream infection in North America and Europe. Clin Microbiol Infect 2013; 19:501–509 [DOI] [PubMed] [Google Scholar]
- 2.Garrouste-Orgeas M, Timsit JF, Tafflet M, et al. ; OUTCOMEREA Study Group: Excess risk of death from intensive care unit-acquired nosocomial bloodstream infections: A reappraisal. Clin Infect Dis 2006; 42:1118–1126 [DOI] [PubMed] [Google Scholar]
- 3.Vincent JL, Rello J, Marshall J, et al. ; EPIC II Group of Investigators: International study of the prevalence and outcomes of infection in intensive care units. JAMA 2009; 302:2323–2329 [DOI] [PubMed] [Google Scholar]
- 4.Vincent JL, Sakr Y, Singer M, et al. ; EPIC III Investigators: Prevalence and outcomes of infection among patients in intensive care units in 2017. JAMA 2020; 323:1478–1487 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Tabah A, Koulenti D, Laupland K, et al. : Characteristics and determinants of outcome of hospital-acquired bloodstream infections in intensive care units: The EUROBACT international cohort study. Intensive Care Med 2012; 38:1930–1945 [DOI] [PubMed] [Google Scholar]
- 6.Zhu S, Kang Y, Wang W, et al. : The clinical impacts and risk factors for non-central line-associated bloodstream infection in 5046 intensive care unit patients: An observational study based on electronic medical records. Crit Care 2019; 23:52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.VanVught LA, Klouwenberg PMCK, Spitoni C, et al. : Prevalence, Risk Factors, and Attributable Mortality of Secondary Infections in the Intensive Care Unit After Admission for Sepsis. JAMA 2016; 315:1469–1479 [DOI] [PubMed] [Google Scholar]
- 8.Pérez-Crespo PMM, Lanz-García JF, Bravo-Ferrer J, et al. ; PROBAC REIPI/GEIH-SEIMC/SAEI Group: Revisiting the epidemiology of bloodstream infections and healthcare-associated episodes: results from a multicentre prospective cohort in Spain (PRO-BAC Study). Int J Antimicrob Agents 2021; 58:106352. [DOI] [PubMed] [Google Scholar]
- 9.Harte J, Soothill G, Samuel JGD, et al. : Hospital-Acquired Blood Stream Infection in an Adult Intensive Care Unit. Crit Care Res Pract 2021; 2021:3652130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Warren DK, Zack JE, Elward AM, et al. : Nosocomial primary bloodstream infections in intensive care unit patients in a non-teaching community medical center: A 21-month prospective study. Clin Infect Dis 2001; 33:1329–1335 [DOI] [PubMed] [Google Scholar]
- 11.Pepper DJ, Demirkale CY, Sun J, et al. : Does obesity protect against death in sepsis? A retrospective cohort study of 55,038 adult patients. Crit Care Med 2019; 47:643–650 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Horan TC, Andrus M, Dudeck MA: CDC/NHSN surveillance definition of health care-associated infection and criteria for specific types of infections in the acute care setting. Am J Infect Control 2008; 36:309–332 [DOI] [PubMed] [Google Scholar]
- 13.Kadri SS, Lai YL, Warner S, et al. ; forming the National Insititutes of Health Antimicrobial Resistance Outcomes Research Initiative (NIH-ARORI): Inappropriate empirical antibiotic therapy for bloodstream infections based on discordant in-vitro susceptibilities: A retrospective cohort analysis of prevalence, predictors, and mortality risk in US hospitals. Lancet Infect Dis 2021; 21:241–251 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Quan H, Sundararajan V, Halfon P, et al. : Coding algorithms for defining comorbidities in ICD-9-CM and ICD-10 administrative data. Med Care 2005; 43:1130–1139 [DOI] [PubMed] [Google Scholar]
- 15.Elixhauser A, Steiner C, Harris DR, et al. : Comorbidity measures for use with administrative data. Med Care 1998; 36:8–27 [DOI] [PubMed] [Google Scholar]
- 16.Moore BJ, White S, Washington R, et al. : Identifying increased risk of readmission and in-hospital mortality using hospital administrative data: The AHRQ Elixhauser comorbidity index. Med Care 2017; 55:698–705 [DOI] [PubMed] [Google Scholar]
- 17.Rhee C, Zhang Z, Kadri SS, et al. ; CDC Prevention Epicenters Program: Sepsis surveillance using adult sepsis events simplified eSOFA criteria versus sepsis-3 sequential organ failure assessment criteria. Crit Care Med 2019; 47:307–314 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Greenberg JA, Hohmann SF, Hall JB, et al. : Validation of a method to identify immunocompromised patients with severe sepsis in administrative databases. Ann Am Thorac Soc 2016; 13:253–258 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.R Core Team: R: A Language and Environment for Statistical Computing. Vienna, Austria, R Foundation for Statistical Computing, 2019. Available at: https://www.r-project.org/. Accessed February 13, 2019 [Google Scholar]
- 20.Subirana I, Sanz H, Vila J: Building Bivariate Tables: The compareGroups Package for R. J Stat Softw 2014; 57:1–1625400517 [Google Scholar]
- 21.Therneau TM: A Package for Survival Analysis in S_. version 2.38. 2015. Available at: https://CRAN.R-project.org/package=survival. Accessed December 23, 2019
- 22.Therneau TM, Grambsch PM: Modeling Survival Data: Extending the Cox Model. Berlin, Germany, Springer Science & Business Media, 2000 [Google Scholar]
- 23.Lucet JC, Bouadma L, Zahar JR, et al. : Infectious risk associated with arterial catheters compared with central venous catheters. Crit Care Med 2010; 38:1030–1035 [DOI] [PubMed] [Google Scholar]
- 24.Prowle JR, Echeverri JE, Ligabo EV, et al. : Acquired bloodstream infection in the intensive care unit: Prevalence and attributable mortality. Crit Care 2011; 15:R100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Weiner LM, Webb AK, Limbago B, et al. : Antimicrobial-resistant pathogens associated with healthcare-associated infections: Summary of data reported to the national healthcare safety network at the centers for disease control and prevention, 2011–2014. Infect Control Hosp Epidemiol 2016; 37:1288–1301 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Noto MJ, Domenico HJ, Byrne DW, et al. : Chlorhexidine bathing and health care-associated infections: A randomized clinical trial. JAMA 2015; 313:369–378 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.El-Solh AA, Hattemer A, Hauser AR, et al. : Clinical outcomes of type III Pseudomonas aeruginosa bacteremia. Crit Care Med 2012; 40:1157–1163 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Gomez-Simmonds A, Uhlemann AC: Clinical implications of genomic adaptation and evolution of carbapenem-resistant Klebsiella pneumoniae. J Infect Dis 2017; 215:S18–S27 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Timsit JF, Ruppé E, Barbier F, et al. : Bloodstream infections in critically ill patients: An expert statement. Intensive Care Med 2020; 46:266–284 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Timsit JF, L’Hériteau F, Lepape A, et al. : A multicentre analysis of catheter-related infection based on a hierarchical model. Intensive Care Med 2012; 38:1662–1672 [DOI] [PubMed] [Google Scholar]
- 31.Carter JH, Langley JM, Kuhle S, et al. : Risk factors for central venous catheter-associated bloodstream infection in pediatric patients: A cohort study. Infect Control Hosp Epidemiol 2016; 37:939–945 [DOI] [PubMed] [Google Scholar]
- 32.Leys L, Weze K, Donaldson S, et al. : Racial and ethnic disparities in healthcare-associated infections in the United States. Chest 2020; 158:A339 [Google Scholar]
- 33.Chen J, Khazanchi R, Bearman G, et al. : Racial/ethnic inequities in healthcare-associated infections under the shadow of structural racism: Narrative review and call to action. Curr Infect Dis Rep 2021; 23:17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Fonseca G, Burgermaster M, Larson E, et al. : The relationship between parenteral nutrition and central line-associated bloodstream infections. JPEN J Parenter Enteral Nutr 2018;42:171–175 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Klompas M: Potential strategies to prevent ventilator-associated events. Am J Respir Crit Care Med 2015; 192:1420–1430 [DOI] [PubMed] [Google Scholar]
- 36.Agbaht K, Diaz E, Muñoz E, et al. : Bacteremia in patients with ventilator-associated pneumonia is associated with increased mortality: A study comparing bacteremic vs. nonbacteremic ventilator-associated pneumonia. Crit Care Med 2007; 35:2064–2070 [DOI] [PubMed] [Google Scholar]
- 37.Wichmann D, Belmar Campos CE, Ehrhardt S, et al. : Efficacy of introducing a checklist to reduce central venous line associated bloodstream infections in the ICU caring for adult patients. BMC Infect Dis 2018; 18:267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Center for Disease Control and Prevention: COVID-19 Impact on HAIs in 2020 [Internet], 2021. Available at: https://www.cdc.gov/hai/data/portal/covid-impact-hai.html. Accessed December 15, 2021
- 39.Fakih MG, Bufalino A, Sturm L, et al. : Coronavirus disease 2019 (COVID-19) pandemic, central-line-associated bloodstream infection (CLABSI), and catheter-associated urinary tract infection (CAUTI): The urgent need to refocus on hardwiring prevention efforts. Infect Control Hosp Epidemiol 2022;43:26–31 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Massart N, Maxime V, Fillatre P, et al. ; COVID ICU Bacteremia Study Group on behalf of the COVID-ICU Investigators: Characteristics and prognosis of bloodstream infection in patients with COVID-19 admitted in the ICU: An ancillary study of the COVID-ICU study. Ann Intensive Care 2021;11:183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.De Santis V, Corona A, Vitale D, et al. : Bacterial infections in critically ill patients with SARS-2-COVID-19 infection: Results of a prospective observational multicenter study. Infection 2022; 50:139–148 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


