Abstract
Objective:
To clarify whether perioperative immunonutrition is effective in adult patients with or without malnutrition undergoing elective surgery for head and neck (HAN) or gastrointestinal (GI) cancers.
Background:
It is important to avoid postoperative complications in patients with cancer as they can compromise clinical outcomes. There is no consensus on the efficacy of perioperative immunonutrition in patients with or without malnutrition undergoing HAN or GI cancer surgery.
Materials and Methods:
We searched MEDLINE (PubMed), MEDLINE (OVID), EMBASE, Cochrane Central Register of Controlled Trials, Web of Science Core Selection, and Emcare from 1981 to 2022 using search terms related to immunonutrition and HAN or GI cancer. We included randomized controlled trials. Intervention was defined as immunonutritional therapy including arginine, n-3 omega fatty acids, or glutamine during the perioperative period. The control was defined as standard nutritional therapy. The primary outcomes were total postoperative and infectious complications, defined as events with a Clavien–Dindo classification grade ≥ II that occurred within 30 days after surgery.
Results:
Of the 4825 patients from 48 included studies, 19 had upper GI cancer, 9 had lower, and 8 had mixed cancer, whereas 12 had HAN cancers. Immunonutrition reduced the total postoperative complications (relative risk ratio: 0.78; 95% CI, 0.66–0.93; certainty of evidence: high) and infectious complications (relative risk ratio: 0.71; 95% CI, 0.61–0.82; certainty of evidence: high) compared with standard nutritional therapy.
Conclusions:
Nutritional intervention with perioperative immunonutrition in patients with HAN and GI cancers significantly reduced total postoperative complications and infectious complications.
Key Words: gastrointestinal cancer, head and neck cancer, immunonutrition, nutritional intervention, perioperative nutrition
Surgical resection is a primary treatment for patients with cancer, and preoperative malnutrition is a risk factor for postoperative complications.1,2 Worsened nutritional status is common in patients with gastrointestinal (GI) cancers, ranging from 20% to 70%, depending on the cancer type and stage.3 Similarly, the prevalence of malnutrition in patients with head and neck (HAN) cancer is ranging from 28.6% to 67%.4 Malnutrition affects the immune response and tissue healing.3 In addition, catabolic reactions because of surgical invasion cause depletion of essential nutrients, leading to immune response dysregulation and risk of infectious complications.1,3 Hence, nutritional interventions to reduce preoperative malnutrition are necessary to decrease infectious and total postoperative complications.
There is no consensus on the efficacy of immunonutrition in patients undergoing elective surgery with or without malnutrition. Immunonutrition therapy containing either arginine, n-3 omega fatty acids or glutamine intended for immunomodulation has been developed and used clinically to reduce the risk of infectious and total postoperative complications, and shorten hospitalization.5 Immunonutrition improves malnutrition and modulates the postoperative inflammatory response, reducing immunosuppression caused by inflammatory cytokines.6 However, its efficacy and the optimal timing, duration, and recipient are clinically unresolved, especially in HAN and GI cancer surgeries. Therefore, we planned a systematic review and meta-analysis of perioperative immunonutrition in patients with HAN and GI cancers.
This study aimed to clarify the effectiveness of perioperative immunonutrition in adult patients undergoing elective surgery for HAN or GI cancer. We also investigated whether the recommendations for patients who are malnourished differed from those who are not malnourished. We hypothesized that perioperative immunonutrition reduces infectious complications in patients with and without malnourishment.
METHODS
We conducted a systematic review of the relevant literature in accordance with the Cochrane Handbook, Preferred Reporting Items for Systematic Reviews and Meta-Analysis 2020 guidelines (PRISMA-2020; Appendix 1, Supplemental Digital Content 1, http://links.lww.com/SLA/E888), and the Minds Manual for Guideline Development 2020.7–9 The protocol was published in PROSPERO (CRD 42022376400).
Eligibility Criteria and Study Selection
We included randomized controlled trials (RCTs) of patients aged over 18 years who underwent elective HAN or GI cancer surgery with perioperative immunonutrition. Intervention was defined as perioperative immunonutritional therapy, including arginine, n-3 omega fatty acids, or glutamine given preoperatively, postoperatively, or both. The control was defined as standard nutritional therapy. We excluded studies in which more than 25% of patients had benign disease or cancer at other sites, review articles, case reports, crossover trials, and cluster-randomized, quasirandomized, and nonrandomized trials.
Search Strategy
Appendix 2 (Supplemental Digital Content 2, http://links.lww.com/SLA/E889) provides the search formulae. We searched MEDLINE (PubMed), MEDLINE (OVID), Embase (OVID), Cochrane Central Register of Controlled Trials (CENTRAL), Web of Science Core Selection, and Emcare (OVID). The period covered by the RCT was 1981–2022.
Study Selection and Data Collection
Two independent reviewers screened the titles and abstracts, assessing their eligibility based on the full texts. The same reviewers performed independent data extraction from the included studies using a standardized data collection form. Reviewer disagreements were resolved through discussion, or with a mediating third reviewer. The original authors were contacted for missing data.
Risk-of-bias Assessment
Two of the 3 researchers carried out risk-of-bias (ROB) assessments using the Cochrane Collaboration ROB tool that has 5 domains: randomization, deviation from intervention, missing data, measurement of outcome, and selective reporting.7 The ratings “high risk,” “some concerns,” and “low risk” were assigned to each domain and overall. Disagreement resolution was as in 2.3.
Outcomes
The primary outcomes were the total postoperative and infectious complications. Secondary outcomes were noninfectious complications, postoperative mortality, severe complications, anastomotic leakage, postoperative pneumonia, nutritional intervention adverse events, and postoperative hospitalization. Postoperative complications were defined as events with a Clavien–Dindo (CD) classification grade of ≥II that occurred within 30 days after surgery. Severe complications were defined as those with a CD grade of ≥III.
Synthesis of Results
We pooled the relative risk ratios (RRs) and 95% CIs for postoperative complications, postoperative mortality, and nutritional intervention adverse events, and the mean differences (MDs) and 95% CIs for postoperative hospitalization in patients with HAN or GI cancer. An intention-to-treat analysis was performed for dichotomous data where possible. We used Review Manager software 5.4.2 and performed meta-analyses with a random-effects model assuming that the true effect would be low owing to many unmeasured or unknown factors and individual differences between studies in accordance with the Cochrane Handbook.7
Statistical heterogeneity was evaluated by visually inspecting forest plots and calculating the I2 statistic (I2 values of 0%–40%, may not be important; 30%–60%, may represent moderate heterogeneity; 50%–90%, may represent substantial heterogeneity; 75%–100%, considerable heterogeneity).7 When there was substantial heterogeneity (I2 > 50%), we assessed the reason.
To elucidate the influence of effect modifiers, subgroup analyses according to malnutrition status (with or without malnutrition), intervention timing (preoperative, postoperative, or perioperative), cancer site (HAN or GI), duration of preoperative administration (<7 days or ≥7 days), duration of postoperative administration (<7 days or ≥7 days), and difference in ingredient (arginine absent or arginine present, 3 ingredient or 1- or 2-ingredient) were performed when sufficient data were available. We also performed a sensitivity analysis for the frequency of malnourishment (>50% or >75%). In one of these analyses, studies using imputed statistics were excluded, whereas the other included only participants who completed the study with complete data.7 Potential publication bias was assessed by visual inspection of the funnel plots for outcomes in more than 10 studies.7
Certainty Assessment
Based on the Cochrane handbook,7 we summarized the findings for total postoperative complications, infectious complications, noninfectious complications, postoperative mortality, severe complications, anastomotic leakage, postoperative pneumonia, nutritional intervention adverse events, and postoperative hospitalization. The summary included grading of certainty of evidence (COE) according to the Grading of Recommendations Assessment, Development, and Evaluation (GRADE) approach.10 We started with “high” COE.10 If there were any serious concerns in any domain, we lowered the grade from “high” COE. The effect estimates displayed in the Summary of Findings table were created using RRs and MD. To determine the inconsistency domain of the GRADE ratings, we examined the consistency of the RR and MD.
RESULTS
Study Selection
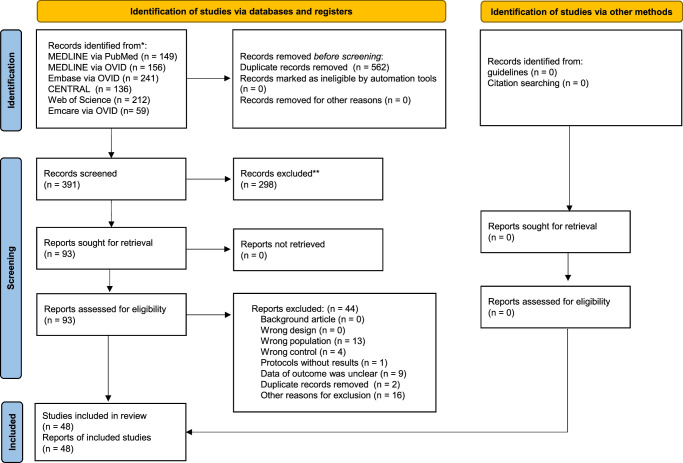
The PRISMA flowchart is shown in Figure 1. A total of 953 records were searched on January 3, 2022. After screening, 48 studies (4825 patients) were included in the qualitative synthesis,11–58 and in the quantitative synthesis .11–58 No unpublished data or ongoing studies were identified. Appendix 3 (Supplemental Digital Content 3, http://links.lww.com/SLA/E890) presents the characteristics of the studies excluded from the qualitative and quantitative syntheses. The reasons for exclusion were incorrect population (n=13), incorrect control (n=4), protocol without results (n=1), insufficient outcome data (n=9), duplicate records (n=2), and other reasons (n=16).
FIGURE 1.
PRISMA 2020 flow diagram of this study. PRISMA 2020 indicates Preferred Reporting Items for Systematic Reviews and Meta-Analysis 2020.
Study and Patient Characteristics
Appendix 4 (Supplemental Digital Content 4, http://links.lww.com/SLA/E891) summarizes the characteristics of the 48 studies included in the quantitative synthesis. Of these studies, 19 were for upper GI cancer,12–16,18–20,24,26,27,30,38,45,46,51,52,56,57 9 were for lower GI,11,21,22,34,35,40,44,48,55 8 were for mixed GI,28,32,36,37,39,43,47,49 and 12 were for HAN cancer.17,23,25,29,31,33,41,42,50,53,54,58 Regarding the nutritional intervention timing, 8 studies were conducted preoperatively,11,18,26,30,32,35,36,49 18 were postoperatively,16,19,24,25,28,29,33,34,37–39,41,42,44,45,47,52,58 and 22 were preoperatively and postoperatively.12–15,17,20–23,27,31,40,43,46,48,50,51,53–57 We did not find any literature including only patients with malnutrition. Table 1 summarizes the findings using the GRADE approach.
TABLE 1.
Summary of Findings
| Impact of perioperative immunonutrition on postoperative outcomes for patients undergoing head and neck or gastrointestinal cancer surgeries | ||||
|---|---|---|---|---|
| Study population: Adults, setting: perioperative, intervention: immunonutrition, comparison: standard nutritional therapy | ||||
| Outcomes | Relative effect (95% CI) | Sample size (studies) | Certainty of the evidence (grade) | Comments |
| Total postoperative complications | RR 0.78 (0.66 to 0.93) | 2336 (24 RCT) | ⊕⊕⊕⊕ High | Immunonutrition reduces total postoperative complications |
| Infectious complications | RR 0.71 (0.61 to 0.82) | 3929 (41 RCT) | ⊕⊕⊕⊕ High | Immunonutrition reduces infectious complications |
| Noninfectious complications | RR 0.96 (0.84 to 1.09) | 2487 (24 RCT) | ⊕⊕⊕⊝ Moderate* | Immunonutrition probably does not reduce noninfectious complications |
| Mortality | RR 0.92 (0.55 to 1.56) | 2812 (27 RCT) | ⊕⊕⊕⊝ Moderate* | Immunonutrition probably does not reduce mortality |
| Severe complications | RR 1.08 (0.76 to 1.53) | 842 (7 RCT) | ⊕⊕⊕⊝ Moderate* | Immunonutrition probably does not reduce severe complications |
| Anastomotic leakage | RR 0.70 (0.53 to 0.91) | 3018 (29 RCT) | ⊕⊕⊕⊕ High | Immunonutrition reduces anastomotic leakage |
| Postoperative pneumonia | RR 0.92 (0.75 to 1.14) | 3109 (30 RCT) | ⊕⊕⊕⊝ Moderate* | Immunonutrition probably does not reduce postoperative pneumonia |
| Adverse events from nutritional interventions | RR 0.88 (0.72 to 1.07) | 825 (15 RCT) | ⊕⊕⊕⊝ Moderate* | Immunonutrition probably does not increase adverse events from nutritional interventions |
| Postoperative hospital stays | MD −1.52 (−2.14 to −0.9) | 3562 (37 RCT) | ⊕⊕⊕⊕ High | Immunonutrition reduces postoperative hospital stays |
Downgraded 1 point because of inconsistency of forest plot.
CI indicates confidence interval; MD mean difference; RCT randomized control trials; RR risk ratio.
Risk-of-Bias
Supplementary Figure 1 (Supplemental Digital Content 5, http://links.lww.com/SLA/E892) summarizes the ROB in the included studies. Regarding postoperative complications, there was a low ROB for incomplete outcome data and selective reporting and a low ROB or “some concerns” for random sequence generation and allocation concealment. The ROB for the participant and personnel blinding and outcome assessment were low, “some concerns” or high, respectively.
Meta-analysis Results
Total Postoperative Complications
Twenty-four studies reported the total postoperative complications.11,12,15–17,20,22,25–27,31,33–40,42–44,55,56 Immunonutrition reduces total postoperative complications compared with standard nutritional therapy (RR: 0.78; 95% CI, 0.66–0.93; I2=48%; n=24; COE, high; Fig. 2A).
FIGURE 2.
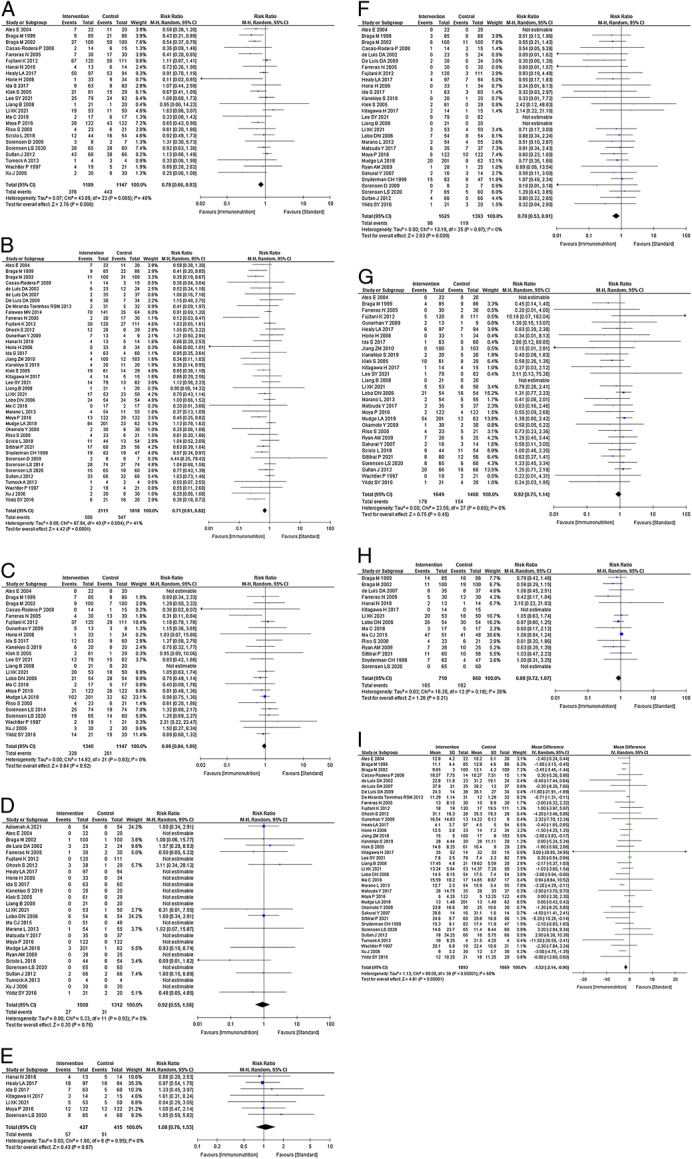
A, Forest plot of total postoperative complications. B, Forest plot of infectious complications. C, Forest plot of noninfectious complications. D, Forest plot of mortality. E, Forest plot of severe complications. F, Forest plot of anastomotic leakage. G, Forest plot of postoperative pneumonia. H, Forest plot of nutritional intervention adverse events. I, Forest plot of postoperative hospital stay.
The subgroup analysis showed no efficacy differences between the groups according to the intervention timing (P=0.33; Supplemental Figure 2a, Supplemental Digital Content 6, http://links.lww.com/SLA/E893), cancer site (P=0.43; Supplemental Figure 2b, Supplemental Digital Content 6, http://links.lww.com/SLA/E893), duration of preoperative administration (P=0.40; Supplemental Figure 2c, Supplemental Digital Content 6, http://links.lww.com/SLA/E893), or duration of postoperative administration (P=0.73; Supplemental Figure 2d, Supplemental Digital Content 6, http://links.lww.com/SLA/E893). Subgroup analysis for ingredient difference showed significantly fewer complications in the group with arginine compared with the group without arginine (P=0.03; Supplemental Figure 2e, Supplemental Digital Content 6, http://links.lww.com/SLA/E893), and fewer complications in the 3 ingredients group compared with the 1-gradient or 2-ingredient group (P=0.05; Supplemental Figure 2f, Supplemental Digital Content 6, http://links.lww.com/SLA/E893).
Infectious Complications
Forty-one studies reported infectious complications.11–18,20,22–44,46,48–50,52–55,58 Immunonutrition reduces infectious complications compared with standard nutritional therapy (RR: 0.71; 95% CI, 0.61–0.82; I 2=41%; n=41; COE, high; Fig. 2B).
The subgroup analysis showed no efficacy differences between the groups according to the intervention timing (P=0.81; Supplemental Figure 3a, Supplemental Digital Content 7, http://links.lww.com/SLA/E894), cancer site (P=0.11; Supplemental Figure 3b, Supplemental Digital Content 7, http://links.lww.com/SLA/E894), duration of preoperative administration (P=0.63; Supplemental Figure 3c, Supplemental Digital Content 7, http://links.lww.com/SLA/E894), duration of postoperative administration (P=0.68; Supplemental Figure 3d, Supplemental Digital Content 7, http://links.lww.com/SLA/E894), arginine (P=0.08; Supplemental Figure 3e, Supplemental Digital Content 7, http://links.lww.com/SLA/E894), or number of ingredients (P=0.10; Supplemental Figure 3f, Supplemental Digital Content 7, http://links.lww.com/SLA/E894).
Noninfectious Complications
Twenty-four studies reported noninfectious complications.11–15,20,22,26,32–40,42–44,46,48,52,55 Immunonutrition is unlikely to reduce noninfectious complications compared with standard nutritional therapy (RR: 0.96; 95% CI, 0.84–1.09; I 2=0%; n=24; COE, moderate; Fig. 2C).
The subgroup analysis showed no efficacy differences between the groups according to the intervention timing (P=0.09; Supplemental Figure 4a, Supplemental Digital Content 8, http://links.lww.com/SLA/E895), cancer site (P=0.33; Supplemental Figure 4b, Supplemental Digital Content 8, http://links.lww.com/SLA/E895), duration of preoperative administration (P=0.57; Supplemental Figure 4c, Supplemental Digital Content 8, http://links.lww.com/SLA/E895), or duration of postoperative administration (P=0.70; Supplemental Figure 4d, Supplemental Digital Content 8, http://links.lww.com/SLA/E895). Subgroup analysis for ingredient difference showed fewer complications in the group with arginine compared with the group without arginine (P=0.06; Supplemental Figure 4e, Supplemental Digital Content 8, http://links.lww.com/SLA/E895), and fewer complications in the 3 ingredients group compared with the 1-gradient or 2-ingredient group (P=0.12; Supplemental Figure 4f, Supplemental Digital Content 8, http://links.lww.com/SLA/E895).
Mortality
Twenty-seven studies reported mortality rates.12–14,16,19,20,22,24–27,34–41,45–47,50,52,55–57 Immunonutrition is unlikely to reduce mortality compared with standard nutritional therapy (RR: 0.92; 95% CI, 0.55–1.56; I2=0%; n=27; COE, moderate; Fig. 2D).
The subgroup analysis showed no efficacy differences between the groups according to the intervention timing (P=0.90; Supplemental Figure 5a, Supplemental Digital Content 9, http://links.lww.com/SLA/E896), cancer site (P=0.21; Supplemental Figure 5b, Supplemental Digital Content 9, http://links.lww.com/SLA/E896), duration of preoperative administration (P=0.32; Supplemental Figure 5c, Supplemental Digital Content 9, http://links.lww.com/SLA/E896), duration of postoperative administration (P=0.11; Supplemental Figure 5d, Supplemental Digital Content 9, http://links.lww.com/SLA/E896), arginine (P=0.93; Supplemental Figure 5e, Supplemental Digital Content 9, http://links.lww.com/SLA/E896), or number of ingredients (P=0.93; Supplemental Figure 5f, Supplemental Digital Content 9, http://links.lww.com/SLA/E896).
Severe Complications
Seven studies reported severe complications.12,17,20,22,55,56 Immunonutrition is unlikely to reduce severe complications compared with standard nutritional therapy (RR: 1.08; 95% CI, 0.76–1.53; I 2=0%; n=7; COE, moderate; Fig. 2E).
The subgroup analysis showed no efficacy differences between the groups according to the duration of preoperative administration (P=0.82; Supplemental Figure 6a, Supplemental Digital Content 10, http://links.lww.com/SLA/E897), arginine (P=0.91; Supplemental Figure 6b, Supplemental Digital Content 10, http://links.lww.com/SLA/E897), or number of ingredients (P=0.91; Supplemental Figure 6c, Supplemental Digital Content 10, http://links.lww.com/SLA/E897). Subgroup analyses were not performed because of the paucity of studies, including the intervention timing, cancer site, malnourished patients, and duration of postoperative administration.
Anastomotic Leakage
Twenty-nine studies reported anastomotic leakages.11–14,18–20,22,24,26,27,29,31,33–35,37–41,43,46,51–53,55–57 Immunonutrition reduces anastomotic leakage compared with standard nutritional therapy (RR: 0.70; 95% CI, 0.53–0.91; I 2=0%; n=29; COE, high; Fig. 2F).
The subgroup analysis showed no efficacy differences between the groups according to the intervention timing (P=0.77; Supplemental Figure 7a, Supplemental Digital Content 11, http://links.lww.com/SLA/E898), cancer site (P=0.49; Supplemental Figure 7b, Supplemental Digital Content 11, http://links.lww.com/SLA/E898), duration of preoperative administration (P=0.69; Supplemental Figure 7c, Supplemental Digital Content 11, http://links.lww.com/SLA/E898), arginine (P=0.39; Supplemental Figure 7d, Supplemental Digital Content 11, http://links.lww.com/SLA/E898), or number of ingredients (P=0.44; Supplemental Figure 7e, Supplemental Digital Content 11, http://links.lww.com/SLA/E898). Subgroup analysis for duration of preoperative administration was not performed due to the paucity of studies.
Postoperative Pneumonia
Thirty studies reported on postoperative pneumonia.11–14,16,18–20,22,24,26–28,30,32,34,35,37–39,42–44,46,51,52,54–57 Immunonutrition is unlikely to reduce postoperative pneumonia compared with standard nutritional therapy (RR: 0.92; 95% CI, 0.75–1.14; I 2=0%; n=30; COE, moderate; Fig. 2G).
The subgroup analysis showed no efficacy differences between the groups according to the intervention timing (P=0.91; Supplemental Figure 8a, Supplemental Digital Content 12, http://links.lww.com/SLA/E899), cancer site (P=0.29; Supplemental Figure 8b, Supplemental Digital Content 12, http://links.lww.com/SLA/E899), duration of preoperative administration (P=0.82; Supplemental Figure 8c, Supplemental Digital Content 12, http://links.lww.com/SLA/E899), duration of postoperative administration (P=0.73; Supplemental Figure 8d, Supplemental Digital Content 12, http://links.lww.com/SLA/E899), arginine (P=0.79; Supplemental Figure 8e, Supplemental Digital Content 12, http://links.lww.com/SLA/E899), or number of ingredients (P=0.86; Supplemental Figure 8f, Supplemental Digital Content 12, http://links.lww.com/SLA/E899).
Nutritional Intervention Adverse Events
Fifteen studies reported nutritional intervention adverse events.12,15,17,18,38,40,42,43,47,52–55,57,58 Immunonutrition is unlikely to increase nutritional intervention adverse events compared with standard nutritional therapy (RR: 0.88; 95% CI, 0.72–1.07; I 2=26%; n=15; COE, moderate; Fig. 2H).
The subgroup analysis showed no efficacy differences between the groups according to the intervention timing (P=0.86; Supplemental Figure 9a, Supplemental Digital Content 13, http://links.lww.com/SLA/E900), cancer site (P=0.51; Supplemental Figure 9b, Supplemental Digital Content 13, http://links.lww.com/SLA/E900), duration of preoperative administration (P=0.15; Supplemental Figure 9c, Supplemental Digital Content 13, http://links.lww.com/SLA/E900), arginine (P=0.44; Supplemental Figure 9d, Supplemental Digital Content 13, http://links.lww.com/SLA/E900), or number of ingredients (P=0.73; Supplemental Figure 9e, Supplemental Digital Content 13, http://links.lww.com/SLA/E900). Subgroup analysis for duration of preoperative administration was not performed due to the paucity of studies.
Postoperative Hospital Stays
Thirty-seven studies reported postoperative hospitalization.11–15,18,19,22,24–30,32–41,43,44,46,49–56,58 Immunonutrition reduces postoperative hospitalization compared with standard nutritional therapy (MD: −1.52; 95% CI, −2.14 to −0.90; I 2=60%; n=37; COE, high; Fig. 2I).
In the subgroup analysis, postoperative hospitalization was reduced for postoperative, or preoperative plus postoperative administration (P=0.002; Supplemental Figure 10a, Supplemental Digital Content 14, http://links.lww.com/SLA/E901). There were no efficacy differences between the groups according to the cancer site (P=0.06; Supplemental Figure 10b, Supplemental Digital Content 14, http://links.lww.com/SLA/E901), duration of preoperative administration (P=0.24; Supplemental Figure 10c, Supplemental Digital Content 14, http://links.lww.com/SLA/E901), or duration of postoperative administration (P=0.65; Supplemental Figure 10d, Supplemental Digital Content 14, http://links.lww.com/SLA/E901). Subgroup analysis for ingredient difference showed significantly fewer complications in the group with arginine compared with the group without arginine (P=0.04; Supplemental Figure 10e, Supplemental Digital Content 14, http://links.lww.com/SLA/E901), and fewer complications in the 3 ingredients group compared with the 1-ingredient or 2-ingredient group (P=0.04; Supplemental Figure 10f, Supplemental Digital Content 14, http://links.lww.com/SLA/E901).
Additional Analysis
Subgroup and sensitivity analysis were not performed because of the paucity of studies that included patients with malnourishment. Funnel plots were visualized as symmetrical, indicating minimal publication bias in the reporting of total postoperative complications (Supplemental Figure 11a, Supplemental Digital Content 15, http://links.lww.com/SLA/E902), infectious complications (Supplemental Figure 11b, Supplemental Digital Content 15, http://links.lww.com/SLA/E902), noninfectious complications (Supplemental Figure 11c, Supplemental Digital Content 15, http://links.lww.com/SLA/E902), mortality (Supplemental Figure 11d, Supplemental Digital Content 15, http://links.lww.com/SLA/E902), anastomotic leakage (Supplemental Figure 11e, Supplemental Digital Content 15, http://links.lww.com/SLA/E902), postoperative pneumonia (Supplemental Figure 11f, Supplemental Digital Content 15, http://links.lww.com/SLA/E902), nutritional intervention adverse events (Supplemental Figure 11g, Supplemental Digital Content 15, http://links.lww.com/SLA/E902), and postoperative hospitalization (Supplemental Figure 11h, Supplemental Digital Content 15, http://links.lww.com/SLA/E902). For severe complications, funnel plots were not prepared because there were less than 10 references, as per the Cochrane Handbook.7
DISCUSSION
The results of the present systematic review and meta-analysis of 48 studies and 4825 patients revealed that immunonutrition probably reduces total postoperative complications in adult patients undergoing elective surgery for HAN or GI cancers. Immunonutrition probably reduces the rates of infectious complications, anastomotic leakage, and the length of postoperative hospitalization. However, immunonutrition is unlikely to reduce the rate of noninfectious complications, postoperative mortality, severe complications, and postoperative pneumonia. Compared with standard nutrition, immunonutrition is unlikely to increase nutritional intervention adverse events. We did not perform sensitivity analysis because of the paucity of studies that included patients with malnourishment. Subgroup analysis showed shorter hospitalization with postoperative administration of immunonutrition but no difference in postoperative complications. Subgroup analysis for ingredient difference showed significantly fewer total complications and shorter hospitalization in the group with arginine compared with the group without arginine, and in the 3 ingredients group compared with the 1-ingredient or 2-ingredient group. This important study shows that compared to standard nutritional therapy, nutritional intervention with immunonutrition can reduce postoperative complications, especially infectious complications, without increasing nutrition-related adverse events.
The interpretation of this study’s results is that immunonutrition for HAN and GI cancer administered mainly in the postoperative period reduces postoperative complications and postoperative hospitalization without increasing nutritional intervention adverse events; however, there is no difference in postoperative mortality. The results should be applied in routine clinical practice for cancer types with common postoperative complications.
The mechanisms by which immunonutrition improves postoperative outcomes include improved nutritional status for malnutrition and resistance to infection by modulating immune function. Surgical stress from highly invasive surgery produces high postoperative inflammation, which leads to immunodeficiency, but immunonutrition has the effect of suppressing excessive inflammation.6 One study reported that preoperative immunonutrition suppressed inflammatory cytokines after pancreaticoduodenectomy, which may be responsible for the decrease in postoperative complications.59 Another study reported that postoperative administration of immunonutrition resulted in lower postoperative C-reactive protein.39 These results suggest that preoperative and postoperative administration may reduce inflammation. It also plays the role of immunostimulation against inflammation after surgical invasion or trauma, which causes immunosuppression and lowers resistance to infection.3 The former should be used preoperatively as modulating and the latter postoperatively as stimulating. In this study, subgroup analysis showed immunonutrition with arginine and containing 3 ingredients was more effective in preventing postoperative complications and reducing hospital stay. The shorter hospitalization with post-operative administration is consistent with an effective immunostimulatory effect of arginine. These may have different results depending on the degree of surgical invasiveness and the extent of resection.
There was no consensus on the impact of immunonutrition on postoperative outcomes because different results have been reported; however, this may be due to the different numbers of studies included in the meta-analysis (Appendix 5, Supplemental Digital Content 16, http://links.lww.com/SLA/E903). Meta-analyses by Mingliang et al60 and Zhuo et al61 for esophageal cancer reported that immunonutrition did not improve postoperative outcomes. On the other hand, Howes et al62 meta-analyzed 19 RCTs of HAN cancer and reported a decrease in fistula formation (RR 0.48; 95% CI, 0.27–0.85). Song et al63 meta-analyzed 11 RCTs in gastric cancer patients and reported a reduction in infectious complications [odds ratio (OR): 0.56; 95% CI, 0.36–0.86], and Xu et al64 in 9 RCTs in colorectal cancer reported a reduction in infectious complications (OR: 0. 33; 95% CI, 0.21–0.53). Despite this clinically meaningful difference, the lack of statistical significance because of the small study numbers is problematic. In this study, the Cochrane team developed a search formula and the guideline members meta-analyzed 48 RCTs. It has the strength of summarizing a much larger number of studies compared with previous reports and showing a possible reduction in infectious complications. In contrast, there was no reduction in noninfectious complications, which is consistent with the results of previous studies.63,65
We were unable to perform subgroup or sensitivity analyses based on the percentage of malnutrition because of the paucity of studies that included more than 50% patients with malnutrition. Riso et al42 compared postoperative outcomes in patients with malnutrition in HAN cancer and reported no severe complications in the immunonutrition group. Immunomodulation by administration of immunonutrition may be necessary in patients with malnutrition because of the possibility of immune impairment, but this was not evident in this study. Further studies on patients with malnutrition are required.
Subgroup analysis by the timing of immunonutrition administration showed a significant difference in postoperative hospitalization for postoperative administration, but few other outcomes. Osland et al66 reported that perioperative and postoperative dosing in patients with GI cancer can reduce postoperative complications. Tian et al67 reported that postoperative administration in patients with esophageal cancer is associated with better control of infectious complications and postoperative pneumonia. In a meta-analysis of patients who underwent pancreaticoduodenectomy, Wang et al68 reported that combined preoperative and postoperative administration was most effective. Pancreaticoduodenectomy is one of the most invasive surgical procedures, and the effect of timing of administration may vary with surgical invasiveness. In other words, preoperative administration is effective in reducing complications when immunosuppression occurs because of postoperative inflammation from highly invasive surgery; however, preoperative administration may not be necessary for less invasive surgery. Although the subgroup analysis in this study did not show statistically significant differences, the forest plot showed a clinical difference with fewer complications after postoperative administration. In the present systematic review, postoperative administration may have been effective because the patients had cancers that are not highly invasive.
This study found no difference in the effect of preoperative and postoperative immunonutrition by duration of nutritional intervention. Most trials administered within 7 days preoperatively or postoperatively were administered for 5 days. Therefore, subgroup analysis implies that postoperative complications do not differ between 5 and 7 days or more. Also, many studies on the postoperative administration of immunonutrition have used the step-up method, with gradual dose increases. This management is in line with the recovery of intestinal peristalsis and is consistent with the concept of “permissive underfeeding” with slow advancement, in which nutritional doses are purposely lowered during the extreme phase of inflammation.69 This may be explained by enhanced autophagy and reduced hyperglycemia. However, few RCTs have calculated the postoperative nutritional dosage based on indirect calorimetry or other methods, and it is questionable whether the dosage varies with the magnitude of invasiveness. Therefore, further studies on the relationship between dosage and postoperative outcomes are required.
This study had several limitations. First, there are few RCTs in patients with malnutrition and further studies are required. Second, the mechanism by which immunonutrition may improve postoperative outcomes in HAN and GI cancers remains unclear. One hypothesis is that it suppresses postoperative inflammation and has an immunostimulatory effect on postoperative immunosuppression. However, further studies are required. Third, the optimal immunonutritional dose remains unknown. Further RCTs with different dosage designs are required. Despite these limitations, the meta-analysis includes large numbers of RCTs, which addresses the problem of previous meta-analyses that were unable to show statistical differences despite clinical differences owing to the small number of RCTs. Our findings have several significant clinical implications.
CONCLUSIONS
Nutritional intervention with perioperative immunonutrition in patients with HAN and GI cancer without malnutrition significantly reduced total and infectious postoperative complications without increasing nutritional intervention adverse events. Immunonutrition with arginine and containing 3 ingredients was more effective in preventing total postoperative complications and reducing hospitalization. Further studies with different dosage designs are required in patients with malnutrition.
Supplementary Material
ACKNOWLEDGMENTS
The authors are grateful to Dr. Satoshi Ida, Dr. Donyarat Ruenmarkkaew, Dr. Jaw-Yuan Wang, Dr. Simon Ladefoged Rasmussen, Dr. Yi Shen, and Dr. Nobuhiro Hanai for responding to our inquiries regarding the outcomes of this study. The authors are grateful to the members of the Guideline Development Committee, Board of Directors, and Secretariat of the Japanese Society for Clinical Nutrition and Metabolism Guideline Development Committee for their kind support of this review. They also appreciate the support of Dr. Norio Watanabe and Cochrane Japan for conducting this systematic literature search.
Footnotes
The Japanese Society for Clinical Nutrition and Metabolism covered the costs of English editing and the submission of this article.
R.M. had full access to all data in the study and took responsibility for the integrity of the data and the accuracy of the data analysis. R.M., Y.U., N.H., and J.K. were involved in acquisition, analysis, or interpretation of data. R.M. was involved in drafting of the article. R.M., Y.U., N.H., and J.K. were involved in critical revision of the article for important intellectual content. R.M. and M.S. were involved in statistical analysis. N.H. and J.K. were involved in administrative, technical, and material support. N.H. and J.K. were involved in supervision. All authors were involved in concept and design.
The authors report no conflicts of interest.
Supplemental Digital Content is available for this article. Direct URL citations are provided in the HTML and PDF versions of this article on the journal’s website, www.annalsofsurgery.com.
Contributor Information
Ryota Matsui, Email: supreme0818@gmail.com.
Masano Sagawa, Email: masanosagawa@yahoo.co.jp.
Akihiko Sano, Email: ak_sano@outlook.jp.
Makoto Sakai, Email: maksakai@gunma-u.ac.jp.
Shin-ichiro Hiraoka, Email: hirashins2@gmail.com.
Isao Tabei, Email: tabei@jikei.ac.jp.
Takayuki Imai, Email: takayuki.imai629@gmail.com.
Hideo Matsumoto, Email: h.matsu2327@gmail.com.
Seiji Onogawa, Email: onochan@xf7.so-net.ne.jp.
Norihiro Sonoi, Email: s-no-08@cc.okayama-u.ac.jp.
Shigeyuki Nagata, Email: punchnagata@live.jp.
Ryo Ogawa, Email: r-ogawa@med.nagoya-cu.ac.jp.
Shigeki Wakiyama, Email: swakiyama@jikei.ac.jp.
Yasuhiro Miyazaki, Email: ymiyazaki02@gh.opho.jp.
Koshi Kumagai, Email: koshi.kumagai@jfcr.or.jp.
Rie Tsutsumi, Email: rtsutsumi@tokushima-u.ac.jp.
Takehiro Okabayashi, Email: tokabaya@gmail.com.
Yu Uneno, Email: yuuneno@kuhp.kyoto-u.ac.jp.
Naoki Higashibeppu, Email: gashibe@hotmail.com.
Joji Kotani, Email: kotanijo0412@gmail.com.
REFERENCES
- 1. Weimann A, Braga M, Carli F, et al. ESPEN guideline: clinical nutrition in surgery. Clin Nutr. 2017;36:623–650. [DOI] [PubMed] [Google Scholar]
- 2. Matsui R, Rifu K, Watanabe J, et al. Impact of malnutrition as defined by the GLIM criteria on treatment outcomes in patients with cancer: a systematic review and meta-analysis. Clin Nutr. 2023;42:615–624. [DOI] [PubMed] [Google Scholar]
- 3. Arends J, Baracos V, Bertz H, et al. ESPEN expert group recommendations for action against cancer-related malnutrition. Clin Nutr. 2017;36:1187–1196. [DOI] [PubMed] [Google Scholar]
- 4. Bossi P, Delrio P, Mascheroni A, et al. The spectrum of malnutrition/cachexia/sarcopenia in oncology according to different cancer types and settings: a narrative review. Nutrients. 2021;13:1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Fukatsu K. Role of nutrition in gastroenterological surgery. Ann Gastroenterol Surg. 2019;3:160–168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Jabłońska B, Mrowiec S. The role of immunonutrition in patients undergoing pancreaticoduodenectomy. Nutrients. 2020;12:2547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Higgins JPT, Thomas J. Cochrane Handbook for Systematic Reviews of Interventions version 6.2, 2021. Cochrane, 2021. Accessed July 3, 2021. https://training.cochrane.org/handbook/current
- 8. Page MJ, McKenzie JE, Bossuyt PM, et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ. 2021;372:n71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Minds Manual Developing Committees . Minds Manual for Guideline Development 2020 ver 30. Tokyo: Japan Council for Quality Health Care; 2021. https://minds.jcqhc.or.jp/docs/various/manual_2020/ver3_0/pdf/all_manual_2020ver3_0.pdf [Google Scholar]
- 10. Guyatt G, Oxman AD, Akl EA, et al. GRADE guidelines: 1. Introduction-GRADE evidence profiles and summary of findings tables. J Clin Epidemiol. 2011;64:383–394. [DOI] [PubMed] [Google Scholar]
- 11. Lee SY, Lee J, Park HM, et al. Impact of preoperative immunonutrition on the outcomes of colon cancer surgery: results from a randomized controlled trial. Ann Surg. 2023;277:381–386. [DOI] [PubMed] [Google Scholar]
- 12. Li XK, Cong ZZ, Wu WJ, et al. Enteral immunonutrition versus enteral nutrition for patients undergoing esophagectomy: a randomized controlled trial. Ann Palliat Med. 2021;10:1351–1361. [DOI] [PubMed] [Google Scholar]
- 13. Kanekiyo S, Takeda S, Iida M, et al. Efficacy of perioperative immunonutrition in esophageal cancer patients undergoing esophagectomy. Nutrition. 2019;59:96–102. [DOI] [PubMed] [Google Scholar]
- 14. Mudge LA Watson DI Smithers BM et al. Australian Immunonutrition Study Group . Multicentre factorial randomized clinical trial of perioperative immunonutrition versus standard nutrition for patients undergoing surgical resection of oesophageal cancer. Br J Surg. 2018;105:1262–1272. [DOI] [PubMed] [Google Scholar]
- 15. Ma C, Tsai H, Su W, et al. Combination of arginine, glutamine, and omega-3 fatty acid supplements for perioperative enteral nutrition in surgical patients with gastric adenocarcinoma or gastrointestinal stromal tumor (GIST): a prospective, randomized, double-blind study. J Postgrad Med. 2018;64:155–163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Scislo L, Pach R, Nowak A, et al. The impact of postoperative enteral immunonutrition on postoperative complications and survival in gastric cancer patients – randomized clinical trial. Nutr Cancer. 2018;70:453–459. [DOI] [PubMed] [Google Scholar]
- 17. Hanai N, Terada H, Hirakawa H, et al. Prospective randomized investigation implementing immunonutritional therapy using a nutritional supplement with a high blend ratio of ω-3 fatty acids during the perioperative period for head and neck carcinomas. Jpn J Clin Oncol. 2018;48:356–361. [DOI] [PubMed] [Google Scholar]
- 18. Kitagawa H, Namikawa T, Yatabe T, et al. Effects of a preoperative immune-modulating diet in patients with esophageal cancer: a prospective parallel group randomized study. Langenbecks Arch Surg. 2017;402:531–538. [DOI] [PubMed] [Google Scholar]
- 19. Matsuda Y, Habu D, Lee S, et al. Enteral diet enriched with ω-3 fatty acid improves oxygenation after thoracic esophagectomy for cancer: a randomized controlled trial. World J Surg. 2017;41:1584–1594. [DOI] [PubMed] [Google Scholar]
- 20. Ida S, Hiki N, Cho H, et al. Randomized clinical trial comparing standard diet with perioperative oral immunonutrition in total gastrectomy for gastric cancer. Br J Surg. 2017;104:377–383. [DOI] [PubMed] [Google Scholar]
- 21. Moya P, Miranda E, Soriano-Irigaray L, et al. Perioperative immunonutrition in normo-nourished patients undergoing laparoscopic colorectal resection. Surg Endosc. 2016;30:4946–4953. [DOI] [PubMed] [Google Scholar]
- 22. Moya P, Soriano-Irigaray L, Ramirez JM, et al. Perioperative Standard Oral Nutrition Supplements Versus Immunonutrition in Patients Undergoing Colorectal Resection in an Enhanced Recovery (ERAS) Protocol: a multicenter randomized clinical trial (SONVI Study). Medicine (Baltimore). 2016;95:e3704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Falewee MN, Schilf A, Boufflers E, et al. Reduced infections with perioperative immunonutrition in head and neck cancer: exploratory results of a multicenter, prospective, randomized, double-blind study. Clin Nutr. 2014;33:776–784. [DOI] [PubMed] [Google Scholar]
- 24. Marano L, Porfidia R, Pezzella M, et al. Clinical and immunological impact of early postoperative enteral immunonutrition after total gastrectomy in gastric cancer patients: a prospective randomized study. Ann Surg Oncol. 2013;20:3912–3918. [DOI] [PubMed] [Google Scholar]
- 25. Turnock A, Calder PC, West AL, et al. Perioperative immunonutrition in well-nourished patients undergoing surgery for head and neck cancer: evaluation of inflammatory and immunologic outcomes. Nutrients. 2013;5:1186–1199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Fujitani K Tsujinaka T Fujita J et al. Osaka Gastrointestinal Cancer Chemotherapy Study Group . Prospective randomized trial of preoperative enteral immunonutrition followed by elective total gastrectomy for gastric cancer. Br J Surg. 2012;99:621–629. [DOI] [PubMed] [Google Scholar]
- 27. Sultan J, Griffin SM, Di Franco F, et al. Randomized clinical trial of omega-3 fatty acid-supplemented enteral nutrition versus standard enteral nutrition in patients undergoing oesophagogastric cancer surgery. Br J Surg. 2012;99:346–355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Jiang ZM, Wilmore DW, Wang XR, et al. Randomized clinical trial of intravenous soybean oil alone versus soybean oil plus fish oil emulsion after gastrointestinal cancer surgery. Br J Surg. 2010;97:804–849. [DOI] [PubMed] [Google Scholar]
- 29. De Luis DA, Izaola O, Cuellar L, et al. High dose of arginine enhanced enteral nutrition in postsurgical head and neck cancer patients. A randomized clinical trial. Eur Rev Med Pharmacol Sci. 2009;13:279–283. [PubMed] [Google Scholar]
- 30. Okamoto Y, Okano K, Izuishi K, et al. Attenuation of the systemic inflammatory response and infectious complications after gastrectomy with preoperative oral arginine and omega-3 fatty acids supplemented immunonutrition. World J Surg. 2009;33:1815–1821. [DOI] [PubMed] [Google Scholar]
- 31. Sorensen D, McCarthy M, Baumgartner B, et al. Perioperative immunonutrition in head and neck cancer. Laryngoscope. 2009;119:1358–1364. [DOI] [PubMed] [Google Scholar]
- 32. Gunerhan Y, Koksal N, Sahin UY, et al. Effect of preoperative immunonutrition and other nutrition models on cellular immune parameters. World J Gastroenterol. 2009;15:467–472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Casas-Rodera P, Gómez-Candela C, Benítez S, et al. Immunoenhanced enteral nutrition formulas in head and neck cancer surgery: a prospective, randomized clinical trial. Nutr Hosp. 2008;23:105–110. [PubMed] [Google Scholar]
- 34. Liang B, Wang S, Ye YJ, et al. Impact of postoperative omega-3 fatty acid-supplemented parenteral nutrition on clinical outcomes and immunomodulations in colorectal cancer patients. World J Gastroenterol. 2008;14:2434–2439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Horie H, Okada M, Kojima M, et al. Favorable effects of preoperative enteral immunonutrition on a surgical site infection in patients with colorectal cancer without malnutrition. Surg Today. 2006;36:1063–1068. [DOI] [PubMed] [Google Scholar]
- 36. Xu J, Zhong Y, Jing D, et al. Preoperative enteral immunonutrition improves postoperative outcome in patients with gastrointestinal cancer. World J Surg. 2006;30:1284–1289. [DOI] [PubMed] [Google Scholar]
- 37. Kłek S, Kulig J, Szczepanik AM, et al. The clinical value of parenteral immunonutrition in surgical patients. Acta Chir Belg. 2005;105:175–179. [PubMed] [Google Scholar]
- 38. Farreras N, Artigas V, Cardona D, et al. Effect of early postoperative enteral immunonutrition on wound healing in patients undergoing surgery for gastric cancer. Clin Nutr. 2005;24:55–65. [DOI] [PubMed] [Google Scholar]
- 39. Ateş E, Yilmaz S, Erkasap S, et al. Perioperative immunonutrition ameliorates the postoperative immune depression in patients with gastrointestinal system cancer (prospective clinical study in 42 patients). Acta Gastroenterol Belg. 2004;67:250–254. [PubMed] [Google Scholar]
- 40. Braga M, Gianotti L, Vignali A, et al. Preoperative oral arginine and n-3 fatty acid supplementation improves the immunometabolic host response and outcome after colorectal resection for cancer. Surgery. 2002;132:805–814. [DOI] [PubMed] [Google Scholar]
- 41. de Luis DA, Aller R, Izaola O, et al. Postsurgery enteral nutrition in head and neck cancer patients. Eur J Clin Nutr. 2002;56:1126–1129. [DOI] [PubMed] [Google Scholar]
- 42. Riso S, Aluffi P, Brugnani M, et al. Postoperative enteral immunonutrition in head and neck cancer patients. Clin Nutr. 2000;19:407–412. [DOI] [PubMed] [Google Scholar]
- 43. Braga M, Gianotti L, Radaelli G, et al. Perioperative immunonutrition in patients undergoing cancer surgery: results of a randomized double-blind phase 3 trial. Arch Surg. 1999;134:428–433. [DOI] [PubMed] [Google Scholar]
- 44. Wachtler P, König W, Senkal M, et al. Influence of a total parenteral nutrition enriched with omega-3 fatty acids on leukotriene synthesis of peripheral leukocytes and systemic cytokine levels in patients with major surgery. J Trauma. 1997;42:191–198. [DOI] [PubMed] [Google Scholar]
- 45. Adiamah A, Rollins KE, Kapeleris A, et al. Postoperative arginine-enriched immune modulating nutrition: long-term survival results from a randomised clinical trial in patients with oesophagogastric and pancreaticobiliary cancer. Clin Nutr. 2021;40:5482–5485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Yildiz SY, Yazicioglu MB, Tiryaki C, et al. The effect of enteral immunonutrition in upper gastrointestinal surgery for cancer: a prospective study. Turk J Med Sci. 2016;46:393. [DOI] [PubMed] [Google Scholar]
- 47. Ma CJ, Wu JM, Tsai HL, et al. Prospective double-blind randomized study on the efficacy and safety of an n-3 fatty acid enriched intravenous fat emulsion in postsurgical gastric and colorectal cancer patients. Nutr J. 2015;14:9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Sorensen LS, Thorlacius-Ussing O, Schmidt EB, et al. Randomized clinical trial of perioperative omega-3 fatty acid supplements in elective colorectal cancer surgery. Br J Surg. 2014;101:33–42. [DOI] [PubMed] [Google Scholar]
- 49. de Miranda Torrinhas RS, Santana R, Garcia T, et al. Parenteral fish oil as a pharmacological agent to modulate post-operative immune response: a randomized, double-blind, and controlled clinical trial in patients with gastrointestinal cancer. Clin Nutr. 2013;32:503–510. [DOI] [PubMed] [Google Scholar]
- 50. Ghosh S, Dempsey G, Skelly R, et al. A double blind, randomised, placebo controlled, feasibility phase III clinical trial of peri-operative immune-enhancing enteral nutrition in patients undergoing surgery for advanced head and neck cancer. ESPEN J. 2012;7:e107–e114. [Google Scholar]
- 51. Sakurai Y, Masui T, Yoshida I, et al. Randomized clinical trial of the effects of perioperative use of immune-enhancing enteral formula on metabolic and immunological status in patients undergoing esophagectomy. World J Surg. 2007;31:2150–2157. [DOI] [PubMed] [Google Scholar]
- 52. Lobo DN, Williams RN, Welch NT, et al. Early postoperative jejunostomy feeding with an immune modulating diet in patients undergoing resectional surgery for upper gastrointestinal cancer: a prospective, randomized, controlled, double-blind study. Clin Nutr. 2006;25:716–726. [DOI] [PubMed] [Google Scholar]
- 53. Snyderman CH, Kachman K, Molseed L, et al. Reduced postoperative infections with an immune-enhancing nutritional supplement. Laryngoscope. 1999;109:915–921. [DOI] [PubMed] [Google Scholar]
- 54. Sittitrai P, Ruenmarkkaew D, Booyaprapa S, et al. Effect of a perioperative immune-enhancing diet in clean-contaminated head and neck cancer surgery: a randomized controlled trial. Int J Surg. 2021;93:106051. [DOI] [PubMed] [Google Scholar]
- 55. Sørensen LS, Rasmussen SL, Calder PC, et al. Long-term outcomes after perioperative treatment with omega-3 fatty acid supplements in colorectal cancer. BJS Open. 2020;4:678–684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Healy LA, Ryan A, Doyle SL, et al. Does prolonged enteral feeding with supplemental omega-3 fatty acids impact on recovery post-esophagectomy: results of a randomized double-blind trial. Ann Surg. 2017;266:720–728. [DOI] [PubMed] [Google Scholar]
- 57. Ryan AM, Reynolds JV, Healy L, et al. Enteral nutrition enriched with eicosapentaenoic acid (EPA) preserves lean body mass following esophageal cancer surgery: results of a double-blinded randomized controlled trial. Ann Surg. 2009;249:355–363. [DOI] [PubMed] [Google Scholar]
- 58. de Luis DA, Izaola O, Cuellar L, et al. Clinical and biochemical outcomes after a randomized trial with a high dose of enteral arginine formula in postsurgical head and neck cancer patients. Eur J Clin Nutr. 2007;61:200–204. [DOI] [PubMed] [Google Scholar]
- 59. Furukawa A, Furukawa K, Suzuki D, et al. Effect of immunonutrition on infectious complications in low skeletal muscle mass patients after pancreaticoduodenectomy. Clin Nutr. 2021;40:103–109. [DOI] [PubMed] [Google Scholar]
- 60. Mingliang W, Zhangyan K, Fangfang F, et al. Perioperative immunonutrition in esophageal cancer patients undergoing esophagectomy: the first meta-analysis of randomized clinical trials. Dis Esophagus. 2020;33:doz111. [DOI] [PubMed] [Google Scholar]
- 61. Zhuo ZG, Luo J, Song HYDTN, et al. Is immunonutrition superior to standard enteral nutrition in reducing postoperative complications in patients undergoing esophagectomy? A meta-analysis of randomized controlled trials. J BUON. 2021;26:204–210. [PubMed] [Google Scholar]
- 62. Howes N, Atkinson C, Thomas S, et al. Immunonutrition for patients undergoing surgery for head and neck cancer. Cochrane Database Syst Rev. 2018;8:CD010954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Song GM, Liu XL, Bian W, et al. Systematic review with network meta-analysis: comparative efficacy of different enteral immunonutrition formulas in patients underwent gastrectomy. Oncotarget. 2017;8:23376–23388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Xu J, Sun X, Xin Q, et al. Effect of immunonutrition on colorectal cancer patients undergoing surgery: a meta-analysis. Int J Colorectal Dis. 2018;33:273–283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Adiamah A, Skořepa P, Weimann A, et al. The impact of preoperative immune modulating nutrition on outcomes in patients undergoing surgery for gastrointestinal cancer: a systematic review and meta-analysis. Ann Surg. 2019;270:247–256. [DOI] [PubMed] [Google Scholar]
- 66. Osland E, Hossain MB, Khan S, et al. Effect of timing of pharmaconutrition (immunonutrition) administration on outcomes of elective surgery for gastrointestinal malignancies: a systematic review and meta-analysis. JPEN J Parenter Enteral Nutr. 2014;38:53–69. [DOI] [PubMed] [Google Scholar]
- 67. Tian X, Jin YF, Liu XL, et al. Network meta-analysis of the optimal time of applying enteral immunonutrition in esophageal cancer patients receiving esophagectomy. Support Care Cancer. 2022;30:7133–7146. [DOI] [PubMed] [Google Scholar]
- 68. Wang SY, Hung YL, Hsu CC, et al. Optimal perioperative nutrition therapy for patients undergoing pancreaticoduodenectomy: a systematic review with a component network meta-analysis. Nutrients. 2021;13:4049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Patel JJ, Martindale RG, McClave SA. Controversies surrounding critical care nutrition: an appraisal of permissive underfeeding, protein, and outcomes. JPEN J Parenter Enteral Nutr. 2018;42:508–515. [DOI] [PubMed] [Google Scholar]