Abstract
The history of intravascular ultrasound (IVUS) and optical coherence tomography (OCT) reflects the relentless pursuit of innovation in interventional cardiology. These intravascular imaging technologies have played a pivotal role in our understanding of coronary atherosclerosis, vascular pathology, and the interaction of coronary stents with the vessel wall. Two decades of clinical investigations demonstrating the clinical efficacy and safety of intravascular imaging modalities have established these technologies as staples in the contemporary cardiac catheterization lab’s toolbox and earning their place in revascularization clinical practice guidelines. In this comprehensive review, we will delve into the historical evolution, mechanisms, and technical aspects of IVUS and OCT. We will discuss the expanding evidence supporting their use in complex percutaneous coronary interventions, emphasizing their crucial roles in optimizing patient outcomes and ensuring procedural success. Furthermore, we will explore the substantial advances that have propelled these imaging modalities to the forefront of contemporary interventional cardiology. Finally, we will survey the latest developments in the field and explore the promising future directions that have the potential to further revolutionize coronary interventions.
Keywords: Coronary artery disease, Intravascular ultrasound, Optical coherence tomography, Percutaneous coronary intervention
INTRODUCTION
Cardiovascular diseases remain a global health challenge, with coronary artery disease (CAD) being a prominent contributor to morbidity and mortality. Percutaneous coronary intervention (PCI) has revolutionized CAD management by enabling the restoration of coronary blood flow and symptom relief in patients with atherosclerotic lesions. Within this context, intravascular ultrasound (IVUS) and optical coherence tomography (OCT) have played pivotal roles, offering unparalleled insights into coronary pathology and optimization of procedural outcomes[1–2].
The history of IVUS and OCT in the PCI domain reflects the relentless pursuit of innovation in interventional cardiology. IVUS, introduced in the early 1990s, marked the inception of intravascular imaging (IVI), providing critical insights into the arterial lumen and vessel wall. Its capacity to deliver cross-sectional images and precise measurements of lesion characteristics, plaque burden, and vessel dimensions swiftly gained prominence. Subsequently, OCT emerged as a high-resolution imaging modality, utilizing light coherence principles to offer micron-level resolution and exceptional visualization of coronary structures. The availability of IVUS and OCT in the contemporary cardiac catheterization lab has reshaped IVI, empowering clinicians with a diverse toolkit for PCI decision-making.
The IVUS and OCT imaging modalities, while distinct, are complementary. IVUS harnesses high-frequency sound waves to generate real-time, cross-sectional images of the coronary vasculature, facilitating assessment of lumen dimensions, plaque morphology, and calcium presence. In contrast, OCT employs near-infrared light to produce detailed, high-resolution images with superior tissue layer delineation, including the identification of thin-cap fibroatheromas and stent apposition. These mechanisms empower clinicians to make informed choices regarding lesion preparation, stent selection, optimization, and post-PCI evaluation.
Significant advances in IVUS and OCT have ensued. IVUS technology has evolved with the introduction of catheters integrating near-infrared spectroscopy (NIRS) and palpography, enhancing imaging capabilities. OCT, on the other hand, has seen the development of faster image acquisition techniques, improved catheter designs, and automated plaque characterization software algorithms. These innovations have not only elevated diagnostic accuracy but have also contributed to safer and more effective PCI procedures.
Furthermore, the indications for IVUS and OCT in PCI continue to expand. Beyond their traditional roles in lesion assessment and stent optimization, these imaging modalities are now employed in complex coronary interventions, including chronic total occlusions, bifurcation lesions, and left main disease. Additionally, their utility extends to post-PCI result evaluation, stent apposition assessment, and the detection of complications such as edge dissections.
HISTORICAL DEVELOPMENT OF INTRAVASCULR IMAGING
The past: early techniques and evolution of IVI
In the era of IVI, two groundbreaking modalities have emerged as gold standards: IVUS and OCT. The development of IVUS is predated by the discovery and eventual medical application of ultrasound when Nobel laureate Pierre Curie discovered the piezoelectric effect, one of the foundations of ultrasound technology[3]. Until the invention and initial clinical investigation of IVUS techniques, angiography was established as the predominant modality to define coronary anatomy for several decades.
In the 1970s, many studies challenged the accuracy of angiography due to high interobserver variability and findings that did not correlate well with autopsy examination of the coronary vessels[4–9]. Angiography displays the coronary arteries in a planar fashion with opacification of the contrast within the arterial lumen defining a silhouette of only two surfaces of the vessel wall. Variations in angiographic projection can therefore display different planar slices of the vessel wall that may distort the perceived degree of luminal stenosis and mislead the operator due to (1) inherent plaque characteristics (eccentric vs. concentric; bifurcation vs. ostial disease; focal vs. diffuse lesions) within the vessel wall; (2) foreshortening of the vessel; or (3) obscuring a lesion due to overlying opacified vessels. In addition, interventions within the coronary artery may result in irregularities that further impair its angiographic appearance[10–12].
Ultrasound offered immense potential advantages when added to angiography. IVUS offers a circumferential vantage point of the vessel wall. Sizing the vessel with ultrasound would not require calibration or correction for radiographic magnification and rather, uses an electronically generated measurement. The concept gave promise to provide more clearly defined images of the layers of the vessel wall, including the atherosclerotic plaque rather than simply defining the borders of the lumen.
In the late 1980s, Yock et al.[13] began experimenting with ultrasound technology for IVI. The initial prototypes were rudimentary, consisting of single-element transducers mounted on catheters and allowed for limited cross-sectional imaging of coronary vessels. By the early 1990s, phased-array catheters were developed, and early investigations compared IVUS image characteristics in peripheral and coronary vessels to histologic specimens, angiographic imaging, and ultimately defining the vascular anatomic ultrasound characteristics of the coronary artery in normal subjects and those with CAD[14–17].
OCT, dating back to 1991, was independently developed by two research teams in Japan and in the United States, almost simultaneously[18]. OCT was initially applied in the clinical setting in ophthalmology in 1996 and was first used in cardiac catheterization in the early 2000s. Since then, innovations have led to more rapid image acquisition as well as a significant improvement in image quality and resolution[19].
Over the years, other notable contending technologies for invasive IVI have risen but failed to gain traction in mainstream clinical practice, when compared to IVUS and OCT. Specifically, angioscopy and NIRS, in the late 1980s and early 1990s, showed potential for improved characterization of the vessel lumen. Angioscopy utilized a miniature fiber optic scope delivered to the coronary arteries by a specialized catheter to assist in visualizing intraluminal structures such as thrombus plaque and dissections. Although it provided a rough indication of the composition of the vessel, it failed in providing information on subluminal structures. This technique additionally required balloon occlusion of the artery and frequent clearance or flushing of blood from the visual field, making it cumbersome to use. Angioscopy ultimately failed to gain traction outside of investigational studies[20].
NIRS utilizes infrared light to assess plaque composition. It was initially used in ex vivo studies to understand the morphology of atherosclerotic plaques. Despite development of an approved intracoronary NIRS catheter to help in identification of lipid-rich vulnerable plaques, the technology offers limited tissue penetration and lacks the ability to clearly define detailed structures in the vessel wall[21]. Nonetheless, with the expanded use and experience with IVUS and OCT in clinical practice, hybrid multimodality imaging catheters represent the next breakthrough in this space[22]. Currently available combined NIRS-IVUS catheters have been validated in assessing plaque composition but are not widely used owing to difficulty in interpretation of the data. Advancement in hybrid imaging catheters has promised to bring combined IVUS-OCT-NIRS catheters to mainstream practice that may, in the future, identify plaques at risk of rupture.
The present: guideline recommendations, consensus statements, and a developing new standard-of-care
Two decades of clinical investigations demonstrating the clinical efficacy and safety of IVUS and OCT established these technologies as staples in the contemporary cardiac catheterization lab’s toolbox.
Due to rising evidence in favor, IVI modalities have certainly earned their place in clinical practice. Indeed, the 2014 European Society of Cardiology (ESC) and the European Association for Cardio-Thoracic Surgery (EACTS) guidelines on myocardial revascularization give a Class IIa recommendation to the use of IVUS and/or OCT to detect stent-related mechanical problems including stent thrombosis or restenosis[23]. In the 2018 update to the European guidelines, IVUS is given a IIa recommendation to assess the severity of unprotected left main coronary artery lesions, IVI (IVUS or OCT) is recommended in selected patients to optimize stent implantation[24]. In 2021, the American College of Cardiology (ACC), American Heart Association (AHA), and Society for Cardiovascular Angiography and Interventions (SCAI) guidelines followed suit and gave a Class IIa recommendation for the use of IVUS for assessment of lesion severity in patients with intermediate angiographic stenosis of the left main artery. Additionally, it is recommended to use IVUS or OCT for procedural guidance and stent implantation. Further, in patients with stent failure, IVI is recommended to determine the mechanism of failure, assessing coronary artery dimensions, optimizing stent placement, and guiding complex PCI[25]. Additionally, many expert consensus documents have been released to standardize the image acquisition and use of IVI in lesion assessment and stent deployment[26–28].
Despite these recommendations, IVI adoption in Europe and in the United States lags with an estimated 5% rate of IVI-guided PCI in the European Union and 20% in the United States[29–31]. However, given the growing body of evidence, many experts expect a higher level of recommendation for their use, particularly in complex coronary and bifurcation lesions, in the coming iterations of the guidelines[32–33]. In this comprehensive review, we will discuss the mechanisms of these technologies, the rapidly building clinical data driving these guideline recommendations as well as future directions to guide the widespread adoption of evidence-based use of IVI in complex PCI.
INTRAVASCULR IMAGING MODALITIES-PRINCIPLES AND METHODOLOGY
IVUS and OCT are two essential advanced coronary imaging modalities, each with distinct principles, technical mechanisms, and methodologies.
Intravascular ultrasound
IVUS relies on the propagation and reflection of high-frequency sound waves within coronary vessels to generate cross-sectional images. A specialized IVUS catheter houses an ultrasound transducer, typically operating at frequencies between 20 and 40 MHz. More recently, high-frequency IVUS transducers emitting 60 to 80 MHz beams have been developed by using asymmetric electrodes for improved beam profiles that may provide high resolution for initial and subintimal structures. Dual-frequency catheters of 30 Hz/80 Hz are also available that aim to combine the high-resolution benefits of high-frequency ultrasound and the higher penetration benefits of lower-frequency ultrasound[34] When advanced into a coronary artery, the catheter emits high-frequency sound waves. These waves travel into the surrounding tissue and vessel structures. When they encounter intravascular structures, the sound waves interface with varying acoustic impedances (e.g., vessel walls, plaque components), and some of the ultrasound waves are reflected toward the transducer, converting them into electrical signals. These signals are then processed to construct cross-sectional images of the vessel[35–36].
These images are displayed in grayscale, with varying shades corresponding to different tissue densities. These grayscale images provide valuable information about vessel dimensions, plaque composition, and stent deployment characteristics. IVUS has played a pivotal role in understanding plaque morphology, optimizing stent placement, and guiding complex interventions.
Optical coherence tomography
OCT catheters, like IVUS catheters, are introduced into the coronary artery. They operate on the principles of low-coherence interferometry. OCT catheters emit near-infrared light. The reflected light is collected and transformed into electrical signals that are used to create high-resolution cross-sectional images of coronary structures[37–38]. The blood absorbs the light emitted by the OCT catheter; thus, intermittent injection of iodinated contrast is necessary to clear the blood in the vessel for image acquisition.
A broadband near-infrared light source emits light waves, typically with wavelengths around 1.3 or 1.9 μm, to achieve micron-level resolution. These beams of light diverge into two arms: one directed toward the coronary tissue under examination (the sample arm) and another toward a reference mirror (the reference arm). Light reflected from both the sample and reference arms recombines, creating an interference pattern. OCT measures the interference pattern to construct detailed cross-sectional images. This pattern is highly sensitive to variations in the path length of the reflected light, allowing OCT to achieve exceptional resolution, typically 10 to 20 μm.
These images are particularly useful for assessing vessel dimensions, plaque morphology and vulnerability, thrombus formation, and stent characteristics. They enable the identification of microstructures and evaluation of stent strut apposition, making OCT a valuable tool in complex PCI procedures.
Similarities and differences
Both IVUS and OCT have significantly enhanced the capabilities of interventional cardiologists by providing real-time, detailed visualization of coronary anatomy and the arterial lumen. These modalities have significant similarities and differences that complement each other well (Table 1). Although IVUS offers a broader penetration depth and has been instrumental in characterizing plaque and optimizing stent placement, OCT stands out with its exceptional resolution, allowing for the precise assessment of microstructures within the coronary vessel wall including evaluation of plaque vulnerability and detection of the most subtle intimal disruptions and edge dissections. These complementary imaging modalities have become indispensable in guiding PCI and advancing our understanding of CAD[39].
Table 1.
Comparison of features of IVUS and OCT
| Vessel features | IVUS | OCT |
|---|---|---|
| Stent apposition/expansion | ++ | +++ |
| Coronary calcification | +++ | ++ |
| Edge dissection | ++ | +++ |
| Ostial/bifurcation lesion | +++ | + |
| Vulnerable plaque/thin-cap atheroma | - | +++ |
| Necrotic core | + | ++ |
| Thrombus | + | +++ |
IVUS: intravascular ultrasound; OCT: optical coherence tomography.
CURRENT USE OF INTRAVASCULR IMAGING IN PERCUTANEOUS CORONARY INTERVENTION
Vessel dimensions, stent sizing, and optimization
Optimal stent sizing is of immense importance for a successful coronary intervention. An undersized stent dramatically increases the risk of in-stent restenosis and target vessel failure (TVF)[40–42]. In addition, operators aim to optimize stent length and placement to cover the entire length of the lesion with both proximal and distal stent landing zones on relatively healthy endothelial segments[43–44].
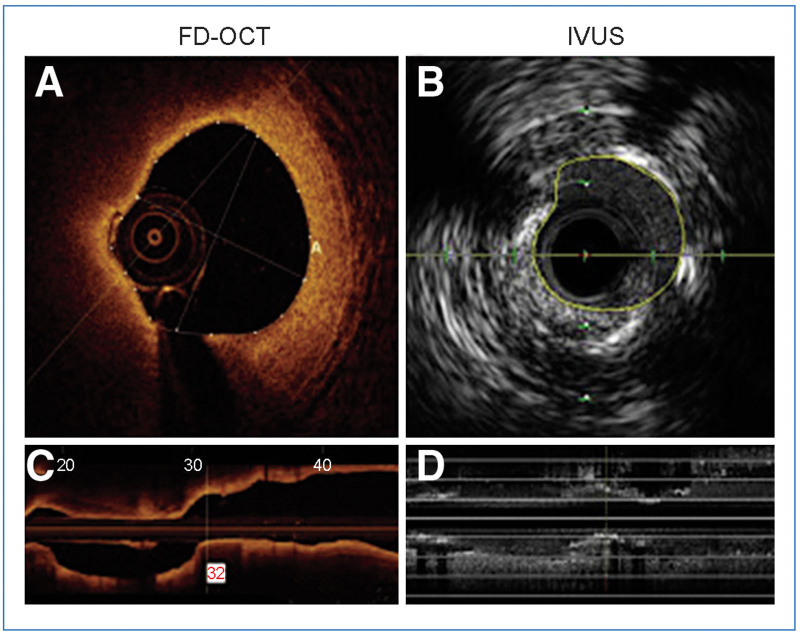
Traditional angiographic methods of estimating the vessel diameter and lesion length are subjective or semi-quantitative, at best. The most traditional method relies heavily on operator experience of visual estimation of angiographic appearance of the vessel size to choose the optimal stent size. Semi-quantitative methods include quantitative coronary analysis which allows the operator to measure the vessel size and area utilizing a reference diameter, usually the guiding catheter. The IVI modalities can provide information on lesion length, percent stenosis by diameter, and percent stenosis by area (Figure 1)[45]. The ability of IVI modalities to quantitatively define vessel and lesion dimensions in the cardiac catheterization lab helps guide accurate decision-making in real-time.
Figure 1.
An example of a comparison between OCT- and IVUS-derived images and measurements of the same lesion in the circumflex coronary artery.
A, The MLA was 2.75 mm2 by OCT; B, The MLA was 3.50 mm2 by IVUS; C, The planar reconstruction of the vessel morphology by OCT; D, The planar reconstruction of the vessel morphology by IVUS.
(Adapted from Baruś et al.[45] under Creative Commons License).
FD-OCT: frequency domain optical coherence tomography; IVUS: intravascular ultrasound; MLA: minimal lumen area; OCT: optical coherence tomography.
Both IVUS and OCT catheters construct cross-sectional images of the coronary arteries in real-time. The catheter is advanced distally to the angiographic lesion. Live imaging while pulling back the catheter proximally across the segment of angiographic stenosis maps the vessel topography and more accurately defines the diameter of the vessel and the length of the lesion[35–38]. Both IVUS and OCT control panels allow the operator to place “bookmarks” to landmark specific areas of interest. These may include the start and end of the lesion, the region of maximal stenosis (minimal luminal area), or the edges of a stent. These images are saved for real-time quantitative analysis of luminal diameter, luminal area, and lesion length. Several prospective studies have validated the use of a minimum luminal area (MLA) cutoff of <6 mm2 in the left main coronary artery and <4 mm2 in the proximal left circumflex or left anterior descending artery to signify severe stenosis, independently of the angiographic appearance of the artery[46–48]. These measurements have been found to correlate reliably with coronary physiological measurements such as instantaneous free-wave ratio (iFR), fractional flow reserve (FFR), and non-invasive stress testing representing ischemic lesions in the right clinical setting[49–51]. The studies showed that left main coronary artery lesions with MLA >6 mm2 by IVUS or >5.4 mm2 on OCT are generally considered a safe cutoff for deferral of revascularization[52]. It is important to note that the MLA measurements obtained from IVUS and OCT can differ significantly, and any cutoff criteria must be assessed in the comparison to a reference segment diameter. Additionally, cutoff values in non-left main vessels are subject to controversy due to lack of adequately powered validation and inconsistent results in various studies. Strictly using these cutoffs is not advised and using this data in practice must be done with caution and in the context of the patient’s clinical picture[52].
Plaque characteristics
Characterization of plaque morphology and vulnerability may assist the interventionalist in procedural planning and employing advanced complex interventions. Angiographically, coronary calcium may be visualized as a radio-dense outline to the arterial lumen, whereas a radiolucent lesion with irregular borders may be perceived as vulnerable soft plaque or thrombus. These findings can often be subtle or obscured in patients with prior coronary interventions or in those with complex coronary lesions. In these scenarios, advanced IVI can assist the interventionalist and provide more definitive information.
IVUS distinguishes between different plaque types based on their echogenicity. Echo-lucent regions often indicate lipid-rich plaques or necrotic cores (Figure 2A), while echo-dense regions may signify calcified or fibrotic plaques (Figure 2B)[53]. OCTs enhanced micron-level resolution may also provide additional valuable information about the plaque. Calcium deposits are highly reflective and appear bright (Figure 2C), whereas lipid-rich regions appear as signal-poor or low-intensity areas (Figure 2D)[53]. OCT offers advantages over IVUS in the assessment, visualization, and characterization of the fibrous cap to determine plaque stability or vulnerability. For example[53], a recently ruptured plaque appears as a disruption or discontinuation in the intimal wall with a cavity (Figure 3A). Alternatively, OCT may demonstrate a protrusion of an eroded plaque with overlying thrombus (Figure 3B), or a calcified nodule with hyperintense signal with disruption of the overlying fibrous cap (Figure 3C). OCT also provides enough resolution for measurement of the fibrous cap (Figure 2D)[53], which is a critical factor in assessing plaque stability[54]. A thin fibrous cap less than 65 μm is associated with an increased risk of plaque rupture[55–56]. Microchannels within the plaque may also be visualized, further characterizing plaque vulnerability and intimal disruption.
Figure 2.
IVUS and OCT images of coronary plaque phenotype.
A, Example of calcified plaque on IVUS, depicted as a bright circumferential structure (white arrow) with deeper shadowing (white star); B, Example of predominantly lipid-laden plaque by IVUS, depicted with less echogenicity than the surrounding hyperechoic adventitia, with a spotty calcification demarcated by the white arrow as a focal hyperechoic signal; C, Example of calcific plaque by OCT with white arrow depicting low-backscattering structure compared to surrounding adventitia with sharply delineated borders; D, Example of thin-cap lipid-laden plaque on OCT, depicted as a low-density structure with diffuse borders covered by a thin fibrous cap with the white arrow identifying signal-rich distinct or confluent punctate regions that exceed intensity of background speckle noise, representing macrophages.
(Adapted from Gurgoglione et al.[53] under Creative Commons License).
OCT: optical coherence tomography; IVUS: intravascular ultrasound.
Figure 3.
Examples of OCT-derived mechanisms of plaque destabilization.
A, Example of a ruptured plaque, characterized by the evidence of a cavity with a clear discontinuity of the fibrous cap (white arrow); B, Example of a definite eroded plaque, characterized by a luminal thrombus (white arrow) overlying a plaque without evidence of fibrous cap disruption; C, Example of a calcified nodule at the white arrow depicted as single of multiple regions of calcium that protrude into the lumen with fibrous cap disruption.
(Adapted from Gurgoglione et al.[53] under Creative Commons License).
OCT: optical coherence tomography.
The experienced interventionalist uses a combination of angiographic appearance and advanced IVI techniques to plan the intervention. A high calcium burden may prompt the interventionalist to use intravascular lithotripsy, orbital or rotational atherectomy depending on the location of the lesion and grade of stenosis.
Evaluation of stent apposition, expansion, and post-PCI complications
After deploying a stent, IVI provides invaluable data about the success of the procedure. Prior to the widespread use of these modalities, detailed stent deployment characteristics were overlooked or underestimated due to lack of adequate resolution of angiography alone.
Stent apposition refers to the contact between the stent struts and the inner vessel wall. Proper apposition is essential to ensure optimal stent function and prevention of thrombosis. Incomplete apposition or malapposition is defined as a gap in contact between at least one strut and the surface of the vessel wall in a specific segment. This segment does not overlay a branching vessel and showcases a gap larger than the thickness of the strut itself[57–58]. Stent expansion is the process of deploying the stent to achieve a desired vessel diameter based on reference vessel size, ensuring adequate luminal gain and restoration of blood flow, and decreasing risk of in-stent restenosis[58–61]. Generally, moderate stent under expansion refers to a stent-to-artery ratio less than 90% while severe under expansion refers to a stent-to-artery ratio less than 70%. It is important to note that these definitions have varied among different studies, guidelines, and clinical practices. In contemporary clinical trials, stent expansion guidance for an adequately optimized stent placement included stent-to-artery ratio of >80%[62].
Using IVUS, real-time cross-sectional images of the adequately apposed stent demonstrate continuous lines (stent struts) with no gaps between the strut and the vessel wall (Figure 4A). Any significant area between the stent struts and the vessel wall indicates a malapposed stent (Figure 4B)[63]. Stent under expansion is identified when the measured stent area is less than the reference vessel area or target stent area defined before the intervention[28]. IVUS is well-suited to evaluate the symmetry of stent expansion by comparing the minimal stent area (MSA) to the reference vessel MLA (Figure 5A)[64]. Identification of malapposed struts or under expansion can often be rectified with high-pressure balloon inflation, providing a more optimized result. IVUS provides broad imaging depth making it particularly well-suited for assessing stent apposition and expansion.
Figure 4.
Matched OCT and IVUS images evaluating stent apposition.
A, A well-apposed stent detected by IVUS; B, A malapposed stent detected by IVUS; C, A well-apposed stent detected by OCT; D, A malapposed stent detected by OCT.
(Adapted from Koganti et al.[63] Under Creative Commons License).
OCT: optical coherence tomography; IVUS: intravascular ultrasound.
Figure 5.
Matched OCT and IVUS images evaluating stent expansion.
A, IVUS images of the same vessel. In the pre-PCI IVUS image (A1–A3), the distal and proximal reference vessel diameters are measures as well as the lesion diameters, demonstrating significant lipid-laden plaque. In the final images (A4–A6), post-PCI result with MSA >80% of the average of the proximal and distal reference MLA, signifying a successful result; B, Post-stent OCT images comparing the proximal half and the distal half MSA demonstrating excellent expansion as compared to the distal and proximal reference mean lumen area.
(Reproduced from Maehara et al.[64] with permission from Elsevier).
IVUS: intravascular ultrasound; MLA: minimum luminal area; MSA: mean stent area; OCT: optical coherence tomography; PCI: percutaneous coronary intervention.
Similarly, OCT excels in visualizing stent struts due to its micron-level resolution. Proper apposition is confirmed when struts are uniformly in contact with the vessel wall without apparent gaps (Figure 4C)[63]. Any gap between the struts and the vessel wall signifies malapposition (Figure 4D)[63] and requires further balloon dilatation. Precise measurement of MSA compared to the MLA of the proximal and distal reference vessel segments may suggest under expansion. Furthermore, comparison of the MSA in the proximal and distal halves of the stent can suggest dissymmetry (Figure 5B)[64].
Another complication of stent deployment and high-pressure balloon expansion is edge dissections. These are tears and separations in the intima at or near the proximal or distal edges of the stent. If detected, this may require intervention. Both IVUS and OCT can visualize the presence, location, and extent of edge dissections. In IVUS images, dissections appear as disruptions or flaps in the vessel wall near the stent edges[28]. OCT offers superior resolution for detecting and characterizing edge dissections, including their length, depth, and location in relation to the stent struts. Due to the high-resolution images produced by OCT, this modality can identify subtle dissections that may be missed by other imaging modalities, including IVUS (Figure 6)[64].
Figure 6.
Matched OCT and IVUS images evaluating stent edge dissection.
A’, Appearance of stent edge dissection by OCT, white arrows depicting medial dissection flap; A’’, The same stent edge dissection, white arrows depicting medial dissection flap seen by IVUS.
(Reproduced from Maehara et al.[64] with permission from Elsevier).
IVUS: intravascular ultrasound; OCT: optical coherence tomography.
Accurate assessment of stent apposition, expansion, and edge dissections is critical in ensuring the success of coronary interventions and preventing downstream complications and re-intervention. Although both IVUS and OCT provide valuable information, understanding their mechanisms, strengths, and weaknesses can help operators utilize the optimal modality individually or in combination with one another to robustly assess the post-intervention result.
COMPARATIVE EFFECTIVENESS AND CLINICAL OUTCOMES
The advent of coronary stenting and the associated complications accelerated the investigation of the role of IVI in a search for tools to help understand the spatial relationship between the stent and the arterial lumen. To understand the nidus that initiated three decades of investigation into IVI, one must begin with a deep familiarity with the landscape and parallelisms in the evolution of coronary interventions over time.
The role of intravascular ultrasound in the revolution of coronary stenting
The early 1990s was a transformative era for coronary interventions. Initial data suggested improved clinical outcomes of intracoronary stents compared to balloon angioplasty. Specifically, stent placement demonstrated reduction in morbidity after acute vessel closure[65–66], as well as significant reductions in the rate of restenosis[40,42,67]. Large, randomized trials ensued and ultimately led to the development of the Palmaz-Schatz balloon-expandable bare metal stent (BMS) which was the first intracoronary stent to be approved by the United States Food and Drug Administration[67–69].
In the years that followed, widespread use of coronary stents was primarily impeded by two major hurdles: an increased risk of stent thrombosis and major bleeding events associated with the antiplatelet and anticoagulation regimens employed to reduce this risk. This led to two pivotal IVUS-guided investigations that revolutionized our understanding of stent mechanics, the pathophysiology of stent thrombosis and restenosis and sparked the cascade of investigations into IVI to optimize stent placement.
First, Nakamura et al.[70] evaluated 65 patients who received Palmaz-Schatz stents with satisfactory angiographic results and who had IVUS imaging post-dilatation. In >80% of these patients, IVUS revealed inadequate stent expansion or strut apposition and required further dilatations. This was consistent with prior data that suggested that larger acute gain in minimum lumen diameter diminishes the rate of restenosis at 6 months after intervention[71]. Several standardized post-dilatation guidelines were proposed in this investigation, however, limitations to a “one-size-fits-all” approach were quickly recognized. The investigators concluded that IVUS images provided data that was unique compared with angiography alone. Angiography was deemed effective at rapidly estimating the luminal diameter longitudinally; however, IVUS provided circumferential assessment that was not obscured by variations in projection, making it ideal for accurately evaluating the stent placement result. This was a revolutionary concept that generated hypotheses that incomplete stent dilatation may be, in part, responsible for the increased risk of stent thrombosis.
A subsequent investigation by Colombo et al.[72] tested the hypothesis that anticoagulation was not necessary when adequate IVUS-guided stent expansion was achieved. Three hundred and fifty-nine consecutive patients with 452 lesions that were treated with Palmaz-Schatz stents and further IVUS-guided balloon dilatation were studied. Those with adequate stent expansion, as confirmed by IVUS, were treated with antiplatelet therapy alone and did not receive anticoagulation after the procedure. At 2-month follow-up, only three stent thromboses were identified, despite the absence of anticoagulation. The use of IVUS-guided high-pressure final balloon dilatations provided assurance that anticoagulation therapy may safely be omitted, reduced hospital time, vascular complications, and resulted in low stent thrombosis rates.
The co-evolution of intravascular ultrasound and the stent
Over the next decade, newer generations of BMS were developed with thinner struts and lower-profile architecture perceived to improve deliverability and further reduce the risk of malapposition and stent thrombosis. Aggressive, post-stent dilatation was widely practiced, independent of the use of IVUS, in attempts to optimize stent placement. Despite initial evidence of the incremental increase in information provided by IVUS, it was not yet widely adopted in the United States. At least seven randomized trials were conducted to evaluate clinical outcomes and cost-effectiveness of IVUS-guided versus angiography-guided BMS placement[73–80].
A metanalysis of these randomized trials demonstrated a significant 36% reduction in the risk of restenosis at 6 months, 38% reduction in the rates of repeat revascularization, and 28% reduction in the risk of major adverse cardiovascular events (MACE). The analysis did not show a significant reduction or increase in the risk of death or myocardial infarction (MI). Only three of the seven randomized trials included in this meta-analysis reported the results of post-PCI IVUS imaging in the angiography-guided arms, therefore no definitive conclusions could be drawn about the mechanism for outcomes improvement in the IVUS-guided arms. Although the overall analysis demonstrated an improvement in clinical outcomes, some of the trials showed no improvement in the rate of restenosis. Furthermore, there was significant heterogeneity in the complexity of the lesions across different trials, the IVUS technique employed by different operators as well as the protocols by which operators chose to use the data generated by IVUS both within and across the participating clinical sites. While this may represent real-life practice, it made the data difficult to generalize across different practice settings. Overall, three of the seven studies reported cost-effectiveness data. Two of the three studies demonstrated that the increased upfront costs associated with IVUS-guided PCI (catheter and additional balloon costs) were offset by the cost of increased downstream revascularization in the angiography-guided arm, resulting in a net economic benefit in IVUS-guided BMS implantation[81].
While the data accumulated to support the use of IVUS-guided BMS implantation to improve clinical outcomes and dramatically reduce the risk of stent thrombosis, a new conundrum arose. Despite all efforts to optimize stent expansion and apposition, BMS restenosis rates remained high. Several studies sought to perform serial IVUS after interventions to help better understand the process. This demonstrated that late lumen loss and in-stent restenosis were the result of neointimal tissue proliferation which was uniformly distributed over the length of the stent[82].
These studies utilized and set the framework for validated volumetric quantitative methods in IVUS[36,83]. This included calculations of changes in the external elastic membrane area (EEM) over time, change in cross-sectional luminal area (CSA) over time, and segments of tissue growth (change in plaque area) over time. It is important to note that the trailing edge of the intima (the internal elastic membrane), despite being highly echogenic, is difficult to define on IVUS. Thus, the EEM is used as a surrogate. Any difference between EEM area and luminal area is estimated as sub-intimal plaque area.
The challenge of high incidence of in-stent restenosis of BMS led to the development of the first generation of drug-eluting stents (DES). These drug-polymer–coated stents incorporated anti-inflammatory and antiproliferative agents (sirolimus and paclitaxel) that reduced endothelial tissue proliferation and thus addressed the proposed mechanism of intimal hyperplasia which was triggered by stent implantation. This advancement came with its own pitfalls. With delayed endothelialization of the stent struts, an immediate improvement in the incidence of in-stent restenosis was realized. However, delayed endothelialization meant that the struts of the stents were exposed to the blood stream for longer. First-generation DES were associated with a higher (albeit small) risk of stent thrombosis compared to BMS[84–85]. Again, IVUS-guided studies to explore the mechanisms behind this phenomenon led to innovative advancement and the advent of newer generations of DES that omitted the use of paclitaxel as an antiproliferative agent, thinner metallic stent struts, more biocompatible durable or biodegradable polymers as well as polymer-free stents[86]. While further innovation in stent technology continues, the momentum of these IVUS studies formed the framework for stent research and development and guided the basis of our current-day stent innovations. Naturally, as the landscape of stent technology evolved, a new wave of randomized trials evaluating the utility of IVUS-guided PCI in the contemporary era of DES came to rise.
Initial randomized trials were underpowered and failed to demonstrate a statistically significant benefit on clinical outcomes[87–90]. Several metanalyses that pooled these trials with large observational studies revealed statistically significant reductions in MACE, death, MI, target lesion revascularization (TLR), and stent thrombosis with IVUS-guided compared to angiography-guided DES[91–94].
The IVUS-XPL trial was the first large-scale, randomized trial to evaluate the efficacy of IVUS-guided PCI compared to angiography-guided PCI in patients receiving newer generation DES for complex coronary lesions (defined as implanted stents ≥28 mm in length)[95]. This trial enrolled 1,400 patients and demonstrated a significant absolute reduction of 2.97% in the MACE composite endpoint with IVUS-guided PCI compared to angiography alone. The MACE composite endpoint included cardiovascular death, target lesion-related MI, or ischemia-driven TLR at 12 months. When evaluating the individual components of the composite endpoint, the benefit was derived primarily from ischemia-driven TLR. Importantly, a stratified analysis within the IVUS-guided group showed that those that met specific IVUS criteria for optimal stent implantation (defined minimal lumen cross-sectional area greater than the lumen cross-sectional area at the distal reference segment) had a significant 69% reduction in the primary composite endpoint, compared to those that did not meet these criteria.
The ULTIMATE trial aimed to replicate the hypothesis of its predecessor in an all-comer patient population who required second-generation DES implantation[96]. Approximately 1,400 patients were randomized to IVUS guidance or angiography guidance, and the primary composite endpoint included cardiac death, target-vessel MI, or target vessel revascularization (TVR) at 12 months. The trial met its primary endpoint, with a statistically significant 47% reduction in the risk of the composite endpoint. This was again driven by the reduction in TVR. Although statistical significance was not achieved on the individual components of the primary endpoint, there was a unanimous numerical reduction. Again, this trial demonstrated that in an all-comer population, optimal PCI (defined by IVUS quantitative analysis) reduced the incidence of primary composite endpoint by 65%. Importantly, this trial utilized different “optimal PCI” criteria compared to prior studies. The ULTIMATE trial defined optimal PCI by three criteria: (1) MLA in the stent segment at least 90% of MLA in the distal reference segments; (2) plaque burden 5 mm proximal or distal to the stent edge <50%; and (3) no edge dissection involves the media with a length >3 mm. Guidance was provided on repeat ballooning with a large balloon at higher pressure to achieve the first criterion. Repeat ballooning using a smaller balloon was recommended to meet the second criterion. The procedure was defined as sub-optimal if any of the three criteria was not met. Importantly, the trial demonstrated that under these stringent criteria, within the IVUS-guided arm, approximately 50% of patients failed to achieve their protocol definition of optimal PCI. The low rate of optimal PCI was driven by inability to achieve <50% plaque burden within 5 mm of the proximal or distal stent edge. A subsequent report of 3-year outcomes of the ULTIMATE trial demonstrated the sustained clinical effectiveness of both IVUS-guided PCI in all-comers as well as the effectiveness of their protocol-defined optimal PCI[97].
While the trial results were positive for IVUS-guided DES implantation in the general population, it also revealed two important truths about this approach: (1) It is difficult to achieve stringent IVUS-defined optimal PCI results; and (2) sub-optimal IVUS-guided PCI had only a modest reduction in clinical outcomes compared to angiography-guided PCI.
A new era for precision coronary interventions
The divergence of clinical outcomes between IVUS-guided PCI and angiography-guided PCI has led operators to question the previous investigations of surgical revascularization versus PCI. Contemporary data overwhelmingly favors surgical revascularization in patients with higher disease burden (multivessel disease), in those with higher lesion complexity, significant left main involvement, and in the presence of diabetes[98–99]. However, with the advent of “precision PCI” guided by advanced IVI modalities, there is a rising unmet need to replicate legacy PCI trials in the contemporary era.
Indeed, there is evidence to support this hypothesis. The BEST trial compared coronary artery bypass graft surgery (CABG) to PCI among patients with non-left main multivessel CAD[100]. As expected, CABG demonstrated a significant reduction in the rate of MACE, compared to PCI. The benefit of CABG was diluted over time and on report of 10-year outcomes, both modalities demonstrated no significant difference in hard clinical endpoints, though patients who underwent PCI had an increased risk of spontaneous MI and repeat revascularization. Upon detailed review of the data, the investigators report a stratified analysis, by those that received IVUS-guided PCI compared to angiography-guided PCI. Interestingly, the subgroup of patients who received angiography-guided PCI had about double the incidence of the primary endpoint as those with IVUS-guided PCI or CABG. In fact, those who underwent IVUS-guided PCI had numerically lower rates of all-cause mortality at 11 years, compared to those who underwent CABG[101]. Although this analysis was not pre-specified, it generates an interesting hypothesis that warrants investigation in an adequately powered randomized trial of contemporary PCI practices.
The clinical development of optical coherence tomography
OCT was first studied and patented simultaneously in Japan and in the United States in 1991[102]. After initial studies of the retina and coronary arteries, the technology was applied clinically in ophthalmology from 1996 onward. In 2002, the first clinical study of OCT in intracoronary imaging began[103]. The potential for higher resolution imaging than IVUS brought forth rapid development of OCT technology to identify thin fibrous caps more accurately, representing unstable atherosclerotic plaques. This study compared matched pairs of OCT and IVUS images and correlated with available histological specimens to characterize plaque characteristics. Experienced IVUS observers, blinded to OCT data, reviewed the IVUS data using standardized criteria and classified plaque types and vessel features. Concurrently, an OCT observer, blinded to all the IVUS data reviewed OCT images and described similar features. Features identified by both modalities were compared to one another and to histological findings in patients who died and underwent autopsy. This study demonstrated that OCT facilitated identification of the elastic lamina, intimal hyperplasia, calcium deposits. OCT also revealed procedural attributes such as stent expansion and apposition. Importantly, OCT successfully allowed for measurement of thin fibrous caps, which were not easily identifiable using IVUS. This study and subsequent replications set the stage for further OCT investigations that would bring this imaging modality to the forefront of a new era of intracoronary imaging[104–106].
Initial experience with OCT was limited by the need to clear the blood field to optimize imaging. In earlier iterations of the technology (time domain-OCT), this was accomplished with sustained low-pressure balloon occlusion. This was replaced by newer non-occlusive, frequency-domain OCT which was approved for clinical use in the European market in 2007 and in the United States in 2010.
Large observational studies and small single-center randomized trials, comparing OCT-guided PCI and angiography-guided PCI ensued, generating consensus that this technology can be used safely[107–109]. Preliminary data showed the promise that OCT may improve clinical outcomes and successfully detect sub-optimal stent deployment as well as impact physician decision-making in more than two-thirds of cases[86,110–113]. There remained an unmet need to conduct large-scale, multicenter randomized trials to prospectively evaluate the effect of this technology on PCI-optimization or clinical outcomes[114–115].
The DOCTORS trial enrolled 240 patients with Non-ST-elevation Acute Coronary Syndrome at nine centers in France. Patients were randomized to OCT-guided versus angiography-guided PCI. Operators performing the procedure in the OCT arm had to acquire at least two sets of OCT images, before and after the angioplasty. The primary endpoint was FFR measurement at the end of the procedure. Secondary endpoints included the safety outcomes such as periprocedural complications or acute kidney injury. Indeed, OCT-guided PCI yielded significantly improved FFR measurements at the end of the procedure, a reduced final diameter of stenosis, and improved stent expansion. Although the procedural duration as well as radiation dose and contrast dose were increased, there was no significant increase in clinical periprocedural complications compared to angiography alone[116].
Most recently, two large multicenter randomized trials were dedicated to compare hard clinical outcomes in patients who underwent OCT versus angiography-guided PCI[117–118]. These trials differed from the DOCTORS trial in that they were adequately powered to detect clinically meaningful differences in hard clinical endpoints and followed up patients for 24 months.
The OCTOBER trial randomized 1,200 patients with stable angina, unstable angina/non-ST-segment elevation myocardial infarction (NSTEMI) with complex bifurcation lesions in a 1:1 ratio to OCT-guided PCI or angiography-guided PCI and followed them for 2 years[117]. The primary endpoint was MACE which consisted of cardiovascular death, target lesion MI, or ischemia-driven TVR. The OCTOBER trial met its primary endpoint, with a statistically significant 30% reduction in MACE with OCT compared to angiography alone. All the components of the primary endpoint were numerically lower with OCT. Additionally, all-cause mortality was a secondary outcome which showed a numerical 44% relative reduction with OCT. In subgroup analysis, the benefit was seen for both left main and non-left main bifurcation PCI.
The ILUMIEN IV trial randomized 2,500 patients with high-risk coronary lesions or medication-treated diabetes in a 1:1 ratio to OCT-guided PCI or angiography-guided PCI and followed them for 2 years[118]. The trial protocol defined a high-risk lesion as an NSTEMI or delayed ST-segment elevation myocardial infarction (STEMI) (>24 hours from symptom onset), long lesions (≥28 mm), diffuse or multi-focal in-stent restenosis, severe calcification, chronic total occlusion, bifurcation lesion with planned two-stent approach, The primary imaging endpoint was a difference in post-PCI MSA assessed by OCT. The primary clinical endpoint was TVF defined as target-vessel MI, or ischemia-driven TVR. Although the trial met its primary imaging endpoint with a significantly larger MSA achieved in the OCT arm, the trial failed to meet its primary clinical endpoint. All the individual components of the primary endpoint were numerically lower in the OCT arm, compared to the angiography arm. Patients in the OCT arm did achieve more acceptable stent expansion (>90% of the reference vessel MLA). In addition, a significant 75% reduction in stent thrombosis was observed, with 95.7% of patients with stent thrombosis experiencing death or MI within 2 years. While this is an important patient-centered endpoint, the negative primary endpoint came as a surprise to many interventionalists.
The disparate outcomes from these trials left the interventional community in a conundrum. The totality of the evidence points toward better PCI results with OCT; however, this fails to consistently translate to improvement in clinical outcomes. Further studies with subgroup analyses and detailed review of the raw images from both trials could help tease out the nuances involved. Additional outcomes-driven trials in specified patient populations are needed to definitively identify a population subgroup that would consistently derive benefit from OCT-guided PCI.
Head-to-head comparisons of intravascular ultrasound and optical coherence tomography
Subsequent randomized trials sought to compare the efficacy of OCT-guided PCI to IVUS, on measures of stent optimization including MLA and MACE[119–120]. Both imaging modalities were comparable on all quantification measures of stent optimization. These results corroborated prior observational studies and demonstrated the robust capabilities of both imaging modalities as viable IVI tools in the contemporary cardiac catheterization lab.
The OCTIVUS trial was the latest randomized trial to compare both modalities’ effect on hard clinical outcomes[121]. Approximately 2,000 patients with significant coronary lesions were randomized in a 1:1 ratio to undergo either OCT-guided or IVUS-guided PCI. The primary endpoint was MACE, consisting of cardiovascular death, target-vessel MI, or ischemia-driven TVR at 1 year. This trial was powered for non-inferiority of OCT compared to IVUS. OCTIVUS met its non-inferiority primary endpoint with an observed reduction in major procedural complications with OCT compared to IVUS although there was no difference in the imaging-related complications. There are several notable features about this population. First, approximately 55% of the patients in this trial had bifurcation lesions, 11% had left main coronary disease, and 60% had diffuse long lesions. Second, the pre-defined imaging-guided stent optimization parameters were met more frequently with IVUS compared to OCT. While there was no significant difference in outcomes between the arms, OCT had numerically lower incidence of the primary endpoint and each of its individual components. Finally, the overall event rate in the study was much lower than expected, so subgroup analyses and individual components of the primary endpoint should be interpreted with caution. Overall, this important trial highlights the interchangeability of these imaging modalities when performed in centers that are well-acquainted with both approaches.
The establishment of a new standard-of-care with intravascular imaging in percutaneous coronary interventions
Thus far, the evidence is overwhelmingly in favor of either IVUS or OCT-guided PCI compared to angiography alone. Studies that compared the modalities to one another demonstrated non-inferiority. The guidelines recommend the use of either modality in selected populations to optimize stent placement. Centers in South Korea and Japan have widespread experience with both modalities and have led the world in investigations and in clinical use of these imaging modalities. The widespread expertise with both modalities marks a particularly fertile environment for the conduct of large, randomized trials that utilize both techniques.
The RENOVATE-COMPLEX PCI trial was a large multicenter trial conducted in South Korea that enrolled patients with complex coronary lesions and randomized in a 2:1 ratio to undergo either IVI-guided PCI or angiography-guided PCI[122]. The choice between IVUS and OCT was at the operator’s discretion. The primary endpoint was a composite endpoint of TVF including cardiovascular death, target-vessel MI, or clinically driven TVR. A total of 1,600 patients underwent randomization. IVI-guided PCI demonstrated a 36% reduction in the incidence of TVF compared to angiography alone. There were no differences in procedural safety events between the groups. This reiterates prior knowledge that IVI enhances clinical outcomes among patients with complex coronary lesions undergoing PCI.
All the major randomized trials using currently widely available second-generation DES or newer, to date, that compared hard clinical endpoints of IVI-guided PCI compared to angiography alone (Table 2), were powered to evaluate a composite endpoint of MACE or TVF and were underpowered to draw any conclusions about cardiovascular death or MI. In a hotline session at the 2023, European Society of Cardiology Congress in Amsterdam, Compelling data were presented on a real-time updated metanalysis comparing OCT, IVUS, and angiography. The study pooled data from 20 randomized trials that collectively enrolled over 12,000 patients. The primary analysis cohort was IVI-guided PCI versus angiography-guided PCI, and secondary analyses included individual comparisons of the different modalities. This included IVUS versus OCT, IVUS versus angiography, OCT versus angiography. IVI-guided PCI demonstrated a 25% reduction in all-cause mortality compared to angiography-guided PCI and 20% reduction in MI, primarily driven by a significant 50% reduction in stent thrombosis. The results of this analysis corroborate prior meta-analyses of randomized trials and confirm the effectiveness of IVI across a variety of patient populations[123].
Table 2.
Summary of all the major randomized trials evaluating clinical outcomes of intravascular imaging in patients receiving second-generation drug-eluting stents or newer
| Trials | N | Patient population | Primary endpoint | Conclusion |
|---|---|---|---|---|
| IVUS versus angiography | ||||
| IVUS-XPL[95] | 1,400 | Long coronary lesions (drug-eluting stent ≥28 mm in length) | Cardiovascular death or, target lesion MI or, ischemia-driven TLR | 50% reduction in MACE with IVUS; Primarily driven by reduction in TLR |
| ULTIMATE[96] | 1,448 | All-comers | Cardiovascular death or target-vessel MI or TVR | 47% reduction in MACE with IVUS; Primarily driven by reduction in TVR |
| OCT versus angiography | ||||
| DOCTORS[116] | 240 | Non–ST-elevation acute coronary syndrome | Change in FFR, before and after procedure | OCT-guided PCI improves FFR measurements; Reduced final diameter of stenosis; Improved stent expansion |
| OCTOBER[117] | 1,201 | Complex bifurcation lesions | Cardiovascular death or, target lesion MI or, ischemia-driven TVR | 30% reduction in MACE with OCT |
| ILUMIEN IV[118] | 2,487 | Diabetes or complex coronary lesions | Target-vessel MI or ischemia-driven TVR | No significant change in the composite TVF; Larger MLA achieved with OCT; 75% reduction in stent thrombosis with OCT |
| IVUS versus OCT | ||||
| OCTIVUS[121] | 2,008 | Significant coronary lesions | Cardiovascular death or, target-vessel MI or, ischemia-driven TVR | OCT and IVUS non-inferior on primary endpoint; No significant difference in individual components; OCT numerically lower events |
| IVI versus angiography | ||||
| RENOVATE-COMPLEX PCI[122] | 1,639 | Complex coronary lesions | Cardiovascular death or, target-vessel MI or, clinically driven TVR | 36% reduction in TVF with IVI; No difference in procedural safety endpoints |
FFR: fractional flow reserve; IVUS: intravascular ultrasound; MACE: major adverse cardiovascular events; MI: myocardial infarction; MLA: minimum luminal area; OCT: optical coherence tomography; PCI: percutaneous coronary intervention; TLR: target lesion revascularization; TVR: target-vessel revascularization.
THE ROLE OF INTRAVASCULAR IMAIGNG IN SPECIFIC LESION TYPES
Heavily calcified lesions
IVUS and OCT play pivotal roles in the assessment of calcification and identification of high-risk features for stent under expansion[30,124–125]. Several society expert position statements have sought to provide guidance on the optimal approach to calcified lesions that have incorporated the use of IVI techniques[27,126–127]. However, there remains an unmet need for dedicated guidelines on the use of IVI to guide lesion modification decisions.
Several scoring systems, based on real-time OCT and IVUS imaging have been developed to predict the need for atherectomy or advanced interventions. For instance, the OCT-based CVI score[128], uses a point-based system, whereby points are assigned to a lesion based on three OCT-derived variables: (1) >180° of calcification; (2) calcium thickness of >0.5 mm; and (3) calcified segment length >5.0 mm. Lesions with a score of 4 or greater are deemed to be at high risk of poor stent expansion and may require complex interventions for lesion preparation.
Similarly, an IVUS calcium score has been developed to aid in lesion preparation decisions[129]. Using IVUS, four variables are assigned up to 1 point each, if present: (1) calcium >270° of the vessel wall in >5.0 mm in length; (2) 360° of calcium, independent of lesion length; (3) presence of focal nodular calcium; (4) vessel diameter <3.5 mm. The higher the score, the greater the likelihood of stent under expansion.
While these integer-based scores aim to provide an objective and simplified way of assessing the risk of stent under expansion and to provide a standardized approach to coronary intervention in calcified lesions, they have not yet been prospectively evaluated or established as part of a clinical practice guideline. Validation and integration of machine learning (ML) tools for a more quantitative real-time risk assessment is highly anticipated as the next breakthrough in real-time imaging-guided intervention.
Bifurcation lesions
Bifurcation lesions represent a large proportion of coronary interventions and are known to carry higher risk of short-term and long-term complications[130–132]. Several of the aforementioned randomized trial investigators have performed subgroup analyses in a subset of patients with bifurcation lesions to evaluate the utility of IVI modalities to guide the management of these complex lesions.
Specifically, subgroup analyses of the all-comer ULTIMATE trial showed that IVUS-guided bifurcation lesion PCI was superior to angiography-guided PCI[95,97]. The OCTOBER trial, on the other hand, utilized OCT in a highly selected population of patients with bifurcation lesions and demonstrated significant improvements over angiography-guided PCI[118]. Analyses of the RENOVATE-COMPLEX PCI trial which utilized both IVUS and OCT compared to angiography, failed to demonstrate a benefit in this subgroup of lesions; however, was not specifically powered for this analyses[122]. When compared to one another in a bifurcation lesion subset, in the OPINION trial, OCT was non-inferior to IVUS[120]. In totality, both IVUS and OCT have individually demonstrated benefit in guiding bifurcation lesion interventions over angiography.
Chronic total occlusions
Chronic total occlusions arguably represent the most complex subset of coronary lesions. IVUS, owing to its superior penetration, is empirically favored over OCT in this subset of lesions. This modality can provide information about the proximal cap and can assist in wire tracking and lesion crossing. OCT, on the other hand, due to its superior spatial resolution, may be optimal for detecting post-implantation complications such as stent edge dissections and malapposition[133].
The use of IVI in chronic occlusion PCI has not been independently studied in a dedicated randomized trial and these lesions have been excluded from most contemporary IVI trials. However, several registry-based studies and subgroup analyses of the trials that did not systematically exclude these patients have been performed.
The current data is mixed, with some large registry data suggesting that the use of IVI may result in higher technical success without an increase in periprocedural complications[134–135], while others failed to demonstrate this association[136–137]. Overall, utilization of IVI in this lesion subset remains below the average for other lesion subsets[137]. As interventional cardiologists gain experience in the use of IVI modalities, further dedicated studies are needed to definitively draw conclusions on the efficacy of these modalities on clinical outcomes in chronic total occlusions.
INNOVATIONS AND FUTURE DIRECTIONS
IVI techniques, such as IVUS and OCT, have revolutionized the field of interventional cardiology by providing high-resolution images of coronary vessels from within. As we look to the future, one of the key areas of innovation lies in the development of hybrid catheters that combine the strengths of both IVUS and OCT, offering a multifaceted approach to IVI. With the rising use of IVI, data continues to grow, there is an increasing need for automated image analysis tools to assist clinicians in extracting valuable information efficiently. In recent years, we have experienced exponential growth in computational power and expertise in ML algorithms that bode well for the integration of automation into the field of IVI. Future developments in these areas are poised to transform the way we acquire and interpret IVUS and OCT images.
Combining intravascular ultrasound and optical coherence tomography modalities
Hybrid catheters aim to overcome the limitations inherent to individual imaging modalities. IVUS excels in providing detailed anatomical information, while OCT offers superior resolution for visualizing microstructures and detecting vulnerable plaques. By integrating these technologies, hybrid catheters promise a comprehensive assessment of coronary lesions[138–139].
These catheters enable simultaneous acquisition of IVUS and OCT data, providing clinicians with a synergistic view of the vessel lumen, plaque composition, and stent apposition. This integrated approach can enhance diagnostic accuracy, guide complex interventions, and improve treatment outcomes. Future advancements in hybrid catheters may also include real-time fusion imaging, where IVUS and OCT data are seamlessly merged to create a composite image. This fusion imaging can offer a more intuitive visualization of vessel structures, making it easier for operators to make informed decisions during procedures. Furthermore, it has the potential to reduce procedure time and radiation exposure.
Automated image analysis for efficiency and precision
ML and artificial intelligence (AI) are becoming integral components of IVI analysis. These technologies can automatically segment vessels, identify plaque types, and quantify stent apposition. ML algorithms can also predict outcomes and assist in treatment planning by analyzing vast datasets of historical cases[140–142]. The integration of AI-driven image analysis into clinical workflows holds promise for optimizing decision-making and reducing inter-operator variability. Automated image analysis can facilitate precise quantification of key parameters, such as minimal lumen diameter, plaque burden, and fibrous cap thickness. This quantitative approach not only aids in treatment planning but also supports risk prediction models for adverse cardiovascular events. Future research may focus on refining these algorithms, enhancing their accuracy, and expanding their applications. This can contribute to personalized medicine by tailoring treatment strategies based on an individual patient’s vessel characteristics and risk profile.
Utilization of intravascular imaging
Despite advances in IVI technologies and accumulating evidence of their clinical efficacy and safety, widespread utilization in Europe and the United States lags. Recent data from the New York PCI Registry show an increase in utilization of IVUS from 13.4% in 2014 to 16.5% in 2018, with significant heterogeneity in practice patterns across centers[29]. On a national scale, an analysis of the National Inpatient Sample shows there was a significant increase in IVUS use from 3.4% in 2008 to 8.7% in 2019, while OCT use went from 0% in 2008 to 0.6% in 2019[143–144]. At two randomly selected hospitals across the United States, there was a more than fourfold contrast in the likelihood of a patient undergoing IVI with PCI, as evidenced by a difference in odds[145–146].
Look to our colleagues in Japan, approximately 91% and 82% of elective and emergent PCI, respectively, between 2008 and 2014[147]. Since the release of the latest data, this trend is expected to a near-ubiquitous use of IVI in almost all PCI performed in Japan.
Now that we have arrived at a time where the totality of the evidence points to a reduction in all-cause mortality with IVI, it becomes imperative to understand and study the barriers to utilization. In a web-based survey distributed to 32,000 individuals, the most reported factors limiting use were the prohibitive cost and prolongation of the procedure[148]. An extensive cost-benefit analysis demonstrates that the increased upfront incremental cost of IVUS derives significant downstream financial benefit[149].
Firstly, data from Japan, where there is increased reimbursement for the use of IVI, suggests that differences in economic perspectives across global healthcare systems may be a key barrier to utilization in the United States. However, this mechanism alone may not explain the reluctance to use IVI, as other public health systems such as the European Union still demonstrate low utilization rates. This suggests other possible mechanisms to explain the observed trends. Secondly, many practicing interventionalists may feel uncomfortable with the technical use of advanced IVI modalities and image interpretation[150]. Finally, interventionalists may lack confidence in the data that supports their widespread use.
To mitigate these hurdles, an update to the guidelines, to reflect the culmination of robust clinical evidence, to date, may motivate interventionalists to participate in continuing medical education and to get comfortable with the use of these modalities. The incorporation of IVI utilization rates into quality metrics used for evaluation of institutional and operator outcomes may also lead to more rapid incorporation into routine clinical practice. Finally, efforts to increase use of IVI at major academic institutions should be prioritized, as new generations of interventional fellows entering the workforce must be trained on the latest evidence-based standard-of-care.
CONCLUSIONS
Two decades of clinical investigations demonstrating the clinical efficacy and safety of IVI modalities have established these technologies as staples in the contemporary cardiac catheterization lab’s toolbox and earning their place in revascularization clinical practice guidelines. The future of IVI with IVUS and OCT holds great promise. Hybrid catheters, automated image analysis, and ongoing research efforts are expected to propel these technologies to new heights, benefiting both clinicians and patients alike. The integration of AI and the miniaturization of devices are shaping a future where IVI plays an increasingly pivotal role in cardiovascular care. Importantly, efforts to increase the global utilization of these modalities should be prioritized. This may be accomplished by dissemination of the latest evidence, continued medical education to practicing interventionalists, systemic efforts to increase reimbursement for IVI-guided PCI as well as incorporation into interventional cardiology fellowship curricula worldwide.
AUTHOR CONTRIBUTIONS
TN collected references, prepared the tablets and figures, wrote the original draft. AS collected references, wrote part of original draft. MF and JSZ collected references, conceived, reviewed, and edited the manuscript. JFO collected references, prepared the figures, conceived, reviewed, and edited the manuscript. All authors reviewed and approved final manuscript.
CONFLICT OF INTEREST STATEMENT
The authors declare that they have no financial conflict of interest with regard to the content of this manuscript.
ACKNOWLEDGMENT
We thank Dr. Kenneth Khaw for the critical review of our manuscript.
DATA SHARING STATEMENT
Data sharing is not applicable to this article as no datasets were generated or analyzed during the current study.
Footnotes
How to cite this article: Nafee T, Shah A, Forsberg M, Zheng JS, Ou JF. State-of-art review: intravascular imaging in percutaneous coronary interventions. Cardiol Plus 2023;8:227–246. doi: 10.1097/CP9.0000000000000069.
REFERENCES
- [1].Mintz GS, Popma JJ, Pichard AD, et al. Patterns of calcification in coronary artery disease. A statistical analysis of intravascular ultrasound and coronary angiography in 1155 lesions. Circulation 1995;91:1959–1965. doi:10.1161/01.cir.91.7.1959. [DOI] [PubMed] [Google Scholar]
- [2].Stone GW, Maehara A, Lansky AJ, et al. PROSPECT Investigators. A prospective natural-history study of coronary atherosclerosis. N Engl J Med 2011;364:226–235. doi:10.1056/NEJMoa1002358. [DOI] [PubMed] [Google Scholar]
- [3].Man K, Sabourin VM, Gandhi CD, et al. Pierre Curie: the anonymous neurosurgical contributor. Neurosurg Focus 2015;39:E7. doi:10.3171/2015.4.FOCUS15102. [DOI] [PubMed] [Google Scholar]
- [4].Galbraith JE, Murphy ML, de Soyza N. Coronary angiogram interpretation interobserver variability. JAMA 1978;240:2053–2056. [PubMed] [Google Scholar]
- [5].Grondin CM, Dyrda I, Pasternac A, et al. Discrepancies between cine angiographic and postmortem findings in patients with coronary artery disease and recent myocardial revascularization. Circulation 1974;49:703–708. doi:10.1161/01.cir.49.4.703. [DOI] [PubMed] [Google Scholar]
- [6].Isner JM, Kishel J, Kent KM, et al. Accuracy of angiographic determination of left main coronary arterial narrowing. Angiographic–histologic correlative analysis in 28 patients. Circulation 1981;63:1056–1064. doi:10.1161/01.cir.63.5.1056. [DOI] [PubMed] [Google Scholar]
- [7].Roberts WC, Jones AA. Quantitation of coronary arterial narrowing at necropsy in sudden coronary death: analysis of 31 patients and comparison with 25 control subjects. Am J Cardiol 1979;44:39–45. doi:10.1016/0002-9149(79)90248-0. [DOI] [PubMed] [Google Scholar]
- [8].Vlodaver Z, Frech R, Van Tassel RA, et al. Correlation of the antemortem coronary arteriogram and the postmortem specimen. Circulation 1973;47:162–169. doi:10.1161/01.cir.47.1.162. [DOI] [PubMed] [Google Scholar]
- [9].Zir LM, Miller SW, Dinsmore RE, et al. Interobserver variability in coronary angiography. Circulation 1976;53:627–632. doi:10.1161/01.cir.53.4.627. [DOI] [PubMed] [Google Scholar]
- [10].Nissen SE, Yock P. Intravascular ultrasound: novel pathophysiological insights and current clinical applications. Circulation 2001;103:604–616. doi:10.1161/01.cir.103.4.604. [DOI] [PubMed] [Google Scholar]
- [11].Topol EJ, Nissen SE. Our preoccupation with coronary luminology. The dissociation between clinical and angiographic findings in ischemic heart disease. Circulation 1995;92:2333–2342. doi:10.1161/01.cir.92.8.2333. [DOI] [PubMed] [Google Scholar]
- [12].Fisher LD, Robertson T, Hughes GM, et al. Research collaboration. J Am Coll Cardiol 1989;14:65A–68A. doi:10.1016/0735-1097(89)90167-8. [DOI] [PubMed] [Google Scholar]
- [13].Yock PG, Linker DT, Angelsen BA. Two-dimensional intravascular ultrasound: technical development and initial clinical experience. J Am Soc Echocardiogr 1989;2:296–304. doi:10.1016/s0894-7317(89)80090-2. [DOI] [PubMed] [Google Scholar]
- [14].Hodgson JM, Graham SP, Savakus AD, et al. Clinical percutaneous imaging of coronary anatomy using an over-the-wire ultrasound catheter system. Int J Card Imaging 1989;4:187–193. doi:10.1007/BF01745149. [DOI] [PubMed] [Google Scholar]
- [15].Nissen SE, Grines CL, Gurley JC, et al. Application of a new phased-array ultrasound imaging catheter in the assessment of vascular dimensions. In vivo comparison to cineangiography. Circulation 1990;81:660–666. doi:10.1161/01.cir.81.2.660. [DOI] [PubMed] [Google Scholar]
- [16].Nissen SE, Gurley JC, Grines CL, et al. Intravascular ultrasound assessment of lumen size and wall morphology in normal subjects and patients with coronary artery disease. Circulation 1991;84:1087–1099. doi:10.1161/01.cir.84.3.1087. [DOI] [PubMed] [Google Scholar]
- [17].Tobis JM, Mallery J, Mahon D, et al. Intravascular ultrasound imaging of human coronary arteries in vivo. Analysis of tissue characterizations with comparison to in vitro histological specimens. Circulation 1991;83:913–926. doi:10.1161/01.cir.83.3.913. [DOI] [PubMed] [Google Scholar]
- [18].Terashima M, Kaneda H, Suzuki T. The role of optical coherence tomography in coronary intervention. Korean J Intern Med 2012;27:1–12. doi:10.3904/kjim.2012.27.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Bouma BE, Yun SH, Vakoc BJ, et al. Fourier-domain optical coherence tomography: recent advances toward clinical utility. Curr Opin Biotechnol 2009;20:111–118. doi:10.1016/j.copbio.2009.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Spears JR, Marais HJ, Serur J, et al. In vivo coronary angioscopy. J Am Coll Cardiol 1983;1:1311–1314. doi:10.1016/s0735-1097(83)80145-4. [DOI] [PubMed] [Google Scholar]
- [21].Jaguszewski M, Klingenberg R, Landmesser U. Intracoronary near-infrared spectroscopy (NIRS) imaging for detection of lipid content of coronary plaques: current experience and future perspectives. Curr Cardiovasc Imaging Rep 2013;6:426–430. doi:10.1007/s12410-013-9224-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Waksman R, Torguson R, Spad MA, et al. The Lipid-Rich Plaque Study of vulnerable plaques and vulnerable patients: study design and rationale. Am Heart J 2017;192:98–104. doi:10.1016/j.ahj.2017.02.010. [DOI] [PubMed] [Google Scholar]
- [23].Windecker S, Kolh P, Alfonso F, et al. Authors/Task Force members. 2014 ESC/EACTS Guidelines on myocardial revascularization: the Task Force on Myocardial Revascularization of the European Society of Cardiology (ESC) and the European Association for Cardio-Thoracic Surgery (EACTS)Developed with the special contribution of the European Association of Percutaneous Cardiovascular Interventions (EAPCI). Eur Heart J 2014;35:2541–2619. doi:10.1093/eurheartj/ehu278. [DOI] [PubMed] [Google Scholar]
- [24].Neumann FJ, Sousa-Uva M, Ahlsson A, et al. ESC Scientific Document Group. 2018 ESC/EACTS Guidelines on myocardial revascularization. Eur Heart J 2019;40:87–165. doi:10.1093/eurheartj/ehy394.30165437 [Google Scholar]
- [25].Lawton JS, Tamis-Holland JE, Bangalore S, et al. 2021 ACC/AHA/SCAI Guideline for coronary artery revascularization: executive summary: a report of the American College of Cardiology/American Heart Association Joint Committee on Clinical Practice Guidelines. Circulation 2022;145:e4–e17. doi:10.1161/CIR.0000000000001039. [DOI] [PubMed] [Google Scholar]
- [26].Leesar MA, Hage FG. IVUS Guidance on optimal stent deployment: new insights and perspectives. JACC Cardiovasc Interv 2022;15:217–219. doi:10.1016/j.jcin.2021.12.013. [DOI] [PubMed] [Google Scholar]
- [27].Räber L, Mintz GS, Koskinas KC, et al. ESC Scientific Document Group. Clinical use of intracoronary imaging Part 1: guidance and optimization of coronary interventions. An expert consensus document of the European Association of Percutaneous Cardiovascular Interventions. Eur Heart J 2018;39:3281–3300. doi:10.1093/eurheartj/ehy285. [DOI] [PubMed] [Google Scholar]
- [28].Saito Y, Kobayashi Y, Fujii K, et al. Clinical expert consensus document on intravascular ultrasound from the Japanese Association of Cardiovascular Intervention and Therapeutics (2021). Cardiovasc Interv Ther 2022;37:40–51. doi:10.1007/s12928-021-00824-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Hannan EL, Zhong Y, Reddy P, et al. Percutaneous coronary intervention with and without intravascular ultrasound for patients with complex lesions: utilization, mortality, and target vessel revascularization. Circ Cardiovasc Interv 2022;15:e011687. doi:10.1161/CIRCINTERVENTIONS.121.011687. [DOI] [PubMed] [Google Scholar]
- [30].Truesdell AG, Alasnag MA, Kaul P, et al. ACC Interventional Council. Intravascular imaging during percutaneous coronary intervention: JACC state-of-the-art review. J Am Coll Cardiol 2023;81:590–605. doi:10.1016/j.jacc.2022.11.045. [DOI] [PubMed] [Google Scholar]
- [31].Vora AN, Swaminathan RV. Posting another win for intravascular imaging: moving away from angiography-only percutaneous coronary intervention toward a more comprehensive approach. Circ Cardiovasc Interv 2022;15:e011670. doi:10.1161/CIRCINTERVENTIONS.121.011670. [DOI] [PubMed] [Google Scholar]
- [32].Leopold JA. Choosing a method for guiding PCI. N Engl J Med 2022;387:840–841. doi:10.1056/NEJMe2209148. [DOI] [PubMed] [Google Scholar]
- [33].Williams DO. When images speak, listen. N Engl J Med 2023;388:1714–1716. doi:10.1056/NEJMe2302129. [DOI] [PubMed] [Google Scholar]
- [34].Sung JH, Jeong JS. Development of high-frequency (>60 MHz) intravascular ultrasound (IVUS) transducer by using asymmetric electrodes for improved beam profile. Sensors (Basel) 2018;18:4414. doi:10.3390/s18124414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].García-García HM, Mintz GS, Lerman A, et al. Tissue characterisation using intravascular radiofrequency data analysis: recommendations for acquisition, analysis, interpretation and reporting. EuroIntervention 2009;5:177–189. doi:10.4244/eijv5i2a29. [DOI] [PubMed] [Google Scholar]
- [36].Mintz GS, Nissen SE, Anderson WD, et al. American College of Cardiology Clinical Expert Consensus Document on Standards for Acquisition, Measurement and Reporting of Intravascular Ultrasound Studies (IVUS). A report of the American College of Cardiology Task Force on Clinical Expert Consensus Documents. J Am Coll Cardiol 2001;37:1478–1492. doi:10.1016/s0735-1097(01)01175-5. [DOI] [PubMed] [Google Scholar]
- [37].Prati F, Regar E, Mintz GS, et al. Expert's OCT Review Document. Expert review document on methodology, terminology, and clinical applications of optical coherence tomography: physical principles, methodology of image acquisition, and clinical application for assessment of coronary arteries and atherosclerosis. Eur Heart J 2010;31:401–415. doi:10.1093/eurheartj/ehp433. [DOI] [PubMed] [Google Scholar]
- [38].Tearney GJ, Regar E, Akasaka T, et al. International Working Group for Intravascular Optical Coherence Tomography (IWG-IVOCT). Consensus standards for acquisition, measurement, and reporting of intravascular optical coherence tomography studies: a report from the International Working Group for Intravascular Optical Coherence Tomography Standardization and Validation. J Am Coll Cardiol 2012;59:1058–1072. doi:10.1016/j.jacc.2011.09.079. [DOI] [PubMed] [Google Scholar]
- [39].Rathod KS, Hamshere SM, Jones DA, et al. Intravascular ultrasound versus optical coherence tomography for coronary artery imaging - apples and oranges. Interv Cardiol 2015;10:8–15. doi:10.15420/icr.2015.10.1.8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Eeckhout E, van Melle G, Stauffer JC, et al. Can early closure and restenosis after endoluminal stenting be predicted from clinical, procedural, and angiographic variables at the time of intervention. Br Heart J 1995;74:592–597. doi:10.1136/hrt.74.6.592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Schühlen H, Kastrati A, Dirschinger J, et al. Intracoronary stenting and risk for major adverse cardiac events during the first month. Circulation 1998;98:104–111. doi:10.1161/01.cir.98.2.104. [DOI] [PubMed] [Google Scholar]
- [42].Strauss BH, Serruys PW, de Scheerder IK, et al. Relative risk analysis of angiographic predictors of restenosis within the coronary Wallstent. Circulation 1991;84:1636–1643. doi:10.1161/01.cir.84.4.1636. [DOI] [PubMed] [Google Scholar]
- [43].Shlofmitz E, Iantorno M, Waksman R. Restenosis of drug-eluting stents: a new classification system based on disease mechanism to guide treatment and state-of-the-art review. Circ Cardiovasc Interv 2019;12:e007023. doi:10.1161/CIRCINTERVENTIONS.118.007023. [DOI] [PubMed] [Google Scholar]
- [44].Zbinden R, von Felten S, Wein B, et al. Impact of stent diameter and length on in-stent restenosis after DES vs BMS implantation in patients needing large coronary stents—a clinical and health-economic evaluation. Cardiovasc Ther 2017;35:19–25. doi:10.1111/1755-5922.12229. [DOI] [PubMed] [Google Scholar]
- [45].Baruś P, Modrzewski J, Gumiężna K, et al. Comparative appraisal of intravascular ultrasound and optical coherence tomography in invasive coronary imaging: 2022 update. J Clin Med 2022;11:4055. doi:10.3390/jcm11144055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [46].de la Torre Hernandez JM, Hernández Hernandez F, Alfonso F, et al. LITRO Study Group (Spanish Working Group on Interventional Cardiology). Prospective application of pre-defined intravascular ultrasound criteria for assessment of intermediate left main coronary artery lesions results from the multicenter LITRO study. J Am Coll Cardiol 2011;58:351–358. doi:10.1016/j.jacc.2011.02.064. [DOI] [PubMed] [Google Scholar]
- [47].Jasti V, Ivan E, Yalamanchili V, et al. Correlations between fractional flow reserve and intravascular ultrasound in patients with an ambiguous left main coronary artery stenosis. Circulation 2004;110:2831–2836. doi:10.1161/01.CIR.0000146338.62813.E7. [DOI] [PubMed] [Google Scholar]
- [48].Kang SJ, Lee JY, Ahn JM, et al. Intravascular ultrasound-derived predictors for fractional flow reserve in intermediate left main disease. JACC Cardiovasc Interv 2011;4:1168–1174. doi:10.1016/j.jcin.2011.08.009. [DOI] [PubMed] [Google Scholar]
- [49].Fassa AA, Wagatsuma K, Higano ST, et al. Intravascular ultrasound-guided treatment for angiographically indeterminate left main coronary artery disease: a long-term follow-up study. J Am Coll Cardiol 2005;45:204–211. doi:10.1016/j.jacc.2004.09.066. [DOI] [PubMed] [Google Scholar]
- [50].Waksman R, Legutko J, Singh J, et al. FIRST: fractional flow reserve and intravascular ultrasound relationship study. J Am Coll Cardiol 2013;61:917–923. doi:10.1016/j.jacc.2012.12.012. [DOI] [PubMed] [Google Scholar]
- [51].Koo BK, Yang HM, Doh JH, et al. Optimal intravascular ultrasound criteria and their accuracy for defining the functional significance of intermediate coronary stenoses of different locations. JACC Cardiovasc Interv 2011;4:803–811. doi:10.1016/j.jcin.2011.03.013. [DOI] [PubMed] [Google Scholar]
- [52].Nogic J, Prosser H, O’Brien J, et al. The assessment of intermediate coronary lesions using intracoronary imaging. Cardiovasc Diagn Ther 2020;10:1445–1460. doi:10.21037/cdt-20-226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [53].Gurgoglione FL, Denegri A, Russo M, et al. Intracoronary imaging of coronary atherosclerotic plaque: from assessment of pathophysiological mechanisms to therapeutic implication. Int J Mol Sci 2023;24:5155. doi:10.3390/ijms24065155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [54].Pedrigi RM, de Silva R, Bovens SM, et al. Thin-cap fibroatheroma rupture is associated with a fine interplay of shear and wall stress. Arterioscler Thromb Vasc Biol 2014;34:2224–2231. doi:10.1161/ATVBAHA.114.303426. [DOI] [PubMed] [Google Scholar]
- [55].Aguirre AD, Arbab-Zadeh A, Soeda T, et al. Optical coherence tomography of plaque vulnerability and rupture: JACC Focus Seminar Part 1/3. J Am Coll Cardiol 2021;78:1257–1265. doi:10.1016/j.jacc.2021.06.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [56].Fujii K, Kawasaki D, Masutani M, et al. OCT assessment of thin-cap fibroatheroma distribution in native coronary arteries. JACC Cardiovasc Imaging 2010;3:168–175. doi:10.1016/j.jcmg.2009.11.004. [DOI] [PubMed] [Google Scholar]
- [57].van der Hoeven BL, Liem SS, Dijkstra J, et al. Stent malapposition after sirolimus-eluting and bare-metal stent implantation in patients with ST-segment elevation myocardial infarction: acute and 9-month intravascular ultrasound results of the MISSION! intervention study. JACC Cardiovasc Interv 2008;1:192–201. doi:10.1016/j.jcin.2008.02.003. [DOI] [PubMed] [Google Scholar]
- [58].Im E, Kim BK, Ko YG, et al. Incidences, predictors, and clinical outcomes of acute and late stent malapposition detected by optical coherence tomography after drug-eluting stent implantation. Circ Cardiovasc Interv 2014;7:88–96. doi:10.1161/CIRCINTERVENTIONS.113.000797. [DOI] [PubMed] [Google Scholar]
- [59].Fujii K, Mintz GS, Kobayashi Y, et al. Contribution of stent underexpansion to recurrence after sirolimus-eluting stent implantation for in-stent restenosis. Circulation 2004;109:1085–1088. doi:10.1161/01.CIR.0000121327.67756.19. [DOI] [PubMed] [Google Scholar]
- [60].Im E, Hong SJ, Ahn CM, et al. Long-term clinical outcomes of late stent malapposition detected by optical coherence tomography after drug-eluting stent implantation. J Am Heart Assoc 2019;8:e011817. doi:10.1161/JAHA.118.011817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [61].Kawamori H, Shite J, Shinke T, et al. Natural consequence of post-intervention stent malapposition, thrombus, tissue prolapse, and dissection assessed by optical coherence tomography at mid-term follow-up. Eur Heart J Cardiovasc Imaging 2013;14:865–875. doi:10.1093/ehjci/jes299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [62].Lee B, Baraki TG, Kim BG, et al. Stent expansion evaluated by optical coherence tomography and subsequent outcomes. Sci Rep 2023;13:3781. doi:10.1038/s41598-023-30717-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [63].Koganti S, Kotecha T, Rakhit RD. Choice of intracoronary imaging: when to use intravascular ultrasound or optical coherence tomography. Interv Cardiol 2016;11:11–16. doi:10.15420/icr.2016:6:1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [64].Maehara A, Matsumura M, Ali ZA, et al. IVUS-guided versus OCT-guided coronary stent implantation: a critical appraisal. JACC Cardiovasc Imaging 2017;10:1487–1503. [DOI] [PubMed] [Google Scholar]
- [65].George BS, Voorhees WD, 3rd, Roubin GS, et al. Multicenter investigation of coronary stenting to treat acute or threatened closure after percutaneous transluminal coronary angioplasty: clinical and angiographic outcomes. J Am Coll Cardiol 1993;22:135–143. doi:10.1016/0735-1097(93)90827-n. [DOI] [PubMed] [Google Scholar]
- [66].Roubin GS, Cannon AD, Agrawal SK, et al. Intracoronary stenting for acute and threatened closure complicating percutaneous transluminal coronary angioplasty. Circulation 1992;85:916–927. doi:10.1161/01.cir.85.3.916. [DOI] [PubMed] [Google Scholar]
- [67].Schatz RA, Goldberg S, Leon M, et al. Clinical experience with the Palmaz-Schatz coronary stent. J Am Coll Cardiol 1991;17:155B–159B. doi:10.1016/0735-1097(91)90952-6. [DOI] [PubMed] [Google Scholar]
- [68].Fischman DL, Leon MB, Baim DS, et al. A randomized comparison of coronary-stent placement and balloon angioplasty in the treatment of coronary artery disease. Stent Restenosis Study Investigators. N Engl J Med 1994;331:496–501. doi:10.1056/NEJM199408253310802. [DOI] [PubMed] [Google Scholar]
- [69].Serruys PW, de Jaegere P, Kiemeneij F, et al. A comparison of balloon-expandable-stent implantation with balloon angioplasty in patients with coronary artery disease. Benestent Study Group. N Engl J Med 1994;331:489–495. doi:10.1056/NEJM199408253310801. [DOI] [PubMed] [Google Scholar]
- [70].Nakamura S, Colombo A, Gaglione A, et al. Intracoronary ultrasound observations during stent implantation. Circulation 1994;89:2026–2034. doi:10.1161/01.cir.89.5.2026. [DOI] [PubMed] [Google Scholar]
- [71].Kuntz RE, Gibson CM, Nobuyoshi M, et al. Generalized model of restenosis after conventional balloon angioplasty, stenting and directional atherectomy. J Am Coll Cardiol 1993;21:15–25. doi:10.1016/0735-1097(93)90712-a. [DOI] [PubMed] [Google Scholar]
- [72].Colombo A, Hall P, Nakamura S, et al. Intracoronary stenting without anticoagulation accomplished with intravascular ultrasound guidance. Circulation 1995;91:1676–1688. doi:10.1161/01.cir.91.6.1676. [DOI] [PubMed] [Google Scholar]
- [73].Gaster AL, Slothuus Skjoldborg U, Larsen J, et al. Continued improvement of clinical outcome and cost effectiveness following intravascular ultrasound guided PCI: insights from a prospective, randomised study. Heart 2003;89:1043–1049. doi:10.1136/heart.89.9.1043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [74].Gaster AL, Slothuus U, Larsen J, et al. Cost-effectiveness analysis of intravascular ultrasound guided percutaneous coronary intervention versus conventional percutaneous coronary intervention. Scand Cardiovasc J 2001;35:80–85. doi:10.1080/140174301750164673. [DOI] [PubMed] [Google Scholar]
- [75].Gil RJ, Pawłowski T, Dudek D, et al. Investigators of Direct Stenting vs Optimal Angioplasty Trial (DIPOL). Comparison of angiographically guided direct stenting technique with direct stenting and optimal balloon angioplasty guided with intravascular ultrasound The multicenter, randomized trial results. Am Heart J 2007;154:669–675. doi:10.1016/j.ahj.2007.06.017. [DOI] [PubMed] [Google Scholar]
- [76].Mudra H, di Mario C, de Jaegere P, et al. OPTICUS (OPTimization with ICUS to reduce stent restenosis) Study Investigators. Randomized comparison of coronary stent implantation under ultrasound or angiographic guidance to reduce stent restenosis (OPTICUS Study). Circulation 2001;104:1343–1349. doi:10.1161/hc3701.096064. [DOI] [PubMed] [Google Scholar]
- [77].Oemrawsingh PV, Mintz GS, Schalij MJ, et al. TULIP Study. Thrombocyte activity evaluation and effects of Ultrasound guidance in Long Intracoronary stent Placement. Intravascular ultrasound guidance improves angiographic and clinical outcome of stent implantation for long coronary artery stenoses: final results of a randomized comparison with angiographic guidance (TULIP Study). Circulation 2003;107:62–67. doi:10.1161/01.cir.0000043240.87526.3f. [DOI] [PubMed] [Google Scholar]
- [78].Russo RJ, Silva PD, Teirstein PS, et al. AVID Investigators. A randomized controlled trial of angiography versus intravascular ultrasound-directed bare-metal coronary stent placement (the AVID Trial). Circ Cardiovasc Interv 2009;2:113–123. doi:10.1161/CIRCINTERVENTIONS.108.778647. [DOI] [PubMed] [Google Scholar]
- [79].Schiele F, Meneveau N, Gilard M, et al. Balloon Equivalent to Stent Study. Intravascular ultrasound-guided balloon angioplasty compared with stent: immediate and 6-month results of the multicenter, randomized Balloon Equivalent to Stent Study (BEST). Circulation 2003;107:545–551. doi:10.1161/01.cir.0000047212.94399.7e. [DOI] [PubMed] [Google Scholar]
- [80].Schiele F, Meneveau N, Vuillemenot A, et al. Impact of intravascular ultrasound guidance in stent deployment on 6-month restenosis rate: a multicenter, randomized study comparing two strategies—with and without intravascular ultrasound guidance RESIST Study Group REStenosis after Ivus guided STenting. J Am Coll Cardiol 1998;32:320–328. doi:10.1016/s0735-1097(98)00249-6. [DOI] [PubMed] [Google Scholar]
- [81].Parise H, Maehara A, Stone GW, et al. Meta-analysis of randomized studies comparing intravascular ultrasound versus angiographic guidance of percutaneous coronary intervention in pre-drug-eluting stent era. Am J Cardiol 2011;107:374–382. doi:10.1016/j.amjcard.2010.09.030. [DOI] [PubMed] [Google Scholar]
- [82].Hoffmann R, Mintz GS, Dussaillant GR, et al. Patterns and mechanisms of in-stent restenosis. A serial intravascular ultrasound study. Circulation 1996;94:1247–1254. doi:10.1161/01.cir.94.6.1247. [DOI] [PubMed] [Google Scholar]
- [83].Mintz GS, Garcia-Garcia HM, Nicholls SJ, et al. Clinical expert consensus document on standards for acquisition, measurement and reporting of intravascular ultrasound regression/progression studies. EuroIntervention 2011;6:1123–1130, 9. doi:10.4244/EIJV6I9A195. [DOI] [PubMed] [Google Scholar]
- [84].Valgimigli M, Campo G, Percoco G, et al. Multicentre Evaluation of Single High-Dose Bolus Tirofiban vs Abciximab With Sirolimus-Eluting Stent or Bare Metal Stent in Acute Myocardial Infarction Study (MULTISTRATEGY) Investigators. Comparison of angioplasty with infusion of tirofiban or abciximab and with implantation of sirolimus-eluting or uncoated stents for acute myocardial infarction: the MULTISTRATEGY randomized trial. JAMA 2008;299:1788–1799. doi:10.1001/jama.299.15.joc80026. [DOI] [PubMed] [Google Scholar]
- [85].Valgimigli M, Percoco G, Malagutti P, et al. STRATEGY Investigators. Tirofiban and sirolimus-eluting stent vs abciximab and bare-metal stent for acute myocardial infarction: a randomized trial. JAMA 2005;293:2109–2117. doi:10.1001/jama.293.17.2109. [DOI] [PubMed] [Google Scholar]
- [86].Piccolo R, Bonaa KH, Efthimiou O, et al. Coronary Stent Trialists' Collaboration. Drug-eluting or bare-metal stents for percutaneous coronary intervention: a systematic review and individual patient data meta-analysis of randomised clinical trials. Lancet 2019;393:2503–2510. doi:10.1016/S0140-6736(19)30474-X. [DOI] [PubMed] [Google Scholar]
- [87].Witzenbichler B, Maehara A, Weisz G, et al. Relationship between intravascular ultrasound guidance and clinical outcomes after drug-eluting stents: the assessment of dual antiplatelet therapy with drug-eluting stents (ADAPT-DES) study. Circulation 2014;129:463–470. doi:10.1161/CIRCULATIONAHA.113.003942. [DOI] [PubMed] [Google Scholar]
- [88].Chieffo A, Latib A, Caussin C, et al. A prospective, randomized trial of intravascular-ultrasound guided compared to angiography guided stent implantation in complex coronary lesions: the AVIO trial. Am Heart J 2013;165:65–72. doi:10.1016/j.ahj.2012.09.017. [DOI] [PubMed] [Google Scholar]
- [89].Jakabcin J, Spacek R, Bystron M, et al. Long-term health outcome and mortality evaluation after invasive coronary treatment using drug eluting stents with or without the IVUS guidance. Randomized control trial HOME DES IVUS. Catheter Cardiovasc Interv 2010;75:578–583. doi:10.1002/ccd.22244. [DOI] [PubMed] [Google Scholar]
- [90].Kim JS, Kang TS, Mintz GS, et al. Randomized comparison of clinical outcomes between intravascular ultrasound and angiography-guided drug-eluting stent implantation for long coronary artery stenoses. JACC Cardiovasc Interv 2013;6:369–376. doi:10.1016/j.jcin.2012.11.009. [DOI] [PubMed] [Google Scholar]
- [91].Ahn JM, Kang SJ, Yoon SH, et al. Meta-analysis of outcomes after intravascular ultrasound-guided versus angiography-guided drug-eluting stent implantation in 26,503 patients enrolled in three randomized trials and 14 observational studies. Am J Cardiol 2014;113:1338–1347. doi:10.1016/j.amjcard.2013.12.043. [DOI] [PubMed] [Google Scholar]
- [92].Jang JS, Song YJ, Kang W, et al. Intravascular ultrasound-guided implantation of drug-eluting stents to improve outcome: a meta-analysis. JACC Cardiovasc Interv 2014;7:233–243. doi:10.1016/j.jcin.2013.09.013. [DOI] [PubMed] [Google Scholar]
- [93].Klersy C, Ferlini M, Raisaro A, et al. Use of IVUS guided coronary stenting with drug eluting stent: a systematic review and meta-analysis of randomized controlled clinical trials and high quality observational studies. Int J Cardiol 2013;170:54–63. doi:10.1016/j.ijcard.2013.10.002. [DOI] [PubMed] [Google Scholar]
- [94].Zhang Y, Farooq V, Garcia-Garcia HM, et al. Comparison of intravascular ultrasound versus angiography-guided drug-eluting stent implantation: a meta-analysis of one randomised trial and ten observational studies involving 19,619 patients. EuroIntervention 2012;8:855–865. doi:10.4244/EIJV8I7A129. [DOI] [PubMed] [Google Scholar]
- [95].Hong SJ, Kim BK, Shin DH, et al. IVUS-XPL Investigators. Effect of intravascular ultrasound-guided vs angiography-guided everolimus-eluting stent implantation: the IVUS-XPL randomized clinical trial. JAMA 2015;314:2155–2163. doi:10.1001/jama.2015.15454. [DOI] [PubMed] [Google Scholar]
- [96].Zhang J, Gao X, Kan J, et al. Intravascular ultrasound versus angiography-guided drug-eluting stent implantation: the ULTIMATE trial. J Am Coll Cardiol 2018;72:3126–3137. doi:10.1016/j.jacc.2018.09.013. [DOI] [PubMed] [Google Scholar]
- [97].Gao XF, Ge Z, Kong XQ, et al. ULTIMATE Investigators. 3-year outcomes of the ULTIMATE trial comparing intravascular ultrasound versus angiography-guided drug-eluting stent implantation. JACC Cardiovasc Interv 2021;14:247–257. doi:10.1016/j.jcin.2020.10.001. [DOI] [PubMed] [Google Scholar]
- [98].Farkouh ME, Domanski M, Sleeper LA, et al. FREEDOM Trial Investigators. Strategies for multivessel revascularization in patients with diabetes. N Engl J Med 2012;367:2375–2384. doi:10.1056/NEJMoa1211585. [DOI] [PubMed] [Google Scholar]
- [99].Serruys PW, Morice MC, Kappetein AP, et al. SYNTAX Investigators. Percutaneous coronary intervention versus coronary-artery bypass grafting for severe coronary artery disease. N Engl J Med 2009;360:961–972. doi:10.1056/NEJMoa0804626. [DOI] [PubMed] [Google Scholar]
- [100].Park SJ, Ahn JM, Kim YH, et al. BEST Trial Investigators. Trial of everolimus-eluting stents or bypass surgery for coronary disease. N Engl J Med 2015;372:1204–1212. doi:10.1056/NEJMoa1415447. [DOI] [PubMed] [Google Scholar]
- [101].Ahn JM, Kang DY, Yun SC, et al. BEST Extended Follow-Up Study Investigators. Everolimus-eluting stents or bypass surgery for multivessel coronary artery disease: extended follow-up outcomes of multicenter randomized controlled BEST trial. Circulation 2022;146:1581–1590. doi:10.1161/CIRCULATIONAHA.122.062188. [DOI] [PubMed] [Google Scholar]
- [102].Huang D, Swanson EA, Lin CP, et al. Optical coherence tomography. Science 1991;254:1178–1181. doi:10.1126/science.1957169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [103].Jang IK, Bouma BE, Kang DH, et al. Visualization of coronary atherosclerotic plaques in patients using optical coherence tomography: comparison with intravascular ultrasound. J Am Coll Cardiol 2002;39:604–609. doi:10.1016/s0735-1097(01)01799-5. [DOI] [PubMed] [Google Scholar]
- [104].Prati F, Cera M, Ramazzotti V, et al. Safety and feasibility of a new non-occlusive technique for facilitated intracoronary optical coherence tomography (OCT) acquisition in various clinical and anatomical scenarios. EuroIntervention 2007;3:365–370. doi:10.4244/eijv3i3a66. [DOI] [PubMed] [Google Scholar]
- [105].van der Sijde JN, Karanasos A, van Ditzhuijzen NS, et al. Safety of optical coherence tomography in daily practice: a comparison with intravascular ultrasound. Eur Heart J Cardiovasc Imaging 2017;18:467–474. doi:10.1093/ehjci/jew037. [DOI] [PubMed] [Google Scholar]
- [106].Yoon JH, Di Vito L, Moses JW, et al. Feasibility and safety of the second-generation, frequency domain optical coherence tomography (FD-OCT): a multicenter study. J Invasive Cardiol 2012;24:206–209. [PubMed] [Google Scholar]
- [107].Antonsen L, Thayssen P, Maehara A, et al. Optical coherence tomography guided percutaneous coronary intervention with nobori stent implantation in patients with non-ST-segment-elevation myocardial infarction (OCTACS) trial: difference in strut coverage and dynamic malapposition patterns at 6 months. Circ Cardiovasc Interv 2015;8:e002446. doi:10.1161/CIRCINTERVENTIONS.114.002446. [DOI] [PubMed] [Google Scholar]
- [108].Prati F, Di Vito L, Biondi-Zoccai G, et al. Angiography alone versus angiography plus optical coherence tomography to guide decision-making during percutaneous coronary intervention: the Centro per la Lotta contro l’Infarto-Optimisation of Percutaneous Coronary Intervention (CLI-OPCI) study. EuroIntervention 2012;8:823–829. doi:10.4244/EIJV8I7A125. [DOI] [PubMed] [Google Scholar]
- [109].Prati F, Romagnoli E, Burzotta F, et al. Clinical impact of OCT findings during PCI: the CLI-OPCI II study. JACC Cardiovasc Imaging 2015;8:1297–1305. doi:10.1016/j.jcmg.2015.08.013. [DOI] [PubMed] [Google Scholar]
- [110].Niccoli G, Montone RA, Di Vito L, et al. Plaque rupture and intact fibrous cap assessed by optical coherence tomography portend different outcomes in patients with acute coronary syndrome. Eur Heart J 2015;36:1377–1384. doi:10.1093/eurheartj/ehv029. [DOI] [PubMed] [Google Scholar]
- [111].Vergallo R, Ren X, Yonetsu T, et al. Pancoronary plaque vulnerability in patients with acute coronary syndrome and ruptured culprit plaque: a 3-vessel optical coherence tomography study. Am Heart J 2014;167:59–67. doi:10.1016/j.ahj.2013.10.011. [DOI] [PubMed] [Google Scholar]
- [112].Wijns W, Shite J, Jones MR, et al. Optical coherence tomography imaging during percutaneous coronary intervention impacts physician decision-making: ILUMIEN I study. Eur Heart J 2015;36:3346–3355. doi:10.1093/eurheartj/ehv367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [113].Porto I, Di Vito L, Burzotta F, et al. Predictors of periprocedural (type IVa) myocardial infarction, as assessed by frequency-domain optical coherence tomography. Circ Cardiovasc Interv 2012;5:89–96, S1. doi:10.1161/CIRCINTERVENTIONS.111.965624. [DOI] [PubMed] [Google Scholar]
- [114].Sawlani NN, Bhatt DL. How to decipher OCT after PCI. JACC Cardiovasc Imaging 2015;8:1306–1308. doi:10.1016/j.jcmg.2015.09.005. [DOI] [PubMed] [Google Scholar]
- [115].Waksman R, Didier R. Intracoronary imaging: see more, better or worse? Eur Heart J 2015;36:3356–3358. doi:10.1093/eurheartj/ehv433. [DOI] [PubMed] [Google Scholar]
- [116].Meneveau N, Souteyrand G, Motreff P, et al. Optical coherence tomography to optimize results of percutaneous coronary intervention in patients with non-ST-elevation acute coronary syndrome: results of the multicenter, randomized DOCTORS study (Does Optical Coherence Tomography Optimize Results of Stenting). Circulation 2016;134:906–917. doi:10.1161/CIRCULATIONAHA.116.024393. [DOI] [PubMed] [Google Scholar]
- [117].Holm NR, Andreasen LN, Neghabat O, et al. OCTOBER Trial Group. OCT or angiography guidance for PCI in complex bifurcation lesions. N Engl J Med 2023;389:1477–1487. doi:10.1056/NEJMoa2307770. [DOI] [PubMed] [Google Scholar]
- [118].Ali ZA, Landmesser U, Maehara A, et al. ILUMIEN IV Investigators. Optical coherence tomography-guided versus angiography-guided PCI. N Engl J Med 2023;389:1466–1476. doi:10.1056/NEJMoa2305861. [DOI] [PubMed] [Google Scholar]
- [119].Ali ZA, Maehara A, Généreux P, et al. ILUMIEN III: OPTIMIZE PCI Investigators. Optical coherence tomography compared with intravascular ultrasound and with angiography to guide coronary stent implantation (ILUMIEN III: OPTIMIZE PCI): a randomised controlled trial. Lancet 2016;388:2618–2628. doi:10.1016/S0140-6736(16)31922-5. [DOI] [PubMed] [Google Scholar]
- [120].Otake H, Kubo T, Takahashi H, et al. OPINION Investigators. Optical frequency domain imaging versus intravascular ultrasound in percutaneous coronary intervention (OPINION trial): results from the OPINION imaging study. JACC Cardiovasc Imaging 2018;11:111–123. doi:10.1016/j.jcmg.2017.06.021. [DOI] [PubMed] [Google Scholar]
- [121].Kang DY, Ahn JM, Yun SC, et al. OCTIVUS Investigators. Optical coherence tomography-guided or intravascular ultrasound-guided percutaneous coronary intervention: the OCTIVUS randomized clinical trial. Circulation 2023;148:1195–1206. doi:10.1161/CIRCULATIONAHA.123.066429. [DOI] [PubMed] [Google Scholar]
- [122].Lee JM, Choi KH, Song YB, et al. RENOVATE-COMPLEX-PCI Investigators. Intravascular imaging-guided or angiography-guided complex PCI. N Engl J Med 2023;388:1668–1679. doi:10.1056/NEJMoa2216607. [DOI] [PubMed] [Google Scholar]
- [123].Shariff M, Kumar A, Kansara T, et al. Network meta-analysis of trials comparing intravascular ultrasound, optical coherence tomography, and angiography-guided technique for drug-eluting stent implantation. J Soc Cardiovasc Angiography Interv 2022;1:100507. doi:10.1016/j.jscai.2022.100507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [124].Petousis S, Skalidis E, Zacharis E, et al. The role of intracoronary imaging for the management of calcified lesions. J Clin Med 2023;12:4622. doi:10.3390/jcm12144622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [125].De Maria GL, Scarsini R, Banning AP. Management of calcific coronary artery lesions: is it time to change our interventional therapeutic approach. JACC Cardiovasc Interv 2019;12:1465–1478. doi:10.1016/j.jcin.2019.03.038. [DOI] [PubMed] [Google Scholar]
- [126].Barbato E, Gallinoro E, Abdel-Wahab M, et al. Management strategies for heavily calcified coronary stenoses: an EAPCI clinical consensus statement in collaboration with the EURO4C-PCR group. Eur Heart J 2023;44:4340–4356. doi:10.1093/eurheartj/ehad342. [DOI] [PubMed] [Google Scholar]
- [127].Riley RF, Henry TD, Mahmud E, et al. SCAI position statement on optimal percutaneous coronary interventional therapy for complex coronary artery disease. Catheter Cardiovasc Interv 2020;96:346–362. doi:10.1002/ccd.28994. [DOI] [PubMed] [Google Scholar]
- [128].Fujino A, Mintz GS, Matsumura M, et al. A new optical coherence tomography-based calcium scoring system to predict stent underexpansion. EuroIntervention 2018;13:e2182–e2189. doi:10.4244/EIJ-D-17-00962. [DOI] [PubMed] [Google Scholar]
- [129].Zhang M, Matsumura M, Usui E, et al. Intravascular ultrasound-derived calcium score to predict stent expansion in severely calcified lesions. Circ Cardiovasc Interv 2021;14:e010296. doi:10.1161/CIRCINTERVENTIONS.120.010296. [DOI] [PubMed] [Google Scholar]
- [130].Baber U, Kini AS, Sharma SK. Stenting of complex lesions: an overview. Nat Rev Cardiol 2010;7:485–496. doi:10.1038/nrcardio.2010.116. [DOI] [PubMed] [Google Scholar]
- [131].Mohamed MO, Polad J, Hildick-Smith D, et al. Impact of coronary lesion complexity in percutaneous coronary intervention: one-year outcomes from the large, multicentre e-Ultimaster registry. EuroIntervention 2020;16:603–612. doi:10.4244/EIJ-D-20-00361. [DOI] [PubMed] [Google Scholar]
- [132].Ninomiya K, Serruys PW, Garg S, et al. SYNTAX Extended Survival Investigators. Predicted and observed mortality at 10 years in patients with bifurcation lesions in the SYNTAX trial. JACC Cardiovasc Interv 2022;15:1231–1242. doi:10.1016/j.jcin.2022.04.025. [DOI] [PubMed] [Google Scholar]
- [133].Xenogiannis I, Pavlidis AN, Kaier TE, et al. The role of intravascular imaging in chronic total occlusion percutaneous coronary intervention. Front Cardiovasc Med 2023;10:1199067. doi:10.3389/fcvm.2023.1199067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [134].Karacsonyi J, Kostantinis S, Simsek B, et al. Intravascular imaging use in percutaneous coronary interventions of chronic total occlusions. J Invasive Cardiol 2023;35:E265–E268. [DOI] [PubMed] [Google Scholar]
- [135].Hong D, Kim SM, Lee SY, et al. RENOVATE-COMPLEX-PCI Investigators. Prognostic impact of intravascular imaging-guided percutaneous coronary intervention in chronic total occlusion. Circulation 2023;148:903–905. doi:10.1161/CIRCULATIONAHA.123.065876. [DOI] [PubMed] [Google Scholar]
- [136].Karacsonyi J, Alaswad K, Jaffer FA, et al. Use of intravascular imaging during chronic total occlusion percutaneous coronary intervention: insights from a contemporary multicenter registry. J Am Heart Assoc 2016;5:e003890. doi:10.1161/JAHA.116.003890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [137].Park DY, Hu JR, Kanitsoraphan C, et al. Trends and inhospital outcomes of intravascular imaging on single-vessel coronary chronic total occlusion treated with percutaneous coronary intervention. Am J Cardiol 2023;206:79–85. doi:10.1016/j.amjcard.2023.08.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [138].Ono M, Kawashima H, Hara H, et al. Advances in IVUS/OCT and future clinical perspective of novel hybrid catheter system in coronary imaging. Front Cardiovasc Med 2020;7:119. doi:10.3389/fcvm.2020.00119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [139].Terashima M, Kaneda H, Honda Y, et al. Current status of hybrid intravascular ultrasound and optical coherence tomography catheter for coronary imaging and percutaneous coronary intervention. J Cardiol 2021;77:435–443. doi:10.1016/j.jjcc.2020.08.012. [DOI] [PubMed] [Google Scholar]
- [140].Carpenter HJ, Ghayesh MH, Zander AC, et al. Automated coronary optical coherence tomography feature extraction with application to three-dimensional reconstruction. Tomography 2022;8:1307–1349. doi:10.3390/tomography8030108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [141].Lv R, Maehara A, Matsumura M, et al. Using optical coherence tomography and intravascular ultrasound imaging to quantify coronary plaque cap thickness and vulnerability: a pilot study. Biomed Eng Online 2020;19:90. doi:10.1186/s12938-020-00832-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [142].Moon IT, Kim SH, Chin JY, et al. Accuracy of artificial intelligence-based automated quantitative coronary angiography compared to intravascular ultrasound: retrospective cohort study. JMIR Cardio 2023;7:e45299. doi:10.2196/45299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [143].Elgendy IY, Ha LD, Elbadawi A, et al. Temporal trends in inpatient use of intravascular imaging among patients undergoing percutaneous coronary intervention in the United States. JACC Cardiovasc Interv 2018;11:913–915. doi:10.1016/j.jcin.2018.01.254. [DOI] [PubMed] [Google Scholar]
- [144].Park DY, Vemmou E, An S, et al. Trends and impact of intravascular ultrasound and optical coherence tomography on percutaneous coronary intervention for myocardial infarction. Int J Cardiol Heart Vasc 2023;45:101186. doi:10.1016/j.ijcha.2023.101186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [145].Mentias A, Sarrazin MV, Saad M, et al. Long-term outcomes of coronary stenting with and without use of intravascular ultrasound. JACC Cardiovasc Interv 2020;13:1880–1890. doi:10.1016/j.jcin.2020.04.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [146].Malik AS, Spertus J, Salisbury A, et al. Hospital-level variability in use of intracoronary imaging for percutaneous coronary intervention in the United States. JSCAI 2023;2:100973. doi:10.1016/j.jscai.2023.100973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [147].Kuno T, Numasawa Y, Sawano M, et al. Real-world use of intravascular ultrasound in Japan: a report from contemporary multicenter PCI registry. Heart Vessels 2019;34:1728–1739. doi:10.1007/s00380-019-01427-9. [DOI] [PubMed] [Google Scholar]
- [148].Koskinas KC, Nakamura M, Räber L, et al. Current use of intracoronary imaging in interventional practice—results of a European Association of Percutaneous Cardiovascular Interventions (EAPCI) and Japanese Association of Cardiovascular Interventions and Therapeutics (CVIT) Clinical Practice Survey. EuroIntervention 2018;14:e475–e484. doi:10.4244/EIJY18M03_01. [DOI] [PubMed] [Google Scholar]
- [149].Shah KB, Cohen DJ. Why is intravascular ultrasound guidance underutilized in percutaneous coronary intervention?: it is not “All About the Benjamins”. Circ Cardiovasc Qual Outcomes 2021;14:e007844. doi:10.1161/CIRCOUTCOMES.121.007844. [DOI] [PubMed] [Google Scholar]
- [150].Flattery E, Rahim HM, Petrossian G, et al. Competency-based assessment of interventional cardiology fellows’ abilities in intracoronary physiology and imaging. Circ Cardiovasc Interv 2020;13:e008760. doi:10.1161/CIRCINTERVENTIONS.119.008760. [DOI] [PubMed] [Google Scholar]