Abstract
Iron is an essential micronutrient for all types of organisms; however, iron has chemical properties that can be harmful to cells. Because iron is both necessary and potentially damaging, insects have homeostatic processes that control the redox state, quantity, and location of iron in the body. These processes include uptake of iron from the diet, intracellular and extracellular iron transport, and iron storage. Early studies of iron-binding proteins in insects suggested that insects and mammals have surprisingly different mechanisms of iron homeostasis, including different primary mechanisms for exporting iron from cells and for transporting iron from one cell to another, and subsequent studies have continued to support this view. This review summarizes current knowledge about iron homeostasis in insects, compares insect and mammalian iron homeostasis mechanisms, and calls attention to key remaining knowledge gaps.
Keywords: insect, iron, heme, ferritin, transferrin, metal transporter
1. OVERVIEW OF IRON IN BIOLOGICAL SYSTEMS
Iron is an essential micronutrient for all types of organisms, mainly because iron functions as a cofactor for many indispensable enzymes (30). In insects, iron is a cofactor for enzymes involved in cellular respiration, DNA synthesis, detoxification of pesticides and plant defense compounds, neural function, and many other physiological processes (13, 81, 91, 102, 106). Some types of insects use iron for geomagnetic orientation (52, 104). A significant difference in the iron requirements between insects and mammals is in the proportion of iron bound to hemoglobin. Most of the iron in mammals is bound to hemoglobin and functions in oxygen transport; in contrast, insects rely on a tracheal system for oxygen delivery, and, thus, have much less hemoglobin-bound iron (14, 30).
Iron in biological systems is present in either the reduced ferrous (Fe2+) form or oxidized ferric (Fe3+) form, and cycling between the two oxidation states is an important aspect of iron homeostasis (62). The need for redox cycling is related to the different properties of the two forms of iron: ferrous iron is soluble under most physiological conditions, but it participates in toxic radical formation; ferric iron is less toxic but is insoluble at physiological pH (62). Iron in biological systems is typically in a protein-bound state that prevents iron precipitation and harmful chemical reactions (30, 63).
Iron homeostasis is better understood in mammals than in insects, thus, information about mammalian iron homeostasis informs iron-related studies in insects (81, 114). However, early studies of insect iron-binding proteins suggested that insects and mammals have quite different mechanisms of iron homeostasis, and subsequent studies continue to support this view (81, 99, 114, 134, 144). This review will highlight similarities and differences (Figure 1).
Figure 1. Iron transport in mammals and insects.
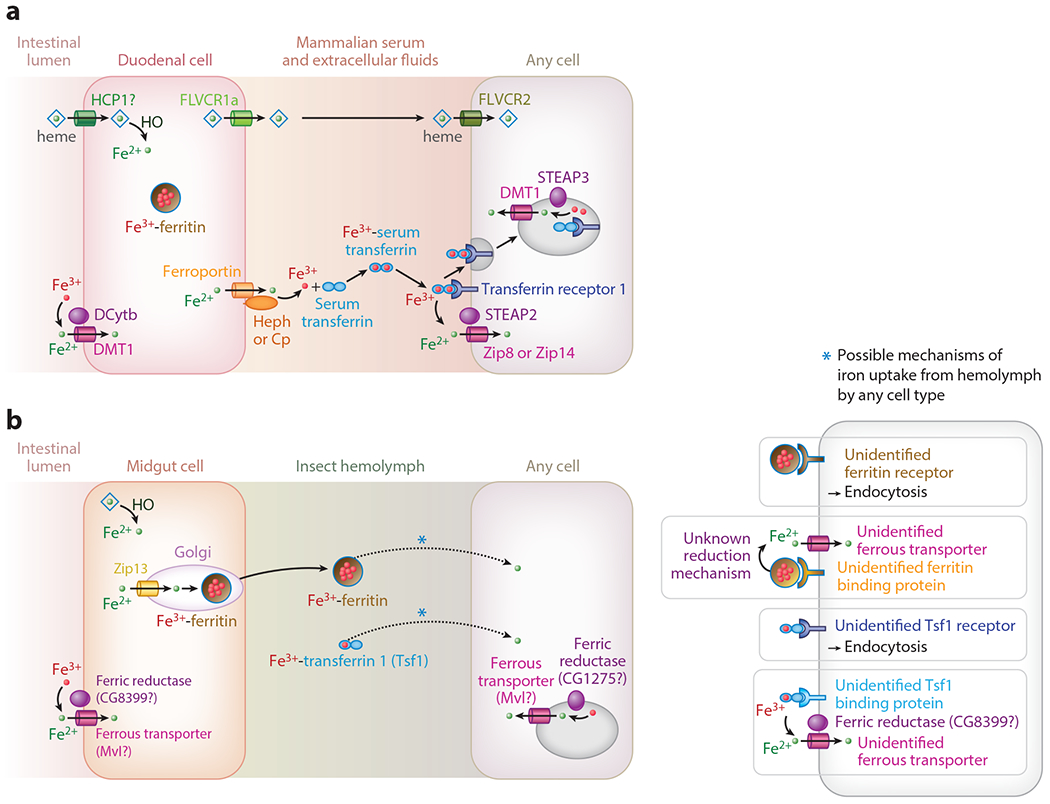
Some key aspects of iron transport in mammals (A) and insects (B) are shown. Abbreviations: Cp, ceruloplasmin; DCytb, duodenal cytochrome b; DMT1, divalent metal transporter 1; FLVCR, feline leukemia virus subgroup C receptor; HCP1, heme carrier protein 1; HO, Heme Oxygenase; Heph, hephaestin; Mvl, Malvolio, Nemy, No extended memory; STEAP, six-transmembrane epithelial antigen of the prostate; Tsf1, Tranferrin 1; Zip, Zinc-regulated, iron-regulated transporter-like protein. Not shown: PCBP, Poly(rC)-binding protein; ZnT, Zinc Transporter.
2. BRIEF SUMMARY OF IRON HOMEOSTASIS IN MAMMALS
The first step in mammalian iron homeostasis is the uptake of heme (an iron-containing porphyrin) and non-heme iron from the diet (Figure 1) (41). Uptake of non-heme iron by intestinal absorptive cells (enterocytes) is better understood than heme uptake, and it occurs in the acidic first section of the small intestine (duodenum) (18, 60). Non-heme iron in the diet is predominantly in the ferric form, and it needs to be reduced before it can be transported across the plasma membrane of an enterocyte (60). A ferric reductase that catalyzes this step is duodenal cytochrome b (DCytb), but at least one other ferric reductase or ferric reduction mechanism is likely to contribute to non-heme iron uptake (18, 30, 70). The resulting ferrous ions are transported across the duodenal cell membrane by the proton symporter divalent metal transporter 1 (DMT1), while the proton gradient needed for ferrous transport is likely to be provided by Na+/H+ exchanger-3 and a gastric H+/K+ ATPase that acidifies the stomach (67, 109, 142). How intestinal cells take up heme from the diet is still uncertain (18, 41). A proton-coupled folate transporter, also known as heme carrier protein 1 (HCP1), appears to be involved, but additional research is needed to fully understand the role of this protein in heme uptake (18, 41, 74).
Once iron is transported into a cell, is can be used, stored, or exported (Figure 1). Heme can remain intact or be degraded by heme oxygenase (HO) to release ferrous ions (23). Ferrous ions transported into the cell via DMT1 or released from heme may be bound by a member of the Poly(rC)-binding protein (PCBP) family, and the PCBPs donate iron to enzymes that require iron as a cofactor, deliver iron to ferritin for storage, and traffic iron to the basal side of the cell for iron export (143). Iron is also delivered to mitochondria, where iron is needed for enzyme metalation, heme synthesis, and iron-sulfur cluster biosynthesis (23, 60, 118). In mammals, iron is stored inside cytosolic ferritin (3). As ferrous ions enter ferritin, they become oxidized, and the resulting ferric ions (up to ~4,000) are stored within the ferritin cavity as ferrihydrite (3, 9).
The best understood iron export mechanism involves efflux of ferrous ions (Figure 1), although iron can also be exported as heme or as ferritin-bound iron. Ferrous ions are exported from enterocytes and other mammalian cells by the ferrous permease ferroportin (24). Once outside the cell, the ferrous ions are oxidized by an extracellular multicopper ferroxidase (e.g., membrane-bound hephaestin (Heph) or soluble ceruloplasmin (Cp)) (49, 98). The resulting ferric ions are bound by serum transferrin, an extracellular protein that binds two ferric ions with high affinity (37). Serum transferrin then traffics iron to other cells (37). Export mechanisms for heme and ferrtin are not well characterized. Only one heme exporter, FLVCR1a, has been studied in detail (16, 23). Mammalian ferritin, although primarily a cytosolic protein, is also released from cells and is then referred to as serum ferritin (127).
Uptake of iron by cells other than enterocytes can occur via multiple pathways (Figure 1). Iron uptake from Fe3+-transferrin mainly occurs via two pathways, both involving transferrin receptor 1 (2, 65). The most widely known mechanism is receptor-mediated endocytic uptake of Fe3+-transferrin. In the acidified endosome, iron is released from serum transferrin, reduced by a ferric reductase (e.g., Six-transmembrane epithelial antigen of the prostate 3 (STEAP3)), and transported across the endosome membrane through a ferrous transporter (e.g., DMT1) (30). A less recognized mechanism for the uptake of transferrin-bound iron involves a conformational change of the receptor-bound transferrin that allows reduction of ferric ions (e.g., by STEAP2) and transport into the cell through a ferrous transporter (e.g., Zinc-regulated, iron-regulated transporter-like protein 8 or 14 (Zip8 or Zip14) (65). Uptake of heme by non-enterocytes is not well understood but is thought to involve the transporters HCP1 and feline leukemia virus subgroup C receptor 2 (FLVCR2) (16, 23). Endocytic uptake of serum ferritin can be mediated by one of several multifunctional receptors (127).
In mammals, the amount of iron in the body is regulated primarily by controlling the export of iron from enterocytes through a process regulated by the hormone hepcidin (18). Hepcidin expression increases under iron replete conditions, which results in the ubiquitination and degradation of ferroportin, leading to less iron efflux (18, 30). The iron content of individual cells is controlled by many mechanisms that affect iron uptake and export (30).
3. UPTAKE OF IRON FROM THE DIET IN INSECTS
Insect diets vary in the quantity and form of iron; for example, herbivores that feed on the leaves of woody plants have a diet containing ~220 - 2,100 μmol L−1 iron, presumably in the form of non-heme iron, whereas hematophagous insects that feed on mammalian blood have a diet containing approximately 10 mmol L−1 iron, mainly in the form of heme bound to hemoglobin (6, 50, 148). Given the diversity of insect diets, it seems likely that significant differences in iron homeostasis exist among types of insects. In fact, multiple mechanisms for handling the large concentration of heme in a blood meal have evolved in hematophagous insects (113, 134). In addition, among various types of insects, a number of differences in other aspects of iron homeostasis have been identified (described in sections below).
The non-heme iron in insect diets is expected to be predominantly in the ferric form, but all known iron transporters in animals are specific for ferrous ions (61, 64); therefore, ferric ions in the midgut lumen must be reduced by some mechanism prior to uptake. How this process occurs in insects is still unknown. A member of the Cytochrome b561 (Cytb561) family of proteins, DCytb, catalyzes ferric reduction in the apical membrane of mammalian enterocytes (Figure 1) (70). Three conserved Cytb561s in insects have been considered candidate ferric reductases: two, named CG1275 and No extended memory (Nemy) in Drosophila melanogaster, are similar to DCytb, and a third, CG8399 in D. melanogaster, is more distantly related (73, 81, 101, 114, 121, 122, 135). Of the three, only CG8399 is known to have ferric reductase activity (101, 121). CG8399 is located in the plasma membrane and is expressed in the midgut, making CG8399 a promising candidate for the role of iron uptake from the midgut lumen (17, 58). CG1275 is expressed in the midgut, but may be present in endosomal and lysosomal membranes rather than the plasma membrane; therefore, CG1275 is more likely to function in the endocytic pathway than in the apical membrane of midgut cells (73). Nemy does not appear to have midgut expression (17).
Ferrous ions that have been generated in the midgut lumen could be transported into midgut cells through a ferrous transporter. Because different ferrous transporters function at either acidic pH or at neutral pH (65), it is likely that the pH of an insect’s digestive system will specify what type of iron transporter is functional in the midgut. Midgut lumenal pH differs among insects and is correlated with insect phylogeny (116). Cyclorrhaphous dipterans, including D. melanogaster, have a specialized, acidic midgut region that is involved in uptake of iron from the diet and is, thus, referred to as the iron region of the midgut (86, 88, 130, 131). The digestive systems of some orthopteran and hemipteran insects may also have restricted regions of iron uptake, although they are not necessarily restricted to acidic areas (131).
Ferrous ion uptake by mammalian enterocytes occurs via DMT1; therefore, an insect homolog of DMT1, Malvolio (Mvl), is predicted to have a similar role in insects (Figure 1) (81, 114). Because these types of transporters are proton symporters, Mvl is predicted to function in low pH environments such as acidic regions of the midgut and in late endosomes and lysosomes (65). Most insect species have one or two Mvl homologs, although Mvl is lacking in culicine mosquitoes (84, 120, 125). Several lines of evidence indicate that Mvl is involved in iron uptake. Anopheles albimanus Mvl was shown to enhance iron uptake by Xenopus laevis oocytes (83). D. melanogaster Mvl is expressed in the acidic region of the midgut where iron uptake occurs, although its presence in the plasma membrane has not yet been verified (12). Recombinant D. melanogaster Mvl was targeted to the plasma membrane and to intracellular sites in cultured cells, but endogenous Mvl was detected only in intracellular locations in the anterior and posterior midgut (29, 111). D. melanogaster Mvl loss of function mutants had less iron in the iron region of the midgut, and this phenotype was not rescued by dietary iron supplementation, indicating that Mvl is important for iron uptake in the iron region (8). Mvl mutants also had phenotypes (low whole body iron content and an abnormal taste preference) that were rescued by iron supplementation, suggesting that iron uptake in the iron region of the midgut is not the only role for Mvl in iron homeostasis (8, 96).
Although Mvl seems likely to transport ferrous ions from the diet in cyclorrhaphous dipterans, a different transporter must function in insects that lack an acidic midgut section. In addition, the well-documented uptake of iron by the anterior midgut of D. melanogaster is expected to involve a transporter that functions at neutral pH (8, 88, 108). The two well-characterized mammalian ferrous importers that function at neutral pH are Zip8 and Zip14 (61). Although Zip family members are present in insects, orthologs of Zip8 and Zip14 have not been identified (103, 120, 125). RNA-seq-based and RNAi-based screens for iron transporters in Aedes aegypti identified candidate iron importers, including Zinc transporter 7 (ZnT7) and Dyspepsia (119, 120). ZnT7, which transports zinc into the Golgi in mammalian and D. melanogaster cells, is expressed in the A. aegypti midgut, and knock down in cultured cells resulted in decreased ferritin-reporter expression, suggesting a role in iron uptake (46, 78, 103, 120). Dyspepsia is a member of the SLC16 family of transporters, which are not known to be iron transporters (43); however, knock down of Dyspepsia in A. aegypti cultured cells resulted in decreased ferritin-reporter expression, suggesting a role in iron uptake (119). Additional studies of ZnT7, Dyspepsia, and other candidate iron transporters will clarify their roles in iron homeostasis.
Heme uptake by the insect midgut has been studied mostly in blood feeding insects, which consume large quantities of heme. In A. aegypti, greater than 95% of the iron transported from a blood meal to various tissues originates from hemoglobin (148). An insect heme transporter has not been definitively identified. The insect ortholog of mammalian HCP1 (CG30345 in D. melanogaster) is expressed in the midgut, but a role in iron homeostasis has not been established (17, 18, 81). An RNAi-based screen, informed by gene expression analyses, identified four candidate heme importers in A. aegypti (AAEL000417, AAEL003318, AAEL004513 and AAEL012440); future studies will resolve their possible role in heme uptake (27). A midgut-associated heme transporter has been identified in ticks, although this protein transports heme not through the apical membrane but through the membrane surrounding digestion vesicles (72). The insect ortholog of this protein, ABCB10, is predicted to be a mitochondrial membrane protein and has not been implicated in heme transport (27, 72, 73, 134). Insects also have an uncharacterized homolog of mammalian heme importer FLVCR2 (CG1358 in D. melanogaster) (73).
4. INTRACELLULAR IRON IN INSECTS
Some of the heme that is transported into cells is degraded by heme oxygenase (HO), a conserved, ubiquitously expressed enzyme that is localized to the endomembrane system and positioned to cleave heme in the cytosol (Figure 1) (39). D. melanogaster and A. gambiae HOs have been biochemically characterized (112, 146). Like mammalian HOs, they cleave heme, leading to the release of a ferrous ion (112, 146). The affinities of A. gambiae HO and human HO for heme are similar (112). The affinity of D. melanogaster HO for heme is somewhat less, and the active site is thought to have structural differences compared with mammalian and other insect HOs (146). Ubiquitous knock down of D. melanogaster HO resulted in larval and pupal lethality, demonstrating that it is an essential gene, and knock down in eye discs resulted in iron accumulation and apoptosis in the adult eye (19, 56). Chemical inhibition or RNAi-mediated knock down of HO in A. gambiae and Rhodnius prolixus females caused a decrease in fecundity, likely due to a disruption of iron homeostasis or possibly heme toxicity in the ovaries (112, 125).
In mammalian cells, some of the ferrous ions that are transported directly into cells or released from heme are bound by chaperones (e.g., PCBPs) and shuttled to various subcellular destinations (100). No iron chaperones have been identified in insects, but a PCBP family member has been identified in D. melanogaster and other insect species (73, 136). The D. melanogaster protein, named Mushroom-body expressed (Mub), influences circadian rhythm in flies, as does PCBP1 in cultured mammalian cells (136). It is not known if the circadian rhythm phenotypes are related to changes in iron homeostasis; disruptions in iron homeostasis can affect circadian rhythms in D. melanogaster, but PCBP proteins have non-iron-related functions (73, 80, 100, 136).
In contrast to mammalian ferritin, which is mainly a cytosolic storage protein, insect ferritin is present in the secretory pathway (Figure 1) (93, 99). In mammals, PCBP-type iron chaperones deliver iron to ferritin in the cytosol, but these chaperones are not expected to cross the membranes of the secretory pathway (100); instead, delivery of iron to insect ferritin is mediated by a ferrous transporter, Zip13, located in the endoplasmic reticulum (ER) and Golgi (138). Phylogenetic analyses have shown that insect Zip13 proteins are orthologous to human Zip13, which functions as a zinc importer (although it may also export iron) (120, 125, 134, 138, 139). Biochemical and genetic evidence that Zip13 exports iron in D. melanogaster and A. aegypti is very convincing (120, 138–140, 147). Biochemical analyses of D. melanogaster Zip13 (Zip99C) demonstrated that it transports radioactive iron, has a DNXXH motif in the fourth transmembrane domain that is essential for iron specificity, and is stabilized by iron through interactions with the amino-domain (138, 140, 147). Ubiquitous knock down of Zip13 in D. melanogaster resulted in less iron in the ER/Golgi and in whole bodies, and midgut-specific knock down resulted in increased activity of a cytosolic iron sensor, aconitase, (indicating iron accumulation) and a decrease in the amount of ferritin-bound iron in the midgut cells; overexpression produced opposite outcomes (138). In A. aegypti, Zip13 is upregulated after a blood meal, particularly in the midgut (120). Knock down in adult A. aegypti females resulted in accumulation of iron in the midgut and decreased iron in the ovaries, suggesting that iron export from midgut cells was hindered by the lack of Zip13; knock down in cultured cells resulted in increased expression of an intracellular iron reporter (120).
Ferritin is the best-studied iron-binding protein in insects, and previous reviews describe the pioneering research on ferritin from many insect species (79, 92, 99, 114). Expression of insect ferritin is ubiquitous (99). Like mammalian ferritin, insect ferritin oxidizes ferrous ions, and the resulting ferric ions (up to ~3,000 ferric ions per molecule of ferritin) are stored as ferrihydrite within the large ferritin shell (3, 15, 123). The 24 ferritin subunits are encoded by two genes, Fer1HCH and Fer2LCH, and the two subunits are often, but not always, present in a 1:1 ratio (3, 42, 44, 94, 99). The Fer1HCH subunit oxidizes ferrous ions, while the Fer2LCH is thought to facilitate the formation of ferrihydrite (3, 44, 88). A. aegypti is unusual in having two Fer2LCH genes, each with a different expression pattern but with unknown functional differences (33, 34). Unlike mammalian ferritin subunits, insect Fer1HCH and Fer2LCH are synthesized with a signal peptide that targets the subunits to the secretory pathway (26, 114). The process of ferritin assembly within the secretory pathway is still not well understood, but it is likely that assembly starts with the formation of heterodimers in the ER, and it is known that temporal regulation of expression is important (44, 105).
Some iron-loaded (holo) ferritin in the secretory pathway is exported into the hemolymph, but some is stored in the Golgi and other membrane-bound compartments (92, 93, 99). Evidence for storage of holo-ferritin in midgut cells is particularly convincing (88, 92). The addition of iron to cultured A. gambiae 4a3b cells resulted in an increase in the amount of ferritin in the Golgi and no detectable ferritin secretion, indicating that the 4a3b cells were storing holo-ferritin in the Golgi (31). In addition, cultured 4a3b and A. aegypti CCL-125 cells were found to have ferritin in punctate vesicles that lacked organelle markers, suggesting a storage form of ferritin (31). Intracellular iron storage is most obvious in xylem-feeding homopterans that have cytosolic and nuclear ferritin (92). The mechanism by which iron is released from ferritin, such that it becomes available to the cell, is not known, but degradation in the lysosome seems likely, since insect ferritin has been observed in lysosomes, and a lysosome-based mechanism of iron release occurs in mammalian cells (31, 76).
A key destination for intracellular iron is the mitochondria, where iron is needed as an enzyme cofactor, and for heme and iron-sulfur cluster synthesis (21). Compared with many aspects of iron homeostasis, the transport and storage of iron in mitochondria appear to be similar between mammals and insects (82, 114). For example, insect mitoferrin and its mammalian homologs transport ferrous ions to the mitochondrial matrix (85, 126). Likewise, insect mitochondrial ferritin (Fer3HCH in D. melanogaster) and mammalian mitochondrial ferritin are both expressed primarily in the testis and appear to have similar iron storage functions (25, 87, 126).
5. IRON EXPORT IN INSECTS
Whereas the primary iron export mechanism for mammalian cells involves the ferrous transporter ferroportin (Figure 1), insects lack an identifiable ferroportin homolog (2, 81, 114). An RNAi-based screen of predicted metal transporters from A. aegypti identified two possible iron exporters: AAEL014762 (Zip13, described in Section 4), which exports iron to the secretory pathway, and AAEL013490, which is orthologous to mammalian Zip11 and insect Zip48C (73, 120, 145). Mammalian Zip11 and D. melanogaster Zip48C influence zinc homeostasis, but their possible roles in iron homeostasis have not yet been investigated (20, 145). Additional research is needed to determine whether AAEL013490 functions as a ferrous exporter in vivo.
In mammals, ferrous ions that are exported through ferroportin are oxidized by one of two ferroxidases in the multicopper oxidase family, hephaestin and ceruloplasmin (Figure 1) (2). Whether insects have a ferroxidase that functions similarly to hephaestin and ceruloplasmin has not been fully resolved. Insects have two conserved multicopper oxidases: MCO1 and MCO2 (22). MCO2 functions as a laccase (22, 71, 97). MCO1 was thought to be a ferroxidase because MCO1 from D. melanogaster has detectable ferroxidase activity, and knock down interfered with iron homeostasis (71). Likewise, MCO1 knock down in A. gambiae and Helicoverpa armigera affected iron content of whole bodies or Malpighian tubules, respectively (77, 97). Like hephaestin, the dipteran MCO1s were detected on the basal surface of tissues (71, 97). However, an analysis of the kinetic properties of MCO1 from D. melanogaster, A. gambiae, Manduca sexta and Tribolium castaneum, using various substrates, demonstrated that MCO1 is unlikely to function as a ferroxidase in vivo (97). When assayed for ferroxidase activity, the enzymes had a high Km relative to the expected concentration of ferrous ions in hemolymph; therefore, the affinity of MCO1 for iron is apparently inadequate for MCO1 to function as a ferroxidase in vivo (97). The catalytic efficiency of the MCO1s as ascorbate oxidases was much higher than their catalytic efficiency as ferroxidases, and Km values were similar to the concentration of ascorbate in hemolymph (66, 97). Taken together, studies of MCO1 suggest that it functions as an ascorbate oxidase rather than a ferroxidase, and that MCO1 influences iron homeostasis through an unknown mechanism involving ascorbate (97). Cyclorrhaphous dipterans have an additional multicopper oxidase, Mco3 (not orthologous to mosquito MCO3) (8, 22, 73, 128). D. melanogaster Mco3 has significant ferroxidase activity, and, like ceruloplasmin, it is a secreted, soluble protein (128). Expression is mainly restricted to pupae (40, 128). Mco3 null phenotypes include increased iron content of the whole body, head, and gut (8, 128). If the affinity of Mco3 for ferrous ions is found to be physiologically pertinent, Mco3 most likely oxidizes ferrous ions in pupal hemolymph.
Iron export from insect cells is primarily accomplished through the secretion of holo-ferritin (Figure 1) (81, 99, 114). The concentration of ferritin in M. sexta and Musca domestica hemolymph is ~100 - 500 nmol L−1, which is hundreds of times higher than a typical concentration of serum ferritin in humans (15, 53). Iron export via holo-ferritin secretion has been carefully documented for A. aegypti CCL-125 cells (31, 32, 36). In D. melanogaster, cells with insufficient ferritin accumulate an abnormal amount of iron, resulting in cellular damage consistent with an excess of iron (89, 114). Studies of ferritin mutants and RNAi-mediated knockdown phenotypes in diverse insect species have shown that ferritin is an essential protein; both subunits were shown to be essential in D. melanogaster and the planthopper Nilaparvata lugens (38, 75, 88, 110, 125).
Some types of mammalian cells export heme through FLVCR1a (Figure 1) (23). Insects have a protein in the FLVCR family, but whether it functions in heme export like FLVCR1a or heme import like FLVCR2 or no heme transport at all is not known (113, 134). A homolog in R. prolixus is upregulated after a blood meal, and knock down results in a reduction in lifespan and in fecundity (125). A combination of gene expression analyses and RNAi-based screens in A. aegypti cultured cells and midgut cells identified one candidate heme exporter (AAEL00864), although the D. melanogaster ortholog of this protein, Pummelig, is a phospholipase with functions related to lipid metabolism; therefore, future studies are needed to establish whether this protein has a role in heme export (27, 48).
Mammalian serum transferrin, which is secreted in its apo form, is not thought to export iron from cells. Like serum transferrin, insect transferrin 1 (Tsf1, described in Section 6), is assumed to be secreted as an an apo-protein, and evidence of apo-Tsf1 in M. sexta hemolymph supports this assumption (53). However, knock down of Tsf1 in the midgut of D. melanogaster resulted in increased iron content of the midgut, and knock down in muscles resulted in iron accumulation in muscle cell mitochondria (137, 141). One explanation for these results is that Tsf1 can be loaded with ferric ions in the ER or Golgi before being secreted as holo-Tsf1.
6. IRON UPTAKE FROM INSECT HEMOLYMPH
The mechanisms of iron uptake from insect hemolymph are still unknown (Figure 1). In mammalian plasma, almost all of the iron is bound to serum transferrin; therefore, most iron uptake involves serum transferrin and transferrin receptor 1 (2). In contrast, based on studies of iron in the plasma of M. sexta larvae, most of the iron in insect plasma appears to be bound to ferritin. This assumption is based on the following calculations: ferritin is present at ~0.2 μmol L−1 and each ferritin molecule contains ~2,000 ferric ions, thus, the concentration of ferritin-bound iron should be ~400 μmol L−1, whereas Tsf1 is present at ~1 μmol L−1 and can bind no more than one ferric ion, thus, the concentration of Tsf1-bound iron should be no higher than ~1 μmol L−1 (11, 53, 132). Non-protein bound iron was undetectable in M. sexta plasma (1). The concentration of heme in M. sexta plasma is not known. Taken together, these studies suggest that iron uptake from hemolymph must involve iron bound to ferritin and/or transferrin.
There is strong evidence that ferritin participates in cellular uptake of iron from hemolymph. Radioactive iron bound to ferritin is transferred to insect tissues, and iron-loaded ferritin is a mitogen for cultured insect cells (75, 115, 148). In addition, knock down of ferritin in midgut cells results in iron deficiency in other cell types; this result indicates that ferritin secreted from midgut cells delivers iron to other cells in the body (115). The mechanism by which ferritin delivers iron to cells is not yet known (Figure 1). Mammalian serum ferritin can be taken up via endocytosis after binding to various receptors (e.g., Transmembrane immunoglobulin and mucin domain 2 (TIM-2) and Scavenger Receptor Class A Member 5 (Scara5)) (65, 127). Orthologs of serum ferritin receptors have not been identified in insects, but endocytic uptake via an unidentified receptor seems likely (114). Alternatively, the ferric ions in ferritin could be reduced and removed from ferritin prior to uptake through a ferrous transporter. This type of mechanism has not been observed in insects or mammals, but it is similar to one utilized by the bacterium Bacillus cereus to remove iron from host ferritin (107).
Insect Tsf1, which arose early in insect evolution, is homologous but not orthologous to serum transferrin (4, 90). Serum transferrin evolved with the placental mammal lineage and arose from a duplication of an ancestral gene encoding lactoferrin, which has immune rather than iron transport functions (28, 54, 129). The amino acid sequence of Tsf1 from D. melanogaster is equally similar to both human serum transferrin and human lactoferrin sequences, consistent with studies demonstrating a role for Tsf1 in both iron transport and immunity (35, 51, 55, 137). Tsf1 and serum transferrin have biochemical and structural differences, including in the way that they coordinate iron; however, like serum transferrin, Tsf1 has a very high affinity for ferric ions (5, 132, 133). In addition, like serum transferrin, Tsf1 releases iron under moderately acidic conditions, suggesting that Tsf1 could release iron in acidified endosomes after endocytic uptake via an unidentified receptor (5, 132).
Several lines of evidence indicate that Tsf1 is involved in iron uptake. Radioactive iron bound to Tsf1 is taken up by insect cells, and 125I-labeled Tsf1 is transported into developing insect eggs (53, 68). In addition, RNAi-mediated knock down of Tsf1 in D. melanogaster results in changes in iron distribution in the insect body, and a Tsf1 null mutation interferes with the immune-induced transfer of iron from hemolymph to fat body (55, 137). On the other hand, Tsf1 null mutant flies have the same amount of iron in fat body, hemolymph, ovaries and thoraxes as control flies, and they are viable and fertile (55). Additional studies are needed to reconcile the differences in phenotypes resulting from RNAi-mediated knock down versus a mutant genotype. Notably, the knockdown and null phenotypes are different from those of mice and humans having a severe deficiency of serum transferrin; affected mice and humans have iron overload of various tissues, and affected mice die shortly after birth (7, 45, 47). Despite most types of insects having a Tsf1 ortholog, the louse, aphid and thrips lineages have lost the Tsf1 gene, and, thus, must have evolved to manage iron without it (90). (Note that insects have three additional orthologous groups of transferrins; however, these transferrins are not likely to have a role in iron homeostasis (90, 117).)
Serum transferrin-mediated iron uptake requires transferrin receptor 1 (Figure 1) (65). Insects lack a mammalian transferrin receptor homolog; therefore, hypothetical endocytic uptake of Tsf1 requires an unidentified receptor (35, 69). In mammalian cells, once serum transferrin is endocytosed, iron is released in the acidic environment of the endosome, reduced by a ferric reductase such as STEAP3, and transported into the cytoplasm through a ferrous transporter such as DMT1 (Figure 1) (30). Assuming endocytic uptake of Tsf1 occurs, iron would prbably be reduced by a ferric reductase prior to export to the cytosol through the ferrous transporter Mvl (described in Section 3) (81, 114). Insects are not known to have homologs of the mammalian STEAP proteins, but candidate ferric reductases include CG1275 and Nemy proteins (described in Section 3) (81, 114). Both enzymes are thought to be present in intracellular membranes (57, 73). The physiological functions of CG1275 have not been studied, whereas Nemy is known to participate in peptide amidation in neuroendocrine neurons and influence olfactory memory, but a possible role in iron homeostasis has not been investigated (57, 59).
Serum transferrin can also deliver iron to cells through a non-endocytic mechanism that involves binding to transferrin receptor 1, ferric reduction by a ferric reductase such as STEAP2, and transport through a ferrous transporter such as Zip8 or Zip14 (Figure 1) (65). As described above, insects lack homologs of transferrin receptor 1 and STEAP proteins and do not have orthologs of Zip8 or Zip14; however, the insect ortholog of mammalian ferric chelate reductase 1 (CG8399 in D. melanogaster) may function as a plasma membrane ferric reductase (101, 103, 121).
Possibly conserved heme uptake mechanisms are described in Section 3. A novel type of heme transport occurs in the hematophagous insect R. prolixus. Rhodnius heme binding protein binds heme that is exported from midgut cells and delivers heme to developing oocytes via receptor-mediated endocytosis (10, 95, 124).
7. REGULATION OF IRON HOMEOSTASIS IN INSECTS
Many pathways that regulate systemic and cellular iron homeostasis in mammals are known, but regulation of iron homeostasis in insects is still poorly understood. In mammals, systemic iron homeostasis is primarily controlled through the action of the hormone hepcidin through its effect on the iron efflux transporter ferroportin (18, 30). Insects do not synthesize hepcidin and do not have a ferroportin homolog; therefore, insects and mammals must regulate systemic iron homeostasis differently (18, 81, 114). At least one aspect of cellular iron regulation is similar: a mechanism that involves the binding of an RNA-binding iron regulatory protein to mRNA iron-response elements when cellular iron levels are low (18, 30). Some of the earliest research on iron homeostasis in insects was centered on this subject, and this work has been reviewed previously (81, 92, 114). At least in some types of insects, excess iron that has been taken up by midgut cells is loaded onto ferritin, which is then secreted into the gut lumen and excreted, and, thus, avoiding iron overload (81, 99, 114). Some blood feeding insects have evolved mechanisms to handle the high concentration of heme in blood, including excretion of heme aggregates, or the synthesis of a heme binding protein in hemolymph that protects against heme toxicity (39, 113, 134). An interesting hypothesis about the possible role of manganese Superoxide dismutase 2 as a mitochondrial iron sensor has been proposed (82). Control of iron homeostasis in the context of immunity was recently reviewed (51).
8. CONCLUSIONS AND REMAINING QUESTIONS
Although there are obvious similarities between mammals and insects in the way iron is transported, stored and regulated, there are also large differences. As examples: mitochondrial iron homeostasis appears to be similar in mammals and insects, but many of the key players in mammalian iron homeostasis are lacking in insects, including hepcidin, ferroportin, conserved ferroxidases, and homologous receptors for transferrin and ferritin. In addition, among various types of insects, significant differences in iron homeostasis have been identified; for example, culicine mosquitoes lack Mvl, and lice, aphids and thrips lack Tsf1. Future studies of iron homeostasis in more diverse insect groups will provide a more complete picture of similarities and differences, and findings that are currently restricted to dipterans may not be applicable to all insect taxa. Future progress in understanding iron homeostasis in insects will require the identification of ferrous and heme transporters, putative Tsf1 and ferritin receptors, iron chaperones, and ferric reductases. Important remaining questions include: how heme is imported, exported, and transported; how ferritin and Tsf1 deliver iron to cells; and how systemic iron homeostasis is regulated. In addition, not covered in this review are interesting studies on the relationship between iron homeostasis and insect-microbe interactions. Related remaining questions include: how mechanisms of iron sequestration inhibit growth of pathogenic but not commensal or symbiotic microbes, and how bacterial symbionts influence insect iron homeostasis.
ACKNOWLEDGEMENTS
Thanks to Emily Ragan and Jessica Holst for information about cytochrome b561 proteins and helpful discussion, and to Diana Najera for a review of Mvl literature. This work was supported by National Science Foundation Grant 1656388 and the National Institute of General Medical Sciences of the National Institutes of Health Award Numbers R37GM041247 and R35GM141859.
REFERENCES
- 1.Adamo SA, Fidler TL, Forestell CA. 2007. Illness-induced anorexia and its possible function in the caterpillar, Manduca sexta. Brain Behav. Immun 21(3):292–300 [DOI] [PubMed] [Google Scholar]
- 2.Anderson GJ, Vulpe CD. 2009. Mammalian iron transport. Cell. Mol. Life Sci. 66(20):3241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Arosio P, Ingrassia R, Cavadini P. 2009. Ferritins: a family of molecules for iron storage, antioxidation and more. Biochim. Biophys. Acta. 1790(7):589–99 [DOI] [PubMed] [Google Scholar]
- 4.Bai L, Qiao M, Zheng R, Deng C, Mei S, Chen W. 2016. Phylogenomic analysis of transferrin family from animals and plants. Comp. Biochem. Physiol. D: Genom. Proteom. 17:1–8 [DOI] [PubMed] [Google Scholar]
- 5.Baker EN, Baker HM, Kidd RD. 2002. Lactoferrin and transferrin: functional variations on a common structural framework. Biochem. Cell Biol. 80(1):27–34 [DOI] [PubMed] [Google Scholar]
- 6.Barbehenn R, Dodick T, Poopat U, Spencer B. 2005. Fenton-type reactions and iron concentrations in the midgut fluids of tree-feeding caterpillars. Arch. Insect Biochem. Physiol. 60(1):32–43 [DOI] [PubMed] [Google Scholar]
- 7.Bernstein SE. 1987. Hereditary hypotransferrinemia with hemosiderosis, a murine disorder resembling human atransferrinemia. J. Lab. Clin. Med. 110(6):690–705 [PubMed] [Google Scholar]
- 8.Bettedi L, Aslam MF, Szular J, Mandilaras K, Missirlis F. 2011. Iron depletion in the intestines of Malvolio mutant flies does not occur in the absence of a multicopper oxidase. J. Exp. Biol. 214(6):971–78 [DOI] [PubMed] [Google Scholar]
- 9.Bou-Abdallah F 2010. The iron redox and hydrolysis chemistry of the ferritins. Biochim. Biophys. Acta. 1800(8):719–31 [DOI] [PubMed] [Google Scholar]
- 10.Braz GRC, Moreira MF, Masuda H, Oliveira PL. 2002. Rhodnius heme-binding protein (RHBP) is a heme source for embryonic development in the blood-sucking bug Rhodnius prolixus (Hemiptera, Reduviidae). Insect Biochem. Mol. Biol. 32(4):361–67 [DOI] [PubMed] [Google Scholar]
- 11.Brummett LM, Kanost MR, Gorman MJ. 2017. The immune properties of Manduca sexta transferrin. Insect Biochem. Mol. Biol. 81:1–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Buchon N, Osman D, David FPA, Fang HY, Boquete J-P, et al. 2013. Morphological and molecular characterization of adult midgut compartmentalization in Drosophila. Cell Rep. 3(5):1725–38 [DOI] [PubMed] [Google Scholar]
- 13.Budnik V, White K. 1987. Genetic dissection of dopamine and serotonin synthesis in the nervous system of Drosophila melanogaster. J. Neurogenet. 4(6):309–14 [PubMed] [Google Scholar]
- 14.Burmester T, Hankeln T. 2007. The respiratory proteins of insects. J. Insect Physiol. 53(4):285–94 [DOI] [PubMed] [Google Scholar]
- 15.Capurro M de L, Iughetti P, Ribolla PE, de Bianchi AG. 1996. Musca domestica hemolymph ferritin. Arch. Insect Biochem. Physiol. 32(2):197–207 [DOI] [PubMed] [Google Scholar]
- 16.Chiabrando D, Vinchi F, Fiorito V, Mercurio S, Tolosano E. 2014. Heme in pathophysiology: a matter of scavenging, metabolism and trafficking across cell membranes. Front. Pharmacol. 5:61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chintapalli VR, Wang J, Dow JAT. 2007. Using FlyAtlas to identify better Drosophila melanogaster models of human disease. Nat. Genet. 39(6):715–20 [DOI] [PubMed] [Google Scholar]
- 18.Coffey R, Ganz T. 2017. Iron homeostasis: An anthropocentric perspective. J. Biol. Chem. 292(31):12727–34 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Cui L, Yoshioka Y, Suyari O, Kohno Y, Zhang X, et al. 2008. Relevant expression of Drosophila heme oxygenase is necessary for the normal development of insect tissues. Biochem. Biophys. Res. Commun. 377(4):1156–61 [DOI] [PubMed] [Google Scholar]
- 20.Dechen K, Richards CD, Lye JC, Hwang JEC, Burke R. 2015. Compartmentalized zinc deficiency and toxicities caused by ZnT and Zip gene over expression result in specific phenotypes in Drosophila. Int. J. Biochem. Cell Biol. 60:23–33 [DOI] [PubMed] [Google Scholar]
- 21.Dietz JV, Fox JL, Khalimonchuk O. 2021. Down the iron path: Mitochondrial iron homeostasis and beyond. Cells. 10(9):2198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Dittmer NT, Kanost MR. 2010. Insect multicopper oxidases: Diversity, properties, and physiological roles. Insect Biochem. Mol. Biol. 40(3):179–88 [DOI] [PubMed] [Google Scholar]
- 23.Donegan RK, Moore CM, Hanna DA, Reddi AR. 2019. Handling heme: The mechanisms underlying the movement of heme within and between cells. Free Radic. Biol. Med. 133:88–100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Donovan A, Lima CA, Pinkus JL, Pinkus GS, Zon LI, et al. 2005. The iron exporter ferroportin/Slc40a1 is essential for iron homeostasis. Cell Metab. 1(3):191–200 [DOI] [PubMed] [Google Scholar]
- 25.Drysdale J, Arosio P, Invernizzi R, Cazzola M, Volz A, et al. 2002. Mitochondrial ferritin: a new player in iron metabolism. Blood Cells Mol. Dis. 29(3):376–83 [DOI] [PubMed] [Google Scholar]
- 26.Dunkov B, Georgieva T. 2006. Insect iron binding proteins: Insights from the genomes. Insect Biochem. Mol. Biol. 36(4):300–309 [DOI] [PubMed] [Google Scholar]
- 27.Eggleston H, Adelman ZN. 2020. Transcriptomic analyses of Aedes aegypti cultured cells and ex vivo midguts in response to an excess or deficiency of heme: a quest for transcriptionally-regulated heme transporters. BMC Genom. 21(1):604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Farnaud S, Evans RW. 2003. Lactoferrin — a multifunctional protein with antimicrobial properties. Mol. Immunol 40(7):395–405 [DOI] [PubMed] [Google Scholar]
- 29.Folwell JL, Barton CH, Shepherd D. 2006. Immunolocalisation of the D. melanogaster Nramp homologue Malvolio to gut and Malpighian tubules provides evidence that Malvolio and Nramp2 are orthologous. J. Exp. Biol. 209(10):1988–95 [DOI] [PubMed] [Google Scholar]
- 30.Frazer DM, Anderson GJ. 2014. The regulation of iron transport. Biofactors. 40(2):206–14 [DOI] [PubMed] [Google Scholar]
- 31.Geiser DL, Conley ZR, Elliott JL, Mayo JJ, Winzerling JJ. 2015. Characterization of Anopheles gambiae (African Malaria Mosquito) ferritin and the effect of iron on intracellular localization in mosquito cells. J. Insect Sci. 15:68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Geiser DL, Mayo JJ, Winzerling JJ. 2007. The unique regulation of Aedes aegypti larval cell ferritin by iron. Insect Biochem. Mol. Biol. 37(5):418–29 [DOI] [PubMed] [Google Scholar]
- 33.Geiser DL, Patel N, Patel P, Bhakta J, Velasquez LS, Winzerling JJ. 2017. Description of a second ferritin light chain homologue from the yellow fever mosquito (Diptera: Culicidae). J. Insect Sci. 17(6):123 [Google Scholar]
- 34.Geiser DL, Thai TN, Love MB, Winzerling JJ. 2019. Iron and ferritin deposition in the ovarian tissues of the yellow fever mosquito (Diptera: Culicidae). J. Insect Sci. 19(5):11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Geiser DL, Winzerling JJ. 2012. Insect transferrins: Multifunctional proteins. Biochim. Biophys. Acta Gen. Subj. 1820(3):437–51 [DOI] [PubMed] [Google Scholar]
- 36.Geiser DL, Zhang DZ, Winzerling JJ. 2006. Secreted ferritin: Mosquito defense against iron overload? Insect Biochem. Mol. Biol. 36(3):177–87 [DOI] [PubMed] [Google Scholar]
- 37.Gkouvatsos K, Papanikolaou G, Pantopoulos K. 2012. Regulation of iron transport and the role of transferrin. Biochim. Biophys. Acta Gen. Subj. 1820(3):188–202 [DOI] [PubMed] [Google Scholar]
- 38.Gonzalez-Morales N, Angel Mendoza-Ortiz M, Blowes LM, Missirlis F, Riesgo-Escovar JR. 2015. Ferritin is required in multiple tissues during Drosophila melanogaster development. PLoS One. 10(7):e0133499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Graça-Souza AV, Maya-Monteiro C, Paiva-Silva GO, Braz GRC, Paes MC, et al. 2006. Adaptations against heme toxicity in blood-feeding arthropods. Insect Biochem. Mol. Biol. 36(4):322–35 [DOI] [PubMed] [Google Scholar]
- 40.Graveley BR, Brooks AN, Carlson JW, Duff MO, Landolin JM, et al. 2011. The developmental transcriptome of Drosophila melanogaster. Nature. 471(7339):473–79 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Gulec S, Anderson GJ, Collins JF. 2014. Mechanistic and regulatory aspects of intestinal iron absorption. Am. J. Physiol. Gastrointest. Liver Physiol. 307(4):G397–409 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Gutierrez L, Zubow K, Nield J, Gambis A, Mollereau B, et al. 2013. Biophysical and genetic analysis of iron partitioning and ferritin function in Drosophila melanogaster. Metallomics. 5(8):997–1005 [DOI] [PubMed] [Google Scholar]
- 43.Halestrap AP. 2013. The SLC16 gene family - structure, role and regulation in health and disease. Mol. Aspects Med. 34(2–3):337–49 [DOI] [PubMed] [Google Scholar]
- 44.Hamburger AE, West AP, Hamburger ZA, Hamburger P, Bjorkman PJ. 2005. Crystal structure of a secreted insect ferritin reveals a symmetrical arrangement of heavy and light chains. J. Mol. Biol. 349(3):558–69 [DOI] [PubMed] [Google Scholar]
- 45.Hamill RL, Woods JC, Cook BA. 1991. Congenital atransferrinemia: A case report and review of the literature. Am. J. Clin. Pathol. 96(2):215–18 [DOI] [PubMed] [Google Scholar]
- 46.Hara T, Takeda T, Takagishi T, Fukue K, Kambe T, Fukada T. 2017. Physiological roles of zinc transporters: molecular and genetic importance in zinc homeostasis. J. Physiol. Sci. 67(2):283–301 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Hayashi A, Wada Y, Suzuki T, Shimizu A. 1993. Studies on familial hypotransferrinemia: unique clinical course and molecular pathology. Am. J. Hum. Genet. 53(1):201–13 [PMC free article] [PubMed] [Google Scholar]
- 48.Hehlert P, Hofferek V, Heier C, Eichmann TO, Riedel D, et al. 2019. The α/β-hydrolase domain-containing 4- and 5-related phospholipase Pummelig controls energy storage in Drosophila. J. Lipid Res. 60(8):1365–78 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Hellman NE, Gitlin JD. 2002. Ceruloplasmin metabolism and function. Annu. Rev. Nutr. 22:439–58 [DOI] [PubMed] [Google Scholar]
- 50.Helmer OM, Emerson CP. 1934. The iron content of the whole blood of normal individuals. J. Biol. Chem. 104(1):157–61 [Google Scholar]
- 51.Hrdina A, Iatsenko I. 2021. The roles of metals in insect-microbe interactions and immunity. Curr. Opin. Insect Sci. 49:71–77 [DOI] [PubMed] [Google Scholar]
- 52.Hsu C-Y, Li C-W. 1994. Magnetoreception in honeybees. Science. 265(5168):95–97 [DOI] [PubMed] [Google Scholar]
- 53.Huebers HA, Huebers E, Finch CA, Webb BA, Truman JW, et al. 1988. Iron binding proteins and their roles in the tobacco hornworm, Manduca sexta (L.). J. Comp. Physiol. B, Biochem. Syst. Environ. Physiol. 158(3):291–300 [DOI] [PubMed] [Google Scholar]
- 54.Hughes AL, Friedman R. 2014. Evolutionary diversification of the vertebrate transferrin multi-gene family. Immunogenetics. 66(11):651–61 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Iatsenko I, Marra A, Boquete J-P, Peña J, Lemaitre B. 2020. Iron sequestration by transferrin 1 mediates nutritional immunity in Drosophila melanogaster. Proc. Natl. Acad. Sci. U. S. A. 117(13):7317–25 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Ida H, Suyari O, Shimamura M, Tien Tai T, Yamaguchi M, Taketani S. 2013. Genetic link between heme oxygenase and the signaling pathway of DNA damage in Drosophila melanogaster. Tohoku J. Exp. Med. 231(2):117–25 [DOI] [PubMed] [Google Scholar]
- 57.Iliadi KG, Avivi A, Iliadi NN, Knight D, Korol AB, et al. 2008. nemy encodes a cytochrome b561 that is required for Drosophila learning and memory. Proc. Natl. Acad. Sci. U. S. A 105(50):19986–91 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Khanna MR, Stanley BA, Thomas GH. 2010. Towards a membrane proteome in Drosophila: a method for the isolation of plasma membrane. BMC Genom. 11:302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Knight D, Iliadi KG, Iliadi N, Wilk R, Hu J, et al. 2015. Distinct regulation of transmitter release at the Drosophila NMJ by different isoforms of nemy. PLoS ONE. 10(8):e0132548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Knutson MD. 2017. Iron transport proteins: Gateways of cellular and systemic iron homeostasis. J. Biol. Chem. 292(31):12735–43 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Knutson MD. 2019. Non-transferrin-bound iron transporters. Free Radic. Biol. Med. 133:101–11 [DOI] [PubMed] [Google Scholar]
- 62.Kosman DJ. 2010. Redox cycling in iron uptake, efflux, and trafficking. J. Biol. Chem. 285(35):26729–35 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Kosman DJ. 2013. Iron metabolism in aerobes: Managing ferric iron hydrolysis and ferrous iron autoxidation. Coord. Chem. Rev.. 257(1):210–17 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Kosman DJ. 2018. The teleos of metallo-reduction and metallo-oxidation in eukaryotic iron and copper trafficking. Metallomics. 10(3):370–77 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Kosman DJ. 2020. A holistic view of mammalian (vertebrate) cellular iron uptake. Metallomics. 12(9):1323–34 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Kramer KJ, Seib PA. 1982. Ascorbic acid and the growth and development of insects. Adv. Chem. Ser. 200:275–91. [Google Scholar]
- 67.Krieg L, Milstein O, Krebs P, Xia Y, Beutler B, Du X. 2011. Mutation of the gastric hydrogen-potassium ATPase alpha subunit causes iron-deficiency anemia in mic. Blood 118(24):6418–6425 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Kurama T, Kurata S, Natori S. 1995. Molecular characterization of an insect transferrin and its selective incorporation into eggs during oogenesis. Eur. J. Biochem. 228(2):229–35 [PubMed] [Google Scholar]
- 69.Lambert LA. 2012. Molecular evolution of the transferrin family and associated receptors. Biochim. Biophys. Acta Gen. Subj. 1820(3):244–55 [DOI] [PubMed] [Google Scholar]
- 70.Lane DJR, Bae D-H, Merlot AM, Sahni S, Richardson DR. 2015. Duodenal cytochrome b (DCYTB) in iron metabolism: an update on function and regulation. Nutrients. 7(4):2274–96 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Lang M, Braun CL, Kanost MR, Gorman MJ. 2012. Multicopper oxidase-1 is a ferroxidase essential for iron homeostasis in Drosophila melanogaster. Proc. Natl. Acad. Sci. U. S. A. 109(33):13337–42 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Lara FA, Pohl PC, Gandara AC, Ferreira J da S, Nascimento-Silva MC, et al. 2015. ATP binding cassette transporter mediates both heme and pesticide detoxification in tick midgut cells. PLoS One. 10(8):e0134779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Larkin A, Marygold SJ, Antonazzo G, Attrill H, dos Santos G, et al. 2021. FlyBase: updates to the Drosophila melanogaster knowledge base. Nucleic Acids Res. 49(D1):D899–907 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Le Blanc S, Garrick MD, Arredondo M. 2012. Heme carrier protein 1 transports heme and is involved in heme-Fe metabolism. Am. J. Physiol. Cell Physiol. 302(12):C1780–85 [DOI] [PubMed] [Google Scholar]
- 75.Li S 2010. Identification of iron-loaded ferritin as an essential mitogen for cell proliferation and postembryonic development in Drosophila. Cell Res. 20(10):1148–57 [DOI] [PubMed] [Google Scholar]
- 76.Linder MC. 2013. Mobilization of stored iron in mammals: a review. Nutrients. 5(10):4022–50 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Liu X, Sun C, Liu X, Yin X, Wang B, et al. 2015. Multicopper oxidase-1 is required for iron homeostasis in Malpighian tubules of Helicoverpa armigera. Sci. Rep. 5(1):14784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Liuzzi JP, Cousins RJ. 2004. Mammalian zinc transporters. Annu. Rev. Nutr. 24:151–72 [DOI] [PubMed] [Google Scholar]
- 79.Locke M, Nichol H. 1992. Iron economy in insects: transport, metabolism, and storage. Annu. Rev. Entomol. 37(1):195–215 [Google Scholar]
- 80.Mandilaras K, Missirlis F. 2012. Genes for iron metabolism influence circadian rhythms in Drosophila melanogaster. Metallomics. 4(9):928–36 [DOI] [PubMed] [Google Scholar]
- 81.Mandilaras K, Pathmanathan T, Missirlis F. 2013. Iron absorption in Drosophila melanogaster. Nutrients. 5(5):1622–47 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Marelja Z, Leimkühler S, Missirlis F. 2018. Iron sulfur and molybdenum cofactor enzymes regulate the Drosophila life cycle by controlling cell metabolism. Front. Physiol. 9:50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Martinez-Barnetche J, Garcia Solache M, Lecona AN, Tello Lopez AT, del Carmen Rodriguez M, et al. 2007. Cloning and functional characterization of the Anopheles albimanus DMT1/NRAMP homolog: Implications in iron metabolism in mosquitoes. Insect Biochem. Mol. Biol. 37(6):532–39 [DOI] [PubMed] [Google Scholar]
- 84.Mehlferber EC, Benowitz KM, Roy-Zokan EM, McKinney EC, Cunningham CB, Moore AJ. 2017. Duplication and Sub/Neofunctionalization of Malvolio, an insect homolog of Nramp, in the subsocial beetle Nicrophorus vespilloides. G3 (Bethesda). 7(10):3393–3403 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Metzendorf C, Wu W, Lind MI. 2009. Overexpression of Drosophila mitoferrin in l(2)mbn cells results in dysregulation of Fer1HCH expression. Biochem. J. 421(3):463–71 [DOI] [PubMed] [Google Scholar]
- 86.Miguel-Aliaga I, Jasper H, Lemaitre B. 2018. Anatomy and physiology of the digestive tract of Drosophila melanogaster. Genetics. 210(2):357–96 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Missirlis F, Holmberg S, Georgieva T, Dunkov BC, Rouault TA, Law JH. 2006. Characterization of mitochondrial ferritin in Drosophila. Proc. Natl. Acad. Sci. U. S. A 103(15):5893–98 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Missirlis F, Kosmidis S, Brody T, Mavrakis M, Holmberg S, et al. 2007. Homeostatic mechanisms for iron storage revealed by genetic manipulations and live imaging of Drosophila ferritin. Genetics. 177(1):89–100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Mumbauer S, Pascual J, Kolotuev I, Hamaratoglu F. 2019. Ferritin heavy chain protects the developing wing from reactive oxygen species and ferroptosis. PLoS Genet. 15(9):e1008396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Najera DG, Dittmer NT, Weber JJ, Kanost MR, Gorman MJ. 2020. Phylogenetic and sequence analyses of insect transferrins suggest that only transferrin 1 has a role in iron homeostasis. Insect Sci. 28(2):495–508 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Neckameyer WS, White K. 1993. Drosophila tyrosine hydroxylase is encoded by the pale locus. J. Neurogenet. 8(4):189–99 [DOI] [PubMed] [Google Scholar]
- 92.Nichol H, Law JH, Winzerling JJ. 2002. Iron metabolism in insects. Annu. Rev. Entomol. 47:535–59 [DOI] [PubMed] [Google Scholar]
- 93.Nichol H, Locke M. 1990. The localization of ferritin in insects. Tissue Cell. 22(6):767–77 [Google Scholar]
- 94.Nichol H, Locke M. 1999. Secreted ferritin subunits are of two kinds in insects - Molecular cloning of cDNAs encoding two major subunits of secreted ferritin from Calpodes ethlius. Insect Biochem. Mol. Biol. 29(11):999–1013 [DOI] [PubMed] [Google Scholar]
- 95.Oliveira P, Kawooya J, Ribeiro J, Meyer T, Poorman R, et al. 1995. A heme-binding protein from hemolymph and oocytes of the bloodsucking insect, Rhodnius prolixus - isolation and characterization. J. Biol. Chem. 270(18):10897–901 [DOI] [PubMed] [Google Scholar]
- 96.Orgad S, Nelson H, Segal D, Nelson N. 1998. Metal ions suppress the abnormal taste behavior of the Drosophila mutant malvolio. J. Exp. Biol. 201(1):115–20 [DOI] [PubMed] [Google Scholar]
- 97.Peng Z, Dittmer NT, Lang M, Brummett LM, Braun CL, et al. 2015. Multicopper oxidase-1 orthologs from diverse insect species have ascorbate oxidase activity. Insect Biochem. Molec. Biol. 59:58–71 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Petrak J, Vyoral D. 2005. Hephaestin - a ferroxidase of cellular iron export. Int. J. Biochem. Cell Biol. 37(6):1173–78 [DOI] [PubMed] [Google Scholar]
- 99.Pham DQD, Winzerling JJ. 2010. Insect ferritins: Typical or atypical? Biochim. Biophys. Acta. 1800(8):824–33 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Philpott CC, Ryu M-S, Frey A, Patel S. 2017. Cytosolic iron chaperones: Proteins delivering iron cofactors in the cytosol of mammalian cells. J. Biol. Chem. 292(31):12764–71 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Picco C, Scholz-Starke J, Naso A, Preger V, Sparla F, et al. 2014. How are cytochrome b561 electron currents controlled by membrane voltage and substrate availability? Antioxid. Redox Signal. 21(3):384–91 [DOI] [PubMed] [Google Scholar]
- 102.Puig S, Ramos-Alonso L, Romero AM, Martínez-Pastor MT. 2017. The elemental role of iron in DNA synthesis and repair. Metallomics. 9(11):1483–1500 [DOI] [PubMed] [Google Scholar]
- 103.Qin Q, Wang X, Zhou B. 2013. Functional studies of Drosophila zinc transporters reveal the mechanism for dietary zinc absorption and regulation. BMC Biol. 11(1):101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Qin S, Yin H, Yang C, Dou Y, Liu Z, et al. 2016. A magnetic protein biocompass. Nature Mater. 15(2):217–26 [DOI] [PubMed] [Google Scholar]
- 105.Rosas-Arellano A, Vasquez-Procopio J, Gambis A, Blowes LM, Steller H, et al. 2016. Ferritin assembly in enterocytes of Drosophila melanogaster. Int. J. Mol. Sci. 17(2):27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Scott JG, Wen Z. 2001. Cytochromes P450 of insects: the tip of the iceberg. Pest Manag. Sci. 57(10):958–67 [DOI] [PubMed] [Google Scholar]
- 107.Segond D, Khalil EA, Buisson C, Daou N, Kallassy M, et al. 2014. Iron acquisition in Bacillus cereus: The roles of IlsA and bacillibactin in exogenous ferritin iron mobilization. PLoS Pathog. 10(2):e1003935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Shanbhag S, Tripathi S. 2005. Electrogenic H+ transport and pH gradients generated by a V-H+ -ATPase in the isolated perfused larval Drosophila midgut. J Membr. Biol. 206(1):61–72 [DOI] [PubMed] [Google Scholar]
- 109.Shawki A, Engevik MA, Kim RS, Knight PB, Baik RA, et al. 2016. Intestinal brush-border Na+/H+ exchanger-3 drives H+-coupled iron absorption in the mouse. Am. J. Physiol. Gastrointest. Liver Physiol. 311(3):G423–430 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Shen Y, Chen Y-Z, Zhang C-X. 2021. RNAi-mediated silencing of ferritin genes in the brown planthopper Nilaparvata lugens affects survival, growth and female fecundity. Pest Manag. Sci. 77(1):365–77 [DOI] [PubMed] [Google Scholar]
- 111.Southon A, Farlow A, Norgate M, Burke R, Camakaris J. 2008. Malvolio is a copper transporter in Drosophila melanogaster. J. Exp. Biol. 211(Pt 5):709–16 [DOI] [PubMed] [Google Scholar]
- 112.Spencer CS, Yunta C, de Lima GPG, Hemmings K, Lian L-Y, et al. 2018. Characterisation of Anopheles gambiae heme oxygenase and metalloporphyrin feeding suggests a potential role in reproduction. Insect Biochem. Molec. Biol. 98:25–33 [DOI] [PubMed] [Google Scholar]
- 113.Sterkel M, Oliveira JHM, Bottino-Rojas V, Paiva-Silva GO, Oliveira PL. 2017. The dose makes the poison: Nutritional overload determines the life traits of blood-feeding arthropods. Trends Parasitol. 33(8):633–44 [DOI] [PubMed] [Google Scholar]
- 114.Tang X, Zhou B. 2013. Iron homeostasis in insects: Insights from Drosophila studies. IUBMB Life. 65(10):863–72 [DOI] [PubMed] [Google Scholar]
- 115.Tang X, Zhou B. 2013. Ferritin is the key to dietary iron absorption and tissue iron detoxification in Drosophila melanogaster. FASEB J. 27(1):288–98 [DOI] [PubMed] [Google Scholar]
- 116.Terra WR, Barroso IG, Dias RO, Ferreira C. 2019. Molecular physiology of insect midgut. Adv. In Insect Phys. 56:117–63 [Google Scholar]
- 117.Tiklová K, Senti K-A, Wang S, Gräslund A, Samakovlis C. 2010. Epithelial septate junction assembly relies on melanotransferrin iron binding and endocytosis in Drosophila. Nat. Cell Biol. 12(11):1071–77 [DOI] [PubMed] [Google Scholar]
- 118.Tsaousis AD. 2019. On the origin of iron/sulfur cluster biosynthesis in eukaryotes. Front. Microbiol. 10:2478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Tsujimoto H, Anderson MAE, Eggleston H, Myles KM, Adelman ZN. 2021. Aedes aegypti dyspepsia encodes a novel member of the SLC16 family of transporters and is critical for reproductive fitness. PLoS Negl. Trop. Dis. 15(4):e0009334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Tsujimoto H, Anderson MAE, Myles KM, Adelman ZN. 2018. Identification of candidate iron transporters from the ZIP/ZnT gene families in the mosquito Aedes aegypti. Front. Physiol. 9:380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Vargas JD, Herpers B, McKie AT, Gledhill S, McDonnell J, et al. 2003. Stromal cell-derived receptor 2 and cytochrome b561 are functional ferric reductases. Biochim. Biophys. Acta Proteins Proteom. 1651(1–2):116–23 [DOI] [PubMed] [Google Scholar]
- 122.Verelst W, Asard H. 2003. A phylogenetic study of cytochrome b561 proteins. Genome Biol. 4(6):R38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Wajnberg E, Alves OC, Perales J, da Rocha SLG, Ferreira AT, et al. 2018. Ferritin from the haemolymph of adult ants: an extraction method for characterization and a ferromagnetic study. Eur. Biophys. J. 47(6):641–53 [DOI] [PubMed] [Google Scholar]
- 124.Walter-Nuno AB, Oliveira MP, Oliveira MF, Gonçalves RL, Ramos IB, et al. 2013. Silencing of maternal heme-binding protein causes embryonic mitochondrial dysfunction and impairs embryogenesis in the blood sucking insect Rhodnius prolixus. J. Biol. Chem. 288(41):29323–32 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Walter-Nuno AB, Taracena ML, Mesquita RD, Oliveira PL, Paiva-Silva GO. 2018. Silencing of iron and heme-related genes revealed a paramount role of iron in the physiology of the hematophagous vector Rhodnius prolixus. Front. Genet. 9:19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Wan Z, Xu J, Huang Y, Zhai Y, Ma Z, et al. 2020. Elevating bioavailable iron levels in mitochondria suppresses the defective phenotypes caused by PINK1 loss-of-function in Drosophila melanogaster. Biochem. Biophys. Res. Commun. 532(2):285–91 [DOI] [PubMed] [Google Scholar]
- 127.Wang W, Knovich MA, Coffman LG, Torti FM, Torti SV. 2010. Serum ferritin: Past, present and future. Biochim. Biophys. Acta. 1800(8):760–69 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Wang X, Yin S, Yang Z, Zhou B. 2018. Drosophila multicopper oxidase 3 is a potential ferroxidase involved in iron homeostasis. Biochim. Biophys. Acta Gen. Subj. 1862(8):1826–34 [DOI] [PubMed] [Google Scholar]
- 129.Ward PP, Mendoza-Meneses M, Cunningham GA, Conneely OM. 2003. Iron status in mice carrying a targeted disruption of lactoferrin. Mol. Cell. Biol. 23(1):178–85 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Waterhouse D, Stay B. 1955. Functional differentiation in the midgut epithelium of blowfly larvae as revealed by histochemical tests. Aust. J. Biol. Sci. 8(2):253 [Google Scholar]
- 131.Waterhouse DF, Day MF. 1953. Function of the gut in absorption, excretion, and intermediary metabolism. In Insect Physiology. New York: John Wiley & Sons [Google Scholar]
- 132.Weber JJ, Kanost MR, Gorman MJ. 2020. Iron binding and release properties of transferrin-1 from Drosophila melanogaster and Manduca sexta: Implications for insect iron homeostasis. Insect Biochem. Mol. Biol. 125:103438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Weber JJ, Kashipathy MM, Battaile KP, Go E, Desaire H, et al. 2020. Structural insight into the novel iron-coordination and domain interactions of transferrin-1 from a model insect, Manduca sexta. Protein Sci. 30(2)408–422 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Whiten SR, Eggleston H, Adelman ZN. 2018. Ironing out the details: Exploring the role of iron and heme in blood-sucking arthropods. Front. Physiol. 8:1134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Winzerling JJ, Pham DQ-D. 2006. Iron metabolism in insect disease vectors: mining the Anopheles gambiae translated protein database. Insect Biochem. Mol. Biol. 36(4):310–21 [DOI] [PubMed] [Google Scholar]
- 136.Wu Y, Zhao H, Zhang EE, Liu N. 2021. Identification of PCBP1 as a novel modulator of mammalian circadian clock. Front. Genet. 12:656571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Xiao G, Liu Z-H, Zhao M, Wang H-L, Zhou B. 2019. Transferrin 1 functions in iron trafficking and genetically interacts with ferritin in Drosophila melanogaster. Cell Reports. 26(3):748–758 [DOI] [PubMed] [Google Scholar]
- 138.Xiao G, Wan Z, Fan Q, Tang X, Zhou B. 2014. The metal transporter ZIP13 supplies iron into the secretory pathway in Drosophila melanogaster. eLife. 3:e03191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Xiao G, Zhou B. 2018. ZIP13: A study of Drosophila offers an alternative explanation for the corresponding human disease. Front. Genet. 8:234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Xu J, Wan Z, Zhou B. 2019. Drosophila ZIP13 is posttranslationally regulated by iron-mediated stabilization. Biochim. Biophys. Acta Mol. Cell Res. 1866(9):1487–97 [DOI] [PubMed] [Google Scholar]
- 141.Xue J, Li G, Ji X, Liu Z-H, Wang H-L, Xiao G. 2022. Drosophila ZIP13 overexpression or transferrin1 RNAi influences the muscle degeneration of Pink1 RNAi by elevating iron levels in mitochondria. J. Neurochem. doi: 10.1111/jnc.15574 [DOI] [PubMed] [Google Scholar]
- 142.Yanatori I, Kishi F. 2019. DMT1 and iron transport. Free Radic. Biol. Med. 133:55–63 [DOI] [PubMed] [Google Scholar]
- 143.Yanatori I, Richardson DR, Toyokuni S, Kishi F. 2020. The new role of poly (rC)-binding proteins as iron transport chaperones: Proteins that could couple with inter-organelle interactions to safely traffic iron. Biochim. Biophys. Acta Gen. Subj. 1864(11):129685. [DOI] [PubMed] [Google Scholar]
- 144.Yoshiga T, Hernandez VP, Fallon AM, Law JH. 1997. Mosquito transferrin, an acute-phase protein that is up-regulated upon infection. Proc. Natl. Acad. Sci. U. S. A. 94(23):12337–42 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Yu Y, Wu A, Zhang Z, Yan G, Zhang F, et al. 2013. Characterization of the GufA subfamily member SLC39A11/Zip11 as a zinc transporter. J. Nutr. Biochem. 24(10):1697–1708 [DOI] [PubMed] [Google Scholar]
- 146.Zhang X, Sato M, Sasahara M, Migita CT, Yoshida T. 2004. Unique features of recombinant heme oxygenase of Drosophila melanogaster compared with those of other heme oxygenases studied. Eur. J. Biochem. 271(9):1713–24 [DOI] [PubMed] [Google Scholar]
- 147.Zhao M, Zhou B. 2020. A distinctive sequence motif in the fourth transmembrane domain confers ZIP13 iron function in Drosophila melanogaster. Biochim. Biophys. Acta Mol. Cell. Res 1867(2):118607. [DOI] [PubMed] [Google Scholar]
- 148.Zhou G, Kohlhepp P, Geiser D, Frasquillo MDC, Vazquez-Moreno L, Winzerling JJ. 2007. Fate of blood meal iron in mosquitoes. J. Insect Physiol. 53(11):1169–78 [DOI] [PMC free article] [PubMed] [Google Scholar]


