ABSTRACT
Interleukin-17 inhibitors are effective treatments for plaque psoriasis. However, these medications have been linked to the development of new-onset inflammatory bowel disease (IBD) and the worsening of existing IBD in some patients. This case report describes a patient with plaque psoriasis who developed new-onset Crohn's disease after treatment with ixekizumab, an interleukin-17A inhibitor. He was then transitioned to ustekinumab, which resulted in successful remission of both psoriasis and Crohn's disease. This case highlights the potential for ustekinumab to be an effective rescue treatment for psoriasis patients with new-onset IBD triggered by medications.
KEYWORDS: ustekinumab, ixekizumab, Crohn's disease, psoriasis
INTRODUCTION
Psoriasis and inflammatory bowel disease (IBD) share a significant overlap in the genetic factors that contribute to their development. They both are chronic inflammatory diseases that are mediated by the immune system.1 Previous studies showed psoriasis and IBD are bidirectionally associated and patients with psoriasis are more likely to develop IBD than the general population.2,3 Ixekizumab is a humanized monoclonal antibody that selectively binds to and neutralizes interleukin-17 (IL-17A). It is a biological agent approved for the treatment of chronic plaque psoriasis, psoriatic arthritis, and ankylosing spondylitis.4–6 IL-17 is also implicated in the pathogenesis of IBD because elevated mRNA levels of IL-17A were found in the intestinal mucosa of patients with IBD.7,8 Inhibition of IL-17 was once hypothesized to be a promising therapeutic strategy for IBD. However, clinical trials of IL-17 inhibitors in IBD have not only failed to demonstrate clinical efficacy but have also raised concerns about the potential to trigger new-onset IBD and relapse.9,10
CASE REPORT
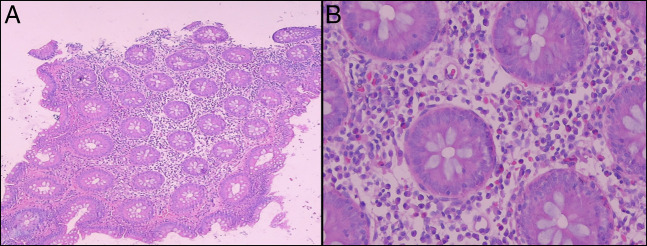
A 40-year-old man presented with a 3-month history of abdominal pain, mucoid diarrhea, undocumented weight loss, and hematochezia. Worsening of the symptoms and more frequent episodes of hematochezia prompted the patient to seek consult at our institution. He was diagnosed with plaque psoriasis 2 years before presentation and was initially started with over-the-counter emollients and betamethasone dipropionate 0.05% cream (Figure 1). After failure of the skin lesions to resolve, treatment was shifted to ixekizumab subcutaneous injection. There was complete resolution of the skin lesions, and therapy was maintained at the dose of 80 mg every 4 weeks for 6 months until the gastrointestinal symptoms began to present. On physical examination, his blood pressure was 90/60 mm Hg; heart rate at 126 bpm; and respiratory rate of 21 cycles per min. He had pale palpebral conjunctivae, whereas the abdomen was soft and nontender. There was fresh blood on digital rectal examination. Blood tests revealed a low hemoglobin at 7 g/dL, a low hematocrit at 0.208, and an elevated white blood cell count at 16.2 × 109/L. Immediate fluid resuscitation and blood transfusion with a unit of packed red blood cells was performed followed by infusion of intravenous ciprofloxacin for empiric bacterial coverage. On the next hospital day, the patient underwent colonoscopy, which showed multiple, deep, varisized ileal ulcers topped with exudates (Figure 2). The ileocecal valve was erythematous and friable. There were also multiple varisized ulcers and superficial aphthous ulcers seen from the cecum until the descending colon. The mucosal surface of the right colon was likewise erythematous with normal intervening mucosa. Normal mucosal surface was visualized at the rectosigmoid area. On inspecting the perianal region, there were anal fissures, perianal fistula, and anal tags. Two hemoclips were deployed on the ileal ulcer with visible vessel (Figure 2). Multiple biopsies were taken from the ileal ulcer edge, ileocecal valve, and right colon. Microsections disclosed fragments of ileal and ileocolonic mucosa with reactive lymphoid hyperplasia and notable expansion of lamina propria by inflammatory cells (Figure 3). On higher magnification, there was severe chronic active inflammation, fibrinopurulent exudate with crypt distortion and cryptitis. No granulomas and dysplasia were noted (Figure 3). Based on the symptoms, endoscopic, and histopathologic findings, the patient was diagnosed with severe Crohn's disease (CD).
Figure 1.
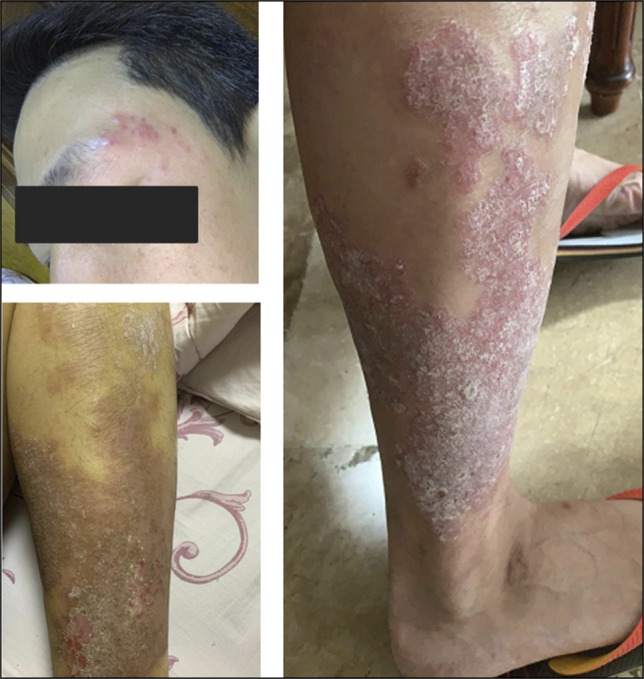
Psoriatic lesions in the face and limbs.
Figure 2.
(A) Ileal ulcers with visible vessel. (2) Hemoclip deployed on the ileal ulcer.
Figure 3.
(A) Ileal mucosa under low-power field. (B) Ileal mucosa under high-power field.
A team discussion was held with the patient to inform him about the various biologics accessible at our facility, which can be used for treating IBD and psoriasis. An agreement was made to proceed with ustekinumab instead of Infliximab because of the concern of possible flare-up of skin lesions using infliximab.11,12 He was treated with ustekinumab 390 mg intravenous for the induction phase followed by a maintenance subcutaneous dose of 90 mg every 8 weeks. There was rapid relief of symptoms after the induction phase of ustekinumab. A repeat colonoscopy after 11 months revealed absence of ileal ulcers with an appearance of ileocolic fistula (Figure 4). There was also noted healing of the previously noted perianal fistula. Histopathology of the ileal biopsies showed only mild chronic inflammation and absence of granuloma, whereas the biopsies from the right colon revealed no inflammation (Figure 5).
Figure 4.
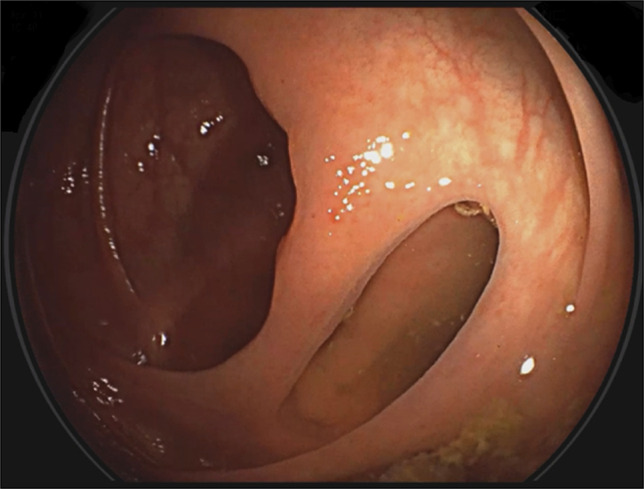
Ileocolonic fistula (after treatment of ustekinumab).
Figure 5.
(A) Ileal mucosa under low-power field (after treatment of ustekinumab). (B) Ileal mucosa under high-power field (after treatment of ustekinumab).
DISCUSSION
Tumor necrosis factor alpha (TNF-α) and interleukin 12/23 (IL-12/23) inhibitors can be effective in the simultaneous treatment of psoriasis and IBD because they target common inflammatory pathways.10 Infliximab and adalimumab are 2 anti–TNF-α monoclonal antibodies that have been shown to be effective in treating psoriasis, psoriatic arthritis, ulcerative colitis, and CD.13 Despite their efficacy, anti–TNF-α monoclonal antibodies can paradoxically worsen psoriasis in patients with IBD or cause new-onset psoriasis in patients without a history of the disease.11,12 Ustekinumab is a fully human IgG1κ monoclonal antibody that binds to the shared p40 protein subunit of IL-12 and IL-23. Ustekinumab is currently approved for the treatment of moderate-to-severe plaque psoriasis, psoriatic arthritis, and CD. It has also shown to be effective in the treatment of ulcerative colitis.13–15 Previous studies also showed the effectiveness of ustekinumab as a rescue drug on patients who have developed paradoxical skin reactions, such as psoriasis, after receiving a TNF-α inhibitor as treatment for IBD.12,16,17 In patients with both psoriasis and IBD, there are a number of effective treatments available that lead to improvement of both conditions. Ustekinumab is a promising treatment and could be a first-line option for patients with IBD without worsening psoriasis. Further large-scale clinical trials are warranted to definitively establish its efficacy and safety in this specific patient population.
This case also highlights the necessity to closely monitor patients receiving IL-17 therapies such as ixekizumab, especially those with preexisting risk factors for inflammatory bowel disease. It is important for physicians to perform in-depth history and physical examination before initiation of treatment because evidences showed IL-17 inhibitors may trigger or exacerbate IBD.9,10,18 Therapeutic interventions for individuals with psoriasis should consider the potential for this uncommon paradoxical reaction.9,10,18 A collaborative multidisciplinary effort between gastroenterologists and dermatologists within the clinical setting is crucial to promote the safe use of medications.
DISCLOSURES
Author contributions: JRL Tan: wrote the manuscript and is the article guarantor. WS Alba: edited the manuscript, Senior Gastroenterology Adviser for the case.
Financial disclosure: None to report.
Previous presentation: Poster Presentation, 3rd World Congress of GI Endoscopy, ENDO 2022, May 13, 2022, Kyoto, Japan.
Informed consent was obtained for this case report.
REFERENCES
- 1.Skroza N, Proietti I, Pampena R, et al. Correlations between psoriasis and inflammatory bowel diseases. Biomed Research International. 2013;2013:983902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Alinaghi F, Tekin HG, Burisch J, Wu JJ, Thyssen JP, Egeberg A. Global prevalence and bidirectional association between psoriasis and inflammatory bowel disease—a systematic review and meta-analysis. J Crohns Colitis. 2020;14(3):351–60. [DOI] [PubMed] [Google Scholar]
- 3.Fu Y, Lee CH, Chi CC. Association of psoriasis with inflammatory bowel disease: A systematic review and meta-analysis. JAMA Dermatol. 2018;154(12):1417–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Leonardi C, Matheson R, Zachariae C, et al. Anti–interleukin-17 monoclonal antibody ixekizumab in chronic plaque psoriasis. New Engl J Med. 2012;366(13):1190–9. [DOI] [PubMed] [Google Scholar]
- 5.Mease PJ, van der Heijde D, Ritchlin CT, et al. , SPIRIT-P1 Study Group. Ixekizumab, an interleukin-17A specific monoclonal antibody, for the treatment of biologic-naive patients with active psoriatic arthritis: Results from the 24-week randomised, double-blind, placebo-controlled and active (adalimumab)-controlled period of the phase III trial SPIRIT-P1. Ann Rheum Dis. 2017;76(1):79–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Strober W, Fuss IJ. Proinflammatory cytokines in the pathogenesis of inflammatory bowel diseases. Gastroenterology. 2011;140(6):1756–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Fujino S, Andoh A, Bamba S, et al. Increased expression of interleukin 17 in inflammatory bowel disease. Gut. 2003;52(1):65–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hueber W, Sands BE, Lewitzky S, et al. , Secukinumab in Crohn's Disease Study Group. Secukinumab, a human anti-IL-17A monoclonal antibody, for moderate to severe Crohn's disease: Unexpected results of a randomised, double-blind placebo-controlled trial. Gut. 2012;61(12):1693–700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Fauny M, Moulin D, d'Amico F, et al. Paradoxical gastrointestinal effects of interleukin-17 blockers. Ann Rheum Dis. 2020;79(9):1132–8. [DOI] [PubMed] [Google Scholar]
- 10.Conforti C, Dianzani C, Zalaudek I, et al. Spotlight on the treatment armamentarium of concomitant psoriasis and inflammatory bowel disease: A systematic review. J Dermatol Treat. 2022;33(3):1279–86. [DOI] [PubMed] [Google Scholar]
- 11.Cleynen I, Vermeire S. Paradoxical inflammation induced by anti-TNF agents in patients with IBD. Nat Rev Gastroenterol Hepatol. 2012;9(9):496–503. [DOI] [PubMed] [Google Scholar]
- 12.Smith MK, Pai J, Panaccione R, Beck P, Ferraz JG, Jijon H. Crohn's-like disease in a patient exposed to anti-interleukin-17 blockade (ixekizumab) for the treatment of chronic plaque psoriasis: A case report. BMC Gastroenterol. 2019;19(1):162–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Whitlock SM, Enos CW, Armstrong AW, et al. Management of psoriasis in patients with inflammatory bowel disease: From the medical board of the national psoriasis foundation. J Am Acad Dermatol. 2018;78(2):383–94. [DOI] [PubMed] [Google Scholar]
- 14.Fiorino G, Allocca M, Correale C, et al. Positioning ustekinumab in moderate-to-severe ulcerative colitis: New kid on the block. Expert Opin Biol Ther. 2020;20(4):421–7. [DOI] [PubMed] [Google Scholar]
- 15.Stelara (Ustekinumab) for Intravenous and Subcutaneous Use (package insert). Horsham, PA: Janssen Biotech; 2017. (https://www.accessdata.fda.gov/drugsatfda_docs/label/2016/761044lbl.pdf). [Google Scholar]
- 16.Andrisani G, Marzo M, Celleno L, et al. Development of psoriasis scalp with alopecia during treatment of Crohn's disease with infliximab and rapid response to both diseases to ustekinumab. Eur Rev Med Pharmacol Sci. 2013;17(20):2831–6. [PubMed] [Google Scholar]
- 17.Matsumoto S, Mashima H. Efficacy of ustekinumab against infliximab-induced psoriasis and arthritis associated with Crohn's disease. Biologics. 2018;12:69–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wright S, Alloo A, Strunk A, Garg A. Real-world risk of new-onset inflammatory bowel disease among patients with psoriasis exposed to interleukin 17 inhibitors. J Am Acad Dermatol. 2020;83(2):382–7. [DOI] [PubMed] [Google Scholar]