Abstract
A nanocomposite membrane incorporating reactive Pd-Fe nanoparticles (NPs) was developed to remediate chlorinated aliphatic hydrocarbons (CAHs) from groundwater. Other than recapturing the produced Fen+ for in-situ regeneration, the functionalized polyanions prevented NPs agglomeration and resulting in a spherical Fe0 core (55 nm, O/Fe = 0.05) and an oxidized shell (4 nm, O/Fe = 1.38). The reactive membranes degraded 92% of target CAHs with a residence time of 1.7 seconds. After long-term treatment and regeneration, reusability was confirmed through recovered reactivity, recurrence of Fe0 in X-ray photoelectron spectroscopy, and >96% remaining of Fe and Pd. The total cost (adjusted present value for 20 years) was estimated to be 13.9% lower than the granular activated carbon system, following an EPA work breakdown structure-based cost model. However, non-target CAHs from groundwater can compete for active sites, leading to decreased surface-area normalized dechlorination rate by 28.2–79.9%. A hybrid nanofiltration (NF)/reactive membrane was proposed to selectively intercept larger competitors, leading to 54% increased dechlorination efficiency and 1.3 to 1.9-fold enlarged . Overall, the practical viability of the developed reactive membranes was demonstrated by the stability, reusability, and cost advantages, while the optional NF strategy could alleviate competitive degradation towards complex water chemistry.
Keywords: Reactive Membrane, Focused Ion Beam, Zero-Valent Iron, Competitive Dechlorination, Cost Estimation
Graphical abstract

1. Introduction
As typical micropollutants, chlorinated aliphatic hydrocarbons (CAHs) are notorious for their mobility and toxicity [1]. Parts of chlorinated methane and ethylene, such as carbon tetrachloride and trichloroethylene, have been regulated and listed in the substance priority list of the US Agency for Toxic Substances and Disease Registry. Although great efforts have been made in remediation [2], conventional incineration, landfill disposal, and bioremediation methods are limited by either the energy demand or the time-consuming process [3–5]. The reductive dechlorination methods using Fe-based nanoparticles (NPs), such as zero-valent iron (Fe0), bimetallic-Fe, and sulfidized-Fe [6–9], have exhibited significant advances in fast kinetic and effective degradation.
The agglomeration tendency of magnetic Fe-based NPs should be controlled to obtain high specific surface area, where polymeric stabilizers, such as carboxymethyl cellulose, were applied to disperse particles [10]. However, the high mobility of nanosized particles raises concerns regarding recycling and leaching. In addition, the reductive dechlorination pathway triggered by Fe-based NPs involves β-elimination, hydrogenolysis, and hydrogenation [6, 11, 12], where Fe0 is a reactant to either donate electrons or produce atomic hydrogen (water corrosion). The further implementation of Fe-based NPs in water treatment was thereby hindered by the consumed reactants and deactivation of Fe0 [13]. Novel methods are anticipated to address the above-mentioned concerns by not only controlling particle size, but also endowing reusability.
With advancements in energy-efficiency, ease of functionalization, and low space usage [14–16], membrane technologies have been regarded as one of the most effective water treatment methods. The potential for versatile applications can be achieved by incorporating nanoparticles into membrane domains due to their porous structure and flexibility [17–20]. Specifically, reactive NPs have been integrated to enable reductive/oxidative water remediation towards micropollutants and/or heavy metals [21–23]. Hybrid polysulfone/NPs membranes were developed to restrict the aggregation of NPs via the wet phase-inversion method, and 98% of NPs were also maintained due to the tightly associated microstructure of the hybrid membranes [24]. Other methods, such as the sol-gel process, ion exchange, in-situ reduction, and post-immobilization, have also been reported to improve the stability of the incorporate NPs into membrane domains [25–27]. Despite these numerous studies, the investigation of the incorporated NPs, especially to those inside the membrane pores, is limited by conventional characterization methods. This limitation not only impedes the observation of individual NPs for their morphology and composition, but also hinders the kinetic studies of the remediation process. Thus, advanced characterizations are anticipated to systematically elucidate the key properties of NPs and to understand the interaction between NPs and membrane domains for rationale design of such reactive membranes.
Moreover, the complex water chemistry and cost estimation are crucial aspects for practical viability of the reactive membranes. The potential competitive dechlorination reactions, with other chlorinated organic competitors from water sources, could significantly raise the reduction demand of Fe0 and harm the dechlorination efficiency of the reactive membranes [4, 28]. Methods are therefore anticipated to endow selectivity towards target contaminants for extended longevity and improved dechlorination efficiency, which requires an evaluation of the differences, in physical-chemical properties, among the target compounds and competitors. In addition, the cost estimation is one of the key considerations in technology transition and implementation, so comparisons between benchmarked remediation methods can not only enhance the practical implications of the reactive membrane but also guide its application in real water remediation.
Herein, the palladium-coated Fe0 (Pd-Fe) NPs incorporated membranes (r-PMA-PVDF) were designed for the remediation of target CAHs from real groundwater. Compared to the benchmarked air-stripping/granular activated carbon (GAC) system, the reactive membranes are designed to reductively degrade CAHs. The main goal of this study focused on (1) endowing reductive dechlorination functionalities on membrane domains for effective removal of target CAHs from the groundwater; (2) understanding the interaction between incorporated NPs and membrane matrix using advanced characterization methods; (3) evaluating the long-term performance and reusability under the real groundwater conditions; (4) elucidating the practical implication of the reactive membrane system via the cost comparison with GAC system, following an EPA work breakdown structure-based cost (WBS) model; and (5) implementing an optional nanofiltration strategy to selectively intercept competitors for alleviating competitive dechlorination.
2. Material and Methods
2.1. Materials
The following chemicals were used as received: sodium methacrylate (99%), potassium tetrachloropalladate (II) (98%), ammonium persulfate (APS, 98%), and N, N’-methylenebisacrylamide (NNMA, 99%) were obtained from Millipore Sigma. The analytical standards of carbon tetrachloride, trichloroethylene, tetrachloroethylene, and hexachloro-1,3-butadiene were purchased from Ultra Scientific. The polyvinylidene fluoride (PVDF) membrane (HVLP09050, pore size 0.45 μm) was also obtained from Millipore Sigma. The nanofiltration membranes (NF 270) were provided by Dow Inc.
2.2. Fabrication Methods
The synthesis method of reactive membranes was modified based on our previously reported methods [29]. The sodium methacrylate solution (8 wt.% of PVDF membranes), with initiator APS (0.5 wt.% of monomer) and cross-linker NNMA (2 wt.% of monomer), was pressurized across the PVDF membranes to rinse the membrane pores. With the thermal activation of radical polymerization (70°C for 1h), the Fe2+ was captured by the introduced carboxyl groups from polymethacrylic acid (PMA) and was then chemically reduced to form Fe0 NPs inside membrane pores by reductive agent NaBH4. The formed Fe0 NPs further reduced Pd2+ to form Pd islets. The designed reactive membranes (denoted as r-PMA-PVDF) were stored in ethanol before the test. To ensure a representative study in the intrinsic dechlorination rate of individual CAHs, The Pd-Fe NPs, with a similar size as the membrane phase, were directly synthesized in water phase and were then tested in a batch mode without competitors [30], where individual target contaminants were spiked into the simulated groundwater (similar pH and ion-strength as the real groundwater).
2.3. Characterization Methods
The surface hydrophilicity was analyzed using a drop shape analyzer (DSA 100, Krüss). The membrane cross-section sample was prepared and characterized using a focused-ion-beam scanning electron microscope (FIB-SEM, FEI Helios Nanolab 660). This preparation aims to create a smooth surface with less distortion of the samples, which enables the accurate observation of pore structures as well as the individual NPs inside the pores of membranes. The morphology and structure of individual Pd-Fe NPs were also analyzed using a transmission electron microscope (TEM, FEI Talos F200X). The introduced carboxylate groups were validated using an attenuated total reflectance-Fourier transform infrared spectroscopy (ATR-FTIR, PerkinElmer). The chemical composition was analyzed using X-ray photoelectron spectroscopy (XPS, ThermoFisher Nexsa), which uses an aluminum mono-chromatic X-ray source (1486.6 eV) and an electron flood gun for charge neutralization. The etching analysis was conducted for 50 levels at a total etching time of 500 s.
2.4. Analytical Methods
The EPA method 624 was applied to analyze CAHs, which includes a gas chromatography mass spectrometry (GC-MS, Varian 3900 GC, and Saturn 2100T MS) and a purge-and-trap auto-sampler. The initial temperature conditions for the oven, inlet, and detector were 40°C, 200°C, and 200°C, respectively. The oven was heated from 40°C to 130 °C at a rate of 8 °C/min and then up to 220 at 20 °C/min. Pentafluorobenzene was added as an internal standard, and the calibration curves of each CAHs were made from 1 to 1000 μg/L (R2 > 0.99). A chloride probe (Fisherband Accumet) was used to monitor the dechlorination results in real-time. A 2 vol.% ionic strength adjuster (5 M NaNO3) was added to each sample before measurement. The inductively coupled plasma mass spectrometry (ICP-MS, Agilent 7800) was used to determine the inorganic ions from the groundwater as well as the loading of reactive Pd-Fe NPs on the reactive membranes, where the reactive membranes were immersed in 20% nitric acid (50°C for 6 h) to dissolve incorporated Pd and Fe for ICP-MS analysis, and 20% nitric acid was also added in membrane permeates to test the leaching potential of Fe and Pd during the groundwater remediation.
2.5. Groundwater Remediation
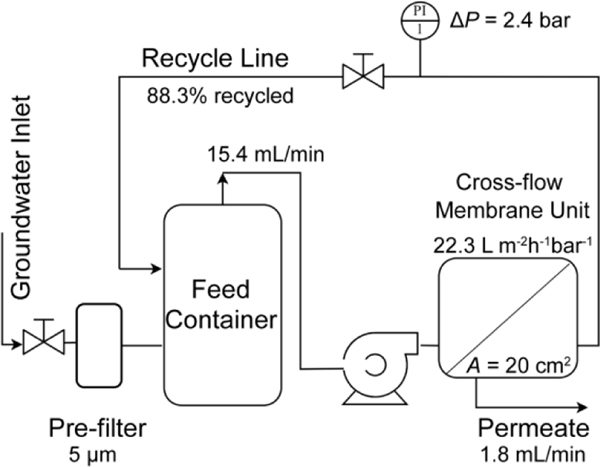
As shown in Fig.1, the reactive membrane system was mainly composed of a pre-filter, a feed container, and a cross-flow reactive membrane unit. A Keystone 5-micron filter cartridge was directly connected to the sampling port of groundwater pumping lines to filter the sediments. The pre-filtered groundwater was then treated by the synthesized reactive membranes (effective surface area: 20 cm2). The permeates were preliminarily analyzed using the chloride probe in real-time and then analyzed via GC-MS. The inlet flow was 15.4 mL/min, and the retentate flow was recycled back to the feed container (at 88.3% recycle rate). The valve was used to control the operating pressure (2.4 bar) through the membranes, which determines the residence time that contaminants spent inside the membrane pores. The removal efficiency was defined as eq.1. Due to the existence of chlorinated competitors in the groundwater, the dechlorination efficiency was defined in eq.2 as the quotient of the corresponding production of chloride ion (the mass balance of the degraded CAHs by the GC-MS) and the total observed addition of .
Figure 1.
The schematic of the developed reactive membrane system for groundwater remediation.
| (1) |
| (2) |
2.6. Regeneration
For the reductive dechlorination, Fe0 could produce atomic hydrogen via water corrosion for the Pd-meditated hydrogenation (eq.3–5) [11, 31, 32]. Hence, the regeneration of reacted Fe0 is essential for long-term usability. The used membranes were in-situ regenerated by passing through the reductive agent NaBH4 solution (10 mM). The deoxygenated water (N2 purging) was then applied to flush away the remained NaBH4. The XPS analysis was further applied to identify the changes in chemical composition during dechlorination and regeneration.
| (3) |
| (4) |
| (5) |
2.7. Cost Estimation
The benchmarked groundwater dechlorination system includes an air-stripping extraction segment and a regenerable granular activated carbon (GAC) adsorption column. Since an air-stripping process is not required for the developed reactive membrane technology, a hypothetical GAC treatment system was designed alone for a consistent comparison of the total cost. The required flows and pressures with the hypothetical design were simulated using the KYPIPE hydraulic simulation program (KYPIPE, 2020). Thus, the cost comparison between the hypothetical GAC and the reactive membrane system was evaluated in the aspects of capital cost as well as operation and maintenance cost (O&M). The work breakdown structure-based cost model (WBS model), developed by the US EPA, was used to evaluate the capital cost of the GAC system [33], where the costs of pumps and internal GAC pressure contactors were included. With the US Inflation calculator and federal discount rates from year 2020 to 2022, a series of inflation rates from of 0 % to 9.9%, and discount rates from 0% to 4.5% were applied to evaluate the total cost. To this regard, the future costs for every year up to 20 years were calculated using eq.6, and the annualized O&M costs were discounted to the present using eq.7.
| (6) |
| (7) |
Where is the adjusted present value, is the future value, and is the present value; is the inflation rate, is the discount rate, and is the number of years.
3. Results and Discussion
3.1. Properties of the Reactive Membranes
The original PVDF membranes, with micron-sized pores (Fig.2a), are suitable to functionalize PMA and NPs inside. The FIB sectioning method was applied to provide a smooth cross-section throughout the whole membrane with a thickness of 84.5 μm (Fig.2b). With the EDS line scan (Fig.2c and Fig.S1), the PMA functionalization decreased the average fluoride/carbon fraction (F/C) from 0.31 to 0.25, which indirectly prove the introduction of PMA since the PVDF backbone is the only source of fluorine. In addition, the F/C ratio was increased from the surface (0.21) to inside the pores (0.25 ± 0.01), which might be attributed to the formation of a PMA-rich zone on the surface of membranes. The characteristic peak of C=O stretching, at 1701 cm−1, occurred after PMA functionalization (Fig.2d), and the C-F stretching peak from the PVDF backbone was maintained at 1172 cm−1 [34]. Furthermore, the chemical composition was analyzed via XPS, which showed that the oxygen/carbon ratio (O/C) increased with a peak of Fe 2p occurring after functionalization (Fig.2e). Various components of carbon, such as C-C (BE at 284.8 eV), C=O (BE at 286.6 eV), O=C-O (BE at 288.8 eV), and C-F (BE at 290.3 eV), were detected as shown in Fig.S2, and the occurrence of C=O and O=C-O was consistent with the PMA functionalization [35]. Like the EDS observation, the XPS etching study also showed an increase in the F/C ratio from top to inside the pores (0.21 to 0.24 ± 0.01). The insignificant deviation in the F/C ratio, inside the membrane pores, further demonstrated the uniform functionalization of PMA inside the membrane domain (Fig.2f).
Figure 2.
SEM imaging of (a) the surface morphology of the pristine PVDF membranes, and (b) the cross-section of the reactive r-PMA-PVDF membranes (with FIB sectioning in the middle). (c) EDS scanning of fluorine/carbon ratio from the top to the bottom. (d) ATR-FTIR and (e) XPS analysis on the chemical compositions of the reactive membranes. (f) XPS etching results of the fluorine/carbon ratio.
The properties of r-PMA-PVDF membranes, such as water permeability and surface hydrophilicity, were included in Table 1. The water permeability was sharply decreased from 4639±299 to 22.3±1.2 L m−2h−1bar−1 (LMH/bar) after the PMA functionalization. Although the PMA-rich zone on the membrane surface was validated by the relatively lower F/C ratio, the insignificant changes in membrane thickness (from 83.4 to 84.5 μm) suggested the absence of a thick PMA layer on the top of the membrane domain. Furthermore, the membrane hydrophilicity was enhanced by the PMA functionalization, where the water contact angle decreased from 63.3° to 43.2° at pH 7.8.
Table 1.
Properties of original PVDF and functionalized r-PMA-PVDF membranes
| Membranes | PMA (wt.%) | Fe loading (mg/cm2) | Thickness (μm) | Water contact angle (°)* | Water permeability (LMH/bar)* |
|---|---|---|---|---|---|
|
| |||||
| Original PVDF | N/A | N/A | 83.4 ± 0.6 | 63.3 ± 1.9 | 4639 ± 299 |
| r-PMA-PVDF | 12.1 | 0.455 | 84.5 ± 0.9 | 43.2 ± 2.4 | 22.3 ± 2.1 |
Both water contact angle and water permeability were measured at similar pH conditions of the groundwater, i.e., pH 7.8.
Due to the constrained pore structure and the electrostatic repulsion among PMA chains, the agglomeration tendency of in-situ generated magnetic Pd-Fe NPs was controlled and the particle size was decreased from the surfaces to inside the membranes (from 169.5±47.3 nm to 58.8±16.3 nm, n=50, Fig.3a-3b and Fig.S3). The composition of NPs was analyzed via XPS in Fig.3c, where the scanned Fe 2p spectra were fit with doubles corresponding to 2p1/2 and 2p3/2. The characteristic peaks of Fe0 (BE: 707.3 eV), FeO (710.2 eV), and Fe2O3 (BE: 712.7 eV) were recognized [36], and the strong peak of Fe(II) and Fe(III) indicated the oxidization of reactive Fe0 [37]. To understand the oxidation of NPs, the chemical composition was also analyzed by using a high-angle annular dark field (HAADF). Since the signal brightness is strongly correlated to the atomic mass in HAADF mode, the core-shell structure was clearly observed with a brighter spherical core (54.8 nm in diameter) and a 3.8 nm thick darker shell (Fig. 3d). This structure was further investigated via EDS mapping (Fig.3e), that a ring of oxygen (matched with the darker shell, O/Fe = 1.38) was closely encircled to the Fe core (brighter core, O/Fe = 0.05). Due to the low fraction of Pd to Fe (0.5 wt.% in dose), insignificant signal of Pd was obtained using both the XPS and EDS analysis. This phenomenon was consistent with our previous study [30], and in which the high fraction of Pd (18.9 wt.% in dose) was intentionally introduced to observe the scattered distribution of Pd islets on Fe NPs. Hence, the Pd loading in the reactive membranes, in this study, was measured via ICP-MS as 0.31 wt.% of Fe.
Figure 3.
SEM imaging of the incorporated NPs (a) on the surface, and (b) inside the pores of membranes. (c) XPS analysis of the Fe 2p spectra. (d) HAADF imaging of the individual NPs, and (e) the corresponding EDS mapping of Fe (red), O (green), and Pd (blue).
3.2. Groundwater Remediation using r-PMA-PVDF Membranes
The key properties of the groundwater were summarized in Table 2. This project focused on three CAHs compounds including chlorinated methane, i.e., carbon tetrachloride (CTC), as well as chlorinated ethylene, i.e., trichloroethylene (TCE) and tetrachloroethylene (PCE). Other CAHs, such as hexachloro-1,3-butadiene (HCBD), were also detected at a concentration level of tens to hundreds μg/L. For dechlorination remediation, the operating pressure controls the residence time that the species across the reactive membrane matrix (eq.8).
Table 2.
Properties of the contaminated groundwater.
| pH | Cl− (mg/L) | Ca2+ (mg/L) | Mg2+ (mg/L) | CTC (μg/L) | TCE (μg/L) | PCE (μg/L) | HCBD (μg/L) |
|---|---|---|---|---|---|---|---|
|
| |||||||
| 7.8 | 22.7±0.3 | 113.2±4.3 | 34.1±2.6 | 58.9±2.3 | 158.9±4.7 | 637.1±7.1 | 221±3.8 |
As shown in Fig.4a, 97% and 91% removal of target CAHs was achieved at operating pressures of 1.0 and 2.4 bar, respectively (= 4.1 s and 1.7 s,), whereas only 51–68% removal was maintained at 5 bar (= 0.8 s). The lower removal efficiency at a higher operating pressure can be attributed to the insufficient residence time for dechlorination. Furthermore, the high linearity between and the residence time (R2>0.96) suggested the pseudo-first-order kinetics (in Fig.4a). Due to the low Reynold number (ranging from 12–63) at the given pressure range, the dechlorination behavior inside the membranes was considered as a plug-flow reactor, and the surface area normalized reaction rate can therefore be derived as eq.9–10.
Figure 4.
(a) The groundwater dechlorination using r-PMA-PVDF membranes at different operating pressures (Fe loading 9.1 mg, Pd was 0.31 wt.% of Fe, permeability 22.3 LMH/bar). The inserted image exhibited the fitting of pseudo-first-order kinetic. (b) The observed surface-area normalized reaction rate of the groundwater dechlorination as well as the intrinsic , the latter was derived from the batch dechlorination studies on the synthetic groundwater with individual target compounds (without competitors).
| (8) |
| (9) |
| (10) |
where is the observed reaction rate (min−1), and the surface area per unit mass was calculated as 12.7 m2/g based on the beforementioned particle size characterization (58.8 ± 16.3 nm in diameter). The loading density of NPs was derived as 53.8 g/L according to the ICP analysis. is the effective membrane area and is the membrane thickness. is the fraction of the void volume, which was derived as 29.4% by the water uptake measurement of the membranes.
As shown in Fig.4b, the observed of groundwater remediation followed the order of PCE < TCE < CTC in the groundwater treatment. The intrinsic was further evaluated in the batch phase and the individual CAHs was dechlorinated by the directly formed Pd-Fe NPs (Fig.S4). Although the same sequence in reaction rate was observed, the intrinsic , for individual CAHs, was found to be 1.4 to 4.8-fold greater than that of the observed . This difference could be attributed to the potential competitive dechlorination reactions. Furthermore, nearly 90% of CTC was firstly transformed to the dechlorination intermediate chloroform, whereas the conversion of dichloromethane remained below 2% (Fig.S4). As for chlorinated ethylene, TCE and dichloroethylene were not yielded in the dechlorination of PCE, and dichloroethylene was also absent in the TCE dechlorination. This dechlorination route was consistent with the reported literature [38], where Pd-Fe enhanced the complete dechlorination (about 88%), thus being converted to ethane.
Due to the difference in among target CAHs, above 98% of CTC was removed continuously during the first-2 h operation period (Fig.5a), while a slight decrease in reactivity was observed for TCE and PCE (from nearly 92% to 85%). The removal efficiency can be recovered after the regeneration process (NaBH4 regeneration), and the feasibility of NaBH4 regeneration was also validated by additional two regeneration cycles. As one of the products of dechlorination remediation, the produced chloride ion showed a similar pattern as the observed removal efficiency (Fig.5b). However, the increase in produced was found to be 3.6-fold higher than the maximum theoretical value (0.7 mg/L, from the mass balance of the complete dechlorination of three target species). The extra chloride production can be attributed to the competitive dechlorination of other CAHs, such as HCBD, from groundwater, which leads to a low dechlorination efficiency of 21.9%.
Figure 5.
(a) Groundwater remediation using r-PMA-PVDF membranes (operating pressure 2.4 bar); the regeneration cycles were denoted as Re-1 and Re-2, respectively. (b) The produced in permeate during remediation (the red dash line indicated the maximum surplus of for the complete dechlorination of the target CAHs in mass balance).
3.3. Integrated Nanofiltration for Competitive Dechlorination
To alleviate competitive dechlorination, methods are anticipated to selectively remove competitors prior to reductive dechlorination. Both target compounds and HCBD are hydrophobic and neutral molecules (Table S1), while HCBD presents a larger molecule equivalent width and higher molecular weight (Mw = 263 g/mol) [39]. The difference in molecule size could be utilized, so that additional nanofiltration membranes (NF 270, molecular weight cutoff 200–400 Da) were stacked on the top of the reactive membranes to intercept the larger CAHs (schematic in Fig.6a). Due to the relatively open pore structure, PMA-PVDF presented insignificant interception (<7%) of the CAHs from the groundwater. With the integrated NF membranes, 94.3% of HCBD was selectively rejected while less than 16% interception was observed for the target compounds with smaller equivalent widths (Fig. 6b). The rejection performance was found to be positively correlated with the molecule size (rejection: HCBD >> PCE > CTC > TCE), this was agreed with the size-exclusive separation mechanism [40]. The size-exclusive rejection of larger chlorinated competitors not only enhanced the by 1.9-, 1.6-, and 1.3-fold for CTC, TCE, and PCE, respectively, but also improved the selectivity towards the target CAHs (Fig. 6c). The produced [Cl-] decreased from 3.2 to 0.9 mg/L, which resulting in an increase of from 21.9% to 76.4%. Furthermore, 78% of Ca2+ and 72% of Mg2+ were also intercepted by the combined NF (Fig. S5), which decreased the water hardness from 401 mg/L to 83.8 mg/L (as CaCO3) and could inhibit the mineral passivation of reactive NPs [41].
Figure 6.
(a) The schematic of the optional nanofiltration strategy to alleviate competitive dechlorination. (b) The rejection of CAHs with and without NF membranes. (c) The enhancement of NF strategy in and dechlorination efficiency. (d) The groundwater remediation with the combined NF strategy (water permeability 11.2 LMH/bar).
It is important to note that the use of the NF strategy could decrease system water permeability (treatment capacity). A 1.9-fold increase in operating pressure was required to achieve a similar treatment capacity as the bare r-PMA-PVDF membranes (Fig. 6d). Although the NF method is effective to enhance the overall selectivity and alleviate the competitive dechlorination, the use of the NF strategy should consider the desired dechlorination performance and additional costs of material and energy, comprehensively. Due to the high removal efficiency achieved by r-PMA-PVDF membranes alone in this study, the NF strategy was not included in the further long-term remediation and cost estimation. However, the NF strategy showed the potential for combination with reactive membranes for improved selectivity and efficiency, especially for scenarios with significant competitors and/or co-contaminants.
3.4. Long-term Performance
The long-term remediation studies were further conducted to evaluate the stability and reusability of the designed r-PMA-PVDF membranes. As shown in Fig.7a, more than 92% removal of CTC, TCE, and PCE was achieved initially, while 24–30% decreases in removal were observed for all the target compounds after a continuous 24 h operation (equivalent to a total treatment capacity of 1273 L per m2 of membranes). This decay in reactivity has been reported to be the consumption of reactant Fe0, owing to both water corrosion (H2 evolution from Fe0 to Fe2+) and oxidation with dissolved oxygen [30, 42]. Although Pd-Fe NPs, with nanocluster or two-dimensional substrates, can be easily separated from liquid phase by a strong magnetic field for recycling, the continuously corrosion could decrease the NPs loading and lead to ineffective regeneration [43, 44]. With the introduced PMA polyanions, the dissolved iron ions were recaptured and were then reduced by NaBH4. As the changes in membrane appearance in Fig.7b, the recovery of high removal rates was present as 99.2%, 93.1%, and 92.1% for CTC, TCE, and PCE, respectively, while < 71% of removal was maintained in the blank control group with deionized (DI) water. The chemical composition was also evaluated via XPS spectra (in Fig.7c): the characteristic peaks of Fe0 disappeared after 24 h treatment, which demonstrated the further oxidation of Fe0 core; while the peaks of Fe0 regained after NaBH4 in-situ regeneration indicating the successful reductive regeneration. In addition, 97.4% of Fe and 96.6% of Pd maintained after 24 h treatment and regeneration (via ICP-MS), which indicated the stability of the in-situ generated NPs and the role of the introduced polyanions.
Figure 7.
(a) Long-term groundwater remediation using r-PMA-PVDF membranes with NaBH4 regeneration (a continuous 24 h treatment equivalent to 1273 L per m2 of membranes). (b) The digit photos for the reactive membranes at different stages during groundwater treatment. (c). The XPS analysis of r-PMA-PVDF membranes at initial, after 24 h treatment, and after regeneration.
The literature on Fe-based reactive membranes (as shown in Table 3) suggests that Fe-based particle agglomeration was controlled either by complexing with precursor Fen+ [45] or by constricting inside membrane pores [24]. However, majority of treatment studies were limited on DI water background, which ignored the impact of ubiquitous competitive degradation/adsorption. To address this issue, r-PMA-PVDF membranes were designed to remediate real groundwater with CAHs. With interferences from competitive dechlorination, the effective r-PMA-PVDF membranes achieved over 90% removal of the target CT, TCE, and PCE. Without dismantling the setup, the impaired reactivity after 24 hours of continuous treatment was recovered by in-situ regeneration. Overall, this long-term evaluation, with real groundwater and regeneration aspects, strengthened the practical implications of the Fe-based reactive membranes.
Table 3.
The comparison of Fe-based reactive membranes in water remediation (“regeneration conditions” was abbreviated as “Regen”).
| Membrane matrix | Fabrication | Contaminants | Particle size (nm) | Stability | Regen | Ref. |
|---|---|---|---|---|---|---|
|
| ||||||
| PVDF/PMA | Ion exchange/reduction | CAHs/groundwater | 58.8±16.3 | <4% leaching in 24 h | NaBH4 | This study |
| PVDF | Dry phase inversion | Nitrobenzene/synthetic water | 0–7000 | >90% removal at 8th cycle | N/A | [46] |
| Polysulfone | Wet phase inversion | Arsenic/DI water | ~67 | >99% uptake at 4th cycle | N/A | [24] |
| Cellulose acetate |
Wet phase inversion | Dye/textile effluent | 25–35 | N/A | N/A | [47] |
| PAN-copolymer | Electrospun | Arsenic/DI water | 70–100 | 90% removal at 3rd cycle | NaOH | [45] |
| Cation exchange | Ion exchange/reduction | TCE/DI water | 30–40 | <5% leaching in 4h | NaBH4 | [48] |
| SiO2 fiber | Calcination | Nimesulide/DI water | Doped | >85% removal at 5th cycle |
N/A | [49] |
3.5. Cost Evaluation
In the benchmarked air-stripping/GAC process, the chlorinated contaminants are stripped from groundwater by air stream, and the contaminants in vapor form are then adsorbed by GAC [50]. 95% removal of TCE vapor can be maintained using modified GAC for treating 20,000 bed volumes of feed, and thermal regeneration was then required [51]. The total cost, as the adjusted present value, is consist of capital cost and operation and maintenance (O&M) cost, where the present value of capital cost is adjusted by the Engineering News Record construction cost index (CCI). The details of cost estimation are summarized in SI section 4.
When considering the required treatment capacity of air-stripping/GAC process (4126 L/min) and the capacity of the developed reactive membrane (0.9 L/min per m2 of the membrane at 2.4 bar), an effective membrane area of 4584 m2 is required which equals the parallel use of 123 commercial spiral wound membrane modules (Synder Filtration, effective area: 37.2 m2). The total capital cost including membrane modules and other core facilities are summarized in Table S5. The membrane modules are associated with 62.6% of the whole capital cost. The design life of the reactive membrane modules is assumed to be 2 years and the NaBH4 regeneration is conducted every single day. This associated O&M cost is listed in Table S6.
The comparison of total cost is listed in Fig.8a, where membrane system is consistently cheaper than the GAC system (12.9%−17.5%) with a series of inflation rates and discount rates. This can be attributed to the 41.5% lower capital cost and comparable O&M cost. For instance, the and were 0.065% and 0.045%, respectively, at December 2022, and the total cost of the membrane system was derived to be 6.52 million US$, which is 13.9% less that of the GAC system (in Fig.8b). The regeneration cost was 16.5% lower in the membrane system compared to that of the GAC system, which is attributed to the mild regeneration conditions (room temperature) in the membrane system. In the GAC route, though, energy-intensive thermal regeneration conditions are required, which usually costs 25–60% of the total O&M cost of a commercial GAC system [52–54]. For membrane systems, the replacement of membrane modules should be more of a concern due to the high ratio (24.6%) in the annual O&M cost. Overall, the economic viability of the developed membrane technology is demonstrated compared with the GAC system.
Figure 8.
(a) The total cost (adjusted present value for 20 years) of both the GAC and membrane systems with a series of inflation rates, , and discount rates, . (b) The total cost of GAC and membrane systems at the specific (0.065%) and (0.045%) of December 2022.
4. Conclusion
Reactive r-PMA-PVDF membranes were developed for CAHs remediation in real groundwater. Above 92% removal of target CAHs was rapidly achieved at a residence time of 1.7 s. The long-term performance and reusability were also confirmed via a continuous 24 h treatment (equivalent treatment capacity of 1273 L per m2 of the membranes) and following in-situ regeneration and reuse cycles. Key findings were summarized as follows:
The stability and reusability were improved by the functionalized polyanions, which prevented NPs agglomeration (58.8±16.3 nm) and recaptured the produced Fen+ that >96% of reactive NPs were maintained after long-term remediation and regeneration cycles.
Compared to GAC system, reactive membranes could not only degrade CAHs to aliphatic hydrocarbons and chloride ions, but also avoided the energy-intensive thermal regeneration, which, as a part, leading to 13.9% decreases in total cost.
The competitive dechlorination, within target CAHs and among other competitors from groundwater, largely decreased the dechlorination rates by 79.9%, 46.8%, and 28.2% for CTC, TCE, and PCE, respectively.
A hybrid NF/reactive membrane option could alleviate competitive dechlorination, necessary in certain realistic water conditions, by selectively intercepting larger competitors, which results in 54.5% increased dechlorination efficiency and 1.3 to 1.9-fold enhanced .
The practical implications of the reactive membrane system were demonstrated by its advantages in cost, stability, and reusability. In addition, the NF strategy showed the potential for combination with reactive membranes for improved selectivity and efficiency, especially for scenarios with significant competitors and/or co-contaminants.
Supplementary Material
Highlights:
Reactive membranes degraded 92% of contaminants at 1.7 s residence time
The Fen+ from Fe0 corrosion was captured by -COOH for in-situ regeneration
Cost lowers 13.9% vs. granular activated carbon in work breakdown structure model
The competitive dechlorination decreased the by 28.2–79.2%.
Nanofiltration layer enhanced by 54.5% via selective rejection
ACKNOWLEDGMENTS
This work was supported by the NIEHS-SRP grant [P42ES007380]; the China Postdoctoral Science Foundation [2020M681204]; and the National Natural Science Foundation of China [22208097 & 22075076].
Footnotes
Hongyi Wan: Conceptualization, Methodology, Writing - Original Draft.; Md. Saiful Islam: Resources, Methodology.; Tahiya Tarannum: Formal analysis, Resources.; Ke Shi: Data Curation, Visualization.; Rollie Mills: Resources, Validation.; Zhiyuan Yi: Visualization.; Fumohan Fang: Data Curation.; Linfeng Lei: Methodology.; Siyao Li: Validation.; Lindell Ormsbee: Conceptualization, Validation.; Zhi Xu: Writing - Review & Editing, Supervision.; Dibakar Bhattacharyya: Conceptualization, Supervision.
Declaration of interests
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
The authors declare no competing financial interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Reference
- [1].Ahmed J, Thakur A, Goyal A, CHAPTER 1 Industrial Wastewater and Its Toxic Effects, Biological Treatment of Industrial Wastewater, The Royal Society of Chemistry, 2022, pp. 1–14. [Google Scholar]
- [2].Xu R, Xie Y, Tian J, Chen L, Adsorbable organic halogens in contaminated water environment: A review of sources and removal technologies, Journal of Cleaner Production, 283 (2021) 124645. [Google Scholar]
- [3].Aken BV, Correa PA, Schnoor JL, Phytoremediation of Polychlorinated Biphenyls: New Trends and Promises, Environmental Science & Technology, 44 (2010) 2767–2776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Ahmad S, Liu X, Tang J, Zhang S, Biochar-supported nanosized zero-valent iron (nZVI/BC) composites for removal of nitro and chlorinated contaminants, Chemical Engineering Journal, 431 (2022) 133187. [Google Scholar]
- [5].Li T, Li H, Li C, A review and perspective of recent research in biological treatment applied in removal of chlorinated volatile organic compounds from waste air, Chemosphere, 250 (2020) 126338. [DOI] [PubMed] [Google Scholar]
- [6].Fan D, Lan Y, Tratnyek PG, Johnson RL, Filip J, O’Carroll DM, Nunez Garcia A, Agrawal A, Sulfidation of iron-based materials: a review of processes and implications for water treatment and remediation, Environmental Science & Technology, 51 (2017) 13070–13085. [DOI] [PubMed] [Google Scholar]
- [7].Bae S, Collins RN, Waite TD, Hanna K, Advances in Surface Passivation of Nanoscale Zerovalent Iron: A Critical Review, Environmental Science & Technology, 52 (2018) 12010–12025. [DOI] [PubMed] [Google Scholar]
- [8].Xu J, Wang Y, Weng C, Bai W, Jiao Y, Kaegi R, Lowry GV, Reactivity, Selectivity, and Long-Term Performance of Sulfidized Nanoscale Zerovalent Iron with Different Properties, Environmental Science & Technology, 53 (2019) 5936–5945. [DOI] [PubMed] [Google Scholar]
- [9].Xu J, Liu X, Lowry GV, Cao Z, Zhao H, Zhou JL, Xu X, Dechlorination Mechanism of 2,4-Dichlorophenol by Magnetic MWCNTs Supported Pd/Fe Nanohybrids: Rapid Adsorption, Gradual Dechlorination, and Desorption of Phenol, ACS Applied Materials & Interfaces, 8 (2016) 7333–7342. [DOI] [PubMed] [Google Scholar]
- [10].He F, Zhao D, Manipulating the Size and Dispersibility of Zerovalent Iron Nanoparticles by Use of Carboxymethyl Cellulose Stabilizers, Environmental Science & Technology, 41 (2007) 6216–6221. [DOI] [PubMed] [Google Scholar]
- [11].Choi H, Al-Abed SR, Agarwal S, Dionysiou DD, Synthesis of Reactive Nano-Fe/Pd Bimetallic System-Impregnated Activated Carbon for the Simultaneous Adsorption and Dechlorination of PCBs, Chemistry of Materials, 20 (2008) 3649–3655. [Google Scholar]
- [12].Liu Y, Majetich SA, Tilton RD, Sholl DS, Lowry GV, TCE Dechlorination Rates, Pathways, and Efficiency of Nanoscale Iron Particles with Different Properties, Environmental Science & Technology, 39 (2005) 1338–1345. [DOI] [PubMed] [Google Scholar]
- [13].Pandey K, Sharma S, Saha S, Advances in design and synthesis of stabilized zero-valent iron nanoparticles for groundwater remediation, Journal of Environmental Chemical Engineering, 10 (2022) 107993. [Google Scholar]
- [14].Lim YJ, Goh K, Kurihara M, Wang R, Seawater desalination by reverse osmosis: Current development and future challenges in membrane fabrication–A review, Journal of Membrane Science, 629 (2021) 119292. [Google Scholar]
- [15].Sengupta A, Jebur M, Kamaz M, Wickramasinghe SR, Removal of Emerging Contaminants from Wastewater Streams Using Membrane Bioreactors: A Review, Membranes, 12 (2022) 60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Wan H, Shi K, Yi Z, Ding P, Zhuang L, Mills R, Bhattacharyya D, Xu Z, Removal of polystyrene nanoplastic beads using gravity-driven membrane filtration: Mechanisms and effects of water matrices, Chemical Engineering Journal, 450 (2022) 138484. [Google Scholar]
- [17].Mansourpanah Y, MXenes and other 2D nanosheets for modification of polyamide thin film nanocomposite membranes for desalination, Separation and Purification Technology, 289 (2022) 120777. [Google Scholar]
- [18].Esfahani MR, Aktij SA, Dabaghian Z, Firouzjaei MD, Rahimpour A, Eke J, Escobar IC, Abolhassani M, Greenlee LF, Esfahani AR, Sadmani A, Koutahzadeh N, Nanocomposite membranes for water separation and purification: Fabrication, modification, and applications, Separation and Purification Technology, 213 (2019) 465–499. [Google Scholar]
- [19].Fang X, Li J, Li X, Pan S, Zhang X, Sun X, Shen J, Han W, Wang L, Internal pore decoration with polydopamine nanoparticle on polymeric ultrafiltration membrane for enhanced heavy metal removal, Chemical Engineering Journal, 314 (2017) 38–49. [Google Scholar]
- [20].Islam MS, Hernández S, Wan H, Ormsbee L, Bhattacharyya D, Role of membrane pore polymerization conditions for pH responsive behavior, catalytic metal nanoparticle synthesis, and PCB degradation, Journal of Membrane Science, 555 (2018) 348–361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Zhang L-P, Liu Z, Faraj Y, Zhao Y, Zhuang R, Xie R, Ju X-J, Wang W, Chu L-Y, High-flux efficient catalytic membranes incorporated with iron-based Fenton-like catalysts for degradation of organic pollutants, Journal of Membrane Science, 573 (2019) 493–503. [Google Scholar]
- [22].Liu P, Wang X, Ma J, Liu H, Ning P, Highly efficient immobilization of NZVI onto bioinspired reagents functionalized polyacrylonitrile membrane for Cr(VI) reduction, Chemosphere, 220 (2019) 1003–1013. [DOI] [PubMed] [Google Scholar]
- [23].Aher A, Papp J, Colburn A, Wan H, Hatakeyama E, Prakash P, Weaver B, Bhattacharyya D, Naphthenic acids removal from high TDS produced water by persulfate mediated iron oxide functionalized catalytic membrane, and by nanofiltration, Chemical Engineering Journal, 327 (2017) 573–583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Georgiou Y, Dimos K, Beltsios K, Karakassides MA, Deligiannakis Y, Hybrid [polysulfone–Zero Valent Iron] membranes: Synthesis, characterization and application for AsIII remediation, Chemical Engineering Journal, 281 (2015) 651–660. [Google Scholar]
- [25].Li X, Sotto A, Li J, Van der Bruggen B, Progress and perspectives for synthesis of sustainable antifouling composite membranes containing in situ generated nanoparticles, Journal of Membrane Science, 524 (2017) 502–528. [Google Scholar]
- [26].Wang X, Wang N, Li X, An Q-F, A review of nano-confined composite membranes fabricated inside the porous support, Advanced Membranes, 1 (2021) 100005. [Google Scholar]
- [27].Saad A, Mills R, Wan H, Ormsbee L, Bhattacharyya D, Thermoresponsive PNIPAm–PMMA-Functionalized PVDF Membranes with Reactive Fe–Pd Nanoparticles for PCB Degradation, Industrial & Engineering Chemistry Research, 59 (2020) 16614–16625. [Google Scholar]
- [28].Wan H, Mills R, Wang Y, Wang K, Xu S, Bhattacharyya D, Xu Z, Gravity-driven electrospun membranes for effective removal of perfluoro-organics from synthetic groundwater, Journal of Membrane Science, 644 (2022) 120180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Wan H, Briot NJ, Saad A, Ormsbee L, Bhattacharyya D, Pore functionalized PVDF membranes with in-situ synthesized metal nanoparticles: Material characterization, and toxic organic degradation, Journal of Membrane Science, 530 (2017) 147–157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Wan H, Islam MS, Qian D, Ormsbee L, Bhattacharyya D, Reductive degradation of CCl4 by sulfidized Fe and Pd-Fe nanoparticles: Kinetics, longevity, and morphology aspects, Chemical Engineering Journal, 394 (2020) 125013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Su J, Lin S, Chen Z, Megharaj M, Naidu R, Dechlorination of p-chlorophenol from aqueous solution using bentonite supported Fe/Pd nanoparticles: Synthesis, characterization and kinetics, Desalination, 280 (2011) 167–173. [Google Scholar]
- [32].Wan H, Islam MS, Briot NJ, Schnobrich M, Pacholik L, Ormsbee L, Bhattacharyya D, Pd/Fe nanoparticle integrated PMAA-PVDF membranes for chloro-organic remediation from synthetic and site groundwater, Journal of Membrane Science, 594 (2020) 117454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].U.S.E.P. Agency, Work Breakdown Structure-Based Cost Model for Granular Activated Carbon Drinking Water Treatment, in, 2017. [Google Scholar]
- [34].Chiao Y-H, Chen S-T, Yap Ang MB, Patra T, Castilla-Casadiego DA, Fan R, Almodovar J, Hung W-S, Wickramasinghe SR, High-Performance Polyacrylic Acid-Grafted PVDF Nanofiltration Membrane with Good Antifouling Property for the Textile Industry, Polymers, 12 (2020) 2443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Zheng Y, Zhang P, Yue H, Xiang G, Qian Z, Li H, Jiang W, Liang B, Pehkonen SO, Yuan S, Poly(methacrylic acid)-graft-Ni3Si2O5(OH)4 multiwalled nanotubes as a novel nanosorbent for effective removal of copper(II) ions, Colloids and Surfaces A: Physicochemical and Engineering Aspects, 502 (2016) 89–101. [Google Scholar]
- [36].Imran MA, Tong Y, Hu Q, Liu M, Chen H, Effects of Persulfate Activation with Pyrite and Zero-Valent Iron for Phthalate Acid Ester Degradation, Water, 12 (2020) 354. [Google Scholar]
- [37].Greenlee LF, Torrey JD, Amaro RL, Shaw JM, Kinetics of Zero Valent Iron Nanoparticle Oxidation in Oxygenated Water, Environmental Science & Technology, 46 (2012) 12913–12920. [DOI] [PubMed] [Google Scholar]
- [38].Han Y, Liu C, Horita J, Yan W, Trichloroethene hydrodechlorination by Pd-Fe bimetallic nanoparticles: Solute-induced catalyst deactivation analyzed by carbon isotope fractionation, Applied Catalysis B: Environmental, 188 (2016) 77–86. [Google Scholar]
- [39].Yangali-Quintanilla V, Kim TU, Kennedy M, Amy G, Modeling of RO/NF membrane rejections of PhACs and organic compounds: a statistical analysis, Drink. Water Eng. Sci, 1 (2008) 7–15. [Google Scholar]
- [40].Shin MG, Choi W, Park S-J, Jeon S, Hong S, Lee J-H, Critical review and comprehensive analysis of trace organic compound (TOrC) removal with polyamide RO/NF membranes: Mechanisms and materials, Chemical Engineering Journal, 427 (2022) 130957. [Google Scholar]
- [41].Fang Y, Wu X, Dai M, Lopez-Valdivieso A, Raza S, Ali I, Peng C, Li J, Naz I, The sequestration of aqueous Cr(VI) by zero valent iron-based materials: From synthesis to practical application, Journal of Cleaner Production, 312 (2021) 127678. [Google Scholar]
- [42].Xu J, Avellan A, Li H, Clark EA, Henkelman G, R.l. Kaegi, G.V. Lowry, Iron and sulfur precursors affect crystalline structure, speciation, and reactivity of sulfidized nanoscale zerovalent iron, Environmental Science & Technology, 54 (2020) 13294–13303. [DOI] [PubMed] [Google Scholar]
- [43].Ma Y, Lv X, Yang QI, Wang Y, Chen X, Reduction of carbon tetrachloride by nanoscale palladized zero-valent iron@ graphene composites: Kinetics, activation energy, effects of reaction conditions and degradation mechanism, Applied Catalysis A: General, 542 (2017) 252–261. [Google Scholar]
- [44].Xu J, Tan L, Baig SA, Wu D, Lv X, Xu X, Dechlorination of 2,4-dichlorophenol by nanoscale magnetic Pd/Fe particles: Effects of pH, temperature, common dissolved ions and humic acid, Chemical Engineering Journal, 231 (2013) 26–35. [Google Scholar]
- [45].Thekkae Padil VV, Filip J, Suresh KI, Wacławek S, Černík M, Electrospun membrane composed of poly[acrylonitrile-co-(methyl acrylate)-co-(itaconic acid)] terpolymer and ZVI nanoparticles and its application for the removal of arsenic from water, RSC Advances, 6 (2016) 110288–110300. [Google Scholar]
- [46].Yang C, Li K, Xu L, Wang Z, Yu L, Wang J, Reduction of nitrobenzene by a zero-valent iron microspheres/polyvinylidene fluoride (mZVI/PVDF) membrane, Separation and Purification Technology, 282 (2022) 120006. [Google Scholar]
- [47].Saranya R, Arthanareeswaran G, Ismail AF, Dionysiou DD, Paul D, Zero-valent iron impregnated cellulose acetate mixed matrix membranes for the treatment of textile industry effluent, RSC Advances, 5 (2015) 62486–62497. [Google Scholar]
- [48].Kim H, Hong H-J, Lee Y-J, Shin H-J, Yang J-W, Degradation of trichloroethylene by zero-valent iron immobilized in cationic exchange membrane, Desalination, 223 (2008) 212–220. [Google Scholar]
- [49].Wei J, Bi J, Zhang L, Han D, Gong J, Gravity-driven Fe-doped CoTiO3/SiO2 fiber membrane with open catalytic network: Activation of peroxymonosulfate and efficient pollutants removal, Separation and Purification Technology, 280 (2022) 119975. [Google Scholar]
- [50].Miyake Y, Sakoda A, Yamanashi H, Kaneda H, Suzuki M, Activated carbon adsorption of trichloroethylene (TCE) vapor stripped from TCE-contaminated water, Water Research, 37 (2003) 1852–1858. [DOI] [PubMed] [Google Scholar]
- [51].Huang L, Yang Z, Li B, Hu J, Zhang W, Ying W-C, Granular activated carbon adsorption process for removing trichloroethylene from groundwater, AIChE Journal, 57 (2011) 542–550. [Google Scholar]
- [52].Adams JQ, Clark RM, Miltner RJ, Controlling organics with GAC: a cost and performance analysis, Journal-American Water Works Association, 81 (1989) 132–140. [Google Scholar]
- [53].Adams JQ, Clark RM, Lykins BW Jr, DeMarco J, Kittredge D, GAC treatment cost experience at two drinking water utilities, Journal of Environmental Engineering, 114 (1988) 944–961. [Google Scholar]
- [54].Lykins BW Jr, Clark RM, US drinking-water regulations: treatment technologies and cost, Journal of Environmental Engineering, 120 (1994) 783–802. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.