Abstract
Objective: Combining adeno-associated virus (AAV)-mediated expression of Cre recombinase with genetically modified floxed animals is a powerful approach for assaying the functional role of genes in regulating behavior and metabolism. Extensive research in diverse cell types and tissues using AAV-Cre has shown it can save time and avoid developmental compensation as compared with using Cre driver mouse line crossings. We initially sought to study the impact of the ablation of corticotropin-releasing hormone (CRH) in the paraventricular hypothalamic nucleus (PVN) using intracranial AAV-Cre injection in adult animals.
Methods: In the present study, we stereotactically injected AAV8-hSyn-Cre or a control AAV8-hSyn-GFP in both Crh-floxed and wild-type mouse PVN to assess behavioral and metabolic impacts. We then used immunohistochemical markers to systematically evaluate the density of hypothalamic peptidergic neurons and glial cells.
Results: We found that delivery of one specific preparation of AAV8-hSyn-Cre in the PVN led to the development of obesity, hyperphagia, and anxiety-like behaviors. This effect occurred independent of sex and in both floxed and wild-type mice. We subsequently found that AAV8-hSyn-Cre led to neuronal cell death and gliosis at the site of viral vector injections. These behavioral and metabolic deficits were dependent on injection into the PVN. An alternatively sourced AAV-Cre did not reproduce the same results.
Conclusions: Our findings reveal that delivery of a specific batch of AAV-Cre could lead to cellular toxicity and lesions in the PVN that cause robust metabolic and behavioral impacts. These alterations can complicate the interpretation of Cre-mediated gene knockout and highlight the need for rigorous controls.
Keywords: AAV, obesity, paraventricular hypothalamus, toxicity
Introduction
Located in the ventral forebrain, the paraventricular nucleus of the hypothalamus (PVN) acts as a major integrative center for diverse regulatory functions, including neuroendocrine response, autonomic tone, and complex behaviors such as food intake and social interaction [1–3]. Dissecting the molecular, morphological, and functional heterogeneity of the PVN has thus far given rise to several distinct cell types involved in the control of these processes.
Refining our understanding of how the PVN integrates and outputs multimodal information across neuroendocrine and autonomic pathways may inform several disorders, including cardiovascular, metabolic, and neuropsychiatric disorders [2,4,5]. However, the PVN contains spatially intermingled but functionally distinct populations that can only be selectively targeted through genetic means, such as conditional knockout (KO) by Cre-lox recombination [6,7]. While much work relies on promoter-driven Cre expression, another standard method relies on adeno-associated virus-mediated Cre expression (AAV-Cre) [8–11]. Methods such as AAV-Cre delivery in adult mice are valuable approaches to bypass transient developmental transcription and ensure high-fidelity expression in specific brain regions [8,12]. However, emerging evidence suggests that there may be deleterious effects associated with AAV-Cre, resulting in morphological and behavioral abnormalities even in the absence of loxP sequences [13,14].
In this study, we assessed the striking phenotype that emerged from the delivery of an AAV-Cre prepared virus in the PVN of adult homozygous floxed corticotropin-releasing hormone (Crhf/f) mice. PVN CRH neurons are tightly linked to energy homeostasis-they respond to food cues, and acute manipulations decrease motivation to seek and consume food [8,15,16]. Chronic manipulations have been shown to confer risk to diet-induced weight gain, as well [17]. While knockout of CRH has not been shown to contribute to changes in metabolism or food intake thus far, these methods have used Cre driver lines or homologous recombination in embryonic stem cells [18–20]. As has been suggested for discrepancies in findings using knockout models of the type-1 CRH receptor, we hypothesized that developmental compensation may mask the metabolic effects of CRH knockout [21,22]. We found that Crhf/f mice with putatively knocked out corticotropin-releasing hormone (CRH) via AAV-mediated expression of Cre recombinase in the PVN rapidly developed obesity and metabolic syndrome, which was an intriguing and unexpected finding. However, subsequent analyses for validation revealed that both CRH- and oxytocin-expressing neuron densities significantly decreased, likely due to cellular toxicity at the injection sites. Our study confirms the importance of the PVN in the control of metabolism and highlights the potential for toxicity due to a batch of a commonly used AAV, which necessitates careful interpretation and proper controls for experiments utilizing viral vectors.
Hypothesis
We hypothesize that the targeted disruption of corticotropin-releasing hormone (CRH) expression within the paraventricular hypothalamic nucleus (PVN) through intracranial AAV8-hSyn-Cre injection in adult animals may result in obesity and metabolic alterations.
Methods
Animals
All experiments involving animals were conducted at the Child Institute of New Jersey, Rutgers University, U.S.A. All protocols involving mice were approved by the Rutgers University Institutional Animal Care and Use Committee (IACUC) (Protocol # PROTO201702609) and by the National Institute of Health (NIH) guidelines. The animals used in the present study were 5 to 7 weeks old at the time of stereotactic surgery. Homozygous Crhf/f mice (gift from Dr. Larry Zweifel) [23], Glp1rf/f mice [24], and wild-type (C57BL/6) mice were used in the present study. For surgical injections, mice were anesthetized with isoflurane. For histological studies, mice were anesthetized with isoflurane and then followed by transcardial perfusion. For each experimental paradigm, littermate mice were randomized to each experimental group on the basis of body weight. Both males and females were used in this study. Mice of both groups were housed together. Investigators were blinded to treatment. The sample size required (n = 4–7) for each group was estimated from previous studies [18].
AAV vectors and stereotactic surgery
The AAVs used in the present study included pAAV-hSyn-EGFP (Addgene, #50465-AAV8, titer 1.6 × 1013 vg/ml), pENN.AAV.hSyn.HI.eGFP-Cre.WPRE.SV40 (Addgene, #105540-AAV8, lot: v107437, titer: 2.4 × 1013 vg/ml), and AAV-hSyn-GFP-Cre (UNC Viral Vector Core, AAV8, lot: AV5053E, titer: 2.5 × 1012 vg/ml). For stereotactic injections, mice were anesthetized with isoflurane and placed in a stereotactic frame. Burr holes were drilled above the injection sites. Approximately 150 nL of the viruses were bilaterally stereotactically injected in the PVN (AP: -0.7, ML: ±0.18, DV: −4.7) at a rate of 1 nL/s (eGFP for control group, Cre for experimental group). For experiments in the dorsolateral septum (dLS), 100 nL of viruses were bilaterally injected in the dLS (AP: +0.5, ML: ±0.45, DV: −2.7) at a rate of 1 nL/s. Mice recovered for at least 3 weeks prior to conducting behavioral experiments. Injection sites were confirmed by post hoc histological examinations.
Metabolic phenotyping
All experiments were conducted during the light cycle unless otherwise stated. Investigators were blinded to conditions during experiments.
Plasma corticosterone measurements
Blood was collected either at the beginning of the ITT test or during transcardiac perfusion. At least 10 μl was collected into EDTA-coated tubes and then centrifuged at 8000 rpm at 4°C for 5 min. Extracted plasma was stored at −80°C until further processing. Corticosterone measurements were obtained with an ELISA as per manufacturer’s instructions (Enzo Life Sciences, ADI-900-097).
Food intake
Mice were singly housed before the experiment with ad libitum access to normal chow (Purina Mouse Diet 5058) and water. Chow remaining in the cage after each day (12-h light/dark cycle) was measured for 5 days and averaged together.
EchoMRI/fat mass measurements
Whole body composition and fat mass were measured by EchoMRI (Echo Medical Systems, Houston, Texas).
Comprehensive Lab Animal Monitoring System (CLAMS) assays
Crhf/f mice were placed into metabolic phenotyping cages (Columbus Instruments, OH, U.S.A.). Data were recorded every 15 min. Data were collected for 3 days (both light and dark cycles) after 2 days of habituation to single housing within the cages.
Glucose tolerance test (GTT) and insulin tolerance test (ITT)
Mice were fasted overnight (GTT) or for 6 h (ITT). To minimize stress, 0.5 g of chow was provided. For GTT, mice were intraperitoneally (IP) administered 20% dextrose solution. Blood glucose measurements were collected at the 0, 15-, 30-, 60-, and 120-min time points. For ITT, mice were IP-administered insulin (1unit/kg). Injection volumes were 0.1 ml/10 g body weight. Blood glucose measurements were collected at the 0-, 15-, 30-, and 60-min time points.
Behavioral assays
Open field test
Mice were placed in the middle of a custom-made 45 × 45 cm square open field arena for 10 min. Analysis was performed using DeepLabCut v2.2 [25]. We labeled 150–200 frames taken from 14 or 10 videos, as resolution changed between recording sessions (95% of frames were used for training, 5% for testing). We used a ResNet50-based network with default parameters for 225,000 or 450,000 training iterations (until loss plateaued). After analyzing open-field videos, we then calculated the time spent in the inner zone (inner 3/5 of the arena, ∼25 × 25 cm) using a region of interest tool. Distance traveled was calculated with custom code. Investigators were blinded to the condition during analysis.
Light-dark box test
Mice were placed in the SmartCage system (AfaSci) light side of a commercial light-dark box apparatus consisting of one dark and one illuminated compartment connected by a door. Time spent in each zone, latency to enter the dark zone, and number of entries were measured for 10 min. Data were analyzed using Excel.
Histology and immunohistochemistry (IHC) analysis
Mice used for IHC analysis of CRH, and oxytocin (OXT) were intracerebroventricularly administered colchicine a day prior to perfusion. Mice were anesthetized with isoflurane and perfused with PBS (pH 7.4) and then 4% paraformaldehyde (PFA). Coronal brain slices were cryosectioned (50 µm). For IHC analyses, brain slices were first incubated in blocking buffer (4% BSA, 1% goat serum and 0.2% Triton X-100 in PBS) for 1 h, then in primary antibody overnight at 4°C. Slices were washed with blocking buffer 3 × 15 min prior to incubation with a secondary antibody at RT for 2 h, followed by further washing with PBS. Sections were mounted with Fluoroshield with DAPI (Sigma-Aldrich, # F6057). Images were collected with a Zeiss LSM700 confocal microscope. A Z-stack captured the entire thickness of the section with fluorescence. NeuN-stained slices were imaged at a 5 µm-thickness interval for a total range of 25 µm. Imaging analysis was performed using NIH-ImageJ and with the Allen Brain Atlas as a reference. The number of OXT- and CRH-cells in the PVN was counted with the Cell Counter plugin. To quantify NeuN density, images were background subtracted (20-pixel radius) before converting to a binary image. Then, segmented particles were filtered by despeckling twice and watershed transforming, followed by final counting (minimum size of 7 µm2). GFAP and Iba1 fluorescence area were calculated by dividing the area of thresholded signal by total PVN area. The quantifications were performed by two independent investigators who were blinded to the condition during analysis.
Antibodies
The primary antibodies used in this study were rabbit Anti-CRH (1:500, BMA Biomedicals, #T-4037, RRID: AB_2314240), mouse Anti-OXT (1:500 Sigma-Aldrich, #MABN844), rabbit Anti-NeuN (1:500, Millipore, #ABN78, RRID: AB_10807945), rabbit Anti-GFAP (1:1000, Dako, #Z0334, RRID: AB_10013382), and chicken Anti-Iba1 (1:500, Aves Labs, #IBA1-0020).
Statistical analysis
Statistical analyses were performed using GraphPad Prism 9. Data were analyzed using Student’s two-tailed t-tests and two-way ANOVA followed by Bonferroni’s multiple comparisons tests. Data are presented as mean ± SEM. A P-value < 0.05 was considered statistically significant.
Results
Delivery of AAV-mediated Cre in the PVN rapidly induces behavioral changes and metabolic syndrome in male Crhf/f mice
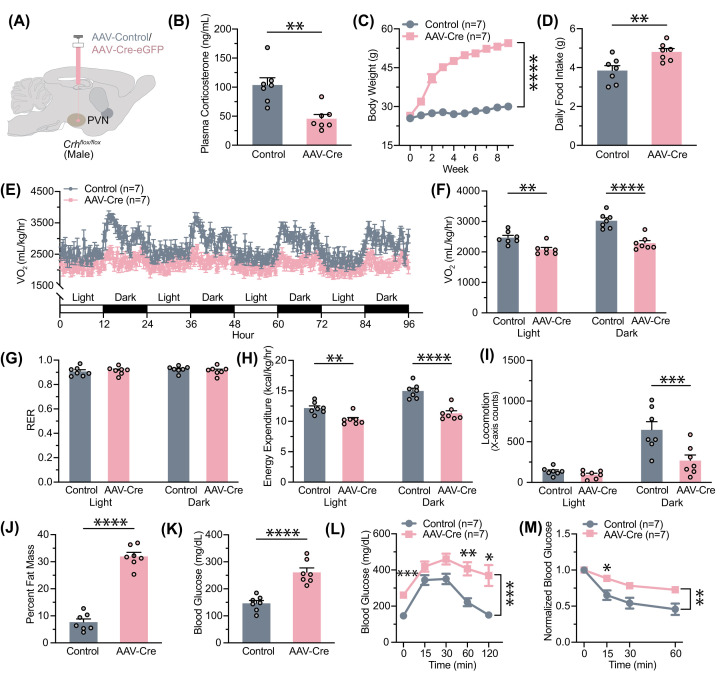
Our initial goal was to appraise the functional role of CRH by knocking it out in the PVN in adult animals. To this end, we bilaterally stereotactically injected AAV8-hSyn-eGFP-Cre in the PVN of 5- to 7-week-old Crhf/f mice, with AAV8-hSyn-eGFP (Control) injected in littermate controls (Figure 1A). After 4 weeks of recovery, we found that plasma corticosterone levels in Cre-injected Crhf/f mice were markedly reduced, which was highly suggestive of possible loss of function of CRH (Figure 1B). Even with normal chow feeding, the Cre-injected mice showed robust and rapid body weight gain within days. Cre-injected mice reached an average of 50.5 ± 0.7 g in 6 weeks, while the average body weight of control animals was 28.2 ± 0.7 g (Figure 1C). The increased body weight in the Cre-injected group might be, at least in part, due to elevated food intake (Figure 1D). We repeated these experiments in an independent cohort of Crhf/f female mice (Supplementary Figure S1A). In this, the Cre-injected group fed with normal chow weighed 45.2 ± 4.2 g 6 weeks after viral injection (Supplementary Figure S1B), indicating that the changes observed thus far occur in a sex-independent manner.
Figure 1. AAV-Cre administration in PVN of male Crhf/f mice results in the development of metabolic syndrome.
(A) Experimental paradigm for virus delivery. AAV-Cre or AAV-eGFP was stereotactically injected into the adult male murine PVN. (B) Plasma corticosterone decreased following AAV-Cre injection (t (12) = 4.011, P=0.0017). (C) Changes in body weight following virus delivery at week 0 (two-way ANOVA, main effect of Group: F (1, 12) = 294.1, P<0.0001; main effect of Time: F (1.378, 16.53) = 228.5, P<0.0001; interaction between Group and Time: F (9, 108) = 138.4, P<0.0001. (D) Daily chow intake following virus injection increased with AAV-Cre (t (12) = 3.152, P=0.0083. (E) Changes in oxygen consumption were observed over the course of 96 h. (F) Quantification of the decrease in oxygen consumption in AAV-Cre-injected mice (two-way ANOVA with Bonferroni’s multiple comparisons test, main effect of Group: F (1, 12) = 25.11, P=0.0003; main effect of Time: F (1, 12) = 208.4, P<0.0001; interaction between Group and Time: F (1, 12) = 43.58, P<0.001. (G) The respiratory exchange ratio (RER) was unaltered (two-way ANOVA, no main effect of Group: F (1, 12) = 0.02021, P=0.8893; no main effect of Time: F (1, 12) = 2.970, P=0.1105; no interaction between Group and Time: F (1, 12) = 3.566, P=0.0834. (H) Energy expenditure decreased following AAV-Cre delivery (two-way ANOVA with Bonferroni’s multiple comparisons test, main effect of Group: F (1, 12) = 26.31, P=0.0002; main effect of Time: F (1, 12) = 207.9, P<0.0001; interaction between Group and Time: F (1, 12) = 45.10, P<0.0001. (I) Locomotion in the X-axis in the CLAMS cage decreased during the dark cycle (two-way ANOVA with Bonferroni’s multiple comparisons test, main effect of Group: F (1, 12) = 8.461, P=0.0131; main effect of Time: F (1, 12) = 41.86, P<0.0001, interaction between Group and Time: F (1, 12) = 10.17, P=0.0078. (J) Proportion of fat mass as determined by EchoMRI increased in AAV-Cre-injected mice (t (12) = 12.67, P<0.0001). (K) Changes in average blood glucose after overnight fasting (t (12) = 5.888, P<0.0001). (L) The glucose tolerance test reveals increased baseline blood glucose levels (two-way ANOVA with Bonferroni’s multiple comparisons test, main effect of Group: F (1, 12) = 22.30, P=0.0005; main effect of Time: F (2.717, 32.60) = 24.72, P<0.0001; interaction between Group and Time: F (4, 48) = 3.111, P=0.0235). (M) The insulin tolerance test reveals insulin tolerance (two-way ANOVA with Bonferroni’s multiple comparisons test, main effect of Group: F (1, 11) = 11.30, P=0.0063; main effect of Time: F (1.712, 18.84) = 55.72, P<0.0001; no interaction between Group and Time: F (2, 22) = 0.6622, P=0.5257). Sample sizes are as shown on graphs (n = 7 mice per group). Data are presented as individual points and mean ± or + SEM. *P<0.05; **P<0.01; ***P<0.001; ****P<0.0001.
As PVN-derived CRH is known to be involved in stress-related behaviors, coping, and relief [16,26–28], we hypothesized that reduced CRH release and corticosterone levels (Figure 1B) in the Cre-injected animals would be anxiolytic. To test this hypothesis, we examined anxiety-like behavior in AAV-Cre injected Crhf/f male mice in the open-field and light/dark box assays (Supplementary Figure S2). Contrary to what we hypothesized, we found that the Cre-injected mice spent less time in the center zone in the open-field assay (Supplementary Figure S2B), and they spent more time in the dark zone during the light/dark box test (Supplementary Figure S2D). These tests suggested that AAV-Cre in the adult PVN may lead to heightened anxiety-like behaviors.
Nevertheless, the drastic increase in body weight of AAV-Cre-injected animals prompted us to further assess their metabolic phenotype. We used CLAMS to measure energy intake and expenditure, as well as to identify any changes in nutrient utilization. Compared with AAV-GFP-injected animals, AAV-Cre-injected mice consumed less oxygen throughout the entire day (Figure 1E,F). The respiratory exchange ratio was equal between both groups, indicating no shift in nutrient utilization accompanying increases in weight (Figure 1G). Energy expenditure was significantly decreased in AAV-Cre-injected mice in both light and dark cycles (Figure 1H). This may be in part due to a decrease in locomotor activity (Figure 1I). These data suggest that alterations in energy expenditure may contribute to the development of obesity alongside hyperphagia in AAV-Cre-injected mice. Consistent with these findings, Cre-injected mice display significantly increased body fat composition (Figure 1J) and significantly increased fasting plasma glucose levels measured (Figure 1K). To evaluate any changes in glucose and insulin homeostasis, we also performed GTT and ITT. GTT revealed that Cre-injected mice had consistently elevated glucose levels, indicative of higher glucose tolerance (Figure 1L), while ITT revealed that they had insulin resistance (Figure 1M). These results indicate that Cre recombinase expression in the PVN region of Crhf/f mice leads to obesity development and maintenance through changes in food intake behavior and energy expenditure and causes metabolic syndrome resembling type 2 diabetes mellitus.
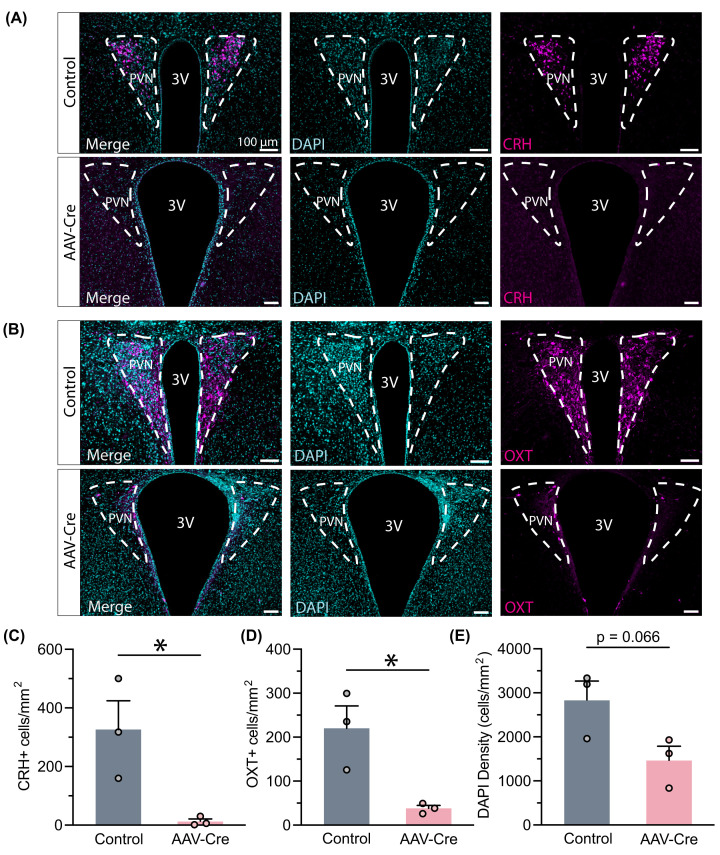
Having observed the development of diabetes and obesity in the PVN Cre-injected Crhf/f mice, we sought to validate these findings as attributable to the depletion of hypothalamic CRH. We examined CRH expression using immunohistochemistry and found decreased CRH-positive cell density in the PVN of AAV-Cre-injected mice (Figure 2A,C). This suggested that CRH expression was suppressed, which supports the hypothesis that PVN CRH participates in regulating metabolism. However, this hypothesis is complicated by the observation of decreased OXT expression in brain sections of the same animals with Cre injections (Figure 2B,D). Decreased expression of both CRH and OXT was accompanied with a trend toward reduced cell density as measured by DAPI staining (Figure 2E). Based on these data, we caution that virally expressed Cre recombinase may be sufficient to deplete CRH from the PVN of Crhf/f animals but may also have an off-target impact on PVN neuronal cells.
Figure 2. AAV-Cre action in PVN of Crhf/f mice depletes CRH and OXT.
(A) Example comparison of CRH expression in PVN between both groups. (B) Example of OXT expression in PVN. (C) CRH-positive cell density decreased following AAV-Cre expression in Crhf/f PVN (t (4) = 3.176, P=0.0337). (D) OXT-positive cell density decreased as well (t (4) = 2.807, P=0.0236). (E) Overall cell density trended toward a decline (t (4) = 2.511, P=0.0660). Sample sizes are as shown on graphs (n = 3 mice per group). Data are presented as individual points and mean + SEM. *P<0.05.
Delivery of AAV-mediated Cre in the PVN rapidly alters behavior and causes metabolic syndrome in wild-type animals
While OXT expression may have decreased due to the development of obesity, the trend of decreased cell density in the AAV-Cre group led us to hypothesize that the AAV-Cre virus may impact PVN neuronal survival. The toxicity of Cre expression on cellular health has been reported previously [13,14,29,30].
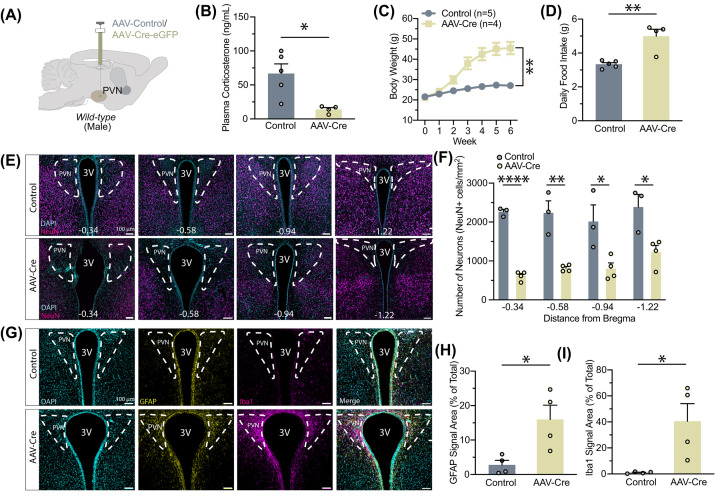
To this end, we examined the impacts of AAV-Cre in C57BL/6 wild-type (WT) mice (Figure 3A). Similar to the AAV-Cre-injected Crhf/f animals, we found a marked reduction in plasma corticosterone levels in WT mice injected with AAV-Cre (Figure 3B). They also gained significant body weight potentially due to hyperphagia (Figure 3C,D). Given that our observations closely mirrored those found in Crhf/f mice, we also assessed changes in anxiety-like behaviors (Supplementary Figure S3A and S3C). AAV-Cre-injected mice spent less time in the center zone during the open field test (Supplementary Figure S3B). Consistent with this, Cre mice spent more time in the dark zone of the light/dark box (Supplementary Figure S3D). These data suggest that the behavioral, metabolic, and cellular alterations observed with Cre injection in the PVN may be due to the expression of the Cre-encoding viral vector.
Figure 3. AAV-Cre action in wild-type PVN induces metabolic changes, neuronal cell death, and recruitment of glia.
(A) Experimental paradigm for virus delivery in wild-type male mice. (B) Plasma corticosterone levels decreased after AAV-Cre injection (t (7) = 3.260, P=0.0139). (C) Body weight changes followed virus delivery (two-way ANOVA, main effect of Group: F (1, 7) = 22.37, P=0.0021; main effect of Time: F (1.134, 7.940) = 191.5, P<0.0001; interaction between Group and Time: F (6, 42) = 79.71, P<0.0001). (D) Daily food intake increased in AAV-Cre-injected mice (Mann–Whitney test, U = 0, P=0.0159). (E) Example images of neuronal nuclei (NeuN) across the PVN following injection in wild-type mice. (F) Quantification of neuron density at different stereotactic coordinates: −0.34 (t (5) = 18.72, P<0.0001), −0.58 (t (5) = 5.343, P=0.0031), −0.94 (t (5) = 3.053, P=0.0283), and −1.22 (t (5) = 3.372, P=0.0199). (G) Example comparison of glial fibrillary acidic protein (GFAP) and ionized calcium binding adaptor molecule 1 (Iba1) expression in the wild-type PVN between the two groups. (H) Expression of astrocytes increased as quantified by GFAP fluorescence area (t (6) = 3.017, P=0.0235). (I) Expression of microglia increased as quantified by Iba1 fluorescence area (t (6) = 2.917, P=0.0267). Sample sizes are as shown on graph: (F) n=3 control, 4 AAV-Cre (H,I) n = 4 per group. Data are presented as individual points and mean ± or + SEM.N numbers are indicated in each plot; ∗p < 0.05; ∗∗p < 0.01; ∗∗∗p < 0.001; ∗∗∗∗p < 0.0001.
We next systematically evaluated the number of neurons in the PVN of WT mice following AAV-Cre or AAV-GFP injections using neuronal nuclei (NeuN) staining (Figure 3E). Expression of NeuN was significantly decreased across most of the PVN with the administration of AAV-Cre, likely due to diffusion (Figure 3F). Again accompanied by the enlarged visual appearance of the third ventricle, the widespread decline in neuronal density in AAV-Cre-expressing mice lends support to the idea of toxic and nonspecific AAV-Cre action, resembling the effects of a chemical lesion for PVN neuronal cells [31,32]. This neuronal cell loss is accompanied by astrogliosis and microglial cell activation (Figure 3G–I).
Together, these data suggest that AAV-Cre injection may cause non-specific cell death at the injection site, with PVN neuronal ablation causing obesity and diabetes. This is consistent with previous reports that PVN lesions result in severe obesity and dysregulated metabolism [31–35].
Delivery of AAV-mediated Cre in the dorsal lateral septum induces neuronal cell lesions without causing metabolic syndrome in Glp1rf/f animals
To further confirm the apparent toxicity of the AAV-Cre that we used, we tested it in another brain region: the dorsal lateral septum (dLS). The glucagon-like peptide-1 receptor (GLP-1R) is highly expressed in dLS and is implicated in feeding and metabolism [36–39]. We assessed if a diluted AAV-Cre could be used to determine endogenous GLP-1R function. To prevent the possibility of cellular toxicity due to high AAV titers, we diluted our AAV to 5 × 1012 vg/mL and only injected 100 nl. We injected this AAV-Cre or a control vector in Glp1rf/f mouse dLS (Supplementary Figure S4A), but we did not observe significant changes in body weight or metabolism within 4 weeks of injection (Supplementary Figure S4B). However, we found significantly decreased NeuN positive cell density (Supplementary Figure S4C,D), suggesting that Cre viral vector injection in the mouse brain causes neuronal cell loss.
Delivery of an alternative preparation of AAV-Cre in PVN avoids cell death and body weight changes in Crhf/f mice
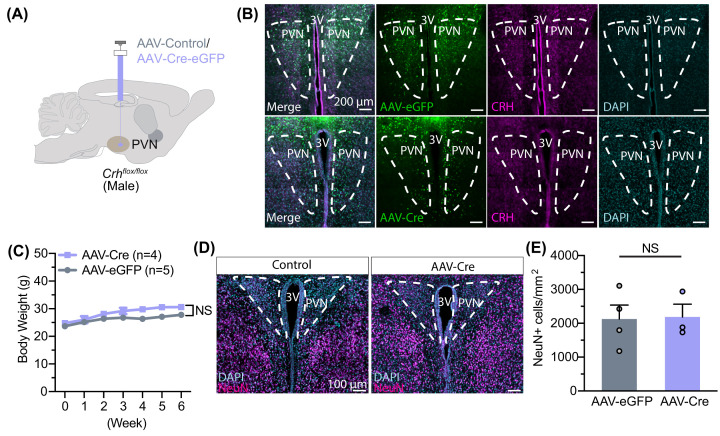
We next tested if our findings were representative of all viral vectors delivering Cre recombinase. We injected a preparation of AAV8-hSyn-Cre-GFP (UNC Vector Core) at the provided titer (2.5 × 1012 vg/mL) in Crhf/f male mouse PVN (Figure 4A,B). We found minimal changes in body weight after 6 weeks of injection (Figure 4C), suggesting that toxicity was attenuated. Accordingly, we found no changes in neuronal cell density (Figure 4D,E; Supplementary Figure S5A,B). Together, these results point toward disparate and variable effects of AAV-Cre viruses, potentially due to preparation or construction of the specific batch of viruses.
Figure 4. An alternative preparation of AAV-Cre does not lead to cell death in PVN.
(A) Experimental paradigm for virus delivery in male Crh-flox mice. (B) Example images of CRH staining in the PVN after AAV-GFP or AAV-Cre injection. (C) Body weight does not change following delivery (two-way ANOVA, main effect of Group: F (1, 7) = 5.304, P=0.0547; main effect of Time: F (6, 42) = 19.71, P<0.0001; interaction between Group and Time: F (6, 42) = 1.729, P=0.1379). (D) Example images of NeuN density in PVN following injection. (E) Neuron density did not change following AAV-Cre injection (t (5) = 0.1005, P=0.9238). Data are presented as individual points and mean ± or + SEM, NS, no statistical significance.
Discussion
In the present study, we showed that delivery of one preparation of AAV-Cre recombinase in the PVN of adult mice can lead to the development of metabolic syndrome and alterations in food-related and anxiety-like behaviors, as well as metabolic phenotype. We demonstrated that Crhf/f mice expressing AAV-Cre in PVN develop obesity largely mediated by hyperphagia and changes in energy expenditure. Moreover, this develops alongside glucose tolerance and insulin resistance, as well as decreased locomotion. We investigated whether CRH was truly and specifically depleted and found off-target effects on OXT expression (Figure 2) and cell loss at sites of Cre viral vector injections in either Crhf/f or WT mice. These data suggested off-target effects of our viral vector and indicate that caution should be used when interpreting the physiological consequences of AAV injection in adult animals. Intriguingly, we found that another preparation of AAV-Cre did not lead to lesions in the PVN. Differences in how these viral vectors (batches, lots, or other procedural variations) were constructed or prepared may potentially be the source of this disparity. Variability in viral vector quality further accentuates the need to validate reagents prior to experimental use.
Specific depletion of PVN-derived CRH has thus far not been shown to induce massive metabolic dysregulation when knocked out using Cre expression driven by either Crhf/f crossed with Sim1-Cre mice or CRISPR [18,40]. Our initial goal was to assess the impact of ablation of PVN CRH in adult animals by using stereotactic injection of AAV-Cre. We observed massive obesity and metabolic syndromes that starkly differ from other models of CRH signaling deficiencies [18,20,40–43]. CRH-deficient mice have intact feeding behavior, even after stress [41]. Deletion of the type-1 CRH receptor yields conflicting results on food intake but largely minimal effects on body weight [22,44]. Global deletion of the type 2 receptor similarly results in no changes in body weight despite significant increases in food consumption, likely due to increased energy expenditure and sympathetic tone [45]. Despite the observation of reduction of CRH expression and plasma corticosterone levels, which may be an indication of impaired regulation of the hypothalamic–pituitary–adrenal axis (HPA), we surmise that any metabolic or behavioral deficits reflected off-target effects of AAV-Cre. Indeed, we confirmed that neuronal cell death and reactive gliosis occurred in the PVN with AAV-Cre expression, resembling a response to central nervous system injury. Therefore, the study cannot conclude whether adult ablation of CRH in the PVN leads to metabolic or behavioral changes in adult animals. While we have preliminarily shown that an alternatively sourced AAV-Cre does not cause significant body weight changes in Crhf/f mice, further behavioral and metabolic phenotyping-as well as validation that CRH is fully knocked out-must be performed in future studies to concretely resolve this aim.
The PVN is speculated to provide top-down control of feeding-related information processing via intrahypothalamic connections or dense projections to midbrain and hindbrain regions [2]. These results could not provide insights on CRH impact on metabolism but highlight the necessity of the PVN in regulating energy homeostasis and body weight. These findings are in accord with prior ablation experiments and more recent work on genetic knockout models specific to the PVN [31,32]. It has been shown that unilateral PVN electrolytic lesions would increase lipid accumulation in white adipose tissue ipsilateral to the side of the PVN [46]. Chronically inhibiting PVN MC4R/PDYN neuron synaptic release by expressing tetanus toxin or ablating those neurons by expressing caspase-3 resulted in pronounced hyperphagia, obesity, and a significant elevation in food consumption [32]. Our metabolic phenotyping data revealed that cellular lesions of PVN develop a strong obesity phenotype largely mediated by hyperphagia and changes in energy expenditure. Moreover, dysfunction of the PVN induces hypersomnia and mediates the diurnal rhythm of metabolism in mice [47,48]. Consistent with this, we also found that changes in energy expenditure were more pronounced in AAV-Cre-injected mice during dark cycles (Figure 1H).
Manipulating gene expression on and off using AAVs in genetically engineered animals is one of the most powerful tools to bypass transient developmental transcription for studying gene function in vivo [8,12]. For example, it has been shown that knockout of Glp1r via AAV-Cre in PVN demonstrated a significant obesity phenotype, which was not observed in Glp1rf/f mice crossed with Sim1- or Nkx2.1-Cre mice [8,49]. Similarly, AAV-CRISPR–Cas9-mediated ablation of leptin receptor in arcuate nucleus demonstrated that agouti-related peptide (AgRP) neurons but not proopiomelanocortin (POMC) neurons are required for the primary action of leptin to regulate both energy balance and glucose homeostasis [12]. The present results add to a growing body of literature suggesting that toxicity occurs irrespective of serotype and brain region, though a systematic comparison is lacking. While we show toxicity with one viral preparation in this study, previous studies have reported varying levels of success with this AAV-Cre and others [9,13,14,48,50]. In the mesolimbic reward system, it has been reported that Cre expression in the ventral tegmental area and substantia nigra can induce toxicity associated with perturbations of dopamine-related behaviors [13,14]. Further work delineating different viral preparations and optimal titers for neuronal manipulations will be needed. Inducible systems of Cre expression, such as those using tamoxifen, offer an appealing alternative to constitutively expressed Cre systems; however, administration of tamoxifen has also been reported to lead to cytotoxicity and off-target effects [51].
Our data confirm that the PVN is an important brain region in the regulation of metabolism; dysfunction of the PVN, such as via neuronal death, causes obesity and Type 2 diabetes. We also conclude that AAV-Cre vector toxicity ablates PVN neurons and results in a phenotype that is not associated with the gene of interest. These results underscore the importance of rigorous controls to ensure virus quality and fidelity of action. Caution must especially be exercised when the observed phenotype closely matches that of lesions in the area of use. Ensuring that expression in wild-type mice results in no observable changes or that neuronal cell densities are unaltered (if they are not expected to be) are two such methods of validating that the AAV is not toxic on its own. Taken together, viral vectors represent a class of worthwhile tools to manipulate the nervous system; however, scientists must take care to ensure that the potential for toxicity in commonly used AAVs is ruled out in experimental design and data interpretation.
Future Directions
In the present study, we exclusively examined the impact of two batches of AAV-hSyn-Cre after injection into the hypothalamus. It remains to be determined if the neurotoxicity stems from the AAV’s preparation in the particular batch. Moreover, it is essential to understand the impact of AAV-Cre on various glial cell types and neurons in different brain regions. Furthermore, the molecular mechanisms behind the neurotoxic effects have not been explored in the present study, warranting future mechanistic research.
Supplementary Material
Acknowledgements
The authors thank Dr Larry Zweifel of the University of Washington for providing us the CRHflox/flox mouse line. The authors would also like to thank Dr. Mark Rossi (Rutgers University) for critical reading of the manuscript and insightful comments.
Abbreviations
- AAV
adeno-associated virus
- CLAMS
Comprehensive Lab Animal Monitoring System
- CRH
corticotropin-releasing hormone
- dLS
dorsal lateral septum
- GLP-1R
glucagon-like peptide-1 receptor
- GTT
glucose tolerance test
- IHC
immunohistochemistry
- ITT
insulin tolerance test
- MC4R
melanocortin-4 receptor
- OXT
oxytocin
- PDYN
prodynorphin
- PFA
paraformaldehyde
- POMC
proopiomelanocortin
- PVN
paraventricular nucleus of hypothalamus
- WT
wild-type
Contributor Information
Le Wang, Email: lw611@rwjms.rutgers.edu.
Zhiping P. Pang, Email: pangzh@rwjms.rutgers.edu.
Data Availability
Code used to analyze open field test data can be found at https://github.com/RohanSavani/OpenFieldAnalysis. The data from the current study are available from the corresponding authors upon reasonable request.
Competing Interests
The authors declare that there are no competing interests associated with the manuscript.
Funding
This study was supported by grants from the Robert Wood Johnson Foundation to the Child Health Institute of New Jersey (RWJF) [#74260] and the NIH NIDDK [R01DK131452]. L.W. was supported by the New Jersey Governor’s Council for Medical Research and Treatment of Autism Postdoctoral Fellowship [CAUT24DFP005] and the NExT-Metabolism Pilot Award [grant number 500301]. R.S. was supported by Rutgers Aresty Research Center’s independent research award.
CRediT Author Contribution
Rohan Savani: Data curation, Formal analysis, Methodology, Writing—original draft. Erin Park: Data curation, Formal analysis. Nidhi Busannagari: Data curation, Formal analysis. Yi Lu: Data curation, Formal analysis. Hyokjoon Kwon: Data curation, Formal analysis. Le Wang: Conceptualization, Data curation, Formal analysis, Supervision, Writing—original draft, Project administration, Writing—review & editing. Zhiping P. Pang: Conceptualization, Resources, Supervision, Funding acquisition, Writing—original draft, Writing—review & editing.
References
- 1.Ferguson A.V., Latchford K.J. and Samson W.K. (2008) The paraventricular nucleus of the hypothalamus - a potential target for integrative treatment of autonomic dysfunction. Expert Opin. Ther. Targets 12, 717–727 10.1517/14728222.12.6.717 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Watts A.G., Kanoski S.E., Sanchez-Watts G. and Langhans W. (2021) The physiological control of eating: signals, neurons, and networks. Physiol. Rev. 102, 689–813 10.1152/physrev.00028.2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Qin C., Li J. and Tang K. (2018) The paraventricular nucleus of the hypothalamus: development, function, and human diseases. Endocrinology 159, 3458–3472 10.1210/en.2018-00453 [DOI] [PubMed] [Google Scholar]
- 4.Manaye K.F., Lei D.L., Tizabi Y., Dávila-García M.I., Mouton P.R. and Kelly P.H. (2005) Selective neuron loss in the paraventricular nucleus of hypothalamus in patients suffering from major depression and bipolar disorder. J. Neuropathol. Exp. Neurol. 64, 224–229 10.1093/jnen/64.3.224 [DOI] [PubMed] [Google Scholar]
- 5.Benarroch E.E. (2005) Paraventricular nucleus, stress response, and cardiovascular disease. Clin. Auton. Res. 15, 254–263 10.1007/s10286-005-0290-7 [DOI] [PubMed] [Google Scholar]
- 6.Sauer B. and Henderson N. (1988) Site-specific DNA recombination in mammalian cells by the Cre recombinase of bacteriophage P1. Proc. Natl. Acad. Sci. 85, 5166–5170 10.1073/pnas.85.14.5166 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Tsien J.Z., Chen D.F., Gerber D., Tom C., Mercer E.H., Anderson D.J.et al. (1996) Subregion- and cell type–restricted gene knockout in mouse brain. Cell 87, 1317–1326 10.1016/S0092-8674(00)81826-7 [DOI] [PubMed] [Google Scholar]
- 8.Liu J., Conde K., Zhang P., Lilascharoen V., Xu Z., Lim B.K.et al. (2017) Enhanced AMPA receptor trafficking mediates the anorexigenic effect of endogenous glucagon-like peptide-1 in the paraventricular hypothalamus. Neuron 96, 897.e5–909.e5 10.1016/j.neuron.2017.09.042 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Deng Y., Dickey J.E., Saito K., Deng G., Singh U., Jiang J.et al. (2022) Elucidating the role of Rgs2 expression in the PVN for metabolic homeostasis in mice. Mol. Metab. 66, 101622 10.1016/j.molmet.2022.101622 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Souza L.A.C., Worker C.J., Li W., Trebak F., Watkins T., Gayban A.J.B.et al. (2019) (Pro)renin receptor knockdown in the paraventricular nucleus of the hypothalamus attenuates hypertension development and AT(1) receptor-mediated calcium events. Am. J. Physiol. Heart Circ. Physiol. 316, H1389–H1405 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Xia H., de Queiroz T.M., Sriramula S., Feng Y., Johnson T., Mungrue I.N.et al. (2015) Brain ACE2 overexpression reduces DOCA-salt hypertension independently of endoplasmic reticulum stress. Am. J. Physiol. Regul. Integr. Comp. Physiol. 308, R370–R378 10.1152/ajpregu.00366.2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Xu J., Bartolome C.L., Low C.S., Yi X., Chien C.-H., Wang P.et al. (2018) Genetic identification of leptin neural circuits in energy and glucose homeostases. Nature 556, 505–509 10.1038/s41586-018-0049-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Erben L., Welday J.P., Murphy R. and Buonanno A. (2022) Toxic and phenotypic effects of AAV_Cre used to transduce mesencephalic dopaminergic neurons. Int. J. Mol. Sci. 23, 9462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Rezai Amin S., Gruszczynski C., Guiard B.P., Callebert J., Launay J.-M., Louis F.et al. (2019) Viral vector-mediated Cre recombinase expression in substantia nigra induces lesions of the nigrostriatal pathway associated with perturbations of dopamine-related behaviors and hallmarks of programmed cell death. J. Neurochem. 150, 330–340 [DOI] [PubMed] [Google Scholar]
- 15.Kim J., Lee S., Fang Y.-Y., Shin A., Park S., Hashikawa K.et al. (2019) Rapid, biphasic CRF neuronal responses encode positive and negative valence. Nat. Neurosci. 22, 576–585 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Yuan Y., Wu W., Chen M., Cai F., Fan C., Shen W.et al. (2019) Reward inhibits paraventricular CRH neurons to relieve stress. Curr. Biol. 29, 1243.e4–1251.e4 [DOI] [PubMed] [Google Scholar]
- 17.Zhu C., Xu Y., Jiang Z., Tian J.B., Cassidy R.M., Cai Z.-L.et al. (2020) Disrupted hypothalamic CRH neuron responsiveness contributes to diet-induced obesity. EMBO Rep. 21, e49210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zhang R., Asai M., Mahoney C.E., Joachim M., Shen Y., Gunner G.et al. (2017) Loss of hypothalamic corticotropin-releasing hormone markedly reduces anxiety behaviors in mice. Mol. Psychiatry 22, 733–744 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Muglia L.J., Jenkins N.A., Gilbert D.J., Copeland N.G. and Majzoub J.A. (1994) Expression of the mouse corticotropin-releasing hormone gene in vivo and targeted inactivation in embryonic stem cells. J. Clin. Invest. 93, 2066–2072 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Muglia L., Jacobson L., Dikkes P. and Majzoub J.A. (1995) Corticotropin-releasing hormone deficiency reveals major fetal but not adult glucocorticoid need. Nature 373, 427–432 [DOI] [PubMed] [Google Scholar]
- 21.Jiang Z. and Tong Q. (2022) Hypothalamic CRH neurons: a crossroad between stress and metabolism. Curr. Opinion Endocrine Metabolic Res. 26, 100384 10.1016/j.coemr.2022.100384 [DOI] [Google Scholar]
- 22.Bradbury M.J., McBurnie M.I., Denton D.A., Lee K.-F. and Vale W.W. (2000) Modulation of urocortin-induced hypophagia and weight loss by corticotropin-releasing factor receptor 1 deficiency in mice*. Endocrinology 141, 2715–2724 10.1210/endo.141.8.7606 [DOI] [PubMed] [Google Scholar]
- 23.Sanford C.A., Soden M.E., Baird M.A., Miller S.M., Schulkin J., Palmiter R.D.et al. (2017) A central amygdala CRF circuit facilitates learning about weak threats. Neuron 93, 164–178 10.1016/j.neuron.2016.11.034 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sisley S., Gutierrez-Aguilar R., Scott M., D'Alessio D.A., Sandoval D.A. and Seeley R.J. (2014) Neuronal GLP1R mediates liraglutide's anorectic but not glucose-lowering effect. J. Clin. Invest. 124, 2456–2463 10.1172/JCI72434 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Nath T., Mathis A., Chen A.C., Patel A., Bethge M. and Mathis M.W. (2019) Using DeepLabCut for 3D markerless pose estimation across species and behaviors. Nat. Protoc. 14, 2152–2176 10.1038/s41596-019-0176-0 [DOI] [PubMed] [Google Scholar]
- 26.Daviu N., Bruchas M.R., Moghaddam B., Sandi C. and Beyeler A. (2019) Neurobiological links between stress and anxiety. Neurobiol. Stress 11, 100191 10.1016/j.ynstr.2019.100191 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sterley T.-L., Baimoukhametova D., Füzesi T., Zurek A.A., Daviu N., Rasiah N.P.et al. (2018) Social transmission and buffering of synaptic changes after stress. Nat. Neurosci. 21, 393–403 10.1038/s41593-017-0044-6 [DOI] [PubMed] [Google Scholar]
- 28.Füzesi T., Daviu N., Wamsteeker Cusulin J.I., Bonin R.P. and Bains J.S. (2016) Hypothalamic CRH neurons orchestrate complex behaviours after stress. Nat. Commun. 7, 11937 10.1038/ncomms11937 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Semprini S., Troup T.J., Kotelevtseva N., King K., Davis J.R., Mullins L.J.et al. (2007) Cryptic loxP sites in mammalian genomes: genome-wide distribution and relevance for the efficiency of BAC/PAC recombineering techniques. Nucleic Acids Res. 35, 1402–1410 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Correa-da-Silva F., Kalsbeek M.J., Gadella F.S., Oppersma J., Jiang W., Wolff S.E.C.et al. (2023) Reduction of oxytocin-containing neurons and enhanced glymphatic activity in the hypothalamic paraventricular nucleus of patients with type 2 diabetes mellitus. Acta Neuropathologica Commun. 11, 107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sims J.S. and Lorden J.F. (1986) Effect of paraventricular nucleus lesions on body weight, food intake and insulin levels. Behav. Brain Res. 22, 265–281 10.1016/0166-4328(86)90071-9 [DOI] [PubMed] [Google Scholar]
- 32.Li M.M., Madara J.C., Steger J.S., Krashes M.J., Balthasar N., Campbell J.N.et al. (2019) The paraventricular hypothalamus regulates satiety and prevents obesity via two genetically distinct circuits. Neuron 102, 653.e6–667.e6 10.1016/j.neuron.2019.02.028 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Leibowitz S.F., Hammer N.J. and Chang K. (1981) Hypothalamic paraventricular nucleus lesions produce overeating and obesity in the rat. Physiol. Behav. 27, 1031–1040 10.1016/0031-9384(81)90366-8 [DOI] [PubMed] [Google Scholar]
- 34.Fukushima M., Tokunaga K., Lupien J., Kemnitz J.W. and Bray G.A. (1987) Dynamic and static phases of obesity following lesions in PVN and VMH. Am. J. Physiol.-Regulatory, Integrative Comparative Physiol. 253, R523–R529 10.1152/ajpregu.1987.253.3.R523 [DOI] [PubMed] [Google Scholar]
- 35.Gold R.M., Jones A.P., Sawchenko P.E. and Kapatos G. (1977) Paraventricular area: critical focus of a longitudinal neurocircuitry mediating food intake. Physiol. Behav. 18, 1111–1119 10.1016/0031-9384(77)90019-1 [DOI] [PubMed] [Google Scholar]
- 36.Terrill S.J., Jackson C.M., Greene H.E., Lilly N., Maske C.B., Vallejo S.et al. (2016) Role of lateral septum glucagon-like peptide 1 receptors in food intake. Am. J. Physiol. Regul. Integr. Comp. Physiol. 311, R124–R132 10.1152/ajpregu.00460.2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Rizzi-Wise C.A. and Wang D.V. (2021) Putting together pieces of the lateral septum: multifaceted functions and its neural pathways. eNeuro 8, 0315–21 10.1523/ENEURO.0315-21.2021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Bales M.B., Centanni S.W., Luchsinger J.R., Fathi P., Biddinger J.E., Le T.D.V.et al. (2022) High fat diet blunts stress-induced hypophagia and activation of Glp1r dorsal lateral septum neurons in male but not in female mice. Mol. Metab. 64, 101571 10.1016/j.molmet.2022.101571 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Terrill S.J., Holt M.K., Maske C.B., Abrams N., Reimann F., Trapp S.et al. (2019) Endogenous GLP-1 in lateral septum promotes satiety and suppresses motivation for food in mice. Physiol. Behav. 206, 191–199 10.1016/j.physbeh.2019.04.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Li S.-B., Borniger J.C., Yamaguchi H., Hédou J., Gaudilliere B. and de Lecea L. (2020) Hypothalamic circuitry underlying stress-induced insomnia and peripheral immunosuppression. Sci. Adv. 6, eabc2590 10.1126/sciadv.abc2590 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Weninger S.C., Muglia L.J., Jacobson L. and Majzoub J.A. (1999) CRH-deficient mice have a normal anorectic response to chronic stress. Regul. Pept. 84, 69–74 [DOI] [PubMed] [Google Scholar]
- 42.Weninger S.C., Dunn A.J., Muglia L.J., Dikkes P., Miczek K.A., Swiergiel A.H.et al. (1999) Stress-induced behaviors require the corticotropin-releasing hormone (CRH) receptor, but not CRH. Proc. Natl. Acad. Sci. 96, 8283–8288 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Dunn A.J. and Swiergiel A.H. (1999) Behavioral responses to stress are intact in CRF-deficient mice. Brain Res. 845, 14–20 [DOI] [PubMed] [Google Scholar]
- 44.Sakamoto R., Matsubara E., Nomura M., Wang L., Kawahara Y., Yanase T.et al. (2013) Roles for corticotropin-releasing factor receptor type 1 in energy homeostasis in mice. Metabolism 62, 1739–1748 [DOI] [PubMed] [Google Scholar]
- 45.Bale T.L., Anderson K.R., Roberts A.J., Lee K.-F., Nagy T.R. and Vale W.W. (2003) Corticotropin-releasing factor receptor-2-deficient mice display abnormal homeostatic responses to challenges of increased dietary fat and cold. Endocrinology 144, 2580–2587 [DOI] [PubMed] [Google Scholar]
- 46.Foster M.T., Song C.K. and Bartness T.J. (2010) Hypothalamic paraventricular nucleus lesion involvement in the sympathetic control of lipid mobilization. Obesity (Silver Spring) 18, 682–689 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Chen C.R., Zhong Y.H., Jiang S., Xu W., Xiao L., Wang Z.et al. (2021) Dysfunctions of the paraventricular hypothalamic nucleus induce hypersomnia in mice. Elife 10, e69909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Kim E.R., Xu Y., Cassidy R.M., Lu Y., Yang Y., Tian J.et al. (2020) Paraventricular hypothalamus mediates diurnal rhythm of metabolism. Nat. Commun. 11, 3794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Burmeister M.A., Ayala J.E., Smouse H., Landivar-Rocha A., Brown J.D., Drucker D.J.et al. (2017) The hypothalamic glucagon-like peptide 1 receptor is sufficient but not necessary for the regulation of energy balance and glucose homeostasis in mice. Diabetes 66, 372–384 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Bozadjieva-Kramer N., Ross R.A., Johnson D.Q., Fenselau H., Haggerty D.L., Atwood B.et al. (2021) The role of mediobasal hypothalamic PACAP in the control of body weight and metabolism. Endocrinology 162, bqab012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Lee C.M., Zhou L., Liu J., Shi J., Geng Y., Liu M.et al. (2020) Single-cell RNA-seq analysis revealed long-lasting adverse effects of tamoxifen on neurogenesis in prenatal and adult brains. Proc. Natl. Acad. Sci. U. S. A. 117, 19578–19589 10.1073/pnas.1918883117 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Code used to analyze open field test data can be found at https://github.com/RohanSavani/OpenFieldAnalysis. The data from the current study are available from the corresponding authors upon reasonable request.