Abstract
After completing treatment for head and neck cancer (HNC), patients often face oral complications like oral pain, limited mouth opening and dry mouth which significantly reduce their oral health related quality of life (OHRQoL). These issues impact their overall well-being, social activities and long-term survival. The primary objective of this study was to evaluate OHRQoL and its association with sociodemographic characteristics, oral hygiene practices and oral clinical parameters such as oral hygiene status and oral mucositis grade in patients who have completed treatment for head and neck cancer. This cross-sectional study involved 79 HNC-treated patients within first year after completion of cancer treatment attending ENT and dental clinics at outpatient department (OPD) setting in Karachi. Data was collected electronically using structured questionnaire comprising of EORTC QLQ H&N – 35 to measure OHRQoL, patients were also examined for oral hygiene status using oral hygiene index- simplified (OHI-s) and oral mucositis grade using WHO oral mucositis scale. Multiple linear regression was used to test OHRQoL associations with the sociodemographic and different clinical factors. The result showed an overall mean score for oral health related quality of life (OHRQoL) of 25.02 ± 15.86 (95% CI 21.46–28.57), with difficulty in mouth opening 53.16 ± 18.88 and dry mouth 45.14 ± 24.48 being predominant concerns for decline in the OHRQoL in the population. Male predilection was observed among participants n = 60 (75.9%), majority of the participants n = 41 (51.9%) were below 52 years of age. n = 63 (80%) participants received radiotherapy alongside surgery and chemotherapy. Most of participants n = 66 (83.5%) experienced moderate to severe oral mucositis with poor oral hygiene status n = 56 (71%). Significant associations were found between OHRQoL and BMI, OH status, marital status, monthly income, gender and fluoride toothpaste (p < 0.05). These findings suggest that Quality of Life (QoL) among HNC treated patients is negatively impacted by their poor oral health, post cancer treatment. Therefore, it is important to evaluate and modify the current treatment modalities and involve multidisciplinary teams, to improve their OHRQoL thereby enhancing overall QoL.
Subject terms: Cancer, Health care, Medical research
Introduction
Head and neck cancer (HNC) is the second most frequently occurring cancer in Pakistan, yet it is vastly underestimated, with only 16,595 reported cases annually1,2. Pakistan is among the top ten countries known for tobacco consumption, with 46% of its population consuming paan and gutka (chewable betel) in their daily lives3. Regions commonly involved in HNC are the oral cavity, tongue, oropharynx, nasopharynx, tonsils, and parotid gland4. Treatment choices for HNC such as surgical resection, radiotherapy (RT) and chemotherapy (CT) vastly depend on the stage of the tumor at the time of diagnosis. For advanced stages of cancer, the use of high-energy radiation can cause certain impairments in the oral cavity that can be severe, such as xerostomia due to reduced salivary secretions, mouth soreness, bacterial and fungal infections, taste disturbance, dysphagia, pain, bleeding from periodontal tissues, and speech problems5,6. Almost 56–80% of the HNC patients often report of having disturbed oral functions leading to various social, economic, and psychological consequences that profoundly impact their overall quality of life (QoL)7.
Due to the aggressiveness of the disease (cancer) as well as its available treatment, patients often suffer harsh side effects (pain, inflammation and systemic fatigue) impairing their willingness to maintain oral hygiene. Absence of regular brushing and interdental cleaning can result in microbial buildup8, also the reduction in the salivary flow following the RT/CT required for the self-cleansing of the oral cavity further aggravates the oral symptoms predisposing patients to oral pain, soreness, periodontal diseases and dental cavities9. Poor oral hygiene is not only the cause of oral diseases but studies have reported, strong association with systemic disorders such as hypertension, diabetes, kidney disease10–12. A study concluded that lack of oral hygiene especially in the cancer patients can be the result of their reduced motivational drive and lack of oral care education by the consulting physician8.
Oral mucositis (OM) is a common acute complication that typically arises around fourth week of radiotherapy (RT). Nearly 80–100% HNC patients report varying degrees of oral mucositis within first three months of treatment13,14. Lesions of oral mucositis are intensely painful, hindering chewing, swallowing and oral hygiene practices. Severe mucositis can result in malnutrition15, necessitating invasive interventions like nasogastric intubation or percutaneous endoscopic gastrostomy16. These interventions not only increase healthcare costs but also reduce QoL due to the associated risks of infection and extended hospital stay. It is worth noting that these symptoms are temporary, lasting for weeks or months after therapy completion17. Several studies have reported patients having chronic oral mucositis 6 months after RT and a longer prevalence of OM in patients receiving concurrent chemo-radiotherapy18,19. QoL assessment is an important tool for evaluating not only the impact of disease and its treatment on an individual level but also helpful in developing and revising the rehabilitative services and patient education material to further improve the clinical outcomes (survival) of patients20. Oral health related quality of life (OHRQoL), specifically focuses on self-report aspects pertaining to oral health, capturing the functional, social, and psychological dimensions affected by oral disease21. In the case of HNC, disruptions in key oral functions poses a higher risk of adversely affecting both OHRQoL and overall QoL.
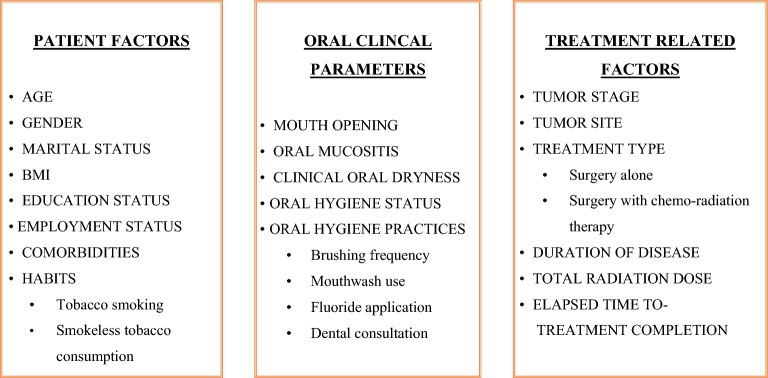
Not much evidence is found in Pakistan related to oral care measures for HNC patients, such as professional care by a dentist and dental hygienist (mechanical or manual removal of mucosal debris, oral hygiene counselling, etc.) at least once a week until the completion of RT19. The patients are often unaware of the potential harm from the cancer treatment, only a proportion of HNC patients seek dental consultation for their complications, and most of them suffer greatly even after the completion of treatment due to disturbed oral functions. To the best of our knowledge, this study represents the first comprehensive assessment of OHRQoL in HNC treated patients who had completed their cancer treatment within the past 12 months. Additionally, it aims to explore the associations between various oral clinical parameters, including oral hygiene status and oral mucositis, and several predictive factors such as sociodemographic characteristics and treatment-related variables in relation to OHRQoL (see Fig. 1). The findings of this study highlights the importance of standardizing oral care and improving oral health support for HNC treated patients to reduce the impact of oral problems which can negatively impact their overall quality of life.
Figure 1.
Factors affecting the OHRQoL among HNC patients, post cancer treatment.
Material and methods
Study design and study setting
A cross sectional study was conducted in outpatient department of ENT and Dental clinics at Aga Khan University Hospital (AKUH), recognized as the largest private tertiary care hospital in Karachi, Pakistan accredited by Joint Commission International (JCi) This facility provides comprehensive care to cancer patients serving diverse ethnic and socioeconomic backgrounds, and has benefitted more than 3 million cancer patients to date22. Additionally, AKUH actively promotes research activities and maintains a well-organized patient registry.
Study population, sampling technique and sample size
The participants in this study were patients who were 18 years and above, treated for HNC at Aga Khan University Hospital (AKUH). The study specifically included HNC survivors who were within the first year after completing their cancer treatment23, coming for their routine follow up at ENT and Dental clinics at AKUH. All the included patients had one of the three molars and a central incisor in upper and lower dentition at the time of follow up (these teeth were required to measure the Oral hygiene status using Oral Hygiene Index- simplified). We excluded patients who didn’t give their consent to participate in the study or had any debilitative conditions or cognitive disabilities confirmed by their medical reports or had trismus i.e. mouth opening < 20 mm (as it hindered the oral examination of the participant)22,24.
Participants were recruited using a non-probability purposive sampling technique. The study aimed to achieve its objectives with a minimum sample of 76 HNC-treated patients, considering an 80% statistical power and a significance level of 0.05. adjustments were made considering a 10% non-response rate, with an anticipated mean score 5 units higher than the hypothesized value of 16.7 in our population, as advised by the subject specialist19.
All patients treated for HNC at AKUH, between January 2021 and September 2022 were screened via their medical records. Patients who were within their first year of post-cancer treatment were contacted by phone to inquire about their upcoming follow-up visit to the ENT or Dental clinics at AKUH. On the scheduled follow-up day, we approached these patients to check for eligibility (i.e. for mouth opening and presence of at least one molar and incisor in each quadrant). Eligible patients were invited to participate in the study after explaining the purpose and scope of the study. Those who provided their written consent were interviewed and examined (Fig. 2). This study was approved by the Ethical review committee of Aga Khan University (2022-7178-21416).
Figure 2.
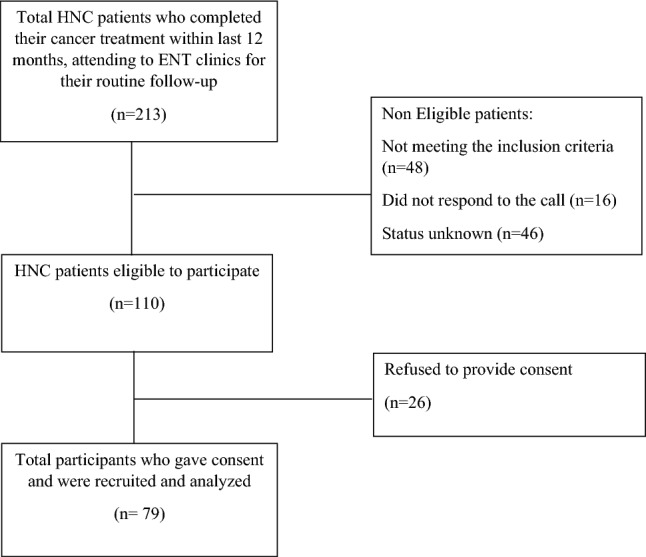
Flowchart of the process of study participant’s recruitment.
Variables and tools
Data was collected through a structured questionnaire, comprising sociodemographic variables, treatment related factors, oral hygiene practices and oral clinical parameters (oral mucositis and oral hygiene status).
Demographic and other variables
Sociodemographic variables included patients age recorded as (< 52 years’ vs ≥ 52 years)25, gender, marital status, BMI recorded as (underweight ≤ 18.5 kg/ m2, normal weight = ≥ 18.5 kg/ m2)26, level of education and monthly income. Treatment related factors included tumor stage at diagnosis according to AJCC stage system (stage I, stage II, stage III, stage IV), type of treatment received (without RT vs with RT), total dose of radiation received, elapsed time since treatment (< 6 months’ vs ≥ 6 months), number of follow up visits and any comorbidities Patients oral hygiene-related details included their practices of brushing and mouthwash use and frequency per day, use of fluoride toothpaste, any instructions received related to oral hygiene maintenance after treatment completion by the consultant, and the current use of dental prostheses.
Oral clinical parameters such as Oral Hygiene status was measured using Oral Hygiene Index – simplified (OHI-S) and categorized as good (0–1.2), fair (1.3- 3.0) and poor (3.1–6.0)27. The Oral Mucositis was assessed using WHO- Oral mucositis scale, and categorized into mild (by combining grade 1 and 2) and moderate to severe (by combining grade 3 and 4) categories28.
Outcome variable
OHRQoL is characterized as a self-report measure that focuses specifically on the oral health. it encompasses a comprehensive assessment of how oral diseases and conditions impact various aspects of an individual’s life. It assess the functionality as well as social and psychological effects stemming from oral health issues, aiming to understand their overall impact on well-being21. For this study we used Urdu version of European Organization of Treatment of Cancer QoL Head and Neck-35 (EORTC QLQ-H&N-35), which has been validated for use in our setting24.
EORTC QLQ-H&N-35 consists 35 questions in total, with 7 multiple item- questions assessing pain and soreness in the mouth and throat (PA) through item 31 -34, swallowing of liquid, pureed and solid food (SW) through item 35–38, senses of taste and smell (SE) from 43–44, social eating with family and friends and enjoying of meal (SO) 49–52 social contact with family and friends (SC) 48, 55–58 and sexuality (SX) 59–60 over the last week and 11 single questions to assess problems with “teeth” “opening of mouth” “dry mouth” ” sticky saliva” “coughing” “feeling ill” “ use of pain killers” “nutritional supplements” or “feeding tube” “weight loss” and “weight gain”. Likert type was used to score the responses as (1 = not at all, 2 = a little, 3 = quite a bit, 4 = very much) item H&N31 – H&N-35 have a 1 = yes, 2 = no response29.
Scoring of tool
First raw scores were calculated. For each multi- item question such as PA, SW, SO etc. an average of the corresponding items and for single- item question (teeth, mouth opening, sticky saliva, dry mouth etc.) the single score of the concerning item was taken as the raw score. Raw score = (I1 + I2 + I3)/n.
Standardization of the raw score was done to obtain mean scores for OHRQoL ranging from 0 to 100. Symptom scale S = [(RS-1)/range] × 100.
Elevated symptom scores for different domains of the tool implied deteriorating oral symptoms contributing to overall high mean score indicating more adversely affected OHRQoL.
Data collection procedure
Hiring of data collector was done prior to data collection. The training of data collector included procedure of taking informed consent, familiarization with the questionnaire and time management. A manual of operations was provided to data collector to facilitate consistency in protocol implementation during data collection. A trained dental hygienist was recruited to perform oral examination of the participants after they were interviewed.
Ethical clearance
An ethical review was sought from the Departmental Review Board (DRC), after its approval, the Ethical Review Committee (ERC) of Aga Khan University, Karachi, was consulted for ethical approval. The ERC approved the study (reference number 2022-7178-21416).
Statistical analysis
OHRQoL, which is the outcome variable is continuous in nature and calculated on the basis of a mean score. Multiple linear regression analysis was performed using STATA version 16.
The descriptive statistics of all independent variables and outcome variable was conducted. For categorical variables such as age, gender, level of education, monthly income, oral hygiene practices and other variables, frequencies and percentages were reported.
Normality of the outcome i.e. OHRQoL was checked using normal probability plot, which showed normal distribution. At the univariate analysis stage, all the categorical variables i.e. age, gender, marital status, BMI, educational status, employment status, monthly income, tumor stage, treatment type, radiation dose, number of follow up, smoking status and frequency, brushing and mouth wash use and their frequency, use of fluoride tooth paste, guidance related to oral complications of cancer treatment, visit to dentist, use of dental prosthesis were individually assessed for a significant association with OHRQoL on a set P value of ≤ 0.25.Variables with p-value greater, were removed and other variables with significant p-values were included in multivariable analysis. In Multivariable analysis, all variables that were significant at univariate analysis level, were assessed using multiple linear regression analysis. All variables with a p-value ≤ 0.05 were added in the model. The presence of biologically plausible interaction and confounding was also assessed.
After finalization of the main effect model, adequacy was assessed using residual plots against fitted values and normal probability plot.
Data management
Data was collected by a web-based structured questionnaire on google forms, which included mandatory questions, thereby, eliminating the issue of missing or incomplete data. The data was assessed for accuracy and completeness on daily basis by the investigator. Built-in range checks and internal consistency checks in google forms further ensured data quality. Backup files were created for data security, with participant information (such as name and contact details) excluded to maintain confidentiality. Each participant was assigned a unique ID number for data organization. The electronic data has been password- protected and will be deleted after seven years as per the policy of Aga Khan University.
Ethics approval and consent to participate
Ethical approval was obtained from Ethical Review Committee of the Aga Khan University Hospital, Karachi, Pakistan (reference number 2022–7178-21,416). Written informed consent was obtained from all the study participants. All methods were performed in accordance with the Declaration of Helsinki.
Results
Patient characteristics
In this study, 79 HNC treated patients who were within their first year of post-cancer treatment were included. Patients’ characteristics are shown in (Table 1). 51.9% (n = 41) participants out of total were below 52 years, 76% (n = 60) were males, showing a male predilection in the study population. 74.68% (n = 59) were married, 75.48%. (n = 60) had normal BMI while 21.52% (n = 17) participants were underweight. 55.7% (n = 44) were unemployed and 51.9% (n = 41) had monthly household income of PKR 50,000 or less.
Table 1.
Sociodemographic, treatment related, habits, oral hygiene related factors of HNC patients within 1 year, post cancer treatment.
| Variables | Frequency n (%) |
|---|---|
| Age | |
| < 52 years | 41 (51.9%) |
| ≥ 52 years | 38 (48.1%) |
| Gender | |
| Male | 60 (75.95%) |
| Female | 19 (24.05%) |
| Marital status | |
| Married | 59 (74.68%) |
| Unmarried | 20 (25.32%) |
| BMI | |
| Underweight | 17 (21.52%) |
| Normal weight | 60 (75.48%) |
| Tumor stage at diagnosis | |
| Stage 1 | 10 (12.66%) |
| Stage 2 | 22 (27.85%) |
| Stage 3 | 46 (58.32%) |
| Stage 4 | 1 (1.27%) |
| Type of the treatment received | |
| Without radiotherapy | 16 (20.25%) |
| With radiotherapy | 63 (79.75%) |
| Total dose of radiation received | |
| < 33 cGy | 21 (33.33%) |
| ≥ 33 cGy | 42 (66.66%) |
| Elapsed time since the treatment | |
| Less than 6 months | 26 (32.9%) |
| 6 months or more | 53 (67.1%) |
| Oral hygiene practices | |
| Brushing or use of applicator for teeth cleaning | |
| Yes | 49 (62.03%) |
| Seldom | 21 (42.8%) |
| Once daily | 18 (36.8%) |
| More than once daily | 10 (20.4%) |
| No | 30 (37.97%) |
| Use of any mouthwash for oral hygiene | |
| Yes | 46 (58.23%) |
| No | 33 (41.77%) |
| Briefing about the oral complications of cancer treatment prior to start of treatment | |
| Yes | 48 (60.7%) |
| No | 31 (39.24%) |
| Instructions related to maintenance of oral hygiene by dental hygienist | |
| Yes | 36 (45.47%) |
| No | 43 (54.43%) |
| Referred to dental clinics for your oral complications during RT? | |
| Yes | 8 (10.13%) |
| No | 71 (89.87%) |
| Visited to dentist after completion of cancer treatment | |
| Yes | 23 (29.1%) |
| No | 56 (70.89%) |
| Use of any supplemental fluoride | |
| Yes | 23 (29.11%) |
| No | 56 (70.89%) |
At the time of diagnosis, 58.23% (n = 46) of the participants had stage III cancer, the majority comprising 79.75% (n = 63) received cancer treatment with radiotherapy along with either surgery or chemotherapy, while 20.2% (n = 16) underwent only surgery. The elapsed time after treatment for majority of the participants 67.1% (n = 53) was more than 6 months. 55.7% (n = 44) patients had attended more than three follow up visits at ENT clinics at Aga Khan University Hospital (AKUH), post treatment completion (Table 1).
Oral hygiene practices among HNC treated patients
Out of participants, 62.03% (n = 49) reported regular teeth brushing, among these individuals, 42.86% (n = 21) reported infrequent daily teeth cleaning. Moreover, 70.89% (n = 56) mentioned that they were not prescribed any fluoride toothpaste or gel during or after cancer treatment. Of the study participants, the majority 60.7% (n = 48) received information about oral complications related to cancer treatment. Consequently, they exhibited a better understanding of associated oral symptoms, such as oral pain, mouth soreness, limited mouth opening and difficulties in eating and speaking recognizing that these concerns typically resolve over time. However, 54.4% (n = 43) of the participants did not receive specific instructions pertaining to the maintenance of oral hygiene during and after their cancer treatment, from either the consulting physician or dental hygienist. Furthermore, only a minor proportion, 10.1% (n = 8) of the participants sought dental consultation to address their oral complications following completion of cancer treatment (Table 1).
Among79 participants, 70.9% (n = 56) exhibited fair oral hygiene index score while 26.58% (n = 21) had poor oral hygiene score. Additionally, all participants included in the study experienced oral mucositis, 83.5% (n = 66) had moderate to severe oral mucositis upon examination (Table 2).
Table 2.
Oral clinical parameters i.e. OH status and OM status and comparisons of OHRQoL symptom scores of HNC patients within 1 year, post cancer treatment.
| Variable | Frequency (%) | QLQ H&N- 35, mean (SD) |
|---|---|---|
| Oral hygiene index score | ||
| Fair | 21 (26.58%) | 10.1 (7.8) |
| Poor | 56 (70.89%) | 16.2 (12.6) |
| Oral mucositis | ||
| Mild | 13 (16.46%) | 23.3 (17.7) |
| Moderate to severe | 66 (83.54%) | 25.3 (15.5) |
OHRQoL and its associated factors
The study findings revealed a mean score for the oral health related quality of life (OHRQoL) was 25.02 ± 15.86 (95% CI 21.46–28.57). Among the 35 items assessed, symptoms score for mouth opening (53.16 ± 18.88), dry month (45.14 ± 24.48), sexuality (39.71 ± 32.51) and speech (37.26 ± 17.61) exhibited higher values compared to other symptoms scores, indicating a more pronounced negative impact of cancer treatment on these functions. These factors collectively contributing significantly to the overall high OHRQoL score (Table 3).
Table 3.
Mean distribution of 18 domains scores of EORTC QLQ H&N -35 35 scores of HNC patients within 1 year, post cancer treatment.
| Symptom score (n = 79) | Mean (SD) |
|---|---|
| PA (pain) | 22.15 (16.28) |
| SW (swallowing) | 17.40 (12.09) |
| PT (teeth) | 19.83 (21.69) |
| MO (opening mouth) | 53.16 (18.88) |
| DM (dry mouth) | 45.14 (24.48) |
| SS (sticky saliva) | 32.91 (24.16) |
| SE (senses) | 9.915 (9.44) |
| CO (coughing) | 19.40 (17.38) |
| FI (feeling ill) | 21.94 (21.93) |
| SO (social eating) | 23.31 (11.43) |
| SP (speech) | 37.26 (17.61) |
| SC (social contact) | 13.58 (13.64) |
| SX (sexuality) | 39.71 (32.51) |
| PK (pain killers) | 12.65 (16.28) |
| MV (supplements) | 21.09 (16.16) |
| FT (feeding tube) | 24.47 (4.810) |
| WL (weight loss) | 13.92 (16.54) |
| WG (weight gain) | 19.40 (16.54) |
*High mean scores suggest more negative impact on OHRQoL.
Analysis of the OHRQoL scores, revealed noteworthy differences across demographic and health parameters. Females exhibited significantly higher score for difficulty in mouth opening (61.4 ± 16.7, p value 0.03) compared to males. Similarly, underweight patients demonstrated more adversely impacted OHRQoL (33.3 ± 13.8, p value 0.01) and experienced increased dry mouth symptoms (56.9 ± 22.9, p value 0.02) Patients with poor oral hygiene status displayed higher scores for OHRQoL (43.2 ± 15.8, p value 0.04) and dry mouth (66.6 ± 18.9, p value 0.03) (Table 4).
Table 4.
Comparisons of OHRQoL symptom scores with few predicting factors of HNC patients within 1 year, post cancer treatment.
| Variable | Mean (SD) | ||
|---|---|---|---|
| QLQ H&N- 35 | MO | DM | |
| Gender | |||
| Male | 23.33 (16.18) | 50.55 (18.90) | 43.33 (24.77) |
| Female | 30.34 (13.88) | 61.40 (16.71) | 50.87 (23.22) |
| BMI | |||
| Underweight | 33.33 (13.81) | 60.78 (13.09) | 56.86 (22.86) |
| Not underweight | 22.74 (15.72) | 51.07 (19.75) | 41.94 (24.1) |
| Treatment type | |||
| Without radiotherapy | 26.67 (18.58) | 50 (24.34) | 19.08 (27.13) |
| With radiotherapy | 34.59 (15.23) | 56.96 (17.38) | 33.38 (23.67) |
| Elapsed time since completion of treatment | |||
| Less than 6 months | 27.11 (16.98) | 55.13 (18.71) | 50 (25.38) |
| Months or more | 23.98 (15.34) | 52.20 (19.1) | 42.76 (23.90) |
| Brushing frequency | |||
| Do not brush | 27.43 (16.03) | 58.89 (18.94) | 47.77 (25.79) |
| Seldom | 24.9 (14.41) | 52.38 (16.90) | 44.44 (19.24) |
| Once daily | 18.74 (16.10) | 35.55 (16.16) | 31.85 (26.13) |
| More than once daily | 11.28 (11.99) | 33.33 (15.71) | 26.66 (21.08) |
| OHI score | |||
| Fair | 17.68 (15.81) | 36.54 (18.98) | 28.21 (19.14) |
| Poor | 26.19 (12.59) | 42.85 (15.43) | 34.92 (16.58) |
| OM grade | |||
| Mild | 23.3 (17.72) | 48.7 (17.3) | 43.59 (28.5) |
| Moderate—severe | 25.3 (15.5) | 54.04 (19.2) | 45.45 (23.8) |
High mean scores suggest a negative impact on OHRQoL.
*DM-dry mouth, *OHI score-Oral Hygiene Index score, OM grade-Oral Mucositis grade, *MO-mouth opening.
Regarding marital status, married patients had lower scores for OHRQoL scores and demonstrated better mouth opening compared to patients who were not married (see Table 5). Notably, individuals brushing teeth more than once daily had a significantly positive impact on OHRQoL (11.3 ± 4.57, p value 0.01), mouth opening (33.3 ± 15.7, p value < 0.01) and dry mouth (26.6 ± 11.9, p value 0.02) (Table 4).
Table 5.
Factors associated with the OHRQoL in HNC patients within one year, post cancer treatment (multivariable model).
| Variables | Adjusted β | 95% CI | P-values |
|---|---|---|---|
| BMI (Ref = not underweight) | |||
| Underweight | 10.37 | 3.31–17.44 | < 0.01 |
| OHI score (ref = good) | |||
| Fair | − 17.43 | − 23.69– − 11.18 | < 0.01 |
| Poor | 30.57 | 39.83–21.30 | < 0.01 |
| Marital status (ref = unmarried) | |||
| Married | − 8.88 | − 15.87– − 1.88 | 0.01 |
| Monthly income (ref = < 50,000/- per month) | |||
| ≥ 50,000/- per months | − 6.69 | − 0.72– − 12.67 | 0.02 |
| Gender (Ref = female) | |||
| Male | − 8.82 | − 16.55– − 1.08 | 0.02 |
| OM grade & fluoride toothpaste use | |||
| OM grade (ref = mild), moderate + severe | 9.42 | − 1.79–20.63 | 0.09 |
| Fluoride toothpaste use (ref = no), yes | − 14.9 | − 1.66– − 28.15 | 0.02 |
| OM grade # fluoride T/P | − 12.84 | − 27.44–1.74 | 0.08 |
CI confidence interval, BMI = body mass index, OM grade oral mucositis grade.
# = Interaction.
*Adjusted β coefficient.
Table 5 shows that BMI, poor oral hygiene status, monthly income, oral mucositis grade were significantly associated with the decline in OHRQoL within one year, post cancer treatment. A significant interaction was observed in the effect model, showing a, lower mean score indicating better OHRQoL in patients with moderate to severe oral mucositis using fluoride toothpaste compared to patients with mild oral mucositis who were not using fluoride tooth paste (Table 5).
Discussion
OHRQoL serves as an indicator of individual comfort with respect to their oral health while performing daily life activities such as eating, sleeping, socializing, etc. HNC patients often experience severe irreversible changes in the maxillofacial region post treatment, impacting crucial oral functions. Despite these changes, the influence on oral health quality of life (OHRQoL) remains insufficiently documented, especially in lower middle-income countries like Pakistan. The present study explored the impact of cancer treatment on oral health-related quality of life using EORTC QLQ-35 and the factors associated with it such as sociodemographic and clinical factors, in HNC treated patients who are within first year after completing their treatment. Our primary finding revealed a high mean score for OHRQoL (25.02 ± 15.86) among these patients. Marital status and gender showed associations with better OHRQoL, while low BMI and low monthly income were associated with poor OHRQoL.
Among previous investigations which have evaluated the OHRQoL in HNC patients6,30, this is the first study to explore the association between clinical characteristics such as oral hygiene practices, oral mucositis status and oral hygiene status and their impact on OHRQoL within 1-year of post cancer treatment using multivariable analysis. Consistent with prior research31,32, our study observed a negative impact on OHRQoL in our population Notably, 79% of the patients underwent surgery combined with chemo-radiotherapy (CTRT), significantly affecting their oral health and impairing oral functions like eating, speaking and maintaining oral hygiene practices, even a year after treatment completion.
Unlike previous studies from India, Sweden and Japan6,30,33 which reported symptoms like fatigue, weight loss, senses, sticky saliva and increased pain killer use as highly rated, our findings highlighted difficulty in mouth opening and dry mouth as most challenging symptoms significantly experienced by the HNC treated patients. These symptoms significantly contributed to lower OHRQoL comparable to a study in China34 Reduced salivary production by the salivary glands is a primary adverse effect of radiotherapy, leading to difficulty in chewing and swallowing along with pain and lesions in the oral cavity35 Another study reported a reduction in mean mouth opening from 45.58 mm to 42.55 mm at 6 months compared to baseline i.e. immediately after completion of cancer treatment19.
Varying degree of oral mucositis was found among all the patients included in the study, high score for OHRQoL 25.3 ± 15.6 (Table 2) was observed in patients with moderate to severe mucositis. These findings were consistent with a study conducted to evaluate the OHRQoL using the OHIP tool, which reported a significant difference in the OHRQoL score in patients with varying degrees of oral mucositis compared to patients without it26 Nevertheless, it is noteworthy that 52% of the patients experiencing moderate to severe OM symptoms had concluded their cancer treatment over six months ago. This finding is unexpected, given the studies have reported that symptoms of OM typically abate or become mild within a three-month period following cancer treatment19,36. A significant association between the oral mucositis severity and use of fluoride was also observed, suggesting improvement in the OHRQoL due to decrease in the severity of mucositis by the use of fluoride toothpaste, this is also supported by37 which recommended that the use of high fluoride tooth paste, fluoride gel or fluoride mouthwash prescribed to patients during and after their cancer treatment could reduce the severity of mucosal inflammation. This recommendation highlights that fluoride toothpastes are cost effective way to improve oral health post radiation treatment.
The current analyses showed a greater proportion of males (75.9%) compared to females (24.1%) similar to other studies35,38, which can be attributed to higher consumption of tobacco among males. The age group more susceptible to developing HNC is below 52 years (51.9%) correlating with other study by Chauhan et al.39 which assessed the prevalence and pattern of tobacco consumption before the development of HNC. However, patients aged above 52 years experienced a greater burden of oral complications following cancer treatment, which was evident through their significantly higher mean score for OHRQoL 26.4 ± 14.8 similar to the findings reported by Barma et al.40 and Lee et al.41 Participants who were underweight i.e. < 18.5 kg/m2 were more adversely affected with a OHRQoL score of 33.33 ± 13.81 which can be possibly due to the disturbance in daily eating and sleeping patterns because of oral complications, similar to findings reported by Huang et al.42 indicating significantly lowered OHRQoL in HNC patients who were underweight. Married participants had lower score of OHRQoL 22.9 ± 15.9 vs unmarried (30.34 ± 13.8) suggesting that patients with care takers may have a higher motivation to recover compared to those without partners or care takers43 in addition, loss of the partner or absence of the partner had a higher negative impact on OHRQoL which agrees with other study result44. Our study also suggested a significant association between low monthly income and decline in OHRQoL among HNC treated patients in Pakistan, which aligns with45 which has demonstrated that socioeconomic factors play a vital role in influencing the oral health of the patients. Also, low socioeconomic status prevents patients from seeking regular dental checkups and attaining specialized products to treat the oral complications post cancer treatment. This further strengthens the urgent need for public health initiatives aimed at improving oral care access and affordability for vulnerable populations in Pakistan.
Studies conducted in Korea41 and Taiwan46 have illuminated the significance of implementing comprehensive oral care programs in the context of head and neck cancer patients, with a focus on enhancing their overall quality of life (QoL). The impact of cancer treatment on oral health has been emphasized in multiple studies stating the challenges caused by the impairment of the oral functions plays pivotal role in the deterioration of the OHRQoL of patients treated with HNC. Our own results findings as presented in Table 4, align with these established patterns, underscoring the substantial role of mouth opening limitation in contributing to the decline in OHRQoL. In the context of Pakistan, it is essential to highlight the scarcity of comprehensive oral care programs, a situation where cancer patients receive minimal or no guidance on maintaining their oral hygiene. According to a study, regular visits to the dentist and professional oral hygiene care during the first 8 weeks of the radiotherapy were effective for maintaining oral health of HNC patients47. Our research revealed that 54.4% of the patients did not receive any guidance or instructions from either the dental hygienist to mitigate the oral complications arising after the cancer treatment, merely 10% of the patients were directed to dental clinics in response to the severity of their complication and rehabilitation needs, 29% patients took the initiative to visit dental clinics independently. This deficiency has resulted in patients enduring substantial distress even after the completion of the treatment, suggesting the imperative significance of oral care programs by proactively addressing the distressing symptoms like dry mouths and swallowing difficulties through tailored interventions such as oral hygiene practices, utilization of substitute saliva and rehabilitation exercises, enhancing OHRQoL.
Some of the study limitations that warrants discussion were, firstly, this study adopted a cross-sectional design, thus providing a glimpse in the OHRQoL of HNC treated patients at a particular moment making it challenging to analyze long-term trends over time. Secondly, it is noteworthy that the research was conducted exclusively within a single center in Pakistan, as a result, caution should be exercised when extending the findings to a broader context or diverse population as the generalizability of the result may be restricted. We suggest that future research involving multiple hospitals, recruiting participants from various regions within Pakistan, would capture a diverse perspective of patient experience related to their oral health, post cancer treatment. Additionally, comparative studies across different countries can highlight the influence of oral care programs and other related factors on OHRQoL among individuals treated for HNC. Another limitation is that it is important to acknowledge that the data collection relied on self-reported information introducing the possibility of result overestimation. However, the potential bias was mitigated through the rigorous training of the data collector ensuring that the patients’ interviews were conducted in a manner conducive to maximize the reliability of the responses.
Implication for practice
This research emphasizes the critical need to incorporate comprehensive oral care protocols into the treatment of the head and neck cancer patients in Pakistan. Education including regular brushing with fluoride toothpaste and judicious use of gentle mouthwash and awareness initiatives are essential to inform patients about the potential oral side effects of the treatment they are taking, emphasizing good oral hygiene practices. Consistent monitoring by the dental professionals is crucial to manage acute oral complications, while interdisciplinary collaboration among healthcare teams is vital to address the multifaceted challenges faced by the HNC patients post treatment. Notably, the study highlights the potential benefits of fluoride toothpaste in mitigating mucositis severity and improving oral health. There is a need to develop and appraise oral care initiatives tailored for individuals affected by oral cancers by conducting longitudinal research to track oral health evolution aiming to enhance the quality of life and overall well-being of HNC patients in Pakistan.
Conclusion
The study findings reveal a substantial negative impact on the oral health related quality of life among HNC treated patients, particularly those who underwent radiotherapy. This negative impact is more pronounced in patients experiencing difficulty in mouth opening and dry mouth caused by hypo salivation. These findings highlight the need to reevaluate the current treatment approaches such as refining treatment protocols through advanced radiation techniques, tailored treatments to each patient’s unique needs and characteristics in terms of health and preferences, better education about treatment process, potential side-effects, involving multidisciplinary teams including oncologist, surgeons, radiation therapist, dentist, speech therapist and mental health professionals to provide a more holistic approach to patient care and integration of oral care programs as a part of HNC treatment to mitigate the adverse effects of treatment on oral heath, to improve OHRQoL and overall QoL in HNC treated patients.
Acknowledgements
We would like to thank our participants who contributed their valuable time to this study. We would like to acknowledge the Director of ASDH Miss Salima Alibhai and her associate Miss Shazia Taimoor for their support and contribution, Dr. Subhan Khan and Dr. Hassan Danish, Sir Iqbal Azam for their clinical and intellectual insight.
Abbreviations
- HNC
Head and neck cancer
- OHRQoL
Oral health related quality of life
- QoL
Quality of life
- EORTC QLQ H&N
European organization of research and treatment of cancer, quality of life questionnaire
- OHI-s
Oral hygiene index-simplified
- OM
Oral mucositis
- CT
Chemotherapy
- RT
Radiotherapy
- JCIA
Joint Commission International Accreditation
Author contributions
Conceptualization, Methodology, Investigation, Data analysis, Manuscript writing: S.Q. Supervision: S.R, S.S. Methodology and Formal analysis: F.R, S.R. Resources and clinical expertise: F.R, S.A, S.A, M.I and A.A. Data collection and manuscript writing: S.Q, S.T. All the authors have overlooked the study and intellectually contributed to the manuscript and have read and approved the final manuscript. Consent was obtained from the participants of the study to publish the study results, online in a scientific journal.
Funding
This is a thesis project of a student of MSc. Epidemiology and Biostatistics. No funding was provided to the student.
Data availability
The datasets generated and analyzed during the current study are not publicly available due to individual privacy could be compromised but are available from the corresponding author on reasonable request.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Ren ZH, Hu CY, He HR, Li YJ, Lyu J. Global and regional burdens of oral cancer from 1990 to 2017: Results from the global burden of disease study. Cancer Commun. (Lond.) 2020;40(2–3):81–92. doi: 10.1002/cac2.12009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Niaz K, Maqbool F, Khan F, Bahadar H, Hassan FI, Abdollahi M. Smokeless tobacco (paan and gutkha) consumption, prevalence, and contribution to oral cancer. Epidemiol. Health. 2017 doi: 10.4178/epih.e2017009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ali A, Manzoor MF, Ahmad N, Aadil RM, Qin H, Siddique R, et al. The burden of cancer, government strategic policies, and challenges in Pakistan: A comprehensive review. Front. Nutr. 2022;9:940514. doi: 10.3389/fnut.2022.940514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Krishnatreya M, Rahman T, Kataki AC, Sharma JD, Nandy P, Baishya N. Pre-treatment performance status and stage at diagnosis in patients with head and neck cancers. Asian Pac. J. Cancer Prev. 2014;15(19):8479–8482. doi: 10.7314/APJCP.2014.15.19.8479. [DOI] [PubMed] [Google Scholar]
- 5.Liu S, Zhao Q, Zheng Z, Liu Z, Meng L, Dong L, et al. Status of treatment and prophylaxis for radiation-induced oral mucositis in patients with head and neck cancer. Front. Oncol. 2021;11:642575. doi: 10.3389/fonc.2021.642575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Shavi GR, Thakur B, Bhambal A, Jain S, Singh V, Shukla A. Oral health related quality of life in patients of head and neck cancer attending cancer hospital of Bhopal City, India. J. Int. Oral Health JIOH. 2015;7(8):21. [PMC free article] [PubMed] [Google Scholar]
- 7.de la Fuente HJ, Díaz F, Vilchis M. Oral health related quality of life. In: Virdi MS, editor. Emerging Trends in Oral Health Sciences and Dentistry. IntechOpen; 2015. [Google Scholar]
- 8.Bundugji MH, Bafarat D, Bundagji N, Arafsha Y, Hassan H. Assessment of oral health and care in head and neck oncology patients in King Faisal Specialist Hospital and Research Center-Jeddah. Saudi Dent. J. 2021;33(8):1174–1183. doi: 10.1016/j.sdentj.2021.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Khaw A, Liberali S, Logan R, Keefe D, Bartold P. Influence of periodontitis on the experience of oral mucositis in cancer patients undergoing head and neck radiotherapy: A pilot study. Support. Care Cancer. 2014;22(8):2119–2125. doi: 10.1007/s00520-014-2186-3. [DOI] [PubMed] [Google Scholar]
- 10.Gil-Montoya JA, de Mello ALF, Barrios R, Gonzalez-Moles MA, Bravo M. Oral health in the elderly patient and its impact on general well-being: A nonsystematic review. Clin. Interv. Aging. 2015;10:461. doi: 10.2147/CIA.S54630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Madjova C, Chokanov S, Negreva M. Correlation between oral hygiene and health status of patients with cardiovascular diseases. J. Union Sci. Varna Med. Ecol. Ser. 2021;25(1):73. [Google Scholar]
- 12.Sirin DA, Ozcelik F. The relationship between COVID-19 and the dental damage stage determined by radiological examination. Oral Radiol. 2021;37(4):600–609. doi: 10.1007/s11282-020-00497-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Al-Ansari S, Zecha JA, Barasch A, de Lange J, Rozema FR, Raber-Durlacher J. Oral mucositis induced by anticancer therapies. Curr. Oral Health Rep. 2015;2(4):202–11. doi: 10.1007/s40496-015-0069-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Brown TJ, Gupta A. Management of cancer therapy–associated oral mucositis. JCO Oncol. Pract. 2020;16(3):103–109. doi: 10.1200/JOP.19.00652. [DOI] [PubMed] [Google Scholar]
- 15.Lalla RV, Sonis ST, Peterson D. Management of oral mucositis in patients who have cancer. Dent. Clin. N. Am. 2008;52(1):61–77. doi: 10.1016/j.cden.2007.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Barrios R, Bravo M, Gil-Montoya JA, Martínez-Lara I, García-Medina B, Tsakos GJH, et al. Oral and general health-related quality of life in patients treated for oral cancer compared to control group. Health Qual. Life Outcomes. 2015;13(1):1–8. doi: 10.1186/s12955-014-0201-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Raber-Durlacher JE, Elad S, Barasch A. Oral mucositis. Oral Oncol. 2010;46(6):452–6. doi: 10.1016/j.oraloncology.2010.03.012. [DOI] [PubMed] [Google Scholar]
- 18.Elad S, Zadik Y. Chronic oral mucositis after radiotherapy to the head and neck: A new insight. Support. Care Cancer. 2016;24(11):4825–30. doi: 10.1007/s00520-016-3337-5. [DOI] [PubMed] [Google Scholar]
- 19.Lalla RV, Treister N, Sollecito T, Schmidt B, Patton LL, Mohammadi K, et al. Oral complications at 6 months after radiation therapy for head and neck cancer. Oral Dis. 2017;23(8):1134–1143. doi: 10.1111/odi.12710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Henry M, Rosberger Z, Bertrand L, Klassen C, Hier M, Zeitouni A, et al. Prevalence and risk factors of suicidal ideation among patients with head and neck cancer: Longitudinal study. Otolaryngol. Head Neck Surg. 2018;159(5):843–852. doi: 10.1177/0194599818776873. [DOI] [PubMed] [Google Scholar]
- 21.Schierz O, Baba K, Fueki K. Functional oral health-related quality of life impact: A systematic review in populations with tooth loss. J. Oral Rehabilit. 2021;48(3):256–270. doi: 10.1111/joor.12984. [DOI] [PubMed] [Google Scholar]
- 22.Zahid N, Zahid W, Khalid W, Azam I, Ikram M, Iftikar H, et al. Resilience and its associated factors in head and neck cancer patients in Pakistan: An analytical cross-sectional study. BMC Cancer. 2021;21(1):888. doi: 10.1186/s12885-021-08624-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Badr H, Lipnick D, Gupta V, Miles B. Survivorship challenges and information needs after radiotherapy for oral cancer. J. Cancer Educ. 2017;32(4):799–807. doi: 10.1007/s13187-016-1048-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zahid N, Khalid W, Ahmad K, Bhamani SS, Azam I, Asad N, et al. Resilience and quality of life (QoL) of head and neck cancer and brain tumour survivors in Pakistan: An analytical cross-sectional study protocol. BMJ Open. 2019;9(9):e029084. doi: 10.1136/bmjopen-2019-029084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Pannu JS, Simpson MC, Donovan CL, Adjei Boakye E, Mass K, Challapalli SD, et al. Sociodemographic correlates of head and neck cancer survival among patients with metastatic disease. Head Neck. 2020;42(9):2505–2515. doi: 10.1002/hed.26284. [DOI] [PubMed] [Google Scholar]
- 26.Ohlsson B, Manjer J. Sociodemographic and lifestyle factors in relation to overweight defined by BMI and "normal-weight obesity". J. Obes. 2020;2020:2070297. doi: 10.1155/2020/2070297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Chowdhury SF, Islam MN, Sony SA. Evaluation of oral health status among pregnant women using oral hygiene index- simplified (OHI-S) score. Int. J. Hum. Health Sci. (IJHHS) 2022 doi: 10.31344/ijhhs.v6i3.462. [DOI] [Google Scholar]
- 28.Erden Y, Ipek CG. Improvement of the comfort perception scale in oral mucositis. Jpn. J. Clin. Oncol. 2021;51(11):1636–1642. doi: 10.1093/jjco/hyab136. [DOI] [PubMed] [Google Scholar]
- 29.Zahid N, Martins RS, Zahid W, Azam I, Ikram M, Hassan A, et al. Psychometric properties of the Urdu version of the EORTC QLQ-H&N35 (European organization for research and treatment of cancer head and neck module) quality of life tool. BMC Psychol. 2022;10(1):1–12. doi: 10.1186/s40359-022-00900-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Loorents V, Rosell J, Salgado Willner H, Börjeson S. Health-related quality of life up to 1 year after radiotherapy in patients with head and neck cancer (HNC) SpringerPlus. 2016;5(1):669. doi: 10.1186/s40064-016-2295-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Maqbool S, Siddique S, Mehak A, Ali Q, Asghar SK, Shahid F. Evaluation of Oral Health Related Quality of Life in Patients Diagnosed with Head and Neck Cancer Receiving Radiotherapy. Ultrasound.3:2.0.
- 32.Sharma Y, Mishra G, Parikh V. Quality of life in head and neck cancer patients. Indian J. Otolaryngol. Head Neck Surg. 2019;71(1):927–932. doi: 10.1007/s12070-019-01620-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Iwanaga K, Ishibashi Y, Maki K, Ura A, Haba K, Sakata T, et al. Two-year evolution of quality of life following radiotherapy and/or chemotherapy in patients with head and neck cancer. Asia-Pac. J. Oncol. Nurs. 2023;10:100301. doi: 10.1016/j.apjon.2023.100301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wan Leung S, Lee T-F, Chien C-Y, Chao P-J, Tsai W-L, Fang F-M. Health-related quality of life in 640 head and neck cancer survivors after radiotherapy using EORTC QLQ-C30 and QLQ-H&N35 questionnaires. BMC Cancer. 2011;11:1–10. doi: 10.1186/1471-2407-11-128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Soldera EB, Ortigara GB, Bonzanini LI, Schulz RE, Danesi CC, Antoniazzi RP, et al. Clinical and sociodemographic factors associated with oral health-related quality of life in survivors of head and neck cancer. Head Neck. 2020;42(5):886–897. doi: 10.1002/hed.26063. [DOI] [PubMed] [Google Scholar]
- 36.Zahid N, Martins RS, Dawood ZS, Zahid W, Azam I, Ikram M, et al. Clinical and psychosocial factors associated with quality of life in patients with head and neck cancer: An analytical cross-sectional study from a lower-middle-income country. BMC Psychol. 2023;11(1):265. doi: 10.1186/s40359-023-01264-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hameed MH, Zafar K, Ghafoor R. Management of oral complications in irradiated head and neck cancer patients: Literature review. IJS Short Rep. 2018;3(1):15–21. doi: 10.4103/ijssr.ijssr_6_18. [DOI] [Google Scholar]
- 38.Das R, Kataki A, Sharma J, Baishya N, Kalita M, Krishnatreya M. A study of head and neck cancer patients with special reference to tobacco use and educational level. Clin. Cancer Investig. J. 2017;6(1):21. doi: 10.4103/ccij.ccij_24_17. [DOI] [Google Scholar]
- 39.Chauhan R, Trivedi V, Rani R, Singh U. A study of head and neck cancer patients with reference to tobacco use, gender, and subsite distribution. South Asian J. Cancer. 2022 doi: 10.1055/s-0041-1740601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Barma MD, Indiran MA, Kumar P, Balasubramaniam A, Kumar MS. Quality of life among head and neck cancer treated patients in South India: A cross-sectional study. J. Oral Biol. Craniofacial Res. 2021;11(2):215–218. doi: 10.1016/j.jobcr.2021.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Lee H-J, Han D-H, Kim J-H, Wu H-G. The effect of comprehensive oral care program on oral health and quality of life in patients undergoing radiotherapy for head and neck cancer: A quasi-experimental case-control study. Medicine. 2021;100(16):e25540. doi: 10.1097/MD.0000000000025540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Huang B-S, Chung C-F, Chang Y-L, Lee L-Y, Peng H-L, Chen S-C. Body mass index and self-care behaviors related to oral health–related quality of life in patients with oral squamous cell carcinoma within three months posttreatment. Support. Care Cancer. 2021;29(4):2239–2248. doi: 10.1007/s00520-020-05737-x. [DOI] [PubMed] [Google Scholar]
- 43.de Melo N-B, de Sousa V-M, de Melo D-P, Bento P-M. Oral health related quality of life and determinant factors in patients with head and neck cancer. Med. Oral Patol. Oral Cir. Bucal. 2019;24(3):e281. doi: 10.4317/medoral.22670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Daume L, Kreis C, Bohner L, Jung S, Kleinheinz J. Clinical characteristics of oral lichen planus and its causal context with dental restorative materials and oral health-related quality of life. BMC Oral Health. 2021;21(1):262. doi: 10.1186/s12903-021-01622-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Hemani A, Rauf F, Noori MY, Faisal A. Barriers to the access of oral health care in individuals from lower socioeconomic communities in Karachi. J. Liaquat Univ. Med. Health Sci. 2017;16(4):218–221. doi: 10.22442/jlumhs.171640537. [DOI] [Google Scholar]
- 46.Wan Leung S, Lee T-F, Chien C-Y, Chao P-J, Tsai W-L, Fang F-M. Health-related quality of life in 640 head and neck cancer survivors after radiotherapy using EORTC QLQ-C30 and QLQ-H&N35 questionnaires. BMC Cancer. 2011;11(1):1–10. doi: 10.1186/1471-2407-11-128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Sohn H-O, Park E-Y, Jung Y-S, Lee E-K, Kim E-K. Effects of professional oral hygiene care in patients with head-and-neck cancer during radiotherapy: A randomized clinical trial. Indian J. Dent. Res. 2018;29(6):700. doi: 10.4103/ijdr.IJDR_226_17. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets generated and analyzed during the current study are not publicly available due to individual privacy could be compromised but are available from the corresponding author on reasonable request.