Abstract
We have examined the antibody response to Helicobacter pylori lipopolysaccharides (LPS) in humans. We used sera from patients with gastroduodenal diseases and healthy adults infected or not infected with H. pylori. Data from the experiments for antibody binding to LPS suggested that the polysaccharide chains from many H. pylori strains showed high immunogenicity in humans. Sera from most (above 70%) H. pylori-infected individuals contained immunoglobulin G (IgG) antibodies against the polysaccharide region highly immunogenic H. pylori LPS. The IgG titers of individual serum samples that reacted strongly with highly immunogenic LPS were quite similar (r2 = 0.84 to 0.98). The results suggest wide distribution among H. pylori strains of a highly antigenic epitope in the polysaccharide moieties of their LPS. Also, the similarity in the titers of individual serum samples against highly immunogenic LPS points to the existence of epitopes sharing a common structural motif. However, some strains showed low antigenicity, even those with polysaccharide-carrying LPS. The dominant subclass of IgG that reacted with the highly immunogenic LPS was IgG2, which was preferentially raised against polysaccharide antigens. Recently, a structure that mimics that of the Lewis antigens was identified in the O-polysaccharide fraction of H. pylori LPS; however, no correlation between antigenicity of the polysaccharide chain in humans and the presence of Lewis antigens was found. The IgA and IgM titers against H. pylori LPS seemed to be mostly nonspecific and directed against lipid A. In a few cases, however, sera from individuals infected with H. pylori gave strong IgA and IgM titers against the highly immunogenic polysaccharide. In conclusion, the LPS of many H. pylori strains possess an antigenic epitope in their polysaccharide regions that is immunogenic in humans. However, our results show that the antigenic epitope is unlikely to be immunologically related to structures mimicking Lewis antigens.
Helicobacter pylori is an emerging candidate for the genesis of chronic gastritis and peptic ulcer (12, 14). Furthermore, H. pylori infection is thought to be one of the causative factors of gastric cancer (10, 15). Recently, extensive structural and immunological studies of H. pylori lipopolysaccharides (LPS) have been carried out. The O-polysaccharide region of H. pylori LPS has been found to be a major antigenic determinant (13), as are those of other typical bacterial LPS. Interestingly, many H. pylori strains have O-polysaccharide containing epitopes that mimic the structures of Lewis antigens, as shown by chemical (6, 7) and immunological studies (2, 3, 18, 20, 23). The Lewis antigens, which are made up of fucosylated lactosamine structures, are known as tumor antigens on cancer cells, and in normal cells they occur as blood-group antigens and a granulocyte marker antigen (CD15). Hence, the immunological response to the Lewis antigen-containing O-polysaccharides is considered to play a role in the pathogenicity of H. pylori through the establishment of an autoimmune response (3). We have been interested in the antigenicity of H. pylori LPS during natural infection in humans. We present data which suggest the existence of an antigenic epitope, immunologically unrelated to the Lewis antigen, in the polysaccharide moiety of the LPS of a wide range of H. pylori strains.
Bacterial strains.
Clinical strains of H. pylori were isolated from the biopsy specimens of lesions obtained from patients with chronic gastritis, gastric ulcer, duodenal ulcer, and gastric cancer (tumor sites and nontumor sites) in the Sapporo Medical University Hospital (Sapporo, Japan). After three to five laboratory subcultures, H. pylori cells were grown on brain heart infusion agar plates supplemented with 10% (vol/vol) horse blood at 37°C for 5 days under microaerophilic conditions by using the GasPak System without a catalyst (BBL, Cockeysville, Md.). The organisms were collected, washed with phosphate-buffered saline (PBS) three times, and lyophilized.
Human sera.
Sera of 25 patients with chronic gastritis, gastric ulcer, duodenal ulcer and gastric cancer and sera of 83 healthy adult volunteers were donated by the Hospitals of Sapporo Medical University and Akita University School of Medicine (Akita, Japan). The status of H. pylori infection was determined by using an enzyme immunoassay kit, Determiner H. pylori Antibody, originally distributed under the name HM-CAP by Enteric Products (Westbury, N.Y.) and purchased from Kyowa Medics (Tokyo, Japan). With this kit, the serum samples of 24 of 25 patients and 21 of 83 healthy adults were found to be positive for H. pylori infection.
IgG response to H. pylori LPS.
We examined the human antibody response to LPS isolated from H. pylori strains by enzyme-linked immunosorbent assay (ELISA). For most ELISA experiments, proteinase K-treated bacterial cells were used as an LPS antigen (2, 24). Briefly, H. pylori cells were suspended in sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) sample buffer (11) at a concentration of 2 mg/ml and incubated at 100°C for 10 min. Two hundred microliters of proteinase K (2.5 mg/ml) was added, and the mixture was incubated at 37°C overnight and then at 65°C for 2 h. The resulting antigen was diluted 50-fold with 50 mM sodium carbonate buffer (pH 9.6), and aliquots were dispensed into a MicroTest III flexible assay plate (Becton Dickinson, Oxnard, Calif.). The plate was incubated at 4°C overnight, and after the reaction was blocked with 1% human serum albumin, the plate was used for ELISA (24). For purified LPS preparations used as coated antigens, an LPS preparation which was purified as described previously (1) was dissolved in 50 mM sodium carbonate buffer (pH 9.8) at a concentration of 5 μg/ml and then dispensed onto an assay plate. Human serum was diluted 3,000-fold with PBS containing 0.05% Tween 20 (PBST) and 2% human serum albumin. Horseradish peroxidase-conjugated goat F(ab′)2 anti-human immunoglobulin G (IgG) antibodies (BioSource International, Camarillo, Calif.) and tetramethylbenzidine peroxidase substrate system (Kirkegaard & Perry Laboratories Inc.) were second antibody and substrate, respectively. Absorbance at 450 nm was measured after the termination of the reaction with 1 M phosphoric acid. The results of two ELISAs, with purified LPS and proteinase K-treated cells used as antigens, and immunoblotting analysis (24) were comparable.
We found that H. pylori strains could be classified into three groups on the basis of the level of antigenicity of their LPS in humans (24). One group possessed high-antigenicity-polysaccharide-carrying smooth LPS. The second group had low-antigenicity-polysaccharide-carrying smooth LPS. Most H. pylori-infected individuals did not have an antibody titer against the low-immunogenicity LPS even though the LPS carried the polysaccharide chains detected by SDS-PAGE and silver staining (24; data not shown). The third group was characterized by the presence of rough LPS (e.g., CG10). We examined samples for correlation between the levels of IgG in individual serum samples for each type of LPS (Table 1). The levels of IgG for highly immunogenic H. pylori LPS derived from strains CG7, CG9, GU2, GU10, DU1, and DU2 were strongly (r2 = 0.84 to 0.98) related. These results indicated that the antigenicities of all the highly immunogenic LPS were nearly the same. However, no significant correlation was observed between the titers of IgG for high-immunogenicity LPS, low-immunogenicity LPS (CA2, CA4, and CA6), and rough LPS (CG10). From the above results, we consider that the antigenicity of H. pylori LPS in humans can be expressed by the mean values of the binding activities to LPS in randomly selected serum samples from H. pylori-infected individuals. The results of ELISA using 10 randomly selected serum samples are shown in Fig. 1. LPS of H. pylori clinical isolates showed various antigenicities. Among them, all the rough strains tested so far showed low antigenicity. The phenomenon is attributable to the antigenic epitope located in the polysaccharide chain. Interestingly, low-immunogenicity LPS, even those with the polysaccharide chain, were frequently found in strains derived from gastric cancer patients. In conclusion, there are high- and low-antigenicity-polysaccharide-carrying LPS among smooth strains of H. pylori.
TABLE 1.
Correlation (r2) of titers of IgG antibody in serum samples tested to LPS fractions from H. pylori strains and S. minnesota Re mutant
| LPS derived from strain indicated | High-immunogenicity LPS
|
Low-immunogenicity LPS
|
Rough LPS
|
||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| CG7 | CG9 | GU2 | GU10 | DU1 | DU2 | CA2 | CA4 | CA6 | CG10 | Re | |
| CG7 | 0.914 | 0.976 | 0.938 | 0.933 | 0.967 | 0.070 | 0.012 | 0.016 | 0.000 | 0.009 | |
| CG9 | 0.914 | 0.877 | 0.873 | 0.922 | 0.140 | 0.053 | 0.070 | 0.002 | 0.007 | ||
| GU2 | 0.914 | 0.944 | 0.959 | 0.082 | 0.021 | 0.021 | 0.001 | 0.009 | |||
| GU10 | 0.836 | 0.900 | 0.105 | 0.022 | 0.021 | 0.016 | 0.037 | ||||
| DU1 | 0.955 | 0.058 | 0.016 | 0.017 | 0.000 | 0.002 | |||||
| DU2 | 0.086 | 0.023 | 0.025 | 0.000 | 0.008 | ||||||
| CA2 | 0.649 | 0.748 | 0.175 | 0.082 | |||||||
| CA4 | 0.837 | 0.093 | 0.004 | ||||||||
| CA6 | 0.101 | 0.024 | |||||||||
| CG10 | 0.390 | ||||||||||
| Re | |||||||||||
FIG. 1.
Antigenicity of H. pylori LPS from clinical isolates. Antigenicity is expressed as the mean values of the ELISA reading (A450) for a random selection of 10 serum samples from patients and healthy adults with H. pylori infection. Smooth and rough chemotypes were discriminated by SDS-PAGE and silver staining as described previously (2).
Many H. pylori strains are known to express Lewis antigen structures as a part of the O-polysaccharide chain of LPS (2, 3, 20, 23). Therefore, we determined if the antigenicity of the LPS correlated with the presence of Lewis antigens. The antigenicity of LPS was determined as described above. The presence of structures mimicking the Lewis antigens in LPS was determined by immunoblotting analysis with monoclonal antibody specific for each Lewis antigen described previously (2). Strains with neither high nor low immunogenicity showed a preference for any Lewis antigens (Table 2). The results indicate that the antigenicity of H. pylori LPS is unrelated to the presence of the Lewis antigens.
TABLE 2.
Comparison of the Lewis antigen distribution in the LPS fraction from 59 smooth strains of H. pylori and the antigenicity of these LPS fractions in humans
| Expression of Lewis antigena | No. of strains | No. of strains showing indicated level of antigenicity in humansb
|
||
|---|---|---|---|---|
| ++ | + | − | ||
| Lex | ||||
| + | 28 | 11 | 7 | 10 |
| − | 31 | 9 | 12 | 10 |
| Ley | ||||
| + | 29 | 10 | 9 | 10 |
| − | 30 | 10 | 10 | 10 |
| Lea | ||||
| + | 5 | 3 | 0 | 2 |
| − | 54 | 17 | 18 | 19 |
| Leb | ||||
| + | 7 | 2 | 3 | 2 |
| − | 48 | 18 | 16 | 18 |
Lewis antigen expression reported in reference 2 was determined by immunoblotting with anti-Lewis antigen monoclonal antibodies. Lex, Ley, Lea, Leb, Lewis X to Lewis b antigens, respectively.
Antigenicity was expressed as the mean value of A450 for 10 human serum samples positive for H. pylori infection: ++, >0.6; +, >0.15 to <0.6; −, <0.15.
IgG subclass of anti-H. pylori LPS antibody.
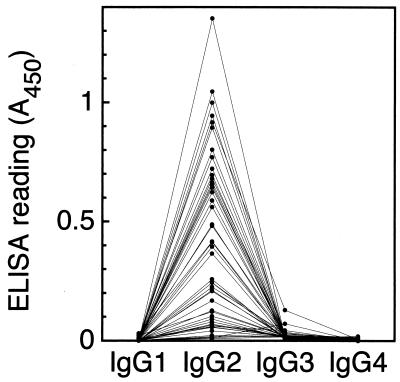
The subclass of IgG specific for high-immunogenicity H. pylori LPS was determined by ELISA using human IgG subclass-specific murine monoclonal antibodies. An LPS preparation derived from H. pylori GU2 was coated onto a microplate as described above. Human serum was diluted 1:1,000 (for IgG1 and IgG2) or 1:300 (for IgG3 and IgG4) with PBST, applied to an LPS-coated plate, and incubated at 37°C for 2 h. After the plate was washed with PBST, murine anti-human IgG subclass-specific monoclonal antibodies (500-fold dilution) were dispensed onto the plate and incubated at 37°C for 2 h. Anti-human IgG1 (Fc) (clone 8c/6-39, murine IgG2a), anti-human IgG2 (Fab) (clone HP6014, murine IgG1), anti-human IgG3 [F(ab)2] (clone HP6050, murine IgG1), and anti-human IgG4 (pFc) (clone HP6023, murine IgG3) were purchased from The Binding Site Ltd. (Birmingham, United Kingdom). After the plate was washed, horseradish peroxidase-conjugated goat anti-mouse IgG (H+L) antibodies (human immunoglobulin adsorbed) (Biosource International) was dispensed onto the plate and incubated at 37°C for 2 h. Antibody binding was developed with the TMB peroxidase substrate system as described above. As shown in Fig. 2, IgG2 was found to be dominant type in almost all the sera. The results suggested that the titer of IgG to high-immunogenicity H. pylori LPS of H. pylori-infected individuals was restricted to the IgG2 subclass. This response is normally attributed to antibodies against bacterial polysaccharide antigens in a T-cell-independent manner (9, 16, 19, 25). Steer et al. (21) determined the IgG subclass response to H. pylori using acid extract as the antigen. IgG1 and IgG4 were significantly present in gastroduodenal patients, and an IgG2 titer was found in 63% of the patients. The value for the IgG2-positive patients was very similar to that of anti-H. pylori LPS IgG2-positive individuals with H. pylori infection described in this study. As mentioned above, many H. pylori LPS share epitopes that mimic the structures of the host carbohydrate antigens, namely, the Lewis antigens. Similarly, Campylobacter jejuni LPS have structures that mimic gangliosides such as GM1 and GQ1b (4, 5, 17, 27, 28). The host carbohydrate antigen-bearing LPS elicit the pathogenic autoantibodies during C. jejuni infection and may result in neuronal diseases such as Guillain-Barré syndrome and Miller-Fisher syndrome. Interestingly, the anti-C. jejuni LPS antibodies which react to host gangliosides in the patients are restricted predominantly to IgG1 and IgG3 classes (22, 26). These are the subclasses normally found as antibodies against protein antigens in T-cell-dependent responses. In the present study, the antibodies that responded to H. pylori LPS were restricted to predominantly the IgG2 class in both gastroduodenal patients and healthy adults. This finding clearly represents a point of distinction between gastroduodenal diseases caused by H. pylori infection and neuronal diseases subsequent to C. jejuni infection.
FIG. 2.
Determination of the IgG subclass of highly immunogenic H. pylori LPS-specific antibodies in human sera. LPS derived from strain GU2 was coated onto a microplate. Human serum (1,000-fold dilution) was applied to the plate. Subsequently, human IgG subclass-specific murine monoclonal antibodies, peroxidase-conjugated goat anti-mouse IgG antibodies, and TMB substrate solution were applied. Binding activity was expressed as absorbance at 450 nm.
IgM and serum IgA responses.
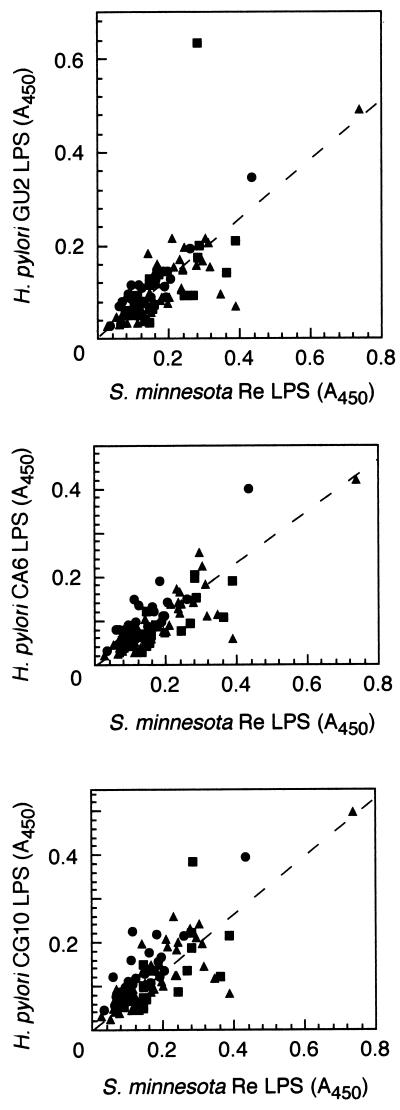
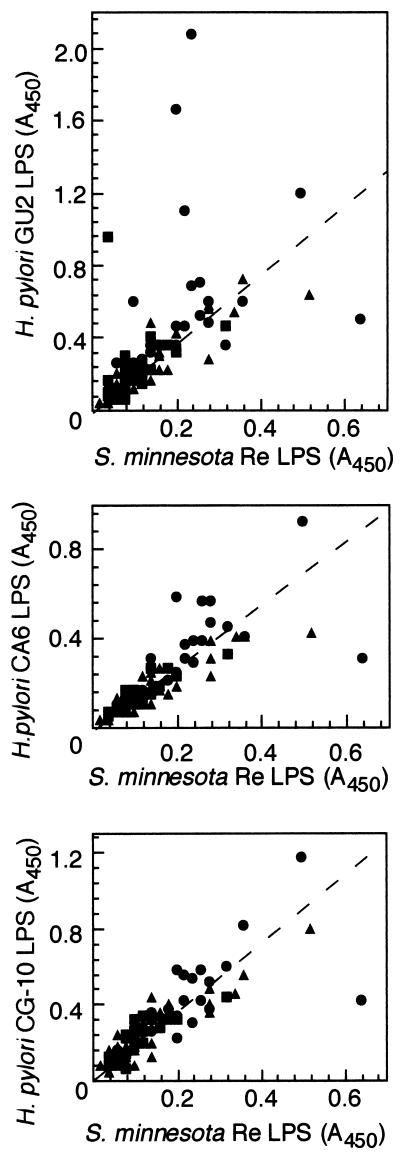
We examined the titers of IgM (Fig. 3) and IgA (Fig. 4) to H. pylori LPS in sera by ELISA. ELISA was carried out as described above, except that 300-fold diluted human serum and horseradish peroxidase-conjugated human IgM- or IgA-specific antibodies (BioSource International) were used as first and second antibodies, respectively. We used Salmonella minnesota Re mutant LPS (Sigma, St. Louis, Mo.) as a control to determine the level of nonspecific anti-LPS antibodies. The Re mutant has a severe defect in its LPS structure; namely, its LPS consists of lipid A and possesses only one 3-deoxyoctulosonic acid residue. Most individual serum samples contained nonspecific anti-LPS antibodies that were mainly IgM and IgA. We succeeded in showing that nonspecific binding to LPS could be discerned by comparison of the antibody titer to that of the Re-type LPS. The IgM and IgA titers of sera to low-immunogenicity LPS (e.g., CA6) and rough LPS (e.g., CG10) could be attributed to such nonspecific anti-LPS antibodies, as the titers were closely related to the antibody titer of Salmonella Re-type LPS (Fig. 3 and 4). However, only 3 of 25 patients and 1 of 21 healthy adults with H. pylori infection were positive for H. pylori LPS-specific IgA, and one healthy adult with H. pylori infection was positive for H. pylori-specific IgM. The specificity of the IgA and IgM titer to H. pylori LPS was similar to that of IgG; namely, the IgA and IgM antibodies that bound to high-immunogenicity LPS did not bind to low-immunogenicity LPS (data not shown). The results indicated that antibody response to the antigenic epitope was not restricted to IgG but that IgA and IgM were detected in some serum samples. An IgM titer to the antigenic epitope of the high-immunogenicity H. pylori LPS was detected in one H. pylori-infected healthy adult, and IgA titers were detected in a few gastroduodenal patients. As the IgM-positive individual had a low titer of IgG to the antigenic epitope, the individual was most probably in an acute phase of infection. A high IgA titer was more frequently found in the patients with gastric diseases. The IgA response seemed to be related to the mucosal immunity in the gastroduodenal disease state during chronic H. pylori infection.
FIG. 3.
Relationship of the IgM titer of human sera to H. pylori LPS derived from a strain with high antigenicity (GU2), a strain with low antigenicity (CA6), and a rough strain (CG10) and S. minnesota rough (Re-type) LPS as determined by ELISA. Human sera were diluted 1:300. The human sera were donated by patients with gastroduodenal disease (•), healthy adults with H. pylori infection (▪), and healthy adults without H. pylori infection (▴).
FIG. 4.
Relationship of the titer of IgA in human sera to H. pylori LPS derived from a strain with high antigenicity (GU2), a strain with low antigenicity (CA6), and a rough strain (CG10) and S. minnesota rough (Re-type) LPS as determined by ELISA. Human serum was diluted 1:300. The human sera were donated by patients with gastroduodenal disease (•), healthy adults with H. pylori infection (▪), and healthy adults without H. pylori infection (▴).
Significant findings of this study.
Recently, H. pylori LPS was shown to have Lewis antigens in the O-polysaccharide chain of its LPS (3, 6–8, 18, 20, 23). The presence of such epitopes mimicking host antigens was suggested to elicit an autoimmune response (3). In this study, we carefully examined the antibody response to H. pylori LPS in H. pylori-infected gastric disease patients and in infected and uninfected healthy adults. We proposed that there was an epitope antigenic to humans on the polysaccharide chain of H. pylori LPS. The antigenic epitope was distributed among many H. pylori strains but not all strains. Strains having the antigenic epitope showed high immunogenicity. In contrast, strains lacking the epitope in the polysaccharide chains and rough strains, namely, strains lacking the polysaccharide chain, showed low antigenicity. We found that few human serum samples contained high titers of antibody specific for the low-immunogenicity LPS but not for the high-immunogenicity ones. The titers of IgG to high-immunogenicity LPS were quite similar to one another. The evidence suggests little variation in the nature of the antigen in the highly immunogenic polysaccharide of LPS among H. pylori strains. The antigenic epitope would appear to be a defined structure in the polysaccharide chain of LPS. However, the structure of the antigenic epitope remains to be defined.
Appelmelk et al. (3) pointed out the presence of an IgG titer to Lewis X antigen (Lex)-bearing LPS in the sera of H. pylori-infected patients. However, they used only three strains, two Lex-positive strains and one Lex-negative strain. We have shown that antigenicity was not related to the existence of any Lewis antigens in about 60 smooth strains of H. pylori. Therefore, we suggest that the mimicry of the Lewis antigen by H. pylori is probably not important for antigenicity in humans. This result is also supported by the observation that an anti-Lewis antigen antibody titer can be detected in human sera independently of H. pylori LPS (2). Indeed, the anti-Lex antibodies were produced by immunization of mice and rabbits with H. pylori cells (3). There may be differences in the immune responses between active immunization in animals and natural infection in humans. On the other hand, we found that the antigenic epitope was related to the clinical sources of the strains. The epitope was frequently lacking in H. pylori strains derived from tumors of gastric cancer patients, an interesting observation described in detail elsewhere (24). However, sera of cancer patients did not contain significantly higher antibody titers to the low immunogenic LPS than those to the high immunogenic LPS. Many of the serum samples from gastric cancer patients contained antibody titers to the antigenic epitope (data not shown).
In conclusion, the epitopes that mimic the structures of the Lewis antigens in H. pylori LPS do not seem to be those that predominate during natural infections in humans. We conclude that the Lewis antigen-related epitopes do not play a major role in the pathogenicity of H. pylori with regard to gastroduodenal diseases, such as chronic gastritis, peptic ulcer, and gastric cancer.
Acknowledgments
We are grateful to T. Ishioka and O. Urayama of Akita University School of Medicine for providing sera from healthy individuals and patients suffering from gastric diseases.
REFERENCES
- 1.Amano K, Fujita M, Suto T. Chemical properties of lipopolysaccharides from spotted fever group rickettsiae and their common antigenicity with lipopolysaccharides from Proteus species. Infect Immun. 1993;61:4350–4355. doi: 10.1128/iai.61.10.4350-4355.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Amano K, Hayashi S, Kubota T, Fujii N, Yokota S. Reactivities of Lewis antigen monoclonal antibodies to the lipopolysaccharides of Helicobacter pylori strains isolated from patients with gastroduodenal diseases in Japan. Clin Diagn Lab Immunol. 1997;4:540–544. doi: 10.1128/cdli.4.5.540-544.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Appelmelk B J, Simoons-Smit I, Negrini R, Moran A P, Aspinall G O, Forte J G, de Vries T, Quan H, Verboom T, Maaskant J J, Ghiara P, Kuipers E J, Bloemena E, Tadema T M, Townsend R R, Tyagarajan K, Crothers J M, Jr, Monterio M A, Savio A, de Graaff J. Potential role of molecular mimicry between Helicobacter pylori lipopolysaccharide and host Lewis blood group antigens in autoimmunity. Infect Immun. 1996;64:2031–2040. doi: 10.1128/iai.64.6.2031-2040.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Aspinall G O, Fujimoto S, McDonald A G, Pang H, Kurjanczyk L A, Penner J L. Lipopolysaccharides from Campylobacter jejuni associated with Guillain-Barré syndrome mimic human gangliosides in structure. Infect Immun. 1994;62:2122–2125. doi: 10.1128/iai.62.5.2122-2125.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Aspinall G O, McDonald A G, Raju T S, Pang H, Mills S D, Kurjanczyk L A, Penner J L. Serological diversity and chemical structures of Campylobacter jejuni low-molecular-weight lipopolysaccharides. J Bacteriol. 1992;174:1324–1332. doi: 10.1128/jb.174.4.1324-1332.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Aspinall G O, Monteiro M A. Lipopolysaccharide of the Helicobacter pylori strains P466 and MO19: structures of the O antigen and core oligosaccharide regions. Biochemistry. 1996;35:2498–2504. doi: 10.1021/bi951853k. [DOI] [PubMed] [Google Scholar]
- 7.Aspinall G O, Monteiro M A, Pang H, Walsh E J, Moran A P. Lipopolysaccharide of the Helicobacter pylori type strain NCTC 11637 (ATCC 43504): structure of the O antigen chain and core oligosaccharide regions. Biochemistry. 1996;35:2489–2497. doi: 10.1021/bi951852s. [DOI] [PubMed] [Google Scholar]
- 8.Aspinall G O, Monteiro M A, Pang H, Walsh E J, Moran A P. O antigen chains in the lipopolysaccharide of Helicobacter pylori NCTC 11637. Carbohydr Lett. 1994;1:151–156. [Google Scholar]
- 9.Barrett D J, Ayoub E M. IgG2 subclass restriction of antibody to pneumococcal polysaccharide. Clin Exp Immunol. 1986;63:127–134. [PMC free article] [PubMed] [Google Scholar]
- 10.The Eurogast Study Group. An international association between Helicobacter pylori infection and gastric cancer. Lancet. 1993;341:1359–1362. [PubMed] [Google Scholar]
- 11.Laemmli U K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970;227:680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- 12.Marshall B J. Unidentified curved bacilli on gastric epithelium in chronic gastritis. Lancet. 1983;i:1273–1313. [PubMed] [Google Scholar]
- 13.Mills S D, Kurjanczyk L A, Penner J L. Antigenicity of Helicobacter pylori lipopolysaccharides. J Clin Microbiol. 1992;30:3175–3180. doi: 10.1128/jcm.30.12.3175-3180.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.NIH Consensus Development Panel on Helicobacter pylori in Peptic Ulcer Diseases. Helicobacter pylori in peptic ulcer diseases. JAMA. 1994;272:65–69. [PubMed] [Google Scholar]
- 15.Parsonnet J, Friedmann G D, Vandersteen D P, Orentreich N, Sibley R K. Helicobacter pylori infection and the risk of gastric carcinoma. N Engl J Med. 1991;325:1127–1131. doi: 10.1056/NEJM199110173251603. [DOI] [PubMed] [Google Scholar]
- 16.Risen W F, Skvaril F, Braun D G. Natural infection of man with group A streptococci. Levels, restriction in class, subclass, and type; and clonal appearance of polysaccharide-group-specific antibodies. Scand J Immunol. 1976;5:383–390. doi: 10.1111/j.1365-3083.1976.tb00292.x. [DOI] [PubMed] [Google Scholar]
- 17.Salloway S, Mermel L A, Seamans M, Aspinall G O, Nam Shin J E, Kurjanczyk L A, Penner J L. Miller-Fisher syndrome associated with Campylobacter jejuni bearing lipopolysaccharide molecules that mimic human ganglioside GD3. Infect Immun. 1996;64:2945–2949. doi: 10.1128/iai.64.8.2945-2949.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sherburne R, Taylor D E. Helicobacter pylori expresses a complex surface carbohydrate, Lewis X. Infect Immun. 1995;63:4564–4568. doi: 10.1128/iai.63.12.4564-4568.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Siber G R, Schur P H, Aisenberg A C, Weitzman S A, Schiffman G. Correlation between serum IgG-2 concentrations and the antibody response to bacterial polysaccharide antigens. N Engl J Med. 1980;303:178–182. doi: 10.1056/NEJM198007243030402. [DOI] [PubMed] [Google Scholar]
- 20.Simoons-Smit I N, Appelmelk B J, Verboom T, Negrini R, Penner J L, Aspinall G O, Moran A P, Fei S H, Bi-Shan S, Rudnica W, Savio A, de Graaff J. Typing of Helicobacter pylori with monoclonal antibodies against Lewis antigens in lipopolysaccharide. J Clin Microbiol. 1996;34:2196–2200. doi: 10.1128/jcm.34.9.2196-2200.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Steer H W, Hawtin P R, Newell D G. An ELISA technique for the serodiagnosis of Campylobacter pyloridis infection in patients with gastric and benign duodenal ulceration. Serodiagn Immunother. 1987;1:253–259. [Google Scholar]
- 22.Willison H J, Veitch J. Immunoglobulin subclass distribution and binding characteristics of anti-GQ1b antibodies in Miller Fisher syndrome. J Neuroimmunol. 1994;50:159–165. doi: 10.1016/0165-5728(94)90042-6. [DOI] [PubMed] [Google Scholar]
- 23.Wirth H-P, Yang M, Karita M, Blaser M J. Expression of the human cell surface glycoconjugates Lewis X and Lewis Y by Helicobacter pylori isolates is related to cagA status. Infect Immun. 1996;64:4598–4605. doi: 10.1128/iai.64.11.4598-4605.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Yokota S, Amano K, Hayashi S, Fujii N. Low antigenicity of the polysaccharide region of Helicobacter pylori lipopolysaccharides derived from tumors of patients with gastric cancer. Infect Immun. 1997;65:3509–3512. doi: 10.1128/iai.65.9.3509-3512.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Yount W J, Dorner M M, Kunkel H G, Kabat E A. Studies on human antibodies. IV. Selective variations in subgroup composition and genetic markers. J Exp Med. 1968;127:633–646. doi: 10.1084/jem.127.3.633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Yuki N, Ichihashi Y, Taki T. Subclass of IgG antibody to GM1 epitope-bearing lipopolysaccharide of Campylobacter jejuni in patients with Guillain-Barré syndrome. J Neuroimmunol. 1995;60:161–164. doi: 10.1016/0165-5728(95)00052-4. [DOI] [PubMed] [Google Scholar]
- 27.Yuki N, Taki T, Inagaki F, Kasama T, Takahashi M, Saito K, Handa S, Miyatake T. A bacterium lipopolysaccharide that elicits Guillian-Barré syndrome has a GM1 ganglioside-like structure. J Exp Med. 1993;178:1771–1775. doi: 10.1084/jem.178.5.1771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Yuki N, Taki T, Takahashi M, Saito K, Yoshino H, Tai T, Handa S, Miyatake T. Molecular mimicry between GQ1b ganglioside and lipopolysaccharides of Campylobacter jejuni isolated from patients with Fisher’s syndrome. Ann Neurol. 1994;36:791–793. doi: 10.1002/ana.410360517. [DOI] [PubMed] [Google Scholar]