Abstract
We identified lbpB, encoding the lipoprotein component of the meningococcal lactoferrin receptor. An LbpB mutant was unable to acquire Fe from lactoferrin and exhibits decreased surface binding to lactoferrin. Primer extension and reverse transcription-PCR analysis indicate that lbpB and lbpA are cotranscribed on a polycistronic Fe-repressible mRNA.
Neisseria meningitidis, one of the most prevalent causative agents of bacterial meningitis in the United States (17), possesses distinct systems for acquiring Fe from host transferrin (TF), lactoferrin (LF), and hemoglobin/hemoglobin-haptoglobin (Hb/Hb-Hp) (1, 8, 10, 12, 14–16). The acquisition of Fe from host Fe-binding compounds is a well-defined determinant of microbial pathogenesis (11, 30, 31). The neisserial receptors required for acquisition of Fe from TF (TbpB/TbpA) and Hb/Hb-Hp (HpuA/HpuB) are two-component TonB-dependent transport systems. Each receptor is composed of a specific outer-membrane receptor that belongs to the well-characterized family of TonB-dependent high-affinity transport proteins (TbpA or HpuB) and a lipoprotein (TbpB or HpuA). The TonB-dependent outer-membrane proteins are believed to function as energy-dependent gated pores through which Fe (derived from TF or Hb) crosses the outer membrane (23). The function of the lipoprotein component is less clear. The lipoprotein component of these neisserial receptors is novel and differentiates the neisserial TonB-dependent transporters from their Escherichia coli counterparts, which do not have a lipoprotein component. The TbpB lipoprotein is surface exposed and is believed to interact with TbpA to form a functional TF receptor with increased specificity for ferrated TF (9).
Although the meningococcal LF receptor was initially described as a single-component receptor consisting of the TonB-dependent LbpA (21, 22, 25, 27), we hypothesized that this receptor was analogous to the TF and Hb/Hb-Hp receptors and consisted of a TonB-dependent protein, LbpA, and a lipoprotein (16). Pettersson et al. (20) noted that the 550 bp 5′ to lbpA shared similarity with tbpB and suggested that this small fragment may be part of a gene, which they designated lbpB. By modifying their original affinity purification protocol, Bonnah et al. (5) have recently demonstrated the presence of a second LF binding protein, which they designated LbpB. To date, there is no evidence that the LbpB identified by Bonnah et al. is encoded by the putative lbpB fragment identified by Pettersson et al. Here we report the complete cloning and sequencing of lbpB, located 5′ to lbpA, and demonstrate that this gene encodes a lipoprotein that is a functional LF receptor involved in the acquisition of Fe from and in binding to LF. Furthermore, lbpB and lbpA are cotranscribed on a polycistronic Fe-repressible mRNA.
Cloning and sequence analysis of lbpA and lbpB.
We previously cloned the 5′ end of lbpA from N. meningitidis DNM2 (15) and demonstrated by insertional activation that this gene encoded the lactoferrin receptor (25). The remainder of lbpA was cloned (pDLGTF7 contains a 2.7-kb fragment of lbpA that was amplified by PCR with primers lbp1 and lbp2 [Fig. 1 and 2 and Table 1], and pDLG11 contains the 5′ end of lbpA isolated by cloning the Campbell insertion from the lbpA mutant strain DNM21 [25] [Fig. 2]) and sequenced on an Applied Biosystems model 373A-01 automated DNA sequencer (7). The predicted LbpA protein of DNM2 appears to be highly conserved, having 99 and 95% identity with the previously published meningococcal LF receptors IroA (22) and LbpA (21) and 95% identity with the gonococcal LbpA (3).
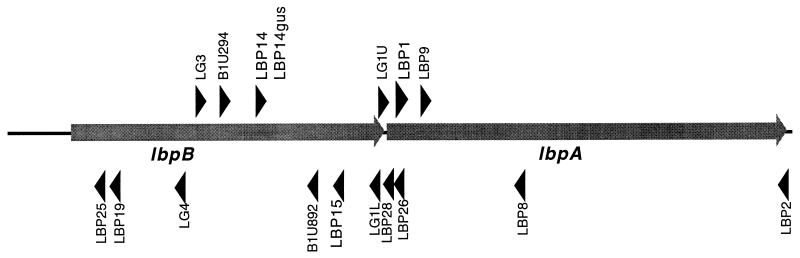
FIG. 1.
Schematic representation of the primers used in the present study.
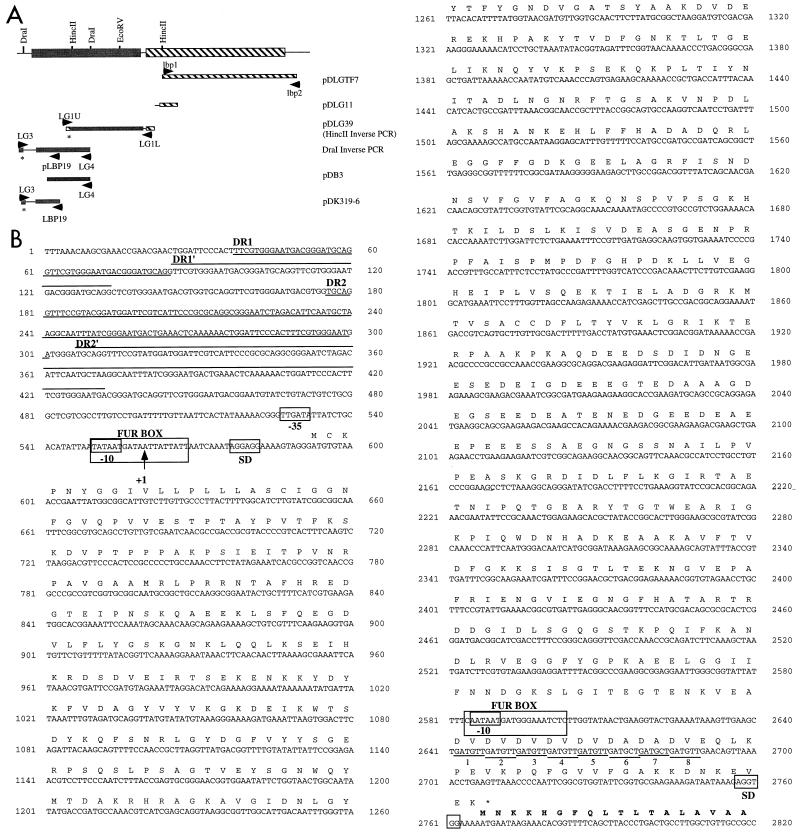
FIG. 2.
(A) A schematic diagram of the lbpBA operon showing restriction sites relevant to generation of the plasmid constructs described. (B) The nucleotide sequence of lbpB and the lbpB promoter region with the transcriptional start site (+1), −10 and −35 regions, putative Fur box, Shine-Dalgarno (SD) sequence, and direct repeat regions (DR1 and DR2; the first repeat is underlined, and the second repeat is overlined) indicated. The nucleotide sequence 5′ of the lbpA coding sequence is also shown. A Fur box consensus, −10 consensus, Shine-Dalgarno, and the hexamer repeats (labeled 1 to 8) are marked. Conceptual translations of LbpB and the N terminus of LbpA (in bold-faced type) are also shown.
TABLE 1.
Primers used in this studya
| Name | Sequence (5′→3′) | Gene location | Annealing temperature (°C) |
|---|---|---|---|
| LG3 | CCGCTGACCATTTACAACATC | lbpB | 60 (with LG4), 55 (with LBP19) |
| LG4 | TTTTGCTTCTCACTGGGTTTG | lbpB | 60 |
| LBP1 | TTGGGCAAAATCGCTAAA | lbpA | 57 |
| LBP2 | AATGACGGCGGAAATCTT | lbpA | 57 |
| B1U892 | CCGTGCGGATACCTTTCAG | lbpB | 45 |
| B1U294 | TATCAGCAACGACAACAGC | lbpB | 45 |
| LG1U | AGGTGGAAAAATGAATAAGAA | lbpB | 55 |
| LG1L | CAACATCAGCATCAGCATCAA | lbpB | 55 |
| LBP9 | GTGTGGCGGTTTCGGTTGAC | lbpA | 60 |
| LBP8 | TGGGCGGACAGGTTTTCGTG | lbpA | 60 |
| LBP26 | GACGGGAAGGCGGCGGCAACAG | lbpA | NAb |
| LBP25 | CCAAAAGTAAGGGCAACAAGACAATG | lbpB | NA |
| LBP19 | TTTGGCAGGGGGCGGAGTGG | lbpB | 55 |
| LBP28 | GGGGTTTAACTTCAGGTTTTAACT | lbpB | NA |
| LBP14 | GCGACTTTTTGACCTATGTGA | lbpB | 55 |
| LBP14gus | GCCGTCTGAAGCGACTTTTTGACCTATGTGA | lbpB | 55 |
| LBP15 | GGCCGTAAAATCCTCCTTCTA | lbpB | 55 |
| APHA1 | CCCCCGCGGTGACTAACTAGGAGGAATAAATGGCTAAAATGAGA | aphA-3 | 45 |
| APHA2 | CCCCCGCGGTCATTATTCCCTCCAGGTACTAAAACAATTCATC | aphA-3 | 45 |
All PCRs were performed with the Boehringer Mannheim PCR kit and standard cycling conditions.
NA, not applicable (primer not used for PCR).
The lbpB open reading frame (ORF), located 5′ to lbpA, was amplified from meningococcal DNA by two inverse PCR experiments. Clone pDLG39 contains a 1.7-kb DNA fragment (in pT7Blue; Novagen) that was amplified from DNM2 by inverse PCR with HincII-digested, ligated chromosomal DNA and primers LG1U and LG1L (Fig. 1 and 2 and Table 1). DNA sequence analysis (performed at The University of Oklahoma Health Science Center Department of Microbiology and Immunology DNA Sequencing Facility on an ALF-express automated DNA sequencer) indicated that pDLG39 did not contain the entire lbpB ORF. A second inverse PCR with DraI-digested, ligated chromosomal DNA and primers LG3 and LG4 (Table 1 and Fig. 1) resulted in amplification of a 1.5-kb DNA fragment (Fig. 2). This fragment could not be cloned into E. coli. Three independent transformations (26) resulted in the isolation of clones containing inserts ranging in size from 700 to 900 bp. The DNA sequence of one deletion clone, pDB3, overlapped the sequence of pDLG39, indicating that this clone resulted from a deletion event and was not the result of cloning an “extraneous” DNA from the PCR. The 5′ end of the lbpB ORF, which was not contained in pDB3, was cloned by using the DraI inverse PCR product as the template in a PCR with the primers LG3 and LBP19 (Fig. 1 and 2 and Table 1). pDK319-6 contains the 782-bp product amplified in pT7Blue (Fig. 2). The cloned lbpB DNA was highly unstable in E. coli, and plasmids frequently suffered deletions. Two large direct repeats were identified 5′ to lbpB (Fig. 2, DR1 [48 nucleotides {nt}] and DR2 [122 nt]). DR2 has identity with sequences of the IS1106 element and may be responsible for the unstable nature of lbpB clones in E. coli. Although a single clone is described for each experiment mentioned above, the DNA sequence of multiple independent PCR clones (and subclones) was determined to obtain the complete lbpB DNA sequence. Southern blot analysis of DNM2 chromosomal DNA confirmed the organization of the cloned DNA (data not shown).
The lbpB ORF extends for 2,175 bp and is predicted to encode a protein of 725 amino acids (GenBank accession no. AF049349). The predicted N terminus of lbpB contains a prokaryotic lipoprotein lipid attachment motif, suggesting that LbpB, like TbpB and HpuA, is a lipoprotein (Fig. 2). The mature LbpB peptide would contain 707 amino acids, with a predicted molecular weight of 77 kDa. LbpB migrates in sodium dodecyl sulfate-polyacrylamide gel electrophoresis with an apparent molecular weight of 95 kDa (the apparent molecular weight was calculated from multiple gels, and a representative gel is shown in Fig. 4A), which could be a consequence of the lipoprotein modification. Fluorographic analysis of DNM2 and DNM221, an LbpB mutant (see below), labeled with [3H]palmitic acid as previously described (16), demonstrated that the 95-kDa Fe-regulated LbpB is a lipoprotein (Fig. 4A).
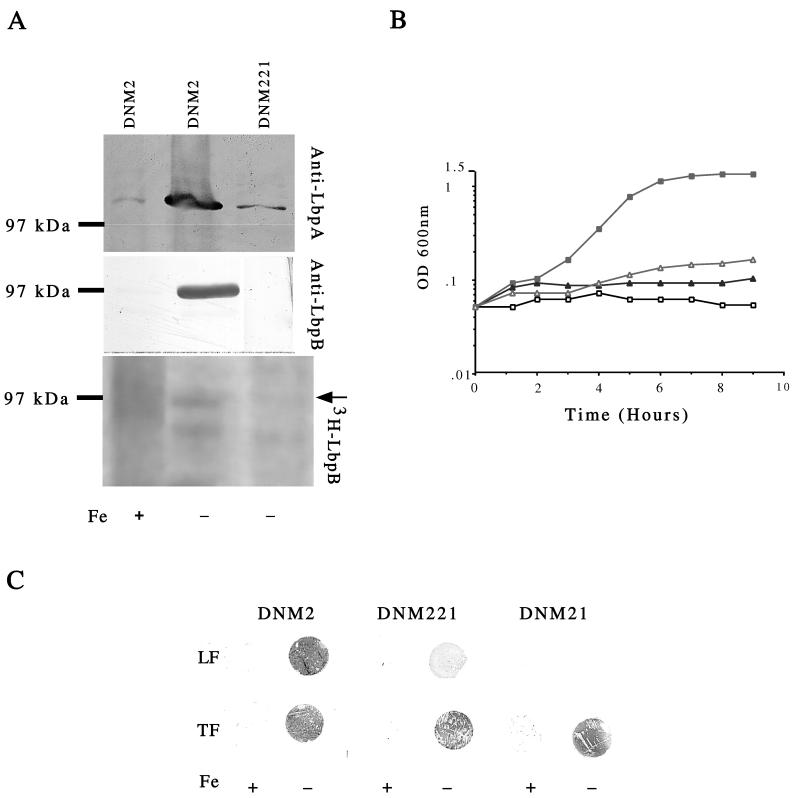
FIG. 4.
Analysis of an LbpB mutant. Western blot of total membrane proteins (40 μg/lane) prepared from DNM2 and DNM221 grown in the presence (+) or absence (−) of Fe. (A) Blots were probed with anti-LbpA (top) and anti-LbpB (middle). [3H]palmitic acid labeling of LbpB. A fluorograph of 3H-labeled extracts prepared from DNM2 and DNM221 grown in the presence (+) or absence (−) of Fe is shown. The position of LbpB is indicated by an arrow (bottom). Blank lanes and lanes containing data not presented in this study were removed from the blots. All lanes within a single panel (top, middle, or bottom) are derived from a single gel. For example, the anti-LbpB DNM221 lane was originally separated from the lane labeled DNM2 without Fe by a blank lane, which was cropped from the figure. (B) Growth of DNM2 (squares) and DNM221 (triangles) with LF (▪ and Δ) or without added Fe (□ and ▴). OD 600 nm, optical density at 600 nm. (C) Dot blot assay to detect binding of LF or TF to intact meningococci grown in the presence (+) or absence (−) of added Fe.
Similarity of LbpB to TbpB.
A BlastP search with the predicted amino acid sequence of the LbpB protein revealed significant similarities to several TbpB lipoproteins (58 and 55% similarity with TbpB proteins from N. meningitidis [GenBank accession no. X78940] and Neisseria gonorrhoeae [GenBank accession no. U65222], respectively). Two unusual domains were identified in LbpB. The first domain spans amino acids 453 to 508 and is particularly hydrophilic, containing 65% acidic amino acids (D and E) (Fig. 2B). The second unusual domain is located near the C terminus of LbpB and contains a repeat of alternating nonpolar (V or A) and acidic (D or E) amino acids (Fig. 2B). Neither of these domains is found in TbpB lipoproteins.
Transcriptional start sites of lbpA and lbpB.
In E. coli, Fe-regulated gene expression occurs by transcriptional repression mediated by the Fur (ferric uptake regulator) protein (6). When the concentration of Fe is sufficient, Fur, with Fe2+ as a corepressor, binds to a 19-bp consensus sequence upstream of Fe-repressible genes, blocking transcription (6). A Fur homolog has been identified in the meningococcus, suggesting that Fe regulation occurs by a similar mechanism (13, 29).
RNA dot blot hybridization (26) with RNA prepared from meningococci (as previously described [16]) demonstrated that expression of LbpA is transcriptionally regulated by Fe (data not shown). Although a −10 consensus sequence and a Fur binding site (74% similarity to the E. coli consensus GATWATGATWATYATTWTC [W = A or T, Y = C or T] [24]) were identified 163 nt 5′ to the lbpA translational start (Fig. 2), a −35 consensus sequence was not observed. Primer extension studies (by using the AMV primer extension system from Promega, according to the manufacturer’s instructions) with primer LBP26 or primer LBP28 (Table 1 and Fig. 1) did not detect a transcriptional start site 5′ to lbpA, suggesting that the promoter-like region 5′ to lbpA (within lbpB) may not direct lbpA transcription.
A Fur box with 89% similarity (17 of 19 nt) to the consensus E. coli Fur box was identified 5′ to the putative ATG start codon of lbpB, suggesting that lbpB may also be transcriptionally regulated by Fe (Fig. 2) (19, 24). Primer extension experiments identified a transcription start site 5′ to lbpB with primer LBP25 (Table 1 and Fig. 1), which is complementary to nt 24 to 50 of the lbpB coding sequence. An 82-nt cDNA was observed (data not shown), placing the transcriptional start at the A nucleotide located 33 nt upstream of lbpB (Fig. 2). This position is in good agreement with the locations of the putative Fur box and the −10 and −35 consensus promoter regions found 5′ to lbpB (Fig. 3). The 82-nt product was not detected in control reaction mixtures in which RNA from meningococci grown in the presence of Fe was used or in reaction mixtures from which RNA was excluded (data not shown).
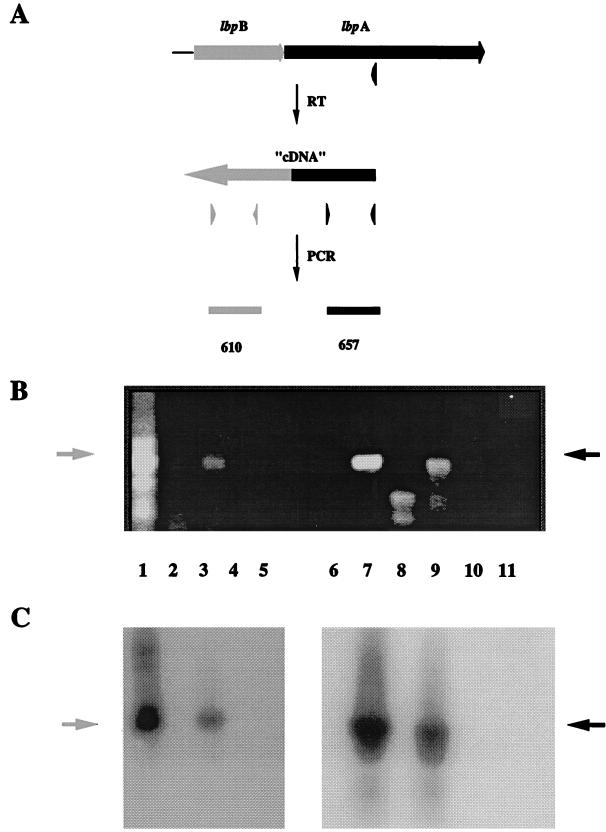
FIG. 3.
(A) RT-PCR analysis of the lbpBA operon. (B) RNA isolated from N. meningitidis DNM2 grown in the presence (lanes 2, 4, 8, and 10) or absence (lanes 3, 5, 9, and 11) of Fe was used as the template for cDNA generation and PCRs with lbpB-specific primers (lane 1 to 5) and lbpA-specific primers (lanes 7 to 11). Chromosomal DNA isolated from DNM2 was used as a positive control (lanes 1 and 7). Reverse transcriptase was omitted from control reaction mixtures (lanes 4, 5, 10, and 11). The ethidium-bromide-stained agarose gel (B) and Southern blot probed with lbpB-specific (lanes 1 to 5) and lbpA-specific (lanes 7 to 11) probes (C) are shown.
Cotranscription of lbpB and lbpA.
The genetic arrangement of the lbpB and lbpA ORFs and the inability to detect a transcription start site 5′ to lbpA suggested that these genes are transcribed as a polycistronic message, beginning at the Fe-regulated promoter identified 5′ to lbpB. Reverse transcription-PCR (RT-PCR) was used to determine if lbpB and lbpA are cotranscribed as a polycistronic Fe-repressible mRNA. Primer LBP8 (Table 1 and Fig. 1), which is complementary to lbpA mRNA, was annealed to total RNA isolated from Fe-starved meningococci, and RT (Superscript II; Gibco BRL) was used to generate cDNA as previously described (16). The cDNA was used as the template for a PCR with primers B1U892 and B1U294, which both anneal within lbpB, 5′ to the putative lbpA promoter (Fig. 1 and 2 and Table 1). If the lbpA message was monocistronic, the cDNA would not contain lbpB sequences and the PCR would not amplify a product. However, if the message was polycistronic, then the cDNA would contain lbpB sequences and a 610-bp PCR product would be amplified. Using this assay, we amplified a product of the correct size and confirmed by Southern hybridization with an lbpB-specific probe that this fragment was lbpB (Fig. 3). The lbpB-specific probe did not react with lbpA sequences (data not shown). Control primers LBP8 and LBP9 (Table 1 and Fig. 1), chosen to amplify a 657-bp internal fragment of lbpA, amplified a product of the correct size (Fig. 3). Amplification from RNA prepared from meningococci grown in the presence of Fe was not observed in either case, confirming that transcription of both lbpB and lbpA is repressed by Fe. As a negative control, RNA annealed to LBP8 and incubated without reverse transcriptase was used as the template for the PCRs described above (Fig. 3). Amplification from this template was not detected, confirming that amplification did not result from trace amounts of DNA contaminating the RNA preparation (Fig. 3). In addition, positive and negative control reaction mixtures contained DNM2 chromosomal DNA (Fig. 3) or double-distilled water (data not shown) as the template; these controls confirmed that the RT-PCR results described above were due to the lbpB and lbpA ORFs contained in a single mRNA.
Mutation of LbpB.
To construct a nonpolar LbpB mutant, an aphA-3 kanamycin resistance cassette without a promoter or transcriptional terminator (18) was ligated into the EcoRV site (Fig. 2) of the cloned lbpB. The mutated lbpB was amplified by PCR with primers LBP14gus and LBP15 (Table 1 and Fig. 1), and the 1.5-kb Qiaex-purified PCR product was used to transform N. meningitidis DNM2 as previously described (2). Transformants were selected on CDM0 agar containing kanamycin (100 μg/ml), and a single transformant, designated DNM221, was isolated. In this construct, aphA-3 transcription is driven from the Fe-regulated lbpB promoter, and transcription of lbpA should not be affected. Translation of lbpB is inhibited by the introduction of a stop codon in each reading frame prior to the translational start site of aphA-3. PCR amplification from DNM221 confirmed both the presence of the aphA-3 cassette in lbpB and the proper orientation of the aphA-3 cassette with respect to the lbpB promoter. Primers LBP14 and LBP15, which flank the aphA-3 insertion, amplified a product of 1.5 kb from DNM221, consistent with the presence of the aphA-3 marker in the lbpB gene. Furthermore, when primers LBP14 and APHA2 (3′ aphA-3 primer) or primers LBP15 and APHA1 (5′ aphA-3 primer; Table 1 and Fig. 1) were used, the amplified products were consistent with the aphA-3 marker oriented such that the lbpB promoter would drive transcription of aphA-3.
Polyclonal antisera was generated to both LbpA and LbpB by immunizing rabbits with keyhole limpet hemocyanin-coupled peptides (LbpA peptide, CEKQYYGTDEAKKFRDKSG; LbpB peptide, CEIHKRDSDVEIRTSELEN). Peptide synthesis and immunization were performed by Alpha Diagnostic International Incorporated, San Antonio, Tex., with a standard 63-day protocol. LbpB was readily detected as a 95-kDa Fe-repressible protein with the anti-LbpB peptide antisera to probe a Western blot of total membrane proteins prepared from DNM2 (Fig. 4A, middle). LbpB was not detected in total-membrane proteins prepared from Fe-starved DNM221 (Fig. 4A, middle), confirming that LbpB was insertionally inactivated in this strain.
The anti-LbpA peptide antisera readily detected LbpA in total membrane proteins prepared from Fe-starved DNM2 (Fig. 4A, top) but did not detect LbpA in total membrane proteins isolated from DNM21 (25), an LbpA mutant (data not shown). Fe-regulated expression of LbpA was also detected in total membrane proteins prepared from DNM221 (Fig. 4A, top), confirming that insertion of the kanamycin marker in lbpB did not abolish Fe-regulated expression of lbpA. However, expression of LbpA in DNM221 was decreased compared to that in strain DNM2. Several methods were used to establish that equivalent amounts of protein were loaded in each lane and that DNM2 and DNM221 were equivalently Fe starved. Each lane shown in Fig. 4A (top and middle) contains 40 μg of total protein (determined as previously described). Furthermore, anti-FrpB and anti-HpuA antisera detected equal quantities of the Fe-repressed proteins, FrpB and HpuA, in membrane proteins prepared from DNM2 and DNM221, confirming equal levels of Fe starvation (data not shown). Thus, the decreased expression of LbpA in DNM221 cannot be attributed to differences in the amounts of protein present or in the levels of Fe starvation.
The ability of strain DNM221 to acquire Fe from LF, TF, Hm, Hb, and ferric nitrate was assessed as previously described (15). Growth of DNM221 was equivalent to that of the parent strain, DNM2, for all Fe sources tested, with the exception of LF (data not shown). Growth with LF was dramatically reduced in DNM221 (Fig. 4B). It is not likely that the decreased expression of LbpA in DNM221 can solely explain the inability to acquire Fe from LF. DNM221 clearly retains the ability to surface bind LF, indicating proper functioning of LbpA (see below). This phenotype is similar to that observed for a meningococcal TbpB mutant which is not able to acquire Fe from TF (12).
Using a solid-phase dot blot assay (4, 15), we determined that intact DNM221 retained the ability to bind LF, although this ability was reduced compared to that of DNM2 (Fig. 4C). This decreased binding is likely due to reduced expression of LbpA. DNM21 (25), an LbpA mutant that expresses wild-type levels of LbpB (data not shown), does not bind LF in this assay (Fig. 4C), suggesting that expression of LbpB alone does not mediate LF binding. Binding of TF to DNM221 or DNM21 was not altered from that of the wild type (Fig. 4C). This phenotype is similar to single knockout mutations in the gonococcal tbpB and tbpA genes, in which binding was abolished by inactivation of the TonB-dependent TbpA and reduced but not eliminated in mutants lacking the lipoprotein component of the receptor (9). The gonococcal LF receptor is probably similarly dependent on an accessory lipoprotein. Blast searches (blastn and tblastn) of the N. gonorrhoeae FA1090 genome database revealed that an lbpB locus was not present (11a). An lbpA locus was identified; however, this locus contains a deletion of ca. 504 nt from the 5′ end and is located 3′ to an ORF with homology to GTP binding proteins but not to lbpB. This observation may explain the inability of strain FA1090 to grow with LF as the sole source of Fe. Thus, the meningococcal LF receptor requires an accessory lipoprotein for full functional activity, as do the TbpBA and HpuAB receptors. These receptors are distinct from HmbR, a second TonB-dependent meningococcal receptor for Hb (28), which lacks a lipoprotein component.
Acknowledgments
These studies were supported by USPHS/NIH grants AI23757 and AI38399 (to D.W.D.).
REFERENCES
- 1.Anderson J E, Sparling P F, Cornelissen C N. Gonococcal transferrin binding protein 2 facilitates but is not essential for transferrin utilization. J Bacteriol. 1994;176:3162–3170. doi: 10.1128/jb.176.11.3162-3170.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Biswas G D, Sox T, Blackman E, Sparling P F. Factors affecting genetic transformation of Neisseria gonorrhoeae. J Bacteriol. 1977;129:983–992. doi: 10.1128/jb.129.2.983-992.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Biswas G D, Sparling P F. Characterization of lbpA, the structural gene for a lactoferrin receptor in Neisseria gonorrhoeae. Infect Immun. 1995;63:2958–2967. doi: 10.1128/iai.63.8.2958-2967.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Blanton K J, Biswas G D, Tsai J, Adams J, Dyer D W, Davis S M, Koch G G, Sen P K, Sparling P F. Genetic evidence that Neisseria gonorrhoeae produces specific receptors for transferrin and lactoferrin. J Bacteriol. 1990;172:5225–5235. doi: 10.1128/jb.172.9.5225-5235.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bonnah R A, Yu R, Schryvers A B. Biochemical analysis of lactoferrin receptors in the Neisseriaceae: identification of a second bacterial lactoferrin receptor protein. Microb Pathog. 1995;19:285–297. doi: 10.1016/s0882-4010(96)80002-7. [DOI] [PubMed] [Google Scholar]
- 6.Bragg A, Neilands J B. Molecular mechanisms of regulation of siderophore-mediated iron assimilation. Microbiol Rev. 1987;51:509–518. doi: 10.1128/mr.51.4.509-518.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chissoe S L, Wang Y F, Clifton S W, Ma N, Sun H J, Lobsinger J S, Kenton S M, White J D, Roe B A. Strategies for rapid and accurate DNA sequencing. Methods Companion Methods Enzymol. 1991;3:55–65. [Google Scholar]
- 8.Cornelissen C, Biswas G D, Tsai J, Paruchuri D K, Thompson S A, Sparling P F. Gonococcal transferrin-binding protein 1 is required for transferrin utilization and is homologous to TonB-dependent outer membrane receptors. J Bacteriol. 1992;174:5788–5797. doi: 10.1128/jb.174.18.5788-5797.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cornelissen C N, Sparling P F. Binding and surface exposure characteristics of the gonococcal transferrin receptor are dependent on both transferrin-binding proteins. J Bacteriol. 1996;178:1437–1444. doi: 10.1128/jb.178.5.1437-1444.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cornelissen C N, Sparling P F. Iron piracy: acquisition of transferrin-bound iron by bacterial pathogens. Mol Microbiol. 1994;14:843–850. doi: 10.1111/j.1365-2958.1994.tb01320.x. [DOI] [PubMed] [Google Scholar]
- 11.Finkelstein R A, Sciortino C V, McIntosh M A. Role of iron in microbe-host interactions. Rev Infect Dis. 1983;5:5759–5777. doi: 10.1093/clinids/5.supplement_4.s759. [DOI] [PubMed] [Google Scholar]
- 11a.Gonococcal genome sequence database.http://dna1.chem.uoknor.edu/.
- 12.Irwin S W, Averil N, Cheng C Y, Schryvers A B. Preparation and analysis of isogenic mutants in the transferrin receptor protein genes, tbpA and tbpB, from Neisseria meningitidis. Mol Microbiol. 1993;8:1125–1133. doi: 10.1111/j.1365-2958.1993.tb01657.x. [DOI] [PubMed] [Google Scholar]
- 13.Karkhoff-Schweizer R R, Schryvers A B, Schweizer H P. Cloning and sequence analysis of the fur gene encoding an iron-regulatory protein of Neisseria meningitidis. Gene. 1994;141:139–140. doi: 10.1016/0378-1119(94)90143-0. [DOI] [PubMed] [Google Scholar]
- 14.Legrain M, Mazarin V, Irwin S W, Bouchon B, Quentin-Millet M J, Jacobs E, Schryvers A B. Cloning and characterization of Neisseria meningitidis genes encoding the transferrin-binding proteins Tbp1 and Tbp2. Gene. 1993;130:73–80. doi: 10.1016/0378-1119(93)90348-7. [DOI] [PubMed] [Google Scholar]
- 15.Lewis L A, Dyer D W. Identification of an iron-regulated outer membrane protein of Neisseria meningitidis involved in the utilization of hemoglobin complexed to haptoglobin. J Bacteriol. 1995;177:1299–1306. doi: 10.1128/jb.177.5.1299-1306.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lewis L A, Gray L, Wang Y P, Roe B A, Dyer D W. Molecular characterization of hpuAB, the hemoglobin-haptoglobin utilization operon of Neisseria meningitidis. Mol Microbiol. 1997;23:737–749. doi: 10.1046/j.1365-2958.1997.2501619.x. [DOI] [PubMed] [Google Scholar]
- 17.Loughlin A M, Marchant C D, Lett S M. The changing epidemiology of invasive bacterial infections in Massachusetts children, 1984 through 1991. Am J Public Health. 1995;85:392–394. doi: 10.2105/ajph.85.3.392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Menard R, Sansonetti P J, Parsot C. Nonpolar mutagenesis of the ipa genes defines IpaB, IpaC and IpaD as effectors of Shigella flexneri entry into epithelial cells. J Bacteriol. 1993;175:5899–5906. doi: 10.1128/jb.175.18.5899-5906.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Neilands J B. Molecular biology and regulation of iron acquisition by Escherichia coli K-12. In: Iglewski B H, Clark V L, editors. Molecular basis of bacterial pathogenesis. San Diego, Calif: Academic Press; 1990. pp. 205–223. [Google Scholar]
- 20.Pettersson A, Klarenbeek V, van Deurzen J, Poolman J T, Tommassen J. Molecular characterization of the structural gene for the lactoferrin receptor of the meningococcal strain H44/76. Microb Pathog. 1994;17:395–408. doi: 10.1006/mpat.1994.1085. [DOI] [PubMed] [Google Scholar]
- 21.Pettersson A, Ley P V D, Poolman J T, Tommassen J. Molecular characterization of the 98-kilodalton iron-regulated outer membrane protein of Neisseria meningitidis. Infect Immun. 1993;61:4724–4733. doi: 10.1128/iai.61.11.4724-4733.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Pettersson A, Maas A, Tommassen J. Identification of the iroA gene product of Neisseria meningitidis as a lactoferrin receptor. J Bacteriol. 1994;176:1764–1766. doi: 10.1128/jb.176.6.1764-1766.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Postle K. TonB protein and energy transduction between membranes. J Bioenerg Biomembr. 1993;25:591–601. doi: 10.1007/BF00770246. [DOI] [PubMed] [Google Scholar]
- 24.Pressler U, Staudenmaier H, Zimmerman L, Braun V. Genetics of the iron dicitrate transport system of Escherichia coli. J Bacteriol. 1988;170:2716–2724. doi: 10.1128/jb.170.6.2716-2724.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Quinn M L, Weyer S J, Lewis L A, Dyer D W, Wagner P M. Insertional inactivation of the gene for the meningococcal lactoferrin binding protein. Microb Pathog. 1994;17:227–237. doi: 10.1006/mpat.1994.1068. [DOI] [PubMed] [Google Scholar]
- 26.Sambrook J, Fritsch E F, Maniatis T. Molecular cloning: a laboratory manual. 2nd ed. Cold Spring Harbor, N.Y: Cold Spring Harbor Laboratory; 1989. [Google Scholar]
- 27.Schryvers A B, Morris L J. Identification and characterization of the human lactoferrin-binding protein from Neisseria meningitidis. Infect Immun. 1988;56:1144–1149. doi: 10.1128/iai.56.5.1144-1149.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Stojiljkovic I, Hwa V, Martain L D S, O’Gaora P, Nassif X, Heffron F, So M. The Neisseria meningitidis haemoglobin receptor: its role in iron utilization and virulence. Mol Microbiol. 1995;15:531–541. doi: 10.1111/j.1365-2958.1995.tb02266.x. [DOI] [PubMed] [Google Scholar]
- 29.Thomas C E, Sparling P F. Identification and cloning of a fur homologue from Neisseria meningitidis. Mol Microbiol. 1994;11:725–737. doi: 10.1111/j.1365-2958.1994.tb00350.x. [DOI] [PubMed] [Google Scholar]
- 30.Weinberg E D. Iron and infection. Microbiol Rev. 1978;42:45–66. doi: 10.1128/mr.42.1.45-66.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Weinberg E D. Iron withholding: a defense against infection and neoplasia. Physiol Rev. 1984;64:65–102. doi: 10.1152/physrev.1984.64.1.65. [DOI] [PubMed] [Google Scholar]