Abstract
Background
The TRUSTY study evaluated the efficacy of second-line trifluridine/tipiracil (FTD/TPI) plus bevacizumab in metastatic colorectal cancer (mCRC).
Objective
This exploratory biomarker analysis of TRUSTY investigated the relationship between baseline plasma concentrations of angiogenesis-related factors and cell-free DNA (cfDNA), and the efficacy of FTD/TPI plus bevacizumab in patients with mCRC.
Patients and Methods
The disease control rate (DCR) and progression-free survival (PFS) were compared between baseline plasma samples of patients with high and low plasma concentrations (based on the median value) of angiogenesis-related factors. Correlations between cfDNA concentrations and PFS were assessed.
Results
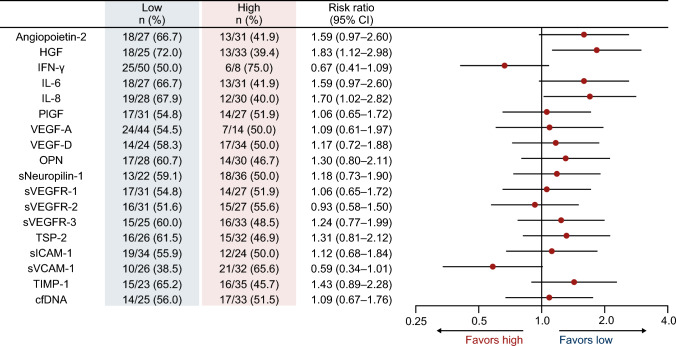
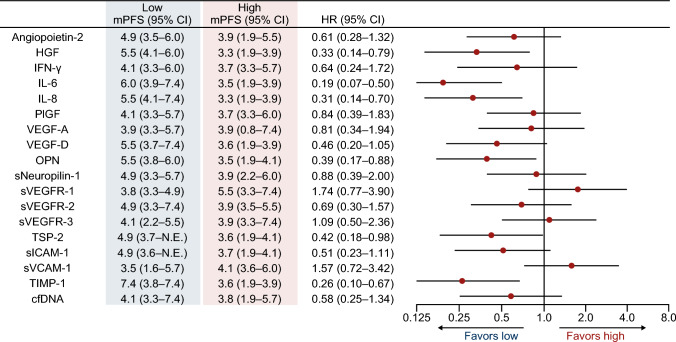
Baseline characteristics (n = 65) were as follows: male/female, 35/30; median age, 64 (range 25–84) years; and RAS status wild-type/mutant, 29/36. Patients in the hepatocyte growth factor (HGF)-low and interleukin (IL)-8-low groups had a significantly higher DCR (risk ratio [95% confidence intervals {CIs}]) than patients in the HGF-high (1.83 [1.12–2.98]) and IL-8-high (1.70 [1.02–2.82]) groups. PFS (hazard ratio {HR} [95% CI]) was significantly longer in patients in the HGF-low (0.33 [0.14–0.79]), IL-8-low (0.31 [0.14–0.70]), IL-6-low (0.19 [0.07–0.50]), osteopontin-low (0.39 [0.17–0.88]), thrombospondin-2-low (0.42 [0.18–0.98]), and tissue inhibitor of metalloproteinase-1-low (0.26 [0.10–0.67]) groups versus those having corresponding high plasma concentrations of these angiogenesis-related factors. No correlation was observed between cfDNA concentration and PFS.
Conclusion
Low baseline plasma concentrations of HGF and IL-8 may predict better DCR and PFS in patients with mCRC receiving FTD/TPI plus bevacizumab, however further studies are warranted.
Clinical Trial Registration Number
jRCTs031180122.
Supplementary Information
The online version contains supplementary material available at 10.1007/s11523-023-01027-8.
Key Points
| This was an exploratory biomarker analysis that investigated the correlation between the concentrations of angiogenesis-related factors and cell-free DNA and the treatment efficacy of trifluridine/tipiracil plus bevacizumab in patients with metastatic colorectal cancer. |
| Patients with below-median baseline plasma hepatocyte growth factor and interleukin-8 levels had significantly higher disease control rate and longer progression-free survival than those with above-median levels. |
Introduction
The 2023 National Comprehensive Cancer Network clinical practice guidelines for colorectal cancer (CRC) recommend chemotherapy (5-fluorouracil [5-FU] and l-leucovorin [l-LV] with oxaliplatin [FOLFOX] or irinotecan [FOLFIRI], or both [FOLFOXIRI]) along with targeted biological agents (e.g., bevacizumab, cetuximab, and panitumumab) as first- or second-line treatment options for metastatic CRC (mCRC) [1]. In addition, the guidelines recommend trifluridine/tipiracil (FTD/TPI) with or without bevacizumab as a treatment option for patients with mCRC who have had progression with standard therapies [1]. The SUNLIGHT trial showed that FTD/TPI plus bevacizumab significantly prolonged survival compared with FTD/TPI alone as third-line or later treatment for patients with mCRC [2]. Furthermore, FTD/TPI plus bevacizumab prolonged survival in patients with mCRC irrespective of the RAS status and is the standard third-line treatment in patients with mCRC [2].
Considering the poor outcome of mCRC, there is a need for reliable measures to predict treatment response [3]. Biomarkers can be useful tools for predicting treatment response in patients with mCRC [4]. RAS gene mutations are established as a negative predictive factor for the efficacy of anti-epidermal growth factor receptor antibodies such as panitumumab and cetuximab in mCRC [5–8].
Plasma concentrations of angiogenesis factors have been used as biomarkers of response to angiogenesis inhibitors used in first- and second-line chemotherapy [9]. In patients with mCRC who received second-line treatment, high levels of plasma vascular endothelial growth factor (VEGF)-D were identified as a potential predictive biomarker for ramucirumab efficacy, and high serum levels of VEGF-A and placental growth factor (PlGF) were found to underlie the development of resistance to bevacizumab [10, 11]. Increased baseline levels of soluble intercellular adhesion molecule-1 (sICAM-1), soluble vascular cell adhesion molecule-1 (sVCAM-1), and interleukin (IL)-8 have been associated with a higher risk of death and shorter progression-free survival (PFS) in patients with CRC than in those who had lower levels of these biomarkers [12, 13]. Cell-free DNA (cfDNA) is a minimally invasive and clinically relevant quantitative biomarker of tumor burden that can predict survival in patients with mCRC [14, 15]. However, biomarkers for the efficacy of FTD/TPI plus bevacizumab have not been clearly established.
The TRiflUridine/tipiracil in Second-line sTudY (TRUSTY) was a phase II/III randomized controlled trial conducted to determine whether FTD/TPI plus bevacizumab was non-inferior to FOLFIRI plus bevacizumab or S-1 and irinotecan plus bevacizumab (control group) in terms of overall survival (OS) among patients with mCRC; the non-inferiority of FTD/TPI plus bevacizumab was not demonstrated [16]. The purpose of this exploratory biomarker study was to investigate the correlation between the concentrations of angiogenesis-related factors and cfDNA and treatment efficacy of FTD/TPI plus bevacizumab using plasma samples obtained from TRUSTY.
Material and Methods
Study Design and Patients
The design of TRUSTY has been reported previously [17]. Briefly, this was an open-label, multicenter, randomized, comparative, phase II/III study conducted at 65 institutions in Japan. The key inclusion criteria were patients aged ≥ 20 years with histologically confirmed mCRC who did not respond to first-line chemotherapy with fluoropyrimidine (5-FU/l-LV, capecitabine, or S-1) plus oxaliplatin combined with bevacizumab, cetuximab, or panitumumab for patients with RAS wild-type tumors; with an Eastern Cooperative Oncology Group performance status of 0 or 1; evaluable lesions, as observed on imaging; and adequate organ function [16]. Written informed consent was obtained from all patients. Eligible patients were subsequently randomized in a 1:1 ratio to either the fluoropyrimidine and irinotecan plus bevacizumab group (control group) or the FTD/TPI plus bevacizumab group (bevacizumab 5 mg/kg by intravenous infusion on days 1 and 15, and oral administration of FTD/TPI 35 mg/m2 twice daily on days 1–5 and 8–12 in a 28-day cycle). TRUSTY was terminated at the interim analysis for futility because of a low possibility for non-inferiority, even if the study would have been completed [16]. This biomarker study was planned after the registration to TRUSTY began. Only patients who provided separate written informed consent were included in this biomarker analysis.
Sample Collection and Evaluation of Angiogenesis-Related Factors and Cell-Free DNA (cfDNA)
Blood samples for the control and FTD/TPI plus bevacizumab groups were prospectively collected before the start of study treatment. The collected blood samples were centrifuged at 10,000g for 10 min to separate the plasma samples. The MILLIPLEX® map assay kit (Merck Millipore, Billerica, MA, USA) was used to evaluate 17 factors associated with angiogenesis in the plasma samples of patients with mCRC: angiopoietin-2, hepatocyte growth factor (HGF), interferon gamma (IFN-γ), IL-6, IL-8, PlGF, VEGF-A, VEGF-D, osteopontin (OPN), soluble neuropilin-1 (sNeuropilin-1), soluble VEGF receptor (sVEGFR)-1, sVEGFR-2, sVEGFR-3, thrombospondin-2 (TSP-2), sICAM-1, sVCAM-1, and tissue inhibitor of metalloproteinase-1 (TIMP-1). All assays for proteins were performed according to the manufacturer’s recommended protocols. The assay plate was then analyzed using the Luminex® 100/200™ instrument (Luminex Corporation, Austin, TX, USA). Total cfDNA was isolated using the QIAamp Circulating Nucleic Acid Kit (50) with the QIAvac (Qiagen, Hilden, Germany). The cfDNA concentration was measured using the TaqMan Copy Number Reference Assay, RNase P with StepOnePlus (Thermo Fisher Scientific, Foster City, CA, USA).
Outcomes
Disease control rate (DCR) and PFS were compared between patients with high and low concentrations of each biomarker in the FTD/TPI plus bevacizumab and control groups. In addition, correlations between the cfDNA concentration and PFS were evaluated. DCR was defined as the proportion of complete or partial responses or stable disease for more than 6 weeks from the initiation of study treatment. PFS was defined as the period from the date of enrollment to the earliest date of disease progression or death due to any cause, whichever occurred first.
Statistical Analysis
Using the median value of each angiogenesis-related factor and cfDNA as the cut-off, patients were divided into the high and low plasma concentration groups. The median value was calculated for all patients, including patients in both the control and FTD/TPI plus bevacizumab groups. For IFN-γ, IL-6, and VEGF-A, patients were divided into high (detectable) and low (undetectable) groups because > 50% of patients had baseline plasma concentrations below the lower limit of detection. For DCR, risk ratio (RR) and the corresponding 95% confidence intervals (CIs) were calculated. PFS was estimated using the Kaplan–Meier method. Hazard ratios (HRs) and CIs were calculated using the Cox proportional hazards model with only category (< median, ≥ median) as a covariate. Correlations between the cfDNA concentration and PFS were assessed using Spearman’s rank correlation coefficient. All statistical analyses were performed using SAS® software, version 9.4 (SAS Institute, Inc., Cary, NC, USA).
Results
Patient Disposition
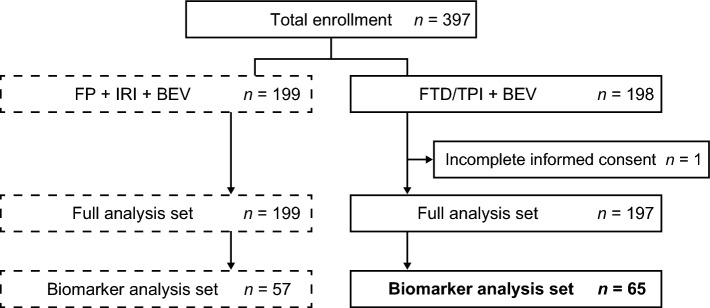
Patients were enrolled in TRUSTY between 2 October 2017 and 16 July 2020 [16]; those who were enrolled after 5 September 2019 were included in this biomarker study. Of the 397 patients enrolled in TRUSTY, 198 were randomized to the FTD/TPI plus bevacizumab group and 199 to the control group. Plasma samples were available for 65 patients in the FTD/TPI plus bevacizumab group and 57 patients in the control group (biomarker analysis population) (Fig. 1).
Fig. 1.
Patient disposition. BEV bevacizumab, FP fluoropyrimidine, FTD/TPI trifluridine/tipiracil, IRI irinotecan
Demographics and Baseline Clinical Characteristics
The biomarker analysis population had similar baseline characteristics to those of the intention-to-treat population [16]. In the FTD/TPI plus bevacizumab group, 35 (53.8%) patients were male and the median (range) age was 64 (25–84) years. RAS mutation was present in 36 (55.4%) patients and 43 (66.2%) patients had two or more metastatic lesions. Bevacizumab was used as a first-line treatment in 48 (73.8%) patients. The demographics and baseline clinical characteristics of patients in the control group were generally similar to those of patients in the FTD/TPI plus bevacizumab group (Table 1). The median values for each angiogenesis-related factor and cfDNA at baseline are presented in electronic supplementary material (ESM) Table S1.
Table 1.
Baseline characteristics of patients in the FTD/TPI plus BEV group and the control group in the biomarker analysis population and the ITT population
| Characteristics | Biomarker analysis population | ITT population | ||
|---|---|---|---|---|
| FTD/TPI plus BEV [n = 65] | Control group [n = 57] |
FTD/TPI plus BEV [n = 197] | Control group [n = 199] |
|
| Sex | ||||
| Male | 35 (53.8) | 32 (56.1) | 94 (47.7) | 99 (49.7) |
| Female | 30 (46.2) | 25 (43.9) | 103 (52.3) | 100 (50.3) |
| Age, years | 64.0 (25–84) | 68.0 (32–81) | 67.0 (25–84) | 68.0 (32–82) |
| < 65 | 33 (50.8) | 23 (40.4) | 80 (40.6) | 75 (37.7) |
| ≥ 65 | 32 (49.2) | 34 (59.6) | 117 (59.4) | 124 (62.3) |
| ECOG performance status | ||||
| 0 | 38 (58.5) | 32 (56.1) | 120 (60.9) | 124 (62.3) |
| 1 | 27 (41.5) | 25 (43.9) | 77 (39.1) | 75 (37.7) |
| RAS status | ||||
| Wild-type | 29 (44.6) | 24 (42.1) | 79 (40.1) | 79 (39.7) |
| Mutant | 36 (55.4) | 33 (57.9) | 118 (59.9) | 120 (60.3) |
| Primary tumor locationa | ||||
| Left side | 52 (80.0) | 44 (77.2) | 150 (76.1) | 149 (74.9) |
| Right side | 13 (20.0) | 13 (22.8) | 47 (23.9) | 50 (25.1) |
| Number of metastatic lesions | ||||
| 0 or 1 | 22 (33.8) | 27 (47.4) | 70 (35.5) | 82 (41.2) |
| ≥ 2 | 43 (66.2) | 30 (52.6) | 127 (64.5) | 117 (58.8) |
| PFS in first-line treatment | ||||
| ≥ 9 months | 44 (67.7) | 42 (73.7) | 130 (66.0) | 131 (65.8) |
| < 9 months | 21 (32.3) | 15 (26.3) | 67 (34.0) | 68 (34.2) |
| Biologics used in first-line treatment | ||||
| Anti-EGFR antibody | 17 (26.2) | 12 (21.1) | 37 (18.8) | 35 (17.6) |
| BEV | 48 (73.8) | 45 (78.9) | 160 (81.2) | 164 (82.4) |
Data are expressed as n (%) or median (range)
BEV bevacizumab, ECOG Eastern Cooperative Oncology Group, EGFR epidermal growth factor receptor, FTD/TPI trifluridine/tipiracil, ITT intention-to-treat, PFS progression-free survival, RAS rat sarcoma virus
aTumors located in the cecum, ascending colon, and transverse colon were considered right-sided; tumors located within the splenic flexure and beyond were considered left-sided
Disease Control Rate (DCR) and Progression-Free Survival (PFS) in the Biomarker Analysis Set
The median duration of follow-up was 4.5 months (range 0.1–9.9) as of 16 July 2020 (data cut-off). For the biomarker analysis population, the DCR was 55.4% (95% CI 42.5–67.7) and the median PFS was 3.9 months (95% CI 3.3–5.7) in the FTD/TPI plus bevacizumab group (ESM Table S2 and ESM Fig. S1).
DCR and PFS According to the Plasma Concentrations of Angiogenesis-Related Factors and cfDNA at Baseline in the Biomarker Analysis Set
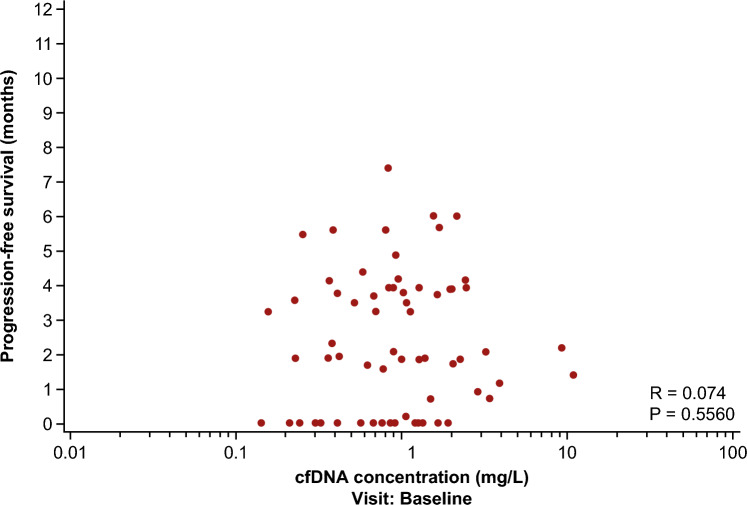
In the FTD/TPI plus bevacizumab group, patients in the HGF-low (RR 1.83, 95% CI 1.12–2.98) and IL-8-low (RR 1.70, 95% CI 1.02–2.82) groups had significantly better DCR than those in the HGF-high and IL-8-high groups. No significant difference was observed between the high and low groups for the remaining angiogenesis-related factors and cfDNA (Fig. 2). Similar to the DCR results, PFS was significantly longer in the HGF-low (5.5 vs. 3.3 months; HR 0.33, 95% CI 0.14–0.79) and IL-8-low groups (5.5 vs. 3.3 months; HR 0.31, 95% CI 0.14–0.70) than in the HGF-high and IL-8-high groups. Patients with low plasma concentrations of IL-6 (6.0 vs. 3.5 months; HR 0.19, 95% CI 0.07–0.50), OPN (5.5 vs. 3.5 months; HR 0.39, 95% CI 0.17–0.88), TSP-2 (4.9 vs. 3.6 months; HR 0.42, 95% CI 0.18–0.98), and TIMP-1 (7.4 vs. 3.6 months; HR 0.26, 95% CI 0.10–0.67) also had longer PFS than those with high plasma concentrations of these biomarkers (ESM Figs. S2–S4; Fig. 3). No correlation was observed between the cfDNA concentration and PFS (Spearman, R = 0.074) (Fig. 4).
Fig. 2.
DCR according to baseline plasma concentrations of angiogenesis-related factors and cfDNA in the FTD/TPI plus bevacizumab group. cfDNA cell-free DNA, CI confidence interval, DCR disease control rate, FTD/TPI trifluridine/tipiracil, HGF hepatocyte growth factor, IFN-γ interferon gamma, IL interleukin, OPN osteopontin, PIGF placental growth factor, sICAM-1 soluble intercellular adhesion molecule-1, sNeuropilin-1 soluble neuropilin-1, sVCAM-1 soluble vascular cell adhesion molecule-1, sVEGFR soluble vascular endothelial growth factor receptor, TIMP-1 tissue inhibitor of metalloproteinase-1, TSP-2 thrombospondin-2, VEGF vascular endothelial growth factor
Fig. 3.
PFS according to baseline plasma concentrations of angiogenesis-related factors and cfDNA in the FTD/TPI plus bevacizumab group. cfDNA cell-free DNA, CI confidence interval, FTD/TPI trifluridine/tipiracil, HGF hepatocyte growth factor, HR hazard ratio, IFN-γ interferon gamma, IL interleukin, mPFS median progression-free survival, N.E. not estimable, OPN osteopontin, PFS progression-free survival, PIGF placental growth factor, sICAM-1 soluble intercellular adhesion molecule-1, sNeuropilin-1 soluble neuropilin-1, sVCAM-1 soluble vascular cell adhesion molecule-1, sVEGFR soluble vascular endothelial growth factor receptor, TIMP-1 tissue inhibitor of metalloproteinase-1, TSP-2 thrombospondin-2, VEGF vascular endothelial growth factor
Fig. 4.
PFS by baseline cfDNA concentration in the FTD/TPI plus bevacizumab group. cfDNA cell-free DNA, FTD/TPI trifluridine/tipiracil, PFS progression-free survival, qPCR quantitative polymerase chain reaction
In the control group, angiopoietin-2 showed a contrary trend to that in the FTD/TPI plus bevacizumab group. Patients in the VEGF-D-low group had a significantly higher DCR than those in the VEGF-D-high group. As for PFS, angiopoietin-2 and sVEGFR-2 showed a contrary trend to that in the FTD/TPI plus bevacizumab group. Patients who had low plasma concentrations of IL-8 and sICAM-1 had a significantly longer PFS than those who had high plasma concentrations of these biomarkers (ESM Fig. S5).
Discussion
This is the first exploratory biomarker analysis to investigate the correlation between baseline concentrations of plasma angiogenesis-related factors and cfDNA with the efficacy of FTD/TPI plus bevacizumab in patients with mCRC. Patients with baseline plasma concentrations of HGF and IL-8 below the median had a higher DCR than those with plasma concentrations above the median. In addition, patients with baseline HGF, IL-6, IL-8, OPN, TSP-2, and TIMP-1 levels below the median had a longer PFS than those with baseline levels above the median. Thus, HGF and IL-8 can be used as predictive biomarkers to preselect patients with mCRC who might respond to FTD/TPI plus bevacizumab.
The angiogenesis-related factors are considered predictive factors for the efficacy of bevacizumab. In this study, although both groups included bevacizumab, the angiogenesis-related factors showing significance differed. Angiogenesis-related factors correlated with bevacizumab might be influenced by the concurrent use of cytotoxic drugs. In the MAX trial, in which bevacizumab was administered in combination with capecitabine and mitomycin to patients with mCRC, patients with a higher VEGF-D expression had a shorter PFS with bevacizumab than those with a lower VEGF-D expression [18]. Furthermore, the predictive value of VEGF-D expression for bevacizumab may depend on the chemotherapy backbone used [18].
The inherent complexity of angiogenesis has been a substantial hurdle in the attempts to develop response-predictive biomarkers to anti-angiogenesis drugs, which may be attributed to the tumor microenvironment comprising tumor-infiltrating cells, vasculature, extracellular matrix, and other matrix-associated molecules [19, 20]. Fibroblasts are the predominant constituents of the tumor stroma, and HGF is a major component of fibroblast secretome [21]. By binding with c-Met (tyrosine-protein kinase Met or HGF receptor), HGF activates a variety of downstream signaling cascades, such as the ERK-MAPK and PI3K-Akt pathways, consequently triggering cancer cell proliferation, invasion, metastasis, and angiogenesis [22]. Overexpression of c-Met at the protein or messenger RNA (mRNA) level is associated with poor prognosis in various cancers, including CRC [23–27]. High levels of circulating HGF have been reported to be a poor prognostic factor in lung cancer, gastric cancer, and melanoma [28–30].
IL-8 is differentially produced by tumor or stromal components, depending on the CRC genetic background [31]. IL-8 binds to the C-X-C chemokine receptor type 2 (CXCR2), thereby promoting tumor angiogenesis, growth, and metastasis [32, 33]. High levels of IL-8 have been reported to be a poor prognostic factor in various types of cancers [34–36]. Furthermore, high IL-8 levels are significantly correlated with a shorter OS and PFS in CRC [31]. Thus far, no previous studies have reported the association between these factors and FTD/TPI plus bevacizumab; this is the first study to investigate this association, however confirmation of the results in further studies is required.
The GI-SCREEN CRC UKIT study evaluated the strength of interactions of pretreatment levels of 17 plasma angiogenesis factors (dichotomized according to the median value) on PFS in patients with mCRC treated with chemotherapy in combination with angiogenesis inhibitors [37]. Patients treated with chemotherapy plus bevacizumab and low PlGF had significantly longer PFS, and patients with high VEGF-A or low VEGF-D tended to have a better PFS [37, 38]. However, no difference in PFS was observed between the high and low groups based on the PlGF, VEGF-A, and VEGF-D levels in this biomarker analysis. In accordance with the findings of the current study, the AFFIRM (Study of Aflibercept and Modified FOLFOX6 as First-Line Treatment in Patients with Metastatic Colorectal Cancer) trial found that high circulating plasma concentrations of IL-8 at baseline were correlated with a reduced PFS [39]. In a retrospective exploratory analysis of the biomarker subgroups in the CORRECT (patients with metastatic COloRectal cancer treated with REgorafenib or plaCebo after failure of standard Therapy) trial, a significant association between plasma IL-8 concentrations and OS was noted in a multivariable analysis after controlling for various clinical factors [40].
In addition to HGF and IL-8, low levels of IL-6, OPN, TSP-2, and TIMP-1 were found to correlate with a better PFS in the present study. During CRC progression, IL-6 is produced mainly by tumor-associated macrophages, mesenchymal stem cells, or colon cancer-associated fibroblasts [41]. Patients with mCRC who had high baseline serum IL-6 concentrations had a shorter median OS than those who had low baseline serum IL-6 concentrations (16.6 vs. 26.0 months, p < 0.001) [42]. OPN is a multifunctional phosphorylated protein widely involved in solid tumor progression, and its expression is negatively correlated with the prognosis of patients with colorectal and head and neck cancers [43]. Thrombospondin is an adhesive protein that stimulates tumor cell invasion into the surrounding tissue [44]. A direct correlation between the plasma thrombospondin levels and degree of venous invasion has been reported in patients with CRC [44]. TIMP-1 is a glycoprotein regulating metalloproteinases and may play a key role in tumor progression [45]. Serum levels of TIMP-1 were elevated in patients with advanced CRC, and patients with high TIMP-1 levels had worse prognosis [46].
The baseline plasma concentration of cfDNA is related to the tumor burden and is a strong prognostic factor in patients with mCRC, with higher levels showing a poor prognosis [47]. However, the current biomarker study found no correlation between cfDNA concentration and PFS. Treatment with FTD/TPI plus bevacizumab may be clinically effective regardless of the tumor volume. Our study suggests that FTD/TPI plus bevacizumab may be the standard of care for patients with mCRC who have a worse prognosis.
This study has some limitations. First, the follow-up was conducted for a short period owing to the early termination of TRUSTY. Therefore, the relationship between OS and the measured biomarkers could not be evaluated. Second, because only a few blood samples were available, it was not considered appropriate to compare the FTD/TPI plus bevacizumab and control groups. Therefore, this biomarker analysis examined only prognostic factors, not predictive factors, for FTD/TPI plus bevacizumab. Third, this biomarker analysis was exploratory in nature, and a validation study is needed. Lastly, although the median was used as the cut-off value for this biomarker analysis, there may be other appropriate cut-off values.
Conclusions
Low baseline plasma concentrations of HGF and IL-8 may better serve as predictors for a higher DCR than high plasma concentrations. Moreover, low plasma concentrations of HGF, IL-6, IL-8, OPN, TSP-2, and TIMP-1 may better serve as predictors of a longer PFS than high plasma concentrations in patients with mCRC treated with second-line FTD/TPI plus bevacizumab, although further studies are warranted.
Supplementary Information
Below is the link to the electronic supplementary material.
Acknowledgments
The authors thank the patients, their families, and the physicians who participated in this study. Medical writing assistance was provided by Sarayu Pai, PhD, CMPP, of Cactus Life Sciences (part of Cactus Communications) and was funded by Taiho Pharmaceutical Co., Ltd.
Declarations
Funding
This study was funded by Taiho Pharmaceutical Co., Ltd. The funding body (Taiho Pharmaceutical Co., Ltd.) participated in the study design, data collection, data management, and data analysis under the guidance of the principal investigator. The corresponding author had full access to all the data in the study and took the final responsibility of making the decision to submit the manuscript for publication.
Conflicts of interest/competing interests
All authors report support for the present manuscript (e.g., funding, provision of study materials, medical writing, and article processing charges) from Taiho Pharmaceutical Co., Ltd K.K. Mitsuru Yokota, Hitoshi Ojima, Naotoshi Sugimoto, Yasushi Tsuji, and Soichiro Ishihara have no other conflicts of interest. Yu Sunakawa has received institutional grants or contracts from Chugai, Taiho, Takeda, Otsuka, and Eli Lilly Japan; payment or honoraria for lectures, presentations, speaker’s bureau, manuscript writing, or educational events from Eli Lilly Japan, Bristol Myers Squibb, Chugai, Takeda, Ono, Merck Biopharma, Taiho, Bayer, Daiichi Sankyo, MSD, Sysmex, and Guardant Health; and has participated on the data safety monitoring board or advisory board of Merck Biopharma, Ono, and Guardant Health. Yasutoshi Kuboki has received institutional grants or contracts from Taiho, Takeda, Ono, AbbVie, Janssen Oncology, Boehringer Ingelheim, Incyte, Amgen, Chugai, GlaxoSmithKline, Genmab, Astellas, Daiichi Sankyo, and Eli Lilly Japan; personal consulting fees from Amgen, Takeda, and Boehringer Ingelheim; and payment or honoraria for lectures, presentations, speaker’s bureau, manuscript writing, or educational events from Taiho, Ono, Bayer, Eli Lilly Japan, Bristol Myers Squibb, and Merck Serono. Jun Watanabe has received institutional grants or contracts from Medtronic, Amco, and Terumo; and payment or honoraria for lectures, presentations, speaker’s bureau, manuscript writing, or educational events from Medtronic, Johnson & Johnson, Eli Lilly Japan, and Takeda. Tetsuji Terazawa has received consulting fees from Chugai, Eli Lilly Japan, Taiho, and Sanofi; has been part of the data safety monitoring board or advisory board of Sanofi; and is an employee of Shionogi. Hisato Kawakami has received institutional grants or contracts from Eisai, Kobayashi, and Bristol Myers Squibb; personal consulting fees from Daiichi Sankyo; and payment or honoraria for lectures, presentations, speaker’s bureau, manuscript writing, or educational events from Bristol Myers Squibb, Eli Lilly Japan, Ono, Daiichi Sankyo, Takeda, Teijin, Otsuka, Bayer, MSD, Chugai, Merck Biopharma, Yakult, and Taiho. Masato Nakamura has received payment or honoraria for lectures, presentations, speaker’s bureau, manuscript writing, or educational events from Eli Lilly Japan, Nihon Servier, Daiichi Sankyo, Ono, Taiho, Yakult, Merck & Co., Bayer, Chugai, Merck Biopharma, Otsuka, Takeda, and AstraZeneca. Masahito Kotaka has received payment or honoraria for lectures, presentations, speaker’s bureau, manuscript writing, or educational events from Chugai, Takeda, Taiho, Yakult, and Eli Lilly Japan. Eiji Oki has received payment or honoraria for lectures, presentations, speaker’s bureau, manuscript writing, or educational events from Taiho, Takeda, Chugai, Bristol Myers Squibb, Bayer, Eli Lilly Japan, and Ono. Takeshi Kajiwara has received payment or honoraria for lectures, presentations, speaker’s bureau, manuscript writing, or educational events from Chugail, Eli Lilly Japan, Bristol Myers Squibb, Taiho, and Ono. Yoshiyuki Yamamoto has received payment or honoraria for lectures, presentations, speaker’s bureau, manuscript writing, or educational events from Ono, Bristol Myers Squibb, Yakult, Chugai, Eli Lilly Japan, Bayer, Taiho, Servier, Takeda, Daiichi Sankyo, AstraZeneca, and Insight. Tadamichi Denda has received institutional grants or contracts from Ono, MSD, Bristol Myers Squibb Foundation, Amgen, and Pfizer; and payment or honoraria for lectures, presentations, speaker’s bureau, manuscript writing, or educational events from Daiichi Sankyo, Sysmex, and Ono. Takao Tamura has received institutional grants or contracts from Chugai, Ltd; and payment or honoraria for lectures, presentations, speaker’s bureau, manuscript writing, or educational events from Takeda, Eli Lilly Japan K.K., Bristol Myers Squibb, Ono, and Chugai. Hiroya Taniguchi has received institutional grants or contracts from Takeda, Daiichi Sankyo, and Ono; and payment or honoraria for lectures, presentations, speaker’s bureau, manuscript writing, or educational events from Takeda, Ono, Eli Lilly Japan, Merck Biopharma, and Chugai. Takako Eguchi Nakajima has received institutional grants or contracts from Guardant Health, Taiho, Takeda, Chugai, Nippon Kayaku Co., AbbVie, Eli Lilly Japan, Shionogi, Otsuka, and Taisho; and payment or honoraria for lectures, presentations, speaker’s bureau, manuscript writing, or educational events from Sumitomo Dainippon, Boehringer Ingelheim, Bristol Myers Squibb, Ono, Taiho, Amgen, Takeda, Chugai, Sanofi, Novartis Japan, Daiichi Sankyo, AstraZeneca, IQVIA, GlaxoSmithKline, NOBEL Pharma, and Parexel. Satoshi Morita has received payment or honoraria for lectures, presentations, speaker’s bureau, manuscript writing, or educational events from Bristol Myers Squibb, Chugai, Taiho, Eli Lilly Japan, and AstraZeneca. Kuniaki Shirao has received institutional grants or contracts from Ono. Naruhito Takenaka and Daisuke Ozawa are employees of Taiho. Takayuki Yoshino has received institutional grants or contracts from Amgen K.K., Chugai, Daiichi Sankyo, Eisai, Falco Biosystems Ltd, Genomedia Inc., Molecular Health GmbH, MSD, Nippon Boehringer Ingelheim, Ono, Pfizer Japan Inc., Roche Diagnostics K.K., Sanofi, Sysmex, and Taiho; personal consulting fees from Sumitomo Corporation; and payment or honoraria for lectures, presentations, speaker’s bureau, manuscript writing, or educational events from Bayer, Chugai, Merck Biopharma, MSD, Ono, and Takeda.
Ethics approval
The study protocol was approved by the National Cancer Center Hospital East Certified Review Board (CRB3180009) and conducted according to the Declaration of Helsinki and Clinical Trials Act. This study is registered with the Japan Registry of Clinical Trials (jRCTs031180122).
Consent to participate
Informed consent was obtained from all individual participants included in this study.
Consent to publish
Not applicable.
Availability of data and material
The datasets used and/or analyzed during the current study are available from the corresponding author upon reasonable request.
Code availability
Not applicable.
Author contributions
YS, YK, TTe, EO, SI, HT, TEN, and TY comprised the steering committee, which coordinated trial implementation at all sites, including coordination of the study protocol and resolution of doubts in its interpretation. YS, YK, JW, TTe, HK, MY, MN, MK, NS, HO, EO, TK, YY, YT, TD, TTa, SI, HT, TEN, and TY collected data. All authors interpreted the data, and SM analyzed the data. YS was responsible for the decision to submit the manuscript.
References
- 1.NCCN Clinical Practice guidelines in Oncology: Colon Cancer. 2023. https://www.nccn.org/professionals/physician_gls/pdf/colon.pdf. Accessed 19 May 2023.
- 2.Prager GW, Taieb J, Fakih M, Ciardiello F, Van Cutsem E, Elez E, et al. Trifluridine-tipiracil and bevacizumab in refractory metastatic colorectal cancer. N Engl J Med. 2023;388:1657–1667. doi: 10.1056/NEJMoa2214963. [DOI] [PubMed] [Google Scholar]
- 3.Mousa L, Salem ME, Mikhail S. Biomarkers of angiogenesis in colorectal cancer. Biomark Cancer. 2015;7:13–19. doi: 10.4137/BIC.S25250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ogunwobi OO, Mahmood F, Akingboye A. Biomarkers in colorectal cancer: current research and future prospects. Int J Mol Sci. 2020;21:5311. doi: 10.3390/ijms21155311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Douillard JY, Oliner KS, Siena S, Tabernero J, Burkes R, Barugel M, et al. Panitumumab-FOLFOX4 treatment and RAS mutations in colorectal cancer. N Engl J Med. 2013;369:1023–1034. doi: 10.1056/NEJMoa1305275. [DOI] [PubMed] [Google Scholar]
- 6.Van Cutsem E, Lenz HJ, Köhne CH, Heinemann V, Tejpar S, Melezínek I, et al. Fluorouracil, leucovorin, and irinotecan plus cetuximab treatment and RAS mutations in colorectal cancer. J Clin Oncol. 2015;33:692–700. doi: 10.1200/JCO.2014.59.4812. [DOI] [PubMed] [Google Scholar]
- 7.Bokemeyer C, Köhne CH, Ciardiello F, Lenz HJ, Heinemann V, Klinkhardt U, et al. FOLFOX4 plus cetuximab treatment and RAS mutations in colorectal cancer. Eur J Cancer. 2015;51:1243–1252. doi: 10.1016/j.ejca.2015.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sorich MJ, Wiese MD, Rowland A, Kichenadasse G, McKinnon RA, Karapetis CS. Extended RAS mutations and anti-EGFR monoclonal antibody survival benefit in metastatic colorectal cancer: a meta-analysis of randomized, controlled trials. Ann Oncol. 2015;26:13–21. doi: 10.1093/annonc/mdu378. [DOI] [PubMed] [Google Scholar]
- 9.Sunakawa Y, Yuki S, Shiozawa M, Masuishi T, Nishina T, Yasui H, et al. Profiling plasma angiogenesis factors after use of biologics in metastatic colorectal cancer (mCRC): update results from GI-SCREEN CRC Ukit study. J Clin Oncol. 2021;39:3529. doi: 10.1200/JCO.2021.39.15_suppl.3529. [DOI] [Google Scholar]
- 10.Tabernero J, Hozak RR, Yoshino T, Cohn AL, Obermannova R, Bodoky G, et al. Analysis of angiogenesis biomarkers for ramucirumab efficacy in patients with metastatic colorectal cancer from RAISE, a global, randomized, double-blind, phase III study. Ann Oncol. 2018;29:602–609. doi: 10.1093/annonc/mdx767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Van Cutsem E, Paccard C, Chiron M, Tabernero J. Impact of prior bevacizumab treatment on VEGF-A and PlGF levels and outcome following second-line aflibercept treatment: biomarker post hoc analysis of the VELOUR trial. Clin Cancer Res. 2020;26:717–725. doi: 10.1158/1078-0432.CCR-19-1985. [DOI] [PubMed] [Google Scholar]
- 12.Ose J, Gigic B, Hardikar S, Lin T, Himbert C, Warby CA, et al. Presurgery adhesion molecules and angiogenesis biomarkers are differently associated with outcomes in colon and rectal cancer: results from the ColoCare study. Cancer Epidemiol Biomarkers Prev. 2022;31:1650–1660. doi: 10.1158/1055-9965.EPI-22-0092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kopetz S, Hoff PM, Morris JS, Wolff RA, Eng C, Glover KY, et al. Phase II trial of infusional fluorouracil, irinotecan, and bevacizumab for metastatic colorectal cancer: efficacy and circulating angiogenic biomarkers associated with therapeutic resistance. J Clin Oncol. 2010;28:453–459. doi: 10.1200/JCO.2009.24.8252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hamfjord J, Guren TK, Dajani O, Johansen JS, Glimelius B, Sorbye H, et al. Total circulating cell-free DNA as a prognostic biomarker in metastatic colorectal cancer before first-line oxaliplatin-based chemotherapy. Ann Oncol. 2019;30:1088–1095. doi: 10.1093/annonc/mdz139. [DOI] [PubMed] [Google Scholar]
- 15.Xu X, Yu Y, Shen M, Liu M, Wu S, Liang L, et al. Role of circulating free DNA in evaluating clinical tumor burden and predicting survival in Chinese metastatic colorectal cancer patients. BMC Cancer. 2020;20:1006. doi: 10.1186/s12885-020-07516-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kuboki Y, Terazawa T, Masuishi T, Nakamura M, Watanabe J, Ojima H, et al. Trifluridine/tipiracil+bevacizumab (BEV) vs. fluoropyrimidine-irinotecan+BEV as second-line therapy for metastatic colorectal cancer: a randomised noninferiority trial. Br J Cancer. 2023;128:1897–1905. doi: 10.1038/s41416-023-02212-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Yoshino T, Oki E, Nozawa H, Eguchi-Nakajima T, Taniguchi H, Morita S, et al. Rationale and design of the TRUSTY study: a randomised, multicentre, open-label phase II/III study of trifluridine/tipiracil plus bevacizumab versus irinotecan, fluoropyrimidine plus bevacizumab as second-line treatment in patients with metastatic colorectal cancer progressive during or following first-line oxaliplatin-based chemotherapy. ESMO Open. 2018;3:e000411. doi: 10.1136/esmoopen-2018-000411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Weickhardt AJ, Williams DS, Lee CK, Chionh F, Simes J, Murone C, et al. Vascular endothelial growth factor D expression is a potential biomarker of bevacizumab benefit in colorectal cancer. Br J Cancer. 2015;113:37–45. doi: 10.1038/bjc.2015.209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ruiz-Bañobre J, Kandimalla R, Goel A. Predictive biomarkers in metastatic colorectal cancer: a systematic review. JCO Precis Oncol. 2019;3:PO.18.00260. doi: 10.1200/PO.18.00260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Peddareddigari VG, Wang D, Dubois RN. The tumor microenvironment in colorectal carcinogenesis. Cancer Microenviron. 2010;3:149–166. doi: 10.1007/s12307-010-0038-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Owusu BY, Galemmo R, Janetka J, Klampfer L. Hepatocyte growth factor, a key tumor-promoting factor in the tumor microenvironment. Cancers (Basel). 2017;9:35. doi: 10.3390/cancers9040035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Faiella A, Riccardi F, Cartenì G, Chiurazzi M, Onofrio L. The emerging role of c-Met in carcinogenesis and clinical implications as a possible therapeutic target. J Oncol. 2022;2022:5179182. doi: 10.1155/2022/5179182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kim JH, Kim HS, Kim BJ. Prognostic value of MET copy number gain in non-small-cell lung cancer: an updated meta-analysis. J Cancer. 2018;9:1836–1845. doi: 10.7150/jca.24980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wang F, Li S, Zhao Y, Yang K, Chen M, Niu H, et al. Predictive role of the overexpression for CXCR4, C-Met, and VEGF-C among breast cancer patients: a meta-analysis. Breast. 2016;28:45–53. doi: 10.1016/j.breast.2016.04.016. [DOI] [PubMed] [Google Scholar]
- 25.Choi J, Lee HE, Lee HS, Han N, Kim MA, Kim WH. Evaluation of intratumoral and intertumoral heterogeneity of MET protein expression in gastric cancer. Appl Immunohistochem Mol Morphol. 2018;26:445–453. doi: 10.1097/PAI.0000000000000448. [DOI] [PubMed] [Google Scholar]
- 26.Liu Y, Yu XF, Zou J, Luo ZH. Prognostic value of c-Met in colorectal cancer: a meta-analysis. World J Gastroenterol. 2015;21:3706–3710. doi: 10.3748/wjg.v21.i12.3706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lee SJ, Lee J, Park SH, Park JO, Lim HY, Kang WK, et al. c-MET overexpression in colorectal cancer: a poor prognostic factor for survival. Clin Colorect Cancer. 2018;17:165–169. doi: 10.1016/j.clcc.2018.02.013. [DOI] [PubMed] [Google Scholar]
- 28.Tsuji T, Sakamori Y, Ozasa H, Yagi Y, Ajimizu H, Yasuda Y, et al. Clinical impact of high serum hepatocyte growth factor in advanced non-small cell lung cancer. Oncotarget. 2017;8:71805–71816. doi: 10.18632/oncotarget.17895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Park DJ, Yoon C, Thomas N, Ku GY, Janjigian YY, Kelsen DP, et al. Prognostic significance of targetable angiogenic and growth factors in patients undergoing resection for gastric and gastroesophageal junction cancers. Ann Surg Oncol. 2014;21:1130–1137. doi: 10.1245/s10434-013-3429-0. [DOI] [PubMed] [Google Scholar]
- 30.Kubo Y, Fukushima S, Inamori Y, Tsuruta M, Egashira S, Yamada-Kanazawa S, et al. Serum concentrations of HGF are correlated with response to anti-PD-1 antibody therapy in patients with metastatic melanoma. J Dermatol Sci. 2019;93:33–40. doi: 10.1016/j.jdermsci.2018.10.001. [DOI] [PubMed] [Google Scholar]
- 31.Bazzichetto C, Milella M, Zampiva I, Simionato F, Amoreo CA, Buglioni S, et al. Interleukin-8 in colorectal cancer: a systematic review and meta-analysis of its potential role as a prognostic biomarker. Biomedicines. 2022;10:2631. doi: 10.3390/biomedicines10102631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Li A, Dubey S, Varney ML, Dave BJ, Singh RK. IL-8 directly enhanced endothelial cell survival, proliferation, and matrix metalloproteinases production and regulated angiogenesis. J Immunol. 2003;170:3369–3376. doi: 10.4049/jimmunol.170.6.3369. [DOI] [PubMed] [Google Scholar]
- 33.Raman D, Baugher PJ, Thu YM, Richmond A. Role of chemokines in tumor growth. Cancer Lett. 2007;256:137–165. doi: 10.1016/j.canlet.2007.05.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Feng L, Qi Q, Wang P, Chen H, Chen Z, Meng Z, et al. Serum levels of IL-6, IL-8, and IL-10 are indicators of prognosis in pancreatic cancer. J Int Med Res. 2018;46:5228–5236. doi: 10.1177/0300060518800588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Sanguinete MMM, Oliveira PH, Martins-Filho A, Micheli DC, Tavares-Murta BM, Murta EFC, et al. Serum IL-6 and IL-8 correlate with prognostic factors in ovarian cancer. Immunol Invest. 2017;46:677–688. doi: 10.1080/08820139.2017.1360342. [DOI] [PubMed] [Google Scholar]
- 36.Tiainen L, Hämäläinen M, Luukkaala T, Tanner M, Lahdenperä O, Vihinen P, et al. Low plasma IL-8 levels during chemotherapy are predictive of excellent long-term survival in metastatic breast cancer. Clin Breast Cancer. 2019;19:e522–e533. doi: 10.1016/j.clbc.2019.03.006. [DOI] [PubMed] [Google Scholar]
- 37.Yuki S, Yamazaki K, Sunakawa Y, Taniguchi H, Masuishi T, Shiozawa M, et al. 349P Analysis of plasma angiogenesis factors on the efficacy of 2nd-line (2L) chemotherapy (chemo) combined with angiogenesis inhibitors (AIs) in metastatic colorectal cancer (mCRC): results from GI-SCREEN CRC Ukit study. Ann Oncol. 2022;33:S695–S696. doi: 10.1016/j.annonc.2022.07.487. [DOI] [Google Scholar]
- 38.Yuki S, Taniguchi H, Masuishi T, Shiozawa M, Bando H, Yamazaki K, et al. 463P impact of plasma angiogenesis factors on the efficacy of 2nd-line chemotherapy combined with biologics in metastatic colorectal cancer (mCRC): early efficacy results from GI-SCREEN CRC Ukit study. Ann Oncol. 2021;32:S563–S564. doi: 10.1016/j.annonc.2021.08.984. [DOI] [Google Scholar]
- 39.Lambrechts D, Thienpont B, Thuillier V, Sagaert X, Moisse M, Peuteman G, et al. Evaluation of efficacy and safety markers in a phase II study of metastatic colorectal cancer treated with aflibercept in the first-line setting. Br J Cancer. 2015;113:1027–1034. doi: 10.1038/bjc.2015.329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Tabernero J, Lenz HJ, Siena S, Sobrero A, Falcone A, Ychou M, et al. Analysis of circulating DNA and protein biomarkers to predict the clinical activity of regorafenib and assess prognosis in patients with metastatic colorectal cancer: a retrospective, exploratory analysis of the CORRECT trial. Lancet Oncol. 2015;16:937–948. doi: 10.1016/S1470-2045(15)00138-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Turano M, Cammarota F, Duraturo F, Izzo P, De Rosa M. A potential role of IL-6/IL-6R in the development and management of colon cancer. Membranes (Basel). 2021;11:312. doi: 10.3390/membranes11050312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Thomsen M, Kersten C, Sorbye H, Skovlund E, Glimelius B, Pfeiffer P, et al. Interleukin-6 and C-reactive protein as prognostic biomarkers in metastatic colorectal cancer. Oncotarget. 2016;7:75013–75022. doi: 10.18632/oncotarget.12601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Tan Y, Zhao L, Yang YG, Liu W. The role of osteopontin in tumor progression through tumor-associated macrophages. Front Oncol. 2022;12:953283. doi: 10.3389/fonc.2022.953283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Yamashita Y, Kurohiji T, Tuszynski GP, Sakai T, Shirakusa T. Plasma thrombospondin levels in patients with colorectal carcinoma. Cancer. 1998;82:632–638. doi: 10.1002/(SICI)1097-0142(19980215)82:4<632::AID-CNCR3>3.0.CO;2-N. [DOI] [PubMed] [Google Scholar]
- 45.Spindler KL, Christensen IJ, Nielsen HJ, Jakobsen A, Brünner N. TIMP-1 and CEA as biomarkers in third-line treatment with irinotecan and cetuximab for metastatic colorectal cancer. Tumour Biol. 2015;36:4301–4308. doi: 10.1007/s13277-015-3069-z. [DOI] [PubMed] [Google Scholar]
- 46.Böckelman C, Beilmann-Lehtonen I, Kaprio T, Koskensalo S, Tervahartiala T, Mustonen H, et al. Serum MMP-8 and TIMP-1 predict prognosis in colorectal cancer. BMC Cancer. 2018;18:679. doi: 10.1186/s12885-018-4589-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Spindler KG, Boysen AK, Pallisgård N, Johansen JS, Tabernero J, Sørensen MM, et al. Cell-free DNA in metastatic colorectal cancer: a systematic review and meta-analysis. Oncologist. 2017;22:1049–1055. doi: 10.1634/theoncologist.2016-0178. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.