Abstract
Antibodies to Staphylococcus aureus heat shock proteins (Hsps) are present in the sera of patients with S. aureus endocarditis (M. W. Qoronfleh, W. Weraarchakul, and B. J. Wilkinson, Infect. Immun. 61:1567–1570, 1993). Although these proteins are immunogenic, their role in infection has not been established. We developed a cell culture system as a model to examine the potential involvement of staphylococcal Hsps in the initial events of infection. This study supports a model in which a clinical endocarditis isolate responds to host cell signals by selectively regulating the synthesis of numerous proteins, including the stress proteins Hsp60 (GroEL homolog) and Hsp70 (DnaK homolog) and a unique 58-kDa protein.
Eukaryotic and prokaryotic cell types respond to a sudden increase in temperature by synthesizing a subset of cellular proteins termed heat shock proteins (Hsps). These proteins were originally described in relation to thermal stress and have subsequently been shown to be inducible by a variety of stresses. The stress response appears to be universal and conserved throughout evolution (20). Hsps, in particular, chaperonins, aid in protein folding and play an important biological role in protein translocation across membranes (33). The heat shock response has been described in various pathogenic bacteria (5, 6, 31, 34–38), including Staphylococcus aureus and Staphylococcus epidermidis (24). The apparent molecular masses of the major staphylococcal Hsps were 84, 76, and 60 kDa, and those of other prominent inducible proteins were 66, 51, 43, and 24 kDa. Studies of Hsp localization in different human pathogens have shown that Hsps are distributed throughout the cytoplasm and periplasm, are found to be associated with the cytoplasmic membrane, and may localize to the bacterial cell surface when the bacteria are intracellular (8, 26, 29, 31). The role of Hsps in pathogenesis is not fully understood. The expression of certain virulence factors in some human pathogens has been shown to be influenced by temperature. For instance, production of adherence factors that mediate colonization by pathogenic Salmonella typhimurium (14) and Escherichia coli (10) are temperature dependent. Likewise, expression of invasion genes by Shigella species (18), Listeria monocytogenes (30), and Yersinia pseudotuberculosis (13) is thermally inducible. Sera from infected patients or immune individuals have been used to identify antigenic constituents of many important pathogens. This approach has shown that some of these antigens are members of stress protein families (25, 31). Buchmeier and Heffron (4) have provided evidence for the induction of Salmonella stress proteins upon infection of a macrophage cell line and the apparent immunodominance of these Hsps, two of which were identified as GroEL and DnaK. Two mutants lacking certain subsets of the Hsps were killed more efficiently in macrophages and were avirulent in mice. Their data suggest a role for these Hsps in infection, particularly in bacterial survival within macrophages. Apparently, a common theme among intracellular bacteria like Brucella abortus, Chlamydia trachomatis, and Legionella pneumophila is the selective synthesis of stress proteins during intracellular growth and their correlation with virulence (8).
Previously, we have demonstrated the production of staphylococcal Hsps under stress conditions (24). We also have shown that sera from seven endocarditis patients contained antibodies which reacted to a range of proteins produced in response to heat shock (25). In this study, we examined if the clinical isolate S. aureus Endo-2 preferentially synthesized Hsps in response to contact with host cells or following intracellular infection. Our present investigation confirms the thermal induction of Hsps and the reactivity of the patient serum against these Hsps with the clinical isolate Endo-2. Additionally, the epithelial cell surface induced staphylococcal proteins presumably involved in bacterial adherence and invasion. To our knowledge, there have been no published reports demonstrating expression of stress proteins early in infection with the gram-positive bacterium S. aureus.
S. aureus Endo-2, an endocarditis isolate, was examined after minimal subculture (25). The strain was maintained at −70°C in 10% skim milk and grown on tryptic soy agar at 37°C overnight. The cells were pregrown before heat shock in 5 ml of Dulbecco’s modified Eagle’s medium (DME)–25 mM HEPES–1% yeast extract (YE) with shaking for 2.5 to 3.5 h at either 30 or 37°C. McCoy A cells, a human epithelial cell line, were used in this study. McCoy A cells were maintained in DME–5% newborn calf serum in 5% CO2 at 37°C. To prepare the cell cultures for infection, the medium was replaced with DME-HEPES-YE (without serum). To inhibit McCoy A cell protein synthesis, cycloheximide (50-μg/ml final concentration) was added to cell cultures 30 min prior to infection. Cycloheximide does not induce staphylococcal Hsps or affect S. aureus adherence or invasion. Aliquots (ca. 0.1 ml; inocula ranged from 2 × 107 to 1 × 108 bacteria) of a bacterial culture grown at either 30 or 37°C were added directly to McCoy cell cultures in 5 ml of DME-YE equilibrated at 37°C and incubated for 30 min (bacterium-host cell ratio, 100:1). Nonadherent cells were removed by gentle washing. The monolayers were then scraped from the flasks at timed intervals (1, 2, and 3 h). The mixed suspensions of bacteria and McCoy A cells were placed on ice for approximately 30 min, and all subsequent procedures were done at 4°C. Aliquots (1 ml) were pelleted in a microcentrifuge at 10,000 × g for 60 s, and the supernatant was discarded. The cell pellet was suspended in 170 μl of lysis buffer (phosphate-buffered saline [pH 7.4] containing DNase at 50 μg/ml and lysostaphin at 100 μg/ml) as previously described (24), with the exception that phenylmethylsulfonyl fluoride (final concentration; 1 mM) was added. After incubation in lysis buffer at 37°C for 2 h, the samples were stored at −20°C until analyzed. Sodium dodecyl sulfate-polyacrylamide gel electrophoresis was carried out as described by Laemmli (16). Western immunoblotting was performed as described previously (25). Serum was obtained from the endocarditis patient from whom Endo-2 was isolated (26). Purified E. coli Hsps GroES, GroEL, and DnaK and rabbit antisera raised against these proteins were obtained from Tony Gatenby (E. I. du Pont Co., Wilmington, Del.).
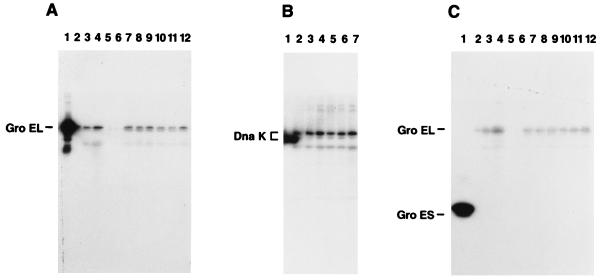
To detect staphylococcal Hsp homologs, rabbit antisera against the GroEL, GroES, and DnaK proteins of E. coli were used to probe the blots. The purified GroEL, GroES, and DnaK proteins used as standards are shown in Fig. 1A, B, and C, lanes 1. The antisera against GroEL and DnaK cross-reacted with the 60- and 76-kDa staphylococcal proteins, respectively, produced in response to heat stress in the absence of McCoy A cells (Fig. 1A, lanes 2 to 4). Thus, antibody (anti-GroEL and anti-DnaK) recognition of Hsp60 and Hsp76 strongly suggests that these proteins are analogous to the E. coli GroEL and DnaK proteins. Although the GroES antisera cross-reacted with the GroEL homolog, it did not react with a unique staphylococcal protein band with a molecular weight similar to that of E. coli GroES (Fig. 1C). This anti-GroES serum did cross-react with the staphylococcal GroEL homolog on the same blot (Fig. 1C), as well as S. typhimurium GroES-like protein and three S. aureus laboratory strains (data not shown). Next, we determined whether Hsp homologs are produced following infection of human epithelial cells. As shown in Fig. 1A (lanes 10 to 12), GroEL homologs were detected in cocultures incubated at 37°C when the staphylococci were pregrown at 30°C. In addition, these same homologs were produced under conditions not involving thermal stress when the staphylococci were pregrown at 37°C and added to McCoy A cultures maintained at 37°C (lanes 7 to 9). Additional samples from the non-thermally induced cocultures probed with antisera against DnaK (Fig. 1B, lanes 5 to 7) detected a staphylococcal DnaK homolog. Based on specific antibody reactivity detected by Western blotting and immunoprecipitation (data not shown), we identified the 60- and 76-kDa proteins as being equivalent to the E. coli GroEL and DnaK proteins, respectively (Fig. 1A and B). These GroEL- and DnaK-like proteins are detectable within 1 h postinfection of cell cultures. Stress proteins Hsp76 and Hsp60 are expressed at elevated levels in McCoy A cells compared to those in nonshocked bacteria. Surprisingly, GroES was not detectable on the gels (Fig. 1C). Densitometry measurements of stressed cells displayed a twofold increase in Hsp60 and Hsp70 synthesis.
FIG. 1.
Detection of GroEL, DnaK, and GroES homologs. Western immunoblots of polyclonal rabbit antiserum against cell lysate proteins. (A) Lanes: 1, purified E. coli GroEL protein; 2, S. aureus maintained at 30°C; 3, S. aureus maintained at 37°C; 4, S. aureus shifted from 30 to 37°C; 5, cycloheximide-treated McCoy cells; 6, heat-killed bacteria added to McCoy cells; 7 to 9, S. aureus grown at 37°C and added to McCoy cells at 37°C (1, 2, and 3 h postinfection, respectively); 10 to 12, S. aureus grown at 30°C and added to McCoy cells at 37°C (1, 2, and 3 h postinfection, respectively). (B) Lanes: 1, purified E. coli DnaK protein; 2, S. aureus maintained at 30°C; 3, S. aureus maintained at 37°C; 4, S. aureus shifted from 30 to 37°C; 5 to 7, S. aureus grown at 37°C and added to McCoy cells at 37°C (1, 2, and 3 h postinfection, respectively). (C) Lanes: 1, purified E. coli GroES protein; 2, S. aureus maintained at 30°C; 3, S. aureus maintained at 37°C; 4, S. aureus shifted from 30 to 37°C; 5, cycloheximide-treated McCoy cells; 6, heat-killed bacteria added to McCoy cells; 7 to 9, S. aureus grown at 30°C and added to McCoy cells at 37°C (1, 2, and 3 h postinfection, respectively); 10 to 12, S. aureus grown at 37°C and added to McCoy cells at 37°C (1, 2, and 3 h postinfection, respectively).
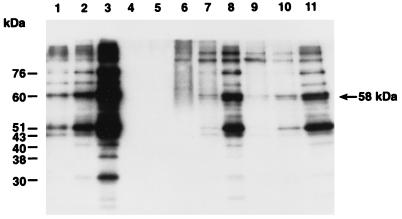
In human epithelial cell cultures, an examination of S. aureus Endo-2 by photomicroscopy revealed that it was internalized by McCoy A cells (7). Through viability plating, we found that less than 21% of the bacterial inoculum was adherent to the cell surface. Adherence was maximal within 2 to 3 h. Infectivity studies with strain Endo-2 showed that it remained viable and virulent over a 6-h period postinfection of cell cultures (7, 28). S. aureus Endo-2 grown at either 30 or 37°C was used to infect cycloheximide-treated McCoy A cell cultures maintained at 37°C. Controls were S. aureus cells in medium without McCoy A cells (i) grown and maintained at 30 or 37°C (non-heat shocked) or (ii) grown at 30°C and shocked at 37°C (Fig. 2, lanes 1 to 3). Hsps belonging to the three major families Hsp90, Hsp70, and Hsp60 are constitutively expressed at normal growth temperatures of 30 and 37°C (Fig. 2, lanes 1 and 2). In response to heat shock, these proteins were abundantly expressed and three unique stress proteins of 40, 38, and 30 kDa were recognized by the patient’s antibodies (Fig. 2, lane 3). In cell culture, as shown in Fig. 2 (lanes 9 to 11), bacteria grown at 30°C and then added to epithelial cells at 37°C produce a pattern of Hsps similar to that of bacteria shifted from 30 to 37°C without McCoy A cells (lane 3). In addition, bacteria grown at 37°C and applied to cell cultures under conditions which do not involve thermal induction produced a full range of Hsps (lanes 6 to 8) similar to those produced under heat stress conditions (lanes 3 and 9 to 11). Our data demonstrate that Endo-2 responds by accelerated synthesis of a set of Hsps within 3 h of cell culture infection. The predominant stress proteins are Hsp84, Hsp76, Hsp60, and Hsp51. While the immunoblots cannot distinguish between newly synthesized and preexisting Hsps, the results suggest that elevated synthesis of these proteins took place in cell culture (Fig. 2, lanes 1, 2, 8, and 11). Densitometry analysis showed three- to fivefold differences in Hsp84, Hsp76, Hsp60, and Hsp51 synthesis at 3 h postinfection. When lanes 1 to 3, 8, and 11 in Fig. 2 are compared, two observations are evident. First, the three stress proteins, Hsp40, Hsp38, and Hsp30, that are unique to Endo-2 and not detected in laboratory strains are not induced in vivo early in infection, although they are antigenic and detectable by the patient’s serum in vitro in heat shock experiments (25). There were very faint bands representing those proteins on autoradiographs. Perhaps these proteins play a role in the later stages of invasion or infection. Second, a novel 58-kDa protein was induced in this culture system. This novel 58-kDa protein has not been reported in other S. aureus strains under stress conditions, nor was it observed in Endo-2 stress experiments using the same serum (24, 25). This protein band was not detected in heat shock lysates of Endo-2 cells, even at low loading. No Hsp synthesis was detected in uninfected McCoy A cell cultures treated with cycloheximide or in McCoy A cell cultures infected with heat-killed S. aureus at 3 h postinfection (Fig. 2, lanes 4 and 5).
FIG. 2.
Demonstration of elevated Hsp synthesis in McCoy cell cultures. Western immunoblotting of the serum of a patient from whom an S. aureus Endo-2 clinical isolate was obtained against bacterial cell lysate proteins. Lanes: 1, S. aureus maintained at 30°C; 2, S. aureus maintained at 37°C; 3, S. aureus shifted from 30 to 37°C; 4, cycloheximide-treated McCoy cells; 5, heat-killed bacteria added to McCoy cells; 6 to 8, S. aureus grown at 37°C and added to McCoy cells at 37°C (1, 2, and 3 h postinfection, respectively); 9 to 11, S. aureus grown at 30°C and added to McCoy cells at 37°C (1, 2, and 3 h postinfection, respectively).
Essential factors in the establishment of a host-parasite relationships are the pathogen’s abilities to invade and colonize the host, multiply, and evade the host defenses. At various stages of this relationship, the pathogen must respond to changing environmental conditions within the host. Our study simulated the in vivo situation more closely by utilizing a cell culture system to investigate the stress response of an S. aureus clinical isolate during infection. Ogawa et al. (21) demonstrated S. aureus adherence to cultured human endothelial cells and subsequent endocytosis of the bacteria within 2 to 3 h. The S. aureus clinical isolates were highly adherent (>21%) to confluent monolayers of human endothelial cells compared to other gram-positive and gram-negative bacterial isolates. Schmidt et al. (27) also showed adhesiveness and invasiveness of various staphylococcal species in HEp-2 cells. Similarly, we observed by photomicroscopy the presence of staphylococci on the surface of McCoy A cells early in infection, adherence, and invagination of the underlying epithelial cell membrane, followed by engulfment. Furthermore, as the infection continued, the McCoy A cells underwent morphological changes and the bacteria were also seen in the same plane of focus as the nuclei (7, 28). Physical or chemical stress induces Hsps in staphylococci. Since many of these proteins appear to be membrane associated (24), we hypothesized that they may be exposed on the cell surface of the microorganism. The observation that stress proteins are antigenic, targets for the host immune response, and immunodominant in many bacterial infections is well documented (11, 22, 25, 32, 37–39). Indeed, we have shown that sera from seven patients recovering from S. aureus endocarditis recognized a range of Hsps produced by the infecting strain, various laboratory S. aureus strains, and E. coli (25). Normal human serum at the same dilution did not detect any S. aureus or E. coli Hsps. Recently, Tang et al. reported similar findings with S. typhi and Hsp reactivity with sera from patients with typhoid fever (31).
In the current study, the expression level of Hsps when S. aureus encounters a host cell was investigated. We demonstrated that staphylococcal Hsps, including GroEL and DnaK homologs, are induced during infection of McCoy A epithelial cells under conditions that do not involve thermal stress. The prominent Hsps were 60- and 76-kDa proteins which were similar to the E. coli GroEL and DnaK Hsps as judged by immunoblots probed with rabbit antisera raised against the purified E. coli GroEL and DnaK proteins and an endocarditis serum. This is further evidence that staphylococcal Hsps are induced during the infection process. Lending support to the involvement of staphylococcal Hsps during infection are cell culture studies with other pathogens. Finlay et al. found that epithelial cell contact induced de novo synthesis of proteins which were required for adherence and invasion by S. typhimurium (9, 19). Buchmeier and Heffron have demonstrated the induction of Salmonella stress proteins upon infection of macrophages. Two of these proteins were homologs of the E. coli GroEL and DnaK Hsps. Furthermore, their experimental evidence suggested that enhanced expression of these Salmonella proteins contributes to bacterial survival within macrophages (4). Others have shown that Hsp60 (GroEL homolog) is expressed in L. pneumophila-infected HeLa cells, human monocytes, and mouse L929 cells (8, 12). Elevated levels of S. aureus Hsp76 (DnaK-like protein) in cell culture infection indicate a contributing role in adherence and invasiveness capacity. This conclusion is corroborated by published reports on DnaK homologs of other microorganisms. In Mycoplasma spp. that are associated with infertility, Hsp70 functions as an adhesin (4). In C. trachomatis, DnaK is exposed on the cell surface (26) and is essential for cell culture infectivity (18). Similarly, in salmonellae, DnaK is essential and a potential adhesin molecule (4, 9, 31). GroES has been shown to be immunogenic in Campylobacter jejuni (36) and Helicobacter pylori (23). Interestingly, anti-GroES serum did not react with the S. aureus Endo-2 GroES-like protein. It is not known whether this is a common feature of clinical isolates. Perhaps the gene is absent or not expressed; alternatively, perhaps the GroES-like protein does not share antigenic epitopes with the corresponding E. coli GroES protein. The induction of a unique 58-kDa stress protein as a result of epithelial cell contact is intriguing. This novel Hsp58 protein was not observed in previous studies (24, 25). The role of Hsp58 in invasion, virulence, and/or pathogenesis requires further investigation. Two studies on S. aureus (2) and Streptococcus faecalis (1) endocarditis are suggestive. Those investigators found that sera from patients contained antibodies to a variety of cell envelope proteins, in addition to recognizing a 58-kDa S. aureus surface antigen. The molecular mass is strikingly similar to that described in this report, even though neither of these groups examined heat-shocked cells. Possibly, this protein is required for adherence and invasion of epithelial cells similar to the Salmonella protein (9). Interestingly, a novel 59-kDa protein of C. trachomatis that is distinct from Hsp60 (common antigen) has been cloned and characterized. This protein is surface exposed and recognized by sera from patients with chlamydial infections. More importantly, it appears to function as an adhesin (15).
The paucity of information on S. aureus Hsps is surprising given the importance of these proteins and given that S. aureus encounters many different environmental stresses. This cell culture model should provide a basis for investigations into the physiological and pathogenic roles of staphylococcal Hsps during infection. Since some Hsps appear to have both common and unique antigenic determinants among bacterial species, they are potential candidates for use in vaccine development or as diagnostic reagents (22, 39).
REFERENCES
- 1.Aitchison E J, Lambert P A, Smith E G, Farrell I D. Serodiagnosis of Streptococcus faecalis endocarditis by immunoblotting of surface protein antigens. J Clin Microbiol. 1987;25:211–215. doi: 10.1128/jcm.25.2.211-215.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Al-Ani B S, Coleman G. Humoral antibody response to extracellular, intracellular and cell envelope proteins of Staphylococcus aureus in cases of staphylococcal endocarditis. Biochem Soc Trans. 1988;16:998–999. [Google Scholar]
- 3.Boulanger J, Faulds D, Eddy E M, Lingwood C A. Members of the 70 kDa heat shock protein family specifically recognize sulfoglycolipids: role in gamate recognition and mycoplasma-related infertility. J Cell Physiol. 1995;165:7–17. doi: 10.1002/jcp.1041650103. [DOI] [PubMed] [Google Scholar]
- 4.Buchmeier N A, Heffron F. Induction of Salmonella stress proteins upon infection of macrophages. Science. 1990;248:730–732. doi: 10.1126/science.1970672. [DOI] [PubMed] [Google Scholar]
- 5.Cluss R G, Boothby J T. Thermoregulation of protein synthesis in Borrelia burgdorferi. Infect Immun. 1990;58:1038–1042. doi: 10.1128/iai.58.4.1038-1042.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Danilition S L, Maclean I W, Peeling R, Winston S, Brunham R C. The 75-kilodalton protein of Chlamydia trachomatis: a member of the heat shock protein 70 family? Infect Immun. 1990;58:189–196. doi: 10.1128/iai.58.1.189-196.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.DeSantis A, Hirtz M M, Qoronfleh M W, Bortner C A. Abstracts of the American Osteopathic Association (AOS) Research Conference. Boston, Mass: American Osteopathic Association; 1993. Photomicroscopy of the interaction between the McCoy cell and Staphylococcus aureus using Hoffman modulation interaction contrast. [Google Scholar]
- 8.Fernandez F C, Logan S M, Lee S H S, Hoffman P S. Elevated levels of Legionella pneumophila stress protein Hsp60 early in infection of human monocytes and L929 cells correlate with virulence. Infect Immun. 1996;64:1968–1976. doi: 10.1128/iai.64.6.1968-1976.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Finlay B B, Heffron F, Falkow S. Epithelial cell surfaces induce Salmonella proteins required for bacterial adherence and invasion. Science. 1989;243:940–943. doi: 10.1126/science.2919285. [DOI] [PubMed] [Google Scholar]
- 10.Goransson M, Uhlin B E. Environmental temperature regulates transcription of a virulence pill operon in E. coli. EMBO J. 1984;3:2855–2888. doi: 10.1002/j.1460-2075.1984.tb02225.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hansen K, Bangsborg J M, Fjordvang H, Pedersen N S, Hindersson P. Immunochemical characterization of and isolation of the gene for a Borrelia burgdorferi immunodominant 60-kilodalton antigen common to a wide range of bacteria. Infect Immun. 1988;56:2047–2053. doi: 10.1128/iai.56.8.2047-2053.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hoffman P S, Houston L, Butler C A. Legionella pneumophila htpAB heat shock operon: nucleotide sequence and expression of the 60-kilodalton antigen in L. pneumophila-infected HeLa cells. Infect Immun. 1990;58:3380–3387. doi: 10.1128/iai.58.10.3380-3387.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Isberg R R, Swain A, Falkow S. Analysis of expression and thermoregulation of the Yersinia pseudotuberculosis inv gene with hybrid proteins. Infect Immun. 1988;56:2133–2138. doi: 10.1128/iai.56.8.2133-2138.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Jones G W, Richardson L A. The attachment to and invasion of HeLa cells by Salmonella typhimurium: the contribution of mannose-sensitive and mannose-resistant haemagglutinating activities. J Gen Microbiol. 1981;127:361–370. doi: 10.1099/00221287-127-2-361. [DOI] [PubMed] [Google Scholar]
- 15.Kahane S, Weinstein Y, Sarov I. Cloning, characterization and sequence of a novel 59-kDa protein of Chlamydia trachomatis. Gene. 1990;90:61–67. doi: 10.1016/0378-1119(90)90439-x. [DOI] [PubMed] [Google Scholar]
- 16.Laemmli U K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature (London) 1970;227:680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- 17.Maclean I W, Peeling R W, Brunham R C. Characterization of Chlamydia trachomatis antigens with monoclonal and polyclonal antibodies. Can J Microbiol. 1988;34:141–147. doi: 10.1139/m88-028. [DOI] [PubMed] [Google Scholar]
- 18.Maurelli A T, Blackmon B, Curtiss R., III Temperature-dependent expression of virulence genes in Shigella species. Infect Immun. 1984;43:195–201. doi: 10.1128/iai.43.1.195-201.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mills S D, Finlay B B. Comparison of Salmonella typhi and Salmonella typhimurium invasion, intracellular growth and localization in cultured human epithelial cells. Microb Pathog. 1994;17:409–423. doi: 10.1006/mpat.1994.1086. [DOI] [PubMed] [Google Scholar]
- 20.Morimoto R I, Tissieres A, Georgopoulos C. The stress response, function of the proteins, and perspectives. In: Morimoto R I, Tissieres A, Georgopoulos C, editors. Stress proteins in biology and medicine. Cold Spring Harbor, N.Y: Cold Spring Harbor Laboratory Press; 1990. pp. 1–36. [Google Scholar]
- 21.Ogawa S K, Yurberg E R, Hatcher V B, Levitt M A, Lowy F D. Bacterial adherence to human endothelial cells in vitro. Infect Immun. 1985;50:218–224. doi: 10.1128/iai.50.1.218-224.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Palen M. Bacterial heat-shock proteins and serodiagnosis. Serodiagn Immunother Infect Dis. 1989;3:149–159. [Google Scholar]
- 23.Perez-Perez G I, Thiberge J M, Labigne A, Blaser M J. Relationship of immune response to heat-shock protein A and characteristics of Helicobacter pylori-infected patients. J Infect Dis. 1996;174:1046–1050. doi: 10.1093/infdis/174.5.1046. [DOI] [PubMed] [Google Scholar]
- 24.Qoronfleh M W, Streips U N, Wilkinson B J. Basic features of the staphylococcal heat shock response. Antonie Leeuwenhoek. 1990;58:79–86. doi: 10.1007/BF00422721. [DOI] [PubMed] [Google Scholar]
- 25.Qoronfleh M W, Weraarchakul W, Wilkinson B J. Antibodies to a range of Staphylococcus aureus and Escherichia coli heat shock proteins in the serum of a patient with S. aureus endocarditis. Infect Immun. 1993;61:1567–1570. doi: 10.1128/iai.61.4.1567-1570.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Raulston J E, Davis C H, Schmiel D H, Morgan M W, Wyrick P B. Molecular characterization and outer membrane association of a Chlamydia trachomatis protein related to the hsp70 family of proteins. J Biol Chem. 1993;268:23139–23147. [PubMed] [Google Scholar]
- 27.Schmidt H, Bukholm G, Holberg-Peterson M. Adhesiveness and invasiveness of staphylococcal species in a cell culture model. APMIS. 1989;97:655–660. doi: 10.1111/j.1699-0463.1989.tb00457.x. [DOI] [PubMed] [Google Scholar]
- 28.Schwartzberg P, Bortner C A, Qoronfleh M W. Abstracts of the 91st General Meeting of the American Society for Microbiology 1991. Washington, D.C: American Society for Microbiology; 1991. S. aureus Hsps induced upon infection of McCoy cells, abstr. D-104; p. 95. [Google Scholar]
- 29.Scorpio A, Johnson P, Laquerre A, Nelson D R. Subcellular localization and chaperone activities of Borrelia burgdorferi Hsp60 and Hsp70. J Bacteriol. 1994;176:6449–6456. doi: 10.1128/jb.176.21.6449-6456.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sokolovic Z, Fuchs A, Goebel W. Synthesis of species-specific stress proteins by virulent strains of Listeria monocytogenes. Infect Immun. 1990;58:3582–3587. doi: 10.1128/iai.58.11.3582-3587.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Tang S-W, Abubakar S, Devi S, Puthucheary S, Pang T. Induction and characterization of heat shock proteins of Salmonella typhi and their reactivity with sera from patients with typhoid fever. Infect Immun. 1997;65:2983–2986. doi: 10.1128/iai.65.7.2983-2986.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Thole J E R, Hindersson P, deBruyn J, Cremers F, van der Zee J, deCock H, Tommassen J, van Eden W, van Embden J D A. Antigenic relatedness of a strongly immunogenic 65 kDa mycobacterial protein antigen with a similar sized ubiquitous bacterial common antigen. Microb Pathog. 1988;4:71–83. doi: 10.1016/0882-4010(88)90049-6. [DOI] [PubMed] [Google Scholar]
- 33.Ulrich-Hartl F. Molecular chaperones in cellular protein folding. Nature (London) 1996;381:571–580. doi: 10.1038/381571a0. [DOI] [PubMed] [Google Scholar]
- 34.Vodkin M H, Williams J C. A heat shock operon in Coxiella burnettii produces a major antigen homologous to a protein in both mycobacteria and Escherichia coli. J Bacteriol. 1988;170:1227–1234. doi: 10.1128/jb.170.3.1227-1234.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Woods M L, II, Bonfiglioli R, McGee Z A, Georgopoulos C. Synthesis of a select group of proteins by Neisseria gonorrhoeae in response to thermal stress. Infect Immun. 1990;58:719–725. doi: 10.1128/iai.58.3.719-725.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Wu Y L, Lee L H, Rollins D M, Ching W M. Heat shock- and alkaline pH-induced proteins of Campylobacter jejuni: characterization and immunological properties. Infect Immun. 1994;62:4256–4260. doi: 10.1128/iai.62.10.4256-4260.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Yamaguchi H, Yamamoto T, Taguchi H, Ogata S. Yersinia enterocolitica immunodominant 60 kDa antigen, common to a broad range of bacteria, is a heat-shock protein. J Gen Microbiol. 1990;136:1091–1097. doi: 10.1099/00221287-136-6-1091. [DOI] [PubMed] [Google Scholar]
- 38.Young D, Lathigra R, Hendrix R, Sweetser D, Young R A. Stress proteins are immune targets in leprosy and tuberculosis. Proc Natl Acad Sci USA. 1988;85:4267–4270. doi: 10.1073/pnas.85.12.4267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Young R A. Stress proteins and immunology. Annu Rev Immunol. 1990;8:401–420. doi: 10.1146/annurev.iy.08.040190.002153. [DOI] [PubMed] [Google Scholar]