Abstract
Background:
Angiotensin II type 1 receptor antibodies (AT1R-Abs) and endothelin-type A receptor antibodies (ETAR-Abs) are G protein-coupled-receptor activating autoantibodies associated with antibody-mediated rejection (AMR), vascular pathology, increased cytokines, allograft dysfunction, and allograft loss in pediatric kidney transplant recipients (KTRs) in the first 2 years post-transplantation. The impact of AT1R-Ab and ETAR-Ab positivity on longer term 5-year transplant outcomes is unknown.
Methods:
One hundred pediatric KTRs were tested for ETAR-Ab and AT1R-Ab on serially collected blood samples in the first 2 years post-transplant. Biopsies were collected per protocol and 6, 12, and 24 months post-transplant and at any time over the 5 year follow-up period for clinical indication. Clinical outcomes including renal dysfunction, rejection, human leukocyte antigen donor specific antibodies (HLA DSA), and allograft loss were assessed through 5 years post-transplantation.
Results:
AT1R-Ab and/or ETAR-Ab were positive in 59% of patients. AT1R-Ab and/or ETAR-Ab positivity was associated with greater declines in eGFR and de novo AT1R-Ab and/or ETAR-Ab was associated with allograft loss in the first 2 years post-transplant. There was no association between antibody positivity and rejection, AMR, or allograft loss in the first 5 years post-transplant. In a model controlled for age, sex, immunosuppression, and HLA mismatch, AT1R-Ab and/or ETAR-Ab positivity was significantly associated with development of HLA DSA at 5 years post-transplant (OR 2.87, p=0.034).
Conclusions:
Our findings suggest temporally distinct clinical complications associated with AT1R-Ab and/or ETAR-Ab positivity in pediatric patients; these patterns of injury are of significant interest for the development of effective treatment strategies.
INTRODUCTION
Antibody-mediated rejection (AMR) remains a leading cause of allograft loss in both adult and pediatric kidney transplant recipients.1,2 Antibodies to human leukocyte antigen donor specific antibodies (HLA DSAs) have been well described in the pathogenesis of AMR. The potential role of non-HLA antibodies in both AMR and allograft dysfunction remains an important area of study. Among the most examined non-HLA antibodies in solid organ transplantation, angiotensin II type 1 receptor antibodies (AT1R-Abs) and endothelin-type A receptor antibodies (ETAR-Abs) are activating autoantibodies to their respective G-protein-coupled receptors (GPCRs). AT1R-Ab and ETAR-Ab have been associated with AMR, vascular pathology, increased cytokines, and allograft dysfunction in kidney transplant recipients.3–10 The mechanisms of injury remain unclear: both classical mechanisms of antibody-mediated allograft injury and overactivation of GPCRs may contribute to the clinical findings.3,11,12
We and others13–16 have recently reported the increased prevalence of AT1R-Ab and ETAR-Ab in pediatric kidney transplant recipients (KTRs). Furthermore, AT1R-Ab and ETAR-Ab are associated with higher risk of allograft loss, decline in eGFR, and elevation of cytokines in pediatric KTRs in the first 2 years post-transplant.13,17,18 The role of additional clinical factors in explaining variations in clinical phenotypes of AT1R-Ab and ETAR-Ab positive transplant recipients remains unclear. Factors such as concurrent HLA DSA (increased risk over time post-transplant) and/or ischemic injury (which is common in the peri-operative period) may potentiate the impact of AT1R-Ab and ETAR-Ab.4,5,19 We therefore sought to evaluate the impact of AT1R-Ab and ETAR-Ab positivity in the first 2 years post-transplant on 5-year clinical outcomes in a longitudinal cohort of pediatric KTRs.
MATERIALS AND METHODS
Patients, Samples, and Study Design
In this single-center study, 100 pediatric kidney transplant patients were monitored for 5 years post-transplant (Figure 1). Patients were transplanted between August 2005 and April 2017 and monitored as part of the UCLA Pediatric Kidney Transplant Immune Monitoring Study. All patients transplanted at our center are offered the opportunity to participate in the study. The patients included in this analysis are the first 100 patients within this time frame who had complete blood sample collection in the first 2 years post-transplantation (maximum of 1 missing study sample). Our previous work reported on 2 year outcomes in the first 65 patients in this cohort.13,18 Study blood samples from pre-transplant and at 6, 12, and 24 months post-transplant and for indication (time of biopsy) in the first 2 years were analyzed for AT1R-Ab and ETAR-Ab. Samples were tested for non-HLA antibodies retrospectively. This study was approved by the UCLA Institutional Review Board (#11–002375) and conforms with the 1964 Helsinki declaration and its later amendments or comparable ethical standards and the Principles of the Declaration of Istanbul. Informed consent and when appropriate patient assent was obtained for all patients. Demographic and clinical data including age, race, ethnicity (Hispanic or Latino vs Not Hispanic or Latino), etiology of end stage renal disease, transplant type (deceased/living donor), sensitization history, time on dialysis, delayed graft function (defined as dialysis in the first week post-transplant), immunosuppression regimen, and blood pressure were collected. Patients were monitored for rejection, allograft loss, and decrease in eGFR as determined by the CKiD Under 25 glomerular filtration rate estimating equation20. Study data were collected and managed using a secure REDCap (Research Electronic Data Capture) electronic data capture tools hosted at UCLA21. Of the 100 patients, 15 suffered graft loss during the follow up period and 1 patient died due to malignancy. The median (IQR) follow-up time for patients without complete follow up due to transfer or care or other reason was 40.5 (29–48) months. Sixty-one patients had surviving allografts and complete follow up at 5 years. Additional allograft loss data for patients without complete clinical follow up to 5 years was collected based on dates of transplant failure recorded in the transplant database. Patients were categorized as “antibody positive” if either AT1R-Ab or ETAR-Ab were positive on a study blood sample. Patients were considered to have preformed antibody if the pre-transplant sample was positive, and they were also positive on a post-transplant sample. Patients were considered to have de novo antibody to AT1R-Ab or ETAR-Ab if the pre-transplant sample was negative and at least one post-transplant sample was positive.
Figure 1: Study Samples and Clinical Data.
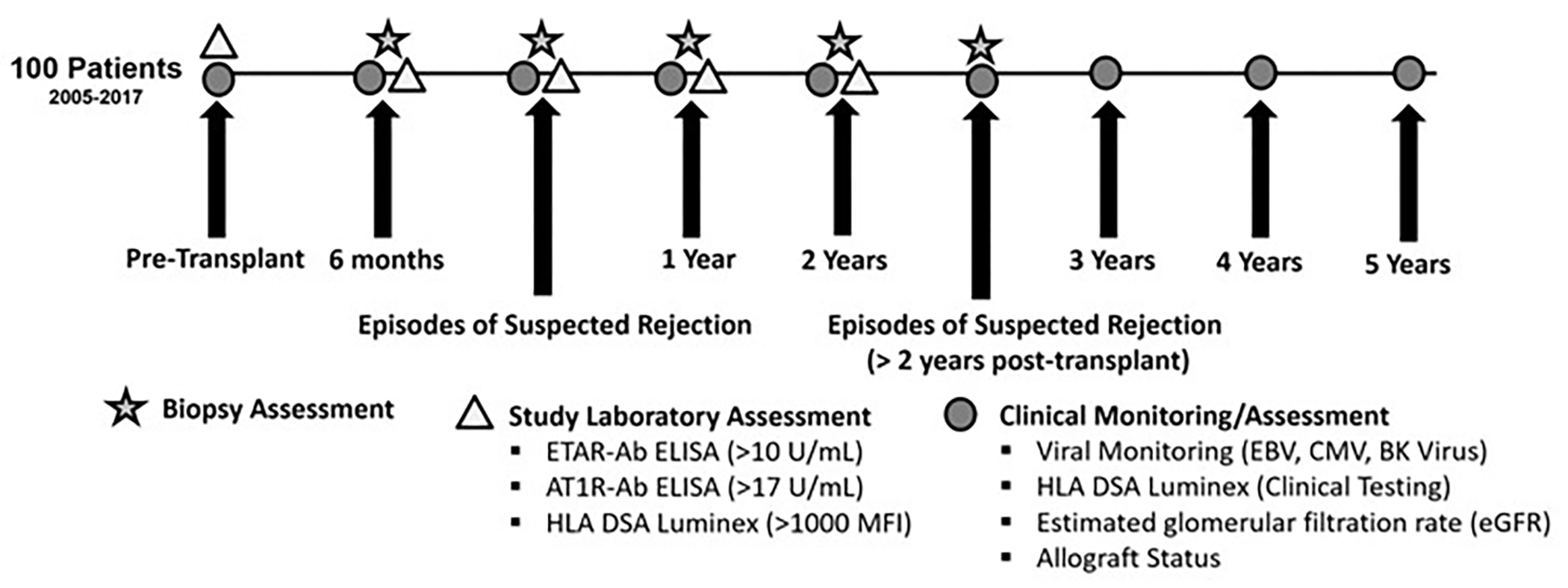
AT1R-Ab and ETAR-Ab were measured in the first 2 years post-transplant at protocolized time points and at time of biopsies for clinical indication. Protocol biopsies were also completed at 6 months, 1 year, and 2 years post-transplant. Clinical data including eGFR and HLA DSA were assessed through 5 years post-transplant yearly and at times of biopsy done for clinical indication.
Clinical Protocols and Biopsy Evaluation
UCLA immunosuppressive regimen for pediatric transplant recipients included induction with either ATG for PRA > 30%, delayed graft function, or rapid-steroid withdrawal protocol or anti-CD25 monoclonal antibody for those with PRA<30%. Maintenance immunosuppression consisted of steroid-free or steroid-based immunosuppression, a calcineurin inhibitor, and an anti-metabolite. Acute and chronic rejection were treated with previously described protocols.22,23
Patients underwent protocol biopsies at 6, 12, and 24 months post-transplantation or for clinical indication (per clinical practice at our center for the duration of the study period). Biopsy data were evaluated based on clinical reports. Patients classified as having AMR and ACR 1a or higher met Banff 2019 criteria for AMR and ACR respectively.24 Patients with borderline ACR were considered positive for rejection in our analysis and were classified as having borderline ACR based on clinical pathology diagnosis at time of biopsy. Diagnoses of borderline ACR were included in our rejection classification because our protocol was to treat these patients with pulse steroids throughout the duration of the study period. Reports that did not include Banff scoring in the clinical report (this included biopsies from prior to 2010 and any additional cases with missing data) or that had an unclear clinical diagnosis were re-reviewed by a pathologist and scored for our analysis. Biopsy findings including interstitial fibrosis and tubular atrophy (IF/TA), acute glomerulitis (g), acute tubulitis (t), acute interstitial inflammation (i), acute intimal arteritis (v), acute peritubular capillaritis (aptc), chronic glomerulopathy (cg), mesangial matrix expansion (mm), tubular atrophy (ct), interstitial fibrosis (ci), arterial fibrous intimal thickening (cv), arteriolar hyalinosis thickening (ah) were assessed. Scores > 0 were considered positive for a given finding. The highest score over time for each patient was used for analysis. One antibody negative and 3 antibody positive patients were missing biopsy scores due to biopsies being performed at outside institutions and were excluded from those analyses.
Antibody Testing
HLA typing of recipient and donor was performed using molecular methods as previously described.22 For patients transplanted prior to 2012, HLA antibodies in the first 2 years post-transplantation were detected using a Luminex single antigen bead (SAB) assay (Immucor, Norcross, GA) on study samples due to lack of protocolized clinical testing. For patients transplanted after 2012, HLA antibodies in the first 2 years post-transplantation were assessed using Luminex single antigen bead (SAB) assay (One Lambda, Canoga Park, CA) and were completed as part of clinical testing. All HLA antibody testing after 2 years post-transplantation for the entire cohort was also completed using Luminex single antigen bead (SAB) assay (One Lambda, Canoga Park, CA) that was completed as part of clinical testing. Clinical samples after 2 years post-transplantation were assessed yearly as part of our clinical protocols and/or for clinical indication. HLA antibodies were quantified by mean fluorescence intensity (MFI). Antibodies were considered positive when MFI was ≥1000 for HLA-A, -B, -DR, -DQ, and ≥2000 for HLA-C and –DP.25
AT1R -Ab and ETAR-Ab were measured by enzyme-linked immunosorbent based assay. Study samples were aliquoted within 8 hours from the time the sample was drawn and then frozen and stored at −80C in the UCLA Immunogenetics Center (UIC). Samples were thawed only once, at the time of testing, to preserve the integrity of the IgG and tested in batches for AT1R-Ab and ETAR-Ab. ELISA tests were conducted rigorously based on the manufacturer’s instructions. Sera were diluted 1:100, tested in duplicate and AT1R-Ab and ETAR-Ab concentrations were determined by a standard curve. AT1R-Ab IgG >17 U/ml and ETAR-Ab > 10 U/mL was considered positive based on other studies,26 recommendations from the test manufacturer, and our previous receiver operating curve analyses.13,18 We did not find any trend in our data to suggest that the age of the sample had an impact on the results.
Statistical Methods
Patients who were ever AT1R-Ab and/or ETAR-Ab positive on any blood sample were compared to patients who were antibody negative on all samples. Categorical variables were compared between groups using chi-square and Fisher’s exact test. Continuous variables were compared between groups using either the Kruskal-Wallis test or one-way ANOVA based on the distribution of the data. Mixed-effect linear regression was used to estimate the effect of AT1R-Ab and/or ETAR-Ab positivity on change in eGFR over time, and logistic regression was used to evaluate the association between antibody positivity and development of HLA DSA by 5 years post-transplant. The odds ratios and regression coefficients are reported, along with 95% confidence intervals. A Cox proportional-hazards regression model was used to compare time to development of HLA DSA up to 5 years post-transplant in patients still at risk at the end of study sample collection (26.5 months). A p-value below 0.05 was considered statistically significant and all tests were two-sided. Analyses were completed in Stata/SE 15.1 and R v4.1.0.
RESULTS
Fifty-nine of 100 patients were positive for AT1R-Ab and/or ETAR-Ab in the first 2 years post-transplant. Of those patients, 41/59 (69%) had both AT1R-Ab and ETAR-Ab, 15/59 (25%) had AT1R-ab alone and 3/59 (5%) had ETAR-Ab alone. The majority of antibody positive patients, 56/59 (95%) were AT1R-Ab positive. The median levels and interquartile ranges (IQR) for AT1R-Ab and ETAR-Ab in all samples in the entire cohort were 14.9 (9.7–22.4) and 6.6 (3.3–15) units/mL respectively. Clinical and demographic characteristics of patients by AT1R-Ab and/or ETAR-Ab status are shown in Table 1. Patients with an available pre-transplant sample (n=85) were categorized as either having preformed (positive both pre- and post-transplant) or de novo (negative pre-transplant and positive post-transplant) antibodies. Younger age was associated with antibody positivity. Deceased donor transplantation was associated with de novo AT1R-Ab and/or ETAR-Ab (Table 1). Five patients received angiotensin receptor blockers (ARBs) during the study period, all in the AT1R-Ab and/or ETAR-Ab positive group (Table 1).
Table 1. Demographic and Clinical Factors by AT1R-Ab and/or ETAR-Ab Status.
Patients were considered AT1R-Ab and/or ETAR-Ab positive if they were positive for AT1R-Ab and/or ETAR-Ab in the first 2 years post-transplant. The entire cohort (n=100) is shown on the left. On the right, patients with a pre-transplant sample available (n=85) are compared by timing of AT1R-Ab and/or ETAR-Ab development.
| AT1R-Ab and ETAR-Ab Negative (N=41) |
AT1R-Ab and/or ETAR-Ab Positive (N=59) |
P-value | AT1R-Ab and ETAR-Ab Negative (N=35) |
De Novo AT1R-Ab and/or ETAR-Ab (N=22) | Preformed AT1R-Ab and/or ETAR-Ab (N=28) |
P-value | |
|---|---|---|---|---|---|---|---|
| Age, Mean (SD) | 15.1 (4.8) | 12.9 (5.0) | 0.034 | 15.7 (4.4) | 14.5 (4.6) | 12.1 (4.8) | 0.011 |
| Race | |||||||
| White | 25 (61.0%) | 37 (62.7%) | 0.415 | 21 (60.0%) | 12 (54.5%) | 17 (60.7%) | 0.707 |
| Black | 2 (4.9%) | 8 (13.6%) | 2 (5.7%) | 4 (18.2%) | 4 (14.3%) | ||
| Asian | 3 (7.3%) | 3 (5.1%) | 2 (5.7%) | 1 (4.5%) | 0 (0.0%) | ||
| Other | 11 (26.8%) | 11 (18.6%) | 10 (28.6%) | 5 (22.7%) | 7 (25.0%) | ||
| Ethnicity, Hispanic or Latino | 25 (61.0%) | 33 (55.9%) | 0.767 | 22 (62.9%) | 13 (59.1%) | 17 (60.7%) | 0.959 |
| Sex, Male | 21 (51.2%) | 36 (61.0%) | 0.442 | 16 (45.7%) | 11 (50.0%) | 20 (71.4%) | 0.106 |
| Etiology of ESRD, Glomerular (vs. non-glomerular) | 6 (14.6%) | 9 (15.3%) | 1.000 | 5 (14.3%) | 5 (22.7%) | 3 (10.7%) | 0.435 |
| Dialysis Prior to Transplant | 31 (79.5%) | 49 (89.1%) | 0.320 | 27 (81.8%) | 21 (95.5%) | 22 (88.0%) | 0.394 |
| Years on Dialysis Prior to Transplant, Mean (SD) | 2.3 (1.7) | 2.4 (1.6) | 0.783 | 2.4 (1.7) | 2.4 (1.6) | 2.4 (1.6) | 0.988 |
| Type of Transplant, Deceased Donor | 21 (51.2%) | 41 (69.5%) | 0.101 | 16 (45.7%) | 18 (81.8%) | 19 (67.9%) | 0.018 |
| Delayed Graft Function | 1 (2.4%) | 4 (6.8%) | 0.646 | 1 (2.9%) | 1 (4.5%) | 2 (7.1%) | 0.817 |
| Cold ischemia time (hours), Mean (SD) | 7.7 (7.6) | 10.1 (8.3) | 0.148 | 7.2 (7.6) | 10.3 (6.8) | 11.0 (9.7) | 0.159 |
| HLA Mismatch (out of 8*), Mean (SD) | 5.1 (1.7) | 5.1 (2.0) | 0.968 | 5.0 (1.7) | 5.3 (2.2) | 5.1 (1.7) | 0.666 |
| Induction Medication, ATG (vs. IL-2 inhibitor) | 3 (7.3%) | 7 (11.9%) | 0.520 | 3 (8.6%) | 2 (9.1%) | 2 (7.1%) | 1.000 |
| Primary Transplant | 40 (97.6%) | 54 (91.5%) | 0.396 | 34 (97.1%) | 19 (86.4%) | 26 (92.9%) | 0.321 |
| Steroid Based Maintenance Immunosuppression | 28 (68.3%) | 36 (61.0%) | 0.594 | 23 (65.7%) | 16 (72.7%) | 17 (60.7%) | 0.673 |
| Angiotensin Receptor Blocker | 0 (0.0%) | 5 (8.5%) | 0.076 | 0 (0.0%) | 2 (9.1%) | 3 (10.7%) | 0.104 |
Eight patients had allograft loss in the first 2 years post-transplant; 6 were from rejection, 1 was from disease recurrence and 1 was from thrombosis. De novo AT1R-Ab and/or ETAR-Ab was associated with allograft loss in the first 2 years post-transplant (p=0.018, Table 2). An additional 7 patients lost their allografts between 2–5 years post-transplant; 6 from rejection and 1 from disease recurrence. There was no association between AT1R-Ab and/or ETAR-Ab and allograft loss at 5 years post-transplant. Any rejection (including borderline ACR) and AMR were more common in the antibody positive group in the first 2 years post-transplantation, but this relationship was not statistically significant (p=0.066 and 0.075 respectively, Table 2). There was no association between any rejection or AMR in the first 5 years post-transplant and antibody positivity. Biopsy findings/scores over 5 years post-transplant were compared by antibody status (Table S1). De novo AT1R-Ab and/or ETAR-Ab was associated with glomerulitis (p=0.025). All cases of intimal arteritis occurred in antibody positive patients, but this did not meet statistical significance (p=0.073, Table S1).
Table 2. Two-and Five-Year Clinical Outcomes by AT1R-Ab and/or ETAR-Ab Status.
De novo AT1R-Ab and/or ETAR-Ab was associated with allograft loss in the first 2 years post-transplant. There was no association with AT1R-Ab and/or ETAR-Ab positivity and any rejection (including borderline acute cellular rejection), AMR, or development of HLA DSA
| AT1R-Ab and ETAR-Ab Negative (N=41) |
AT1R-Ab and/or ETAR-Ab Positive (N=59) |
P-value | AT1R-Ab and ETAR-Ab Negative (N=35) |
De Novo AT1R-Ab and/or ETAR-Ab (N=22) | Preformed AT1R-Ab and/or ETAR-Ab (N=28) |
P-value | |
|---|---|---|---|---|---|---|---|
| 2 Year Outcomes | |||||||
| Rejection | 12 (30.0%) | 29 (50.9%) | 0.066 | 10 (29.4%) | 9 (42.9%) | 16 (57.1%) | 0.089 |
| AMR | 0 (0.0%) | 5 (8.8%) | 0.075 | 0 (0.0%) | 2 (9.5%) | 3 (10.7%) | 0.083 |
| Allograft Loss | 1 (2.4%) | 7 (11.9%) | 0.136 | 1 (2.9%) | 4 (18.2%) | 0 (0.0%) | 0.018 |
| HLA DSA | 8 (19.5%) | 19 (32.2%) | 0.239 | 7 (20.0%) | 7 (31.8%) | 10 (35.7%) | 0.353 |
| 5 Year Outcomes | |||||||
| Rejection | 16 (40.0%) | 33 (57.9%) | 0.126 | 13 (38.2%) | 12 (57.1%) | 17 (60.7%) | 0.167 |
| AMR | 2 (5.0%) | 9 (15.8%) | 0.117 | 2 (5.9%) | 4 (19.0%) | 5 (17.9%) | 0.205 |
| Allograft Loss | 4 (9.8%) | 11 (18.6%) | 0.347 | 4 (11.4%) | 5 (22.7%) | 3 (10.7%) | 0.455 |
| HLA DSA | 11 (26.8%) | 27 (45.8%) | 0.087 | 10 (28.6%) | 12 (54.5%) | 13 (46.4%) | 0.120 |
AMR; antibody mediated rejection, HLA DSA; human leukocyte antigen donor specific antibody
Change in eGFR over time was evaluated by antibody status at both 2 and 5 years post-transplant. Models were adjusted for sex, induction regimen, steroid free vs. steroid based immunosuppression, and number of HLA mismatches. Antibody positivity was associated with decline in eGFR in the first 2 years post-transplantation (slope coefficient, CI of −0.58 (−0.96, −0.21) per month, p=0.002). When broken down by preformed and de novo antibody, de novo antibody positivity remained significantly associated with decline in eGFR over the first 2 years post-transplantation (slope coefficient, CI of −0.51( −0.99, −0.04) per month, p=0.035). However, on extended follow up, neither overall antibody positivity nor de novo antibody development was associated with reduction in eGFR over time at 5 years post-transplantation (Figure 2). Younger age was also significantly associated with greater declines in eGFR slope in all models (Table S2).
Figure 2: EGFR over time by AT1R-Ab and/or ETAR-Ab Status.
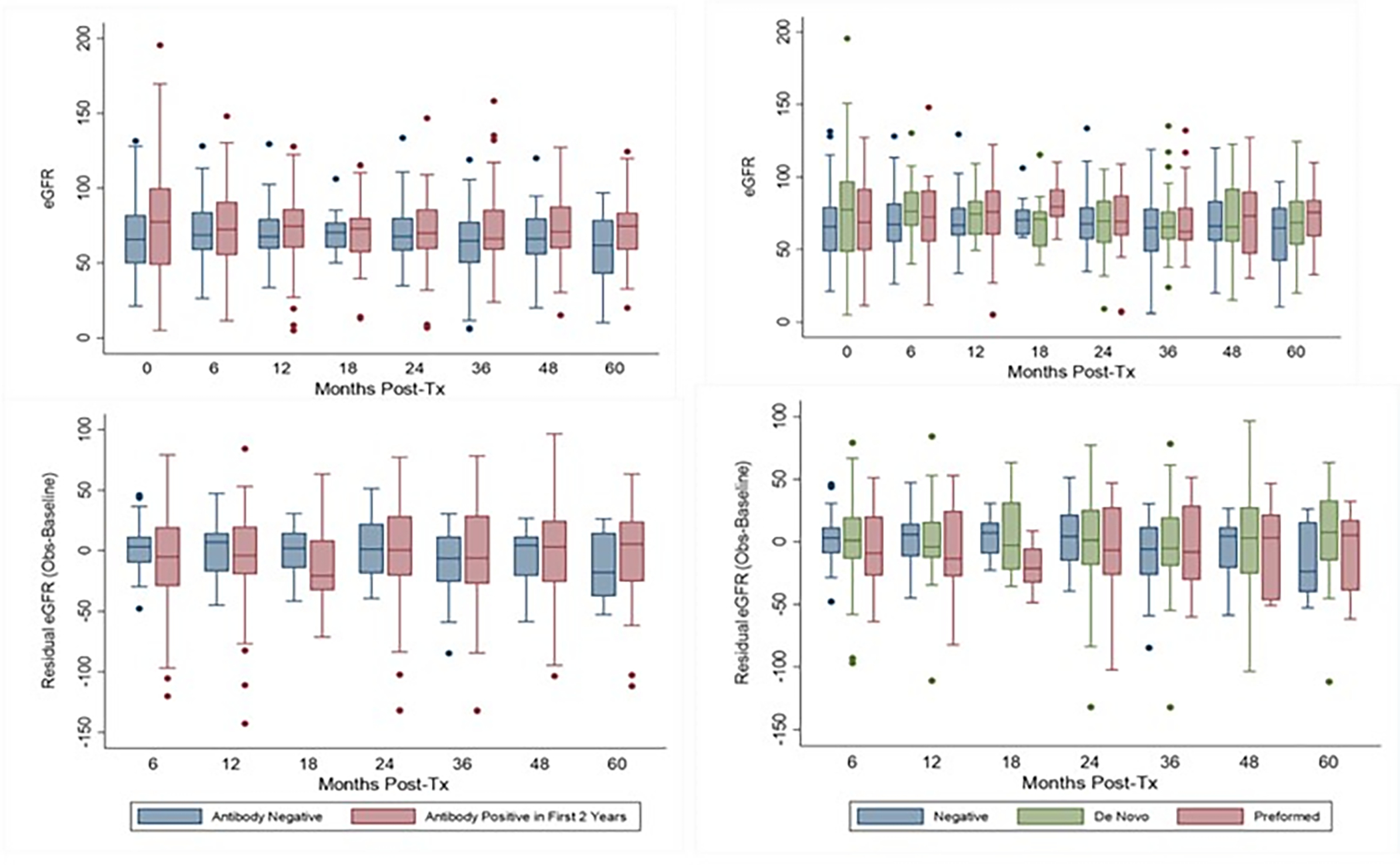
The upper panels showing median (IQR) eGFRs by antibody group. Lower panels show residual eGFR (observed eGFR at the given time point minus the “baseline” eGFR at hospital discharge) to illustrate change over time. AT1R-Ab and/or ETAR-Ab positive patients, had greater residual eGFR declines in time points prior to 24 months post-transplant compared to antibody negative patients. However, these changes stabilized in time points after 24 months post-transplant and there was significant variability in the data.
Out of 27 patients with both HLA DSA and AT1R-Ab and/or ETAR-Ab, 19 (70%) developed AT1R-Ab/ETAR-Ab before HLA DSA, 2 (7%) developed HLA DSA before AT1R-Ab/ETAR-Ab, and 6 (22%) developed HLA DSA and AT1R-Ab/ETAR-Ab simultaneously. There was a borderline association between AT1R-Ab/ETAR-Ab positivity and the development of HLA DSA in the first 5 years post-transplantation (p=0.087, Table 2) on unadjusted analysis. In a model (Table 3) adjusted for age, sex, steroid free vs. steroid based immunosuppression, and HLA mismatch (out of 8), AT1R-Ab and/or ETAR-Ab positivity was significantly associated with development of HLA DSA at 5 years post-transplant (OR 2.87, p=0.034). As expected, HLA Mismatch was also associated with HLA DSA in this model (OR 1.31, p=0.033). Interestingly, this relationship appeared not to be impacted by pre-formed vs. de novo AT1R-Ab/ETAR-Ab (Table 3). We found no interaction effect between HLA DSA and AT1R-Ab and/or ETAR-Ab positivity on likelihood of allograft loss or change in eGFR (data not shown). On survival analysis of patients who had functioning grafts and remained HLA DSA negative at 2 years post-transplant, patients who were AT1R-Ab or ETAR-Ab positive in the first 2 years post-transplant had greater risk of developing HLA DSA between 2–5 years post-transplant, but this result was not statistically significant (HR 3.28 (95% CI 0.64–16.82), p=0.155, Figure S1)
Table 3. Odds of developing HLA DSA by 5 Years Post-Transplant.
AT1R-Ab and/or ETAR-Ab positivity in the first 2 years post-transplant was associated with development of HLA DSA in the first 5 years post-transplant. This relationship did not appear to be specific for preformed or de novo AT1R-Ab and/or ETAR-Ab.
| Predictor | Odds Ratio (OR) | 95% Confidence Interval (CI) Lower | 95% Confidence Interval (CI) Upper | P-value |
|---|---|---|---|---|
| Model 1: | ||||
| Ever AT1R-Ab and/or ETAR-Ab Positive | 2.87 | 1.08 | 7.61 | 0.034 |
| Age (+1 year) | 1.05 | 0.96 | 1.16 | 0.269 |
| Female | 1.28 | 0.50 | 3.26 | 0.61 |
| Steroids at Baseline | 1.08 | 0.42 | 2.78 | 0.872 |
| HLA Mismatch (out of 8) | 1.31 | 1.02 | 1.68 | 0.033 |
| Model 2: | ||||
| De Novo AT1R-Ab and/or ETAR-Ab * | 2.75 | 0.81 | 9.27 | 0.104 |
| Preformed AT1R-Ab and/or ETAR-Ab * | 2.44 | 0.74 | 8.01 | 0.143 |
| Age (+1 year) | 1.02 | 0.92 | 1.14 | 0.701 |
| Female | 1.55 | 0.57 | 4.18 | 0.391 |
| Steroids at Baseline | 0.84 | 0.31 | 2.30 | 0.74 |
| HLA Mismatch (out of 8) | 1.25 | 0.96 | 1.64 | 0.100 |
Reference group: AT1R-Ab and ETAR-Ab Negative
DISCUSSION
On extended follow up of our expanded pediatric kidney transplant cohort, pediatric KTRs remained at high risk of AT1R-Ab and/or ETAR-Ab positivity in the first 2 years post-transplantation. AT1R-Ab and/or ETAR-Ab positivity was associated with allograft loss, decline in allograft function, and a trend towards increased risk of rejection in the first 2 years post-transplant, but these findings were not found upon extended follow up at 5 years. Interestingly, at 5 years post-transplant, there was an association between early AT1R-Ab and/or ETAR-Ab and the development of HLA DSA. We did not observe any interaction between HLA DSA, AT1R-Ab and/or ETAR-Ab positivity and either eGFR or allograft loss at 5 years post-transplantation.
After initially being described in HLA DSA negative patients with steroid resistant AMR and hypertension in the landmark publication by Dragun et.al, AT1R-Ab has been associated with poor kidney transplant outcomes in both adult4,5,7,10,19,27–30 and pediatric13,14,18,31 studies. Translational work in animal models and on cultured endothelial cells suggests AT1R-Ab and ETAR-Ab cause endothelial injury by hyperactivation of the AT1R and ETAR receptors leading to upregulation in extracellular-signal regulated kinase (ERK) and mechanistic target of rapamycin signaling (mTOR).3,11 Overall, this results in a pro-inflammatory and pro-coagulant environment and derangements in endothelial cell repair mechanisms that may preferentially impact the transplanted organ for reasons that are not currently well understood. Clinical studies examining the association between AT1R-Ab and clinical outcomes have been highlighted in a recent review32 and metanalysis.8 Most studies have focused on pre-transplant AT1R-Ab and short term allograft outcomes. Interestingly, one adult study found that patients with high pre-transplant AT1R-Ab had a higher risk of rejection in the first 4 months post-transplant, but higher risk of allograft loss only after 3 years post-transplant.10 The higher risk of rejection weighted towards the early post-transplant period is consistent with our data. Though the higher risk of allograft loss in this study was statistically significant after 3 years post-transplant, the separation in the survival curves primarily occurred after 6 years post-transplant. The differences in our findings, therefore, may be related to the differences in the follow-up period which was significantly longer in the adult study (mean 6.9 years, maximum 13.3 years) as well as sample size.
We found an increased risk of HLA DSA at 5 years post-transplant on analysis controlled for age, sex, steroid free vs. steroid based immunosuppression, and HLA mismatch in patients with AT1R-Ab and/or ETAR-Ab in the first 2 years post-transplant. This is consistent with other studies that have found a relationship between AT1R-Ab and the development of HLA DSAs.4,28,33,34 The mechanism of this association is not yet understood. It is possible that increased HLA expression in the context of endothelial cell inflammation provoked by AT1R and ETAR activation predisposes to the formation of HLA DSA, however, this is an area in need of additional study. There is also evidence that HLA DSA and AT1R-Ab may act synergistically in allograft injury.4,5,19,35 De novo HLA DSA is a well known risk factor for shortened allograft survival, but requires longer follow up periods to fully assess. Though we did not find an increased risk of allograft loss on 5 year follow-up in patients with AT1R-Ab/ETAR-Ab alone or with concomitant HLA DSA, the association of AT1R-Ab/ETAR-Ab with the development of de novo HLA DSA in our cohort suggests allograft loss risk may be increased in these patients after the 5 year time point.
The relationship between AT1R-Ab and/or ETAR-Ab and clinical outcomes may be significantly impacted by concurrent factors including time post-transplantation. It is worth emphasizing that our findings do not support the idea that later development of AT1R-Ab or ETAR-Ab is benign. Fichtner et al have reported that pediatric kidney transplant patients with AT1R-Ab or ETAR-Ab at time of indication biopsies (completed an average of 53.5 months post-transplant) had significantly higher odds of allograft loss and renal dysfunction in the subsequent 5 years.14,31 Our data suggest that in patients with preformed or early post-transplant AT1R-Ab or ETAR-Ab on screening over the first 2 years post-transplant, the risk of associated allograft dysfunction may be higher in the early post-transplant period. The differences in study design yield distinct and complementary information. In the case of our study, factors that may be involved in AT1R-Ab or ETAR-Ab potentiating and/or propagating early allograft injury include ischemia at the time of surgery and or high calcineurin inhibitor levels early post-transplantation.36 These interactions, however, require further investigation.
Our study unfortunately could not evaluate the non-HLA antibody status of patients after the 2 year time point. This is a significant weakness in evaluating risk for ongoing allograft injury. Follow up was also variable due to patients transferring or aging out of our health system, which resulted in missing data. We did not have enough patients who received ARBs to evaluate the impact of this medication on our findings. Strengths of our study include the availability of protocolized blood and biopsy samples in the first 2 years, relatively large size for a pediatric cohort, and comprehensive biopsy scoring. More detailed information regarding both donor and recipient AT1R-Ab genotype may be of significant interest.37 This was not available as part of this study, but would be an intriguing area for future investigation. Our study along with others highlights the gap between the clinical data and our understanding of how and why AT1R-Ab and ETAR-Ab may be detrimental in some clinical scenarios and not in others. To move the field forward, understanding factors that determine the impact of AT1R-Ab and ETAR-Ab on the allograft in vivo by in depth examination of biopsy tissue will inform a more nuanced understanding of this clinical conundrum.
Pediatric KTRs with AT1R-Ab and/or ETAR-Ab positivity in the first 2 years post-transplantation, had higher risk of eGFR decline and allograft loss in the first 2 years post-transplantation that did not extend to 5 years post-transplantation. Antibody positivity, however, was a risk factor for development of HLA DSA at 5 years post-transplantation. Given the frequency of AT1R-Ab and/or ETAR-Ab positivity in our pediatric patients and the significant implications of immune complications on future transplants, strategies for evaluating and treating these antibodies are needed. A randomized control trial assessing the impact of early treatment with AT1R and/or ETAR blockade will be central in answering this open question.
Supplementary Material
Acknowledgments:
The authors would like to thank Katherine Noche for her data collection work on this project.
Funding:
This study was supported by the National Institute of Allergy and Infectious Diseases Grant 5K23AI139335 (M.H.P); Ruth L. Kirschstein National Research Service Award T32 DK104687 UCLA Translational Research Grant in Pediatric Nephrology Program (M.H.P); the National Kidney Foundation (M.H.P); the American Society of Nephrology (M.H.P); the Casey Lee Ball Foundation (E.T.C and M.H.P); the National Institute of Allergy and Infectious Diseases Grant R01AI135201, NIH PO1 AI120944; 5U19AI128913; R21AI56592 (E.F.R.); and by the National Center for Advancing Translational Sciences UL1 TR000124
Abbreviations:
- ACR
acute cellular rejection
- AMR
antibody-mediated rejection
- AT1R
angiotensin II type 1 receptor
- ATG
anti-thymocyte globulin
- DSA
donor specific antibody
- eGFR
estimated glomerular filtration rate
- ETAR
endothelin-type A receptor
- HLA
human leukocyte antigen
- IQR
interquartile range
- KTRs
kidney transplant recipients
- MFI
mean fluorescence intensity
Footnotes
Disclosure: The authors declare no conflict of interest.
References
- 1.Gaston RS, Cecka JM, Kasiske BL, et al. Evidence for antibody-mediated injury as a major determinant of late kidney allograft failure. Transplantation. Jul 15 2010;90(1):68–74. doi: 10.1097/TP.0b013e3181e065de [DOI] [PubMed] [Google Scholar]
- 2.Chua A, Cramer C, Moudgil A, et al. Kidney transplant practice patterns and outcome benchmarks over 30 years: The 2018 report of the NAPRTCS. Pediatr Transplant. Dec 2019;23(8):e13597. doi: 10.1111/petr.13597 [DOI] [PubMed] [Google Scholar]
- 3.Dragun D, Muller DN, Brasen JH, et al. Angiotensin II type 1-receptor activating antibodies in renal-allograft rejection. N Engl J Med. Feb 10 2005;352(6):558–69. doi: 10.1056/NEJMoa035717 [DOI] [PubMed] [Google Scholar]
- 4.Taniguchi M, Rebellato LM, Cai J, et al. Higher risk of kidney graft failure in the presence of anti-angiotensin II type-1 receptor antibodies. Am J Transplant. Oct 2013;13(10):2577–89. doi: 10.1111/ajt.12395 [DOI] [PubMed] [Google Scholar]
- 5.Lefaucheur C, Viglietti D, Bouatou Y, et al. Non-HLA agonistic anti-angiotensin II type 1 receptor antibodies induce a distinctive phenotype of antibody-mediated rejection in kidney transplant recipients. Kidney Int. Jul 2019;96(1):189–201. doi: 10.1016/j.kint.2019.01.030 [DOI] [PubMed] [Google Scholar]
- 6.Philogene MC, Bagnasco S, Kraus ES, et al. Anti-Angiotensin II Type 1 Receptor and Anti-Endothelial Cell Antibodies: A Cross-Sectional Analysis of Pathological Findings in Allograft Biopsies. Transplantation. May 24 2016;doi: 10.1097/TP.0000000000001231 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lee J, Huh KH, Park Y, et al. The clinicopathological relevance of pretransplant anti-angiotensin II type 1 receptor antibodies in renal transplantation. Nephrol Dial Transplant. Nov 5 2015;doi: 10.1093/ndt/gfv375 [DOI] [PubMed] [Google Scholar]
- 8.Kang ZY, Liu C, Liu W, et al. Effect of anti-angiotensin II type 1 receptor antibodies on the outcomes of kidney transplantation: a systematic review and meta-analysis. Nephrol Dial Transplant. May 25 2022;37(6):1171–1180. doi: 10.1093/ndt/gfab344 [DOI] [PubMed] [Google Scholar]
- 9.Malheiro J, Tafulo S, Dias L, et al. Deleterious Effect of Anti-Angiotensin II Type 1 Receptor Antibodies Detected Pretransplant on Kidney Graft Outcomes is Both Proper and Synergistic with Donor-Specific Anti-HLA Antibodies. Nephrology (Carlton). Feb 2018;doi: 10.1111/nep.13239 [DOI] [PubMed] [Google Scholar]
- 10.Giral M, Foucher Y, Dufay A, et al. Pretransplant sensitization against angiotensin II type 1 receptor is a risk factor for acute rejection and graft loss. Am J Transplant. Oct 2013;13(10):2567–76. doi: 10.1111/ajt.12397 [DOI] [PubMed] [Google Scholar]
- 11.Catar RA, Wischnewski O, Chen L, et al. Non-HLA antibodies targeting angiotensin II Type 1 receptor and endothelin-1 Type A receptors induce endothelial injury via β2-arrestin link to mTOR pathway. Kidney Int. Mar 2022;101(3):498–509. doi: 10.1016/j.kint.2021.09.029 [DOI] [PubMed] [Google Scholar]
- 12.Louis K, Loupy A, Lefaucheur C. mTOR signaling cascade: novel clinical implications in HLA and non-HLA antibody-mediated vasculopathies? Kidney Int. Mar 2022;101(3):451–454. doi: 10.1016/j.kint.2021.12.008 [DOI] [PubMed] [Google Scholar]
- 13.Pearl MH, Zhang Q, Palma Diaz MF, et al. Angiotensin II Type 1 receptor antibodies are associated with inflammatory cytokines and poor clinical outcomes in pediatric kidney transplantation. Kidney Int. Jan 2018;93(1):260–269. doi: 10.1016/j.kint.2017.06.034 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fichtner A, Süsal C, Schröder C, et al. Association of angiotensin II type 1 receptor antibodies with graft histology, function and survival in paediatric renal transplant recipients. Nephrol Dial Transplant. Feb 2018;doi: 10.1093/ndt/gfy008 [DOI] [PubMed] [Google Scholar]
- 15.Bjerre A, Tangeraas T, Heidecke H, et al. Angiotensin II type 1 receptor antibodies in childhood kidney transplantation. Pediatr Transplant. Jun 1 2016;doi: 10.1111/petr.12728 [DOI] [PubMed] [Google Scholar]
- 16.Hesemann LE, Subramanian V, Mohanakumar T, et al. De novo development of antibodies to kidney-associated self-antigens angiotensin II receptor type I, collagen IV, and fibronectin occurs at early time points after kidney transplantation in children. Pediatr Transplant. Aug 2015;19(5):499–503. doi: 10.1111/petr.12531 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Pearl MH, Grotts J, Rossetti M, et al. Cytokine Profiles Associated With Angiotensin II Type 1 Receptor Antibodies. Kidney Int Rep. Apr 2019;4(4):541–550. doi: 10.1016/j.ekir.2018.12.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Pearl MH, Chen L, ElChaki R, et al. Endothelin Type A Receptor Antibodies Are Associated With Angiotensin II Type 1 Receptor Antibodies, Vascular Inflammation, and Decline in Renal Function in Pediatric Kidney Transplantation. Kidney Int Rep. Nov 2020;5(11):1925–1936. doi: 10.1016/j.ekir.2020.09.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Malheiro J, Tafulo S, Dias L, et al. Deleterious effect of anti-angiotensin II type 1 receptor antibodies detected pretransplant on kidney graft outcomes is both proper and synergistic with donor-specific anti-HLA antibodies. Nephrology (Carlton). Mar 2019;24(3):347–356. doi: 10.1111/nep.13239 [DOI] [PubMed] [Google Scholar]
- 20.Pierce CB, Muñoz A, Ng DK, et al. Age- and sex-dependent clinical equations to estimate glomerular filtration rates in children and young adults with chronic kidney disease. Kidney Int. 04 2021;99(4):948–956. doi: 10.1016/j.kint.2020.10.047 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Harris PA, Taylor R, Thielke R, et al. Research electronic data capture (REDCap)--a metadata-driven methodology and workflow process for providing translational research informatics support. J Biomed Inform. Apr 2009;42(2):377–81. doi: 10.1016/j.jbi.2008.08.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Pearl MH, Nayak AB, Ettenger RB, et al. Bortezomib may stabilize pediatric renal transplant recipients with antibody-mediated rejection. Pediatr Nephrol. Aug 2016;31(8):1341–8. doi: 10.1007/s00467-016-3319-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Pearl M, Weng PL, Chen L, et al. Long term tolerability and clinical outcomes associated with tocilizumab in the treatment of refractory antibody mediated rejection (AMR) in pediatric renal transplant recipients. Clin Transplant. 08 2022;36(8):e14734. doi: 10.1111/ctr.14734 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Haas M, Loupy A, Lefaucheur C, et al. The Banff 2017 Kidney Meeting Report: Revised diagnostic criteria for chronic active T cell-mediated rejection, antibody-mediated rejection, and prospects for integrative endpoints for next-generation clinical trials. Am J Transplant. Feb 2018;18(2):293–307. doi: 10.1111/ajt.14625 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Blumberg JM, Gritsch HA, Reed EF, et al. Kidney paired donation in the presence of donor-specific antibodies. Kidney international. Nov 2013;84(5):1009–16. doi: 10.1038/ki.2013.206 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Reinsmoen NL, Mirocha J, Ensor CR, et al. A 3-Center Study Reveals New Insights Into the Impact of Non-HLA Antibodies on Lung Transplantation Outcome. Transplantation. 06 2017;101(6):1215–1221. doi: 10.1097/TP.0000000000001389 [DOI] [PubMed] [Google Scholar]
- 27.Banasik M, Boratynska M, Koscielska-Kasprzak K, et al. The influence of non-HLA antibodies directed against angiotensin II type 1 receptor (AT1R) on early renal transplant outcomes. Transpl Int. Oct 2014;27(10):1029–38. doi: 10.1111/tri.12371 [DOI] [PubMed] [Google Scholar]
- 28.Gareau AJ, Wiebe C, Pochinco D, et al. Pre-transplant AT1R antibodies correlate with early allograft rejection. Transpl Immunol. 02 2018;46:29–35. doi: 10.1016/j.trim.2017.12.001 [DOI] [PubMed] [Google Scholar]
- 29.Kim D, Gimferrer I, Warner P, et al. Preformed Angiotensin II Type-1 Receptor Antibodies Are Associated With Rejection After Kidney Transplantation: A Single-Center, Cohort Study. Transplant Proc. Dec 2018;50(10):3467–3472. doi: 10.1016/j.transproceed.2018.05.022 [DOI] [PubMed] [Google Scholar]
- 30.Sorohan BM, Ismail G, Berechet A, et al. The early impact of preformed angiotensin II type 1 receptor antibodies on graft function in a low immunological risk cohort of kidney transplant recipients. Transpl Immunol. Jun 2021;66:101389. doi: 10.1016/j.trim.2021.101389 [DOI] [PubMed] [Google Scholar]
- 31.Fichtner A, Süsal C, Höcker B, et al. Association of non-HLA antibodies against endothelial targets and donor-specific HLA antibodies with antibody-mediated rejection and graft function in pediatric kidney transplant recipients. Pediatr Nephrol. Aug 2021;36(8):2473–2484. doi: 10.1007/s00467-021-04969-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lefaucheur C, Louis K, Philippe A, Loupy A, Coates PT. The emerging field of non-human leukocyte antigen antibodies in transplant medicine and beyond. Kidney Int. Oct 2021;100(4):787–798. doi: 10.1016/j.kint.2021.04.044 [DOI] [PubMed] [Google Scholar]
- 33.Cuevas E, Arreola-Guerra JM, Hernandez-Mendez EA, et al. Pretransplant angiotensin II type 1-receptor antibodies are a risk factor for earlier detection of de novo HLA donor-specific antibodies. Nephrol Dial Transplant. May 24 2016;doi: 10.1093/ndt/gfw204 [DOI] [PubMed] [Google Scholar]
- 34.Crespo M, Llinàs-Mallol L, Redondo-Pachón D, et al. Non-HLA Antibodies and Epitope Mismatches in Kidney Transplant Recipients With Histological Antibody-Mediated Rejection. Front Immunol. 2021;12:703457. doi: 10.3389/fimmu.2021.703457 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wiebe C, Gibson IW, Blydt-Hansen TD, et al. Evolution and clinical pathologic correlations of de novo donor-specific HLA antibody post kidney transplant. Am J Transplant. May 2012;12(5):1157–67. doi: 10.1111/j.1600-6143.2012.04013.x [DOI] [PubMed] [Google Scholar]
- 36.Zhang Q, Reed EF. The importance of non-HLA antibodies in transplantation. Nat Rev Nephrol. Aug 2016;12(8):484–95. doi: 10.1038/nrneph.2016.88 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Zhang Z, Menon MC, Zhang W, et al. Genome-wide non-HLA donor-recipient genetic differences influence renal allograft survival via early allograft fibrosis. Kidney Int. Sep 2020;98(3):758–768. doi: 10.1016/j.kint.2020.04.039 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


