Abstract
In nature, Legionella pneumophila replicates exclusively as an intracellular parasite of amoebae, but it also persists in the environment as a free-living microbe. Studies of how this opportunistic pathogen recognizes and responds to distinct extracellular and intracellular environments identified a link between the growth phase and expression of traits previously correlated with virulence. When cultured in broth, only post-exponential-phase L. pneumophila was sodium sensitive, cytotoxic, osmotically resistant, competent to evade macrophage lysosomes, infectious, and motile. Likewise, the L. pneumophila phenotype changed during growth in macrophages. During the intracellular replication period, this bacterium was sodium resistant and lacked flagella; concomitant with macrophage lysis, L. pneumophila became sodium sensitive and flagellated. Expression of the virulent phenotype was a response to starvation, since exponential-phase L. pneumophila became cytotoxic, sodium sensitive, and motile after incubation in broth from stationary-phase cultures, except when it was supplemented with amino acids. Together, these data indicate that while nutrients are plentiful, intracellular L. pneumophila organisms are dedicated to replication; when amino acids become limiting, the progeny express virulence factors to escape the spent host, to disperse and survive in the aquatic environment, and to reestablish a protected intracellular niche favorable for growth.
A hallmark of microbes is their remarkable capacity to alter cellular structure and metabolism in response to changes in the environment. As a pathogen of freshwater protozoa, Legionella pneumophila must adapt to survive in water as a free-living microbe and to replicate in amoebae. At least a subset of the traits which contribute to this microbe’s fitness in the environment also facilitate its growth in alveolar macrophages (7, 12), which can result in the severe pneumonia known as Legionnaires’ disease.
Expression of some L. pneumophila attributes has been correlated with growth conditions. For example, entry into macrophages and amoebae by coiling phagocytosis (18) is enhanced by prior growth in amoebae (9). In addition, there are marked morphological and biochemical differences between amoebae- and broth-grown L. pneumophila (1, 9, 30). Neither the environmental conditions nor the cellular machinery that regulates expression of L. pneumophila virulence traits has been identified.
For many microbes, the transition from the exponential to the post-exponential growth phase is marked by dramatic phenotypic changes (38). In nature, L. pneumophila replicates exclusively in intracellular vacuoles, where bacterial density increases and nutrient levels presumably decrease with time. The aim of the present study was to determine whether bacterial density or nutrient levels determine the phenotype of L. pneumophila. To this end, the effect of culture conditions on expression of six L. pneumophila traits, including five correlated previously with virulence (5, 17, 19, 20, 29), was examined. The results of this analysis suggest a model for how L. pneumophila alternately adapts to intracellular and extracellular environments.
MATERIALS AND METHODS
Macrophage cultures.
Mouse macrophages were derived from bone marrow exudate cells obtained from femurs of A/J mice (Jackson Laboratory) as described previously (33). For cytotoxicity and infectivity assays, 2.5 × 105 macrophages were cultured in 0.5 ml of RPMI medium containing 10% fetal bovine serum (RPMI-FBS; GIBCO/BRL) per well of 24-well culture dishes. For immunofluorescence localization of flagella, 105 macrophages were cultured in 0.5 ml of RPMI-FBS on 12-mm-diameter coverslips of no. 1 thickness (Fisher Scientific).
Bacterial cultures.
L. pneumophila Lp02, a virulent thymine auxotroph (2), was cultured in N-(2-acetamido)-2-aminoethanesulfonic acid (ACES; Sigma)-buffered yeast extract broth supplemented with 100 μg of thymidine per ml (AYET) at 37°C with agitation. The solid medium was ACES-buffered charcoal-yeast extract agar supplemented with 100 μg of thymidine per ml (CYET) (10, 11).
To test whether post-exponential-phase supernatants induced virulence, exponential-phase cells were collected by centrifugation for 5 min at 2,700 × g and then resuspended with supernatants obtained by centrifugation of post-exponential-phase cultures for 5 min at 9,400 × g. To test whether amino acid depletion induced virulence, cultures prepared in the manner just described were diluted with the equivalent of 1/10 of their volume with H2O, 100 mg of yeast extract per ml, or a cocktail of five amino acids judged most likely to be limiting (24) (6.5 mg of serine, 0.75 mg of tyrosine, 1.5 mg of asparagine, 0.94 mg of proline, and 0.75 mg of threonine per ml of H2O). After incubation with aeration at 37°C for various periods of time, the optical density at 600 nm (OD600), cytotoxicity, sodium sensitivity, and motility of each culture were determined as described below.
Sodium sensitivity.
To measure the sodium sensitivity of broth-grown L. pneumophila, AYET cultures grown to various ODs were diluted into H2O and then plated on CYET which did or did not contain 100 mM NaCl. To measure the sodium sensitivity of macrophage-grown L. pneumophila, pooled macrophage lysates and culture supernatants were collected as described previously (33) and then plated on CYET which did or did not contain 100 mM NaCl. The percentage of bacteria that were sodium resistant was calculated with the following formula: (CFU on CYET + 100 mM NaCl)/(CFU on CYET) × 100.
Cytotoxicity.
To measure L. pneumophila cytotoxicity, bacteria suspended in RPMI-FBS were added to cultured macrophages at various ratios. After incubation for 1 h at 37°C, the bacteria were washed from the monolayers, and then the macrophages were incubated with 0.5 ml of 10% (vol/vol) Alamar blue (AccuMed, Inc.) in RPMI-FBS for from 4 h to overnight to allow the viable macrophages to convert the Alamar blue to its reduced form. Because an L. pneumophila thymine auxotroph was used for this study, the potential contribution of intracellular replication to cytopathicity was eliminated by omitting exogenous thymidine (2). Therefore, this assay reports the fraction of macrophages killed by L. pneumophila during the 1-h infection period. The percentage of macrophages that were viable was calculated from triplicate supernatants by the following equation: (A570 − A600)infected/(A570 − A600)uninfected) × 100 (25).
Osmotic sensitivity.
To measure the osmotic sensitivity, aliquots of cultures grown in AYET to various ODs were transferred to AYET which did or did not contain 0.3 M KCl and then incubated for 1 h at 37°C. Next, cultures were diluted by a factor of 50 or more into H2O, agitated with a vortex mixer, and then plated on CYET to quantify colony formation. The percentage of bacteria that were osmotically resistant was calculated by the following formula: (CFU of KCl-treated samples)/(CFU of untreated samples) × 100.
Phagosome-lysosome fusion.
The ability of phagosomes harboring L. pneumophila to evade macrophage lysosomes was quantified by fluorescence microscopy 2 h after infection, using the endocytic probe Texas red-ovalbumin as described previously (34).
Infectivity.
To measure the entry and survival of L. pneumophila in macrophages, bacteria and macrophages were cocultured at a 1:1 ratio for 2 h at 37°C. Extracellular bacteria were killed by a 30-min treatment with 10 μg of gentamicin per ml of RPMI-FBS, and then intracellular CFU were quantified by lysing monolayers by trituration with phosphate-buffered saline (PBS) and plating duplicate aliquots on CYET. CFU added at 0 h was determined by diluting the infection medium into PBS and plating the diluted medium on CYET. The percentage of infectious bacteria was calculated from triplicate samples by the following equation: (gentamicin-resistant CFU at 2 h)/(CFU added at 0 h) × 100.
Motility.
Motility was scored qualitatively by examining wet mounts of L. pneumophila broth cultures by phase-contrast microscopy at a magnification of ×320. A culture was defined as motile if at least half of the bacteria in a field of at least 100 cells were judged to exhibit rapid, directed movement.
Flagellum production.
Flagellum production by intracellular L. pneumophila was analyzed by immunofluorescence microscopy. Macrophages cultured on coverslips were infected for 1 h, washed twice to remove the majority of extracellular bacteria, and then incubated in fresh medium for various periods of time. Duplicate samples were fixed and stained essentially as described previously (33), using the following reagents: supernatant containing a mouse anti-L. pneumophila flagellum monoclonal antibody (a gift from N. C. Engleberg) was diluted 1:100; Texas red-conjugated goat anti-mouse immunoglobulin G (Molecular Probes) was diluted 1:1,500; and bacteria were stained with 0.1 μg of the DNA stain 4,6-diamidino-2-phenylindole (DAPI; Sigma) per ml of PBS. Preparations were examined with a Zeiss Axioplan 2 microscope equipped with a 100× Plan-Neofluar objective lens with a numerical aperture of 1.3. L. pneumophila vacuoles which contained at least one flagellum were scored as positive; therefore, both flagellum production and stability are reported (see Fig. 2b). Because necrotic macrophages often detached and were washed from the coverslips during the immunostaining procedure, it is likely that the number of flagellin-positive vacuoles was underestimated in the samples analyzed 22 h postinfection. Kodak T-Max ASA 400 film negatives were converted to digital images with an Archimboldi Leafscan-45 film scanner; the size, contrast, and brightness of each image were optimized by using Adobe Photoshop software (Adobe Systems, Inc.).
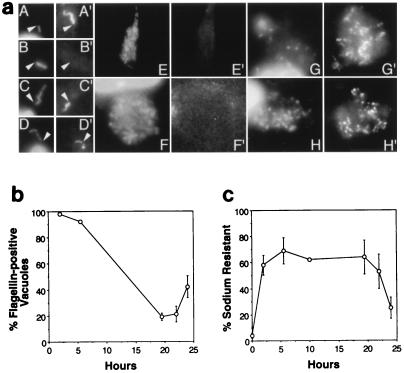
FIG. 2.
Characteristics of L. pneumophila cells growing in macrophages. (a) Representative images of macrophages at the initial (A and B), replicative (C to F), and necrotic stages (G and H) of L. pneumophila infection. After infection for 2 h (A), 6 h (C and D), 19.5 h (G), 22 h (F and H), and 24 h (E), bacteria were stained with DAPI (A to H) and flagella were labeled with a flagellum-specific mouse monoclonal antibody and a Texas red-conjugated anti-mouse immunoglobulin G secondary antibody (A′ to H′). Because initiation of intracellular replication was not synchronous, by 19.5 h postinfection, samples included both the replicative and necrotic stages of infection. Nonmotile bacteria obtained from cultures grown to an OD600 of <2 and incubated with macrophages for 2 h served as a negative control (B). Arrowheads indicate junctions between cells and flagella and serve as reference points. (b) Flagellum production by intracellular L. pneumophila was scored by immunofluorescence microscopic analysis of macrophages infected with motile and sodium-sensitive bacteria obtained from post-exponential-phase broth cultures. Because L. pneumophila vacuoles which contained one or more flagellum were scored as positive, the percentage of positive vacuoles reflects both flagellum production and stability. Because necrotic macrophages often detached and were washed from the coverslips during the immunostaining procedure, flagellin-positive vacuoles in the samples analyzed 22 h postinfection were likely underestimated. Shown are the means and standard errors determined by scoring at least 30 phagosomes at each time point in each of three experiments. (c) L. pneumophila sodium resistance was determined by plating dilutions of the infection medium (0 h) or pooled macrophage lysates and culture supernatants (2 to 24 h) on CYET medium which did or did not contain 100 mM NaCl. Shown are the means and standard errors of values from four to six experiments performed in triplicate.
RESULTS
To begin to understand how L. pneumophila adapts to distinct intracellular and extracellular environments, we examined six attributes of bacteria obtained from exponential- and post-exponential-phase broth cultures.
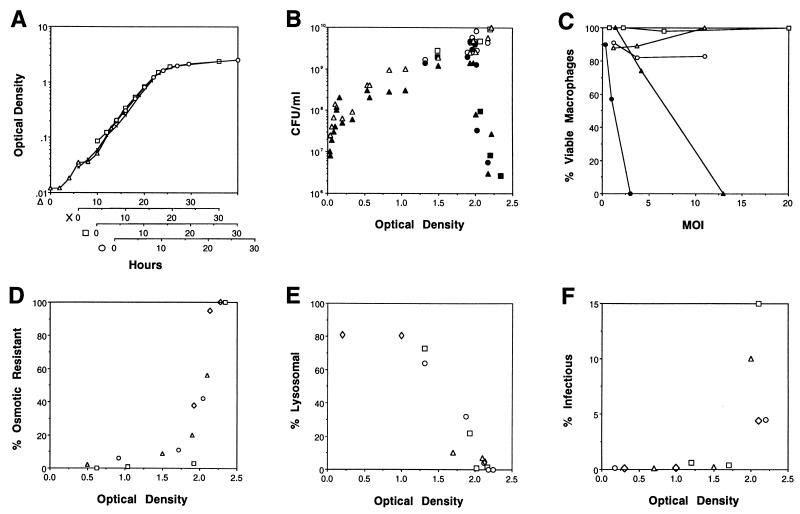
By some undefined mechanism, sodium inhibits the growth of virulent, but not avirulent, strains of L. pneumophila (5, 31, 36). To examine the effects of growth phase on sodium sensitivity, bacteria collected from cultures at different growth stages were plated on a microbiological medium which did or did not contain 100 mM NaCl, and colony formation was quantified. Exponential-phase L. pneumophila was resistant to sodium, a trait previously correlated genetically with avirulence (5, 31). However, the transition to post-exponential phase was marked by a rapid 1,000-fold increase in sodium sensitivity (Fig. 1A and B). Thus, expression of sodium sensitivity depended on the growth phase of L. pneumophila.
FIG. 1.
Characteristics of L. pneumophila exiting the exponential phase of growth. (A) Correspondence between the OD600 and the growth phase of L. pneumophila AYET broth cultures. An exponential-phase culture was diluted 1:350 (▵), 1:120 (×), 1:35 (□), or 1:16 (○) and incubated at 37°C for 15 h; then the OD600 of each subculture was determined at the times shown. Results from four representative cultures were superimposed such that their growth curves overlapped; similar results were obtained in several other experiments. (B) L. pneumophila sodium sensitivity was determined by plating cultures with the OD600s shown on CYET medium without (open symbols) and with (closed symbols) 100 mM NaCl. Three experiments performed in duplicate are represented by different symbols (triangles, circles, and squares). (C) L. pneumophila cytotoxicity was judged by the capacity of viable macrophages to reduce the colorimetric dye Alamar blue after a 1-h incubation with bacteria obtained from AYET cultures grown to OD600s of 0.374 (○), 1.519 (▵), 1.882 (□), 2.151 (•), and 2.231 (▴). The means of values for triplicate samples are shown; standard errors ranged from 1.5 to 8%. The multiplicity of infection (MOI) was calculated by plating the respective broth culture on CYET. The means of values for triplicate samples are shown; standard errors ranged from 2.5 to 14%. (D) Osmotic resistance of L. pneumophila cultures grown to the OD600s indicated was determined by incubation for 1 h in AYET that did or did not contain 0.3 M KCl, dilution into water, and then plating of duplicate samples on CYET to quantify the CFU. Results from each of four experiments are represented by different symbols (triangles, squares, circles, and diamonds). (E) The ability of L. pneumophila grown to the indicated OD600s to evade macrophage lysosomes was quantified by fluorescence microscopy, using the endocytic probe Texas red-ovalbumin. Data were obtained from four experiments, in which the number of phagosomes scored was 35 (◊), 75 (□), 50 (○), or 100 (▵). As a positive control, bacteria grown to an OD600 of >2 and then killed with formalin were analyzed; a mean of 67% (standard error = 13) of such particles colocalized with Texas red-ovalbumin. (F) The capacity of L. pneumophila grown to the OD600s indicated to enter and survive in macrophages was defined as protection from gentamicin added 2 h after infection. The mean percentage of infectious L. pneumophila was determined for duplicate or triplicate samples in four experiments, each represented by a different symbol.
Virulent strains of L. pneumophila kill eukaryotic cells by an undefined mechanism that is independent of intracellular bacterial replication; in contrast, at least some avirulent strains are not cytopathic (20). To determine whether growth phase affects expression of cytotoxicity by L. pneumophila, cultured macrophages were incubated for 1 h with bacteria obtained from cultures at different cell densities, and then macrophage viability was measured as the capacity to reduce the colorimetic dye Alamar blue. Post-exponential-phase L. pneumophila killed macrophages, while exponential-phase bacteria did not (Fig. 1C). However, unlike its sodium sensitivity, L. pneumophila cytotoxicity was expressed for a limited period in the post-exponential phase. In one representative experiment, only 9% of macrophages survived a 1-h incubation with bacteria grown to an OD600 of 2.06 and added at a ratio of 8:1. After an additional 3 h in broth culture, the bacteria were no longer cytotoxic; 80% of a macrophage culture survived a 1-h incubation with bacteria at a ratio of 15:1. Thus, expression of cytotoxicity by L. pneumophila was growth phase dependent.
As an intracellular parasite of freshwater amoebae, L. pneumophila presumably alternates between environments of high and low osmolarity. To test whether resistance to osmotic shock is regulated by growth conditions, bacteria collected from cultures at different growth stages were transferred to broth containing 0.3 M KCl and diluted into distilled water, and then colony formation was quantified. The plating efficiency of exponential-phase cells decreased more than 10-fold after osmotic shock, whereas post-exponential-phase cells were unaffected (Fig. 1D). Therefore, similar to Escherichia coli (21), L. pneumophila became osmotically resistant in the post-exponential growth phase.
The ability of phagosomal L. pneumophila to avoid degradation by macrophage lysosomes is a key to its pathogenesis (2, 15, 17, 34). To judge whether growth phase affected the intracellular fate of L. pneumophila, colocalization of phagosomes harboring exponential- or post-exponential-phase bacteria with a fluorescent endocytic probe was quantified by fluorescence microscopy. Approximately 80% of exponential-phase bacteria, but less than 5% of post-exponential-phase organisms, resided in the lysosomes, as judged by colocalization with Texas red-ovalbumin (Fig. 1E). Thus, consistent with an earlier report (34), the capacity of L. pneumophila to evade lysosomal degradation was acquired in the post-exponential phase.
As an independent measure of relative virulence, the abilities of exponential- and post-exponential-phase cultures of L. pneumophila to enter and survive in macrophages were compared. Two hours after addition of bacteria to cultured macrophages, less than 0.2% of an exponential-phase inoculum was intracellular and viable, as judged by resistance to exogenous gentamicin. In contrast, more than 5% of a post-exponential-phase inoculum entered macrophages and survived (Fig. 1F). Thus, as predicted by their more efficient evasion of lysosomes, post-exponential-phase L. pneumophila organisms were more infectious than exponential-phase bacteria. Whether exponential- and post-exponential-phase bacteria bind and enter macrophages with the same efficiency was not investigated here.
L. pneumophila virulence and flagellum expression are correlated genetically, and yet motility and flagella per se are not required for intracellular growth (26, 29). Instead, flagella and other virulence factors may be coordinately regulated (29). To determine the effect of growth phase on L. pneumophila motility, bacteria obtained from cultures at different cell densities were examined by phase-contrast microscopy. Post-exponential-phase L. pneumophila cells were highly motile, but exponential-phase cells were nonmotile (data not shown). Thus, consistent with previous studies, motility and some L. pneumophila virulence factors appear to be coordinately regulated in response to growth conditions (29, 30).
We next examined whether the L. pneumophila phenotype switched during growth in macrophages. Like in post-exponential-phase broth cultures, L. pneumophila released from host cells at the end of the intracellular replication period are motile (30). Therefore, we determined by immunofluorescence microscopy whether flagellum production is regulated during growth in macrophages. As expected, exponential-phase bacteria, which are not motile, did not have flagella (Fig. 2a, panel B, and data not shown), whereas 2 h after motile post-exponential-phase L. pneumophila cells were added to macrophages, nearly 100% of the intracellular bacteria were flagellated (Fig. 2a, panel A, and Fig. 2b). However, by 6 h postinfection, phagosomes contained four to eight bacteria but only a single flagellum (Fig. 2a, panels C and D, and Fig. 2b). After 19.5 h, vacuoles typically contained dozens of bacteria but no flagella (Fig. 2a, panels E and F, and Fig. 2b). In contrast, 22 h after infection, necrotic macrophages containing dozens of flagellated L. pneumophila cells were frequently observed (Fig. 2a, panels G and H, and Fig. 2b). Thus, consistent with the expression of motility in the post-exponential phase, L. pneumophila produced flagella exclusively in the final phase of its intracellular life cycle.
L. pneumophila sodium sensitivity was measured throughout the primary cycle of macrophage infection. Concordant with the phenotype of exponential-phase broth cultures, during the replication period in macrophages, L. pneumophila was sodium resistant. Concomitant with macrophage lysis and release of bacteria, sodium resistance decreased significantly (Fig. 2c). Thus, expression of at least two L. pneumophila traits depends on growth phase not only in broth but also in macrophages.
Interestingly, macrophages appeared to convert sodium-sensitive L. pneumophila to the sodium-resistant phenotype. When incubated for 24 h in RPMI-FBS alone, post-exponential-phase bacteria remained sodium sensitive (data not shown). In contrast, when an inoculum containing 4% sodium-resistant L. pneumophila was incubated with macrophages for 2 h, nearly 60% of the cell-associated bacteria were sodium resistant (Fig. 2c). It did not appear that a subpopulation of sodium-resistant bacteria selectively associated with macrophages, because the total number of sodium-resistant bacteria typically increased threefold or more during the 2-h incubation (data not shown). We favor a model by which macrophages induce the L. pneumophila sodium resistance phenotype, most likely in the absence of bacterial division, because the L. pneumophila generation time in broth or cells is at least 2 h, L. pneumophila does not replicate in tissue culture medium, and intracellular growth is generally not observed until 4 h after infection (16, 19, 33, 37).
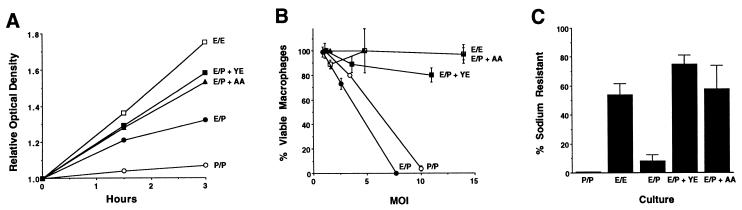
During growth in broth and in macrophages, L. pneumophila apparently regulated expression of a number of virulence traits in response to environmental conditions. By analogy to other microbial regulation systems, this phenotypic switch might be triggered by the accumulation or the depletion of a particular factor from the local environment (13, 22). Accordingly, exponential-phase cells should become virulent when transferred to post-exponential-phase culture supernatant, independent of the culture density. As expected, when exponential-phase cells were incubated for 3 h in broth obtained from post-exponential-phase cultures, cytotoxicity (Fig. 3B), sodium sensitivity (Fig. 3C), and motility (data not shown) were induced. Importantly, supplementation of the conditioned medium with either a complex or a defined supply of amino acids prevented the expression of all three traits (Fig. 3 and data not shown). In particular, media which appeared to contain a limiting supply of amino acids induced exiting from the exponential phase and stimulated expression of the virulent phenotype, whereas media which supported bacterial replication did not (compare Fig. 3A to Fig. 3B and C). Thus, when amino acids were limiting, L. pneumophila exited the exponential phase and expressed the virulent phenotype.
FIG. 3.
Growth, cytotoxicity, and sodium resistance of exponential-phase L. pneumophila cultured in post-exponential-phase supernatants. Cells collected from an exponential-phase culture with an OD600 of 0.62 were incubated for the periods of time shown in supernatant prepared from the identical exponential-phase culture (E/E; OD600 = 0.57 at 0 h) (open squares) or from a post-exponential-phase culture with an OD600 of 2.09 supplemented with H2O (E/P; OD600 = 0.65 at 0 h) (closed circles), with yeast extract (E/P + YE; OD600 = 0.65 at 0 h) (closed squares), or with a cocktail of five amino acids (E/P + AA; OD600 = 0.62 at 0 h) (closed triangles). As a positive control for the virulent phenotype, cells collected from a post-exponential-phase culture with an OD600 of 2.09 were incubated for the periods of times shown in supernatant prepared from the identical post-exponential-phase culture (P/P; OD600 = 1.94 at 0 h) (open circles). (A) The relative level of bacterial growth under each set of conditions is represented as the ratio of the OD600 at the indicated time to the OD600 at 0 h. (B) The cytotoxicity of L. pneumophila cultured for 3 h as described above was determined as described in the legend to Fig. 1C. Shown are the averages of values for duplicate samples; error bars indicate the ranges of the values. The cytoxicity observed was cell dependent, since post-exponential-phase culture supernatants alone were not cytopathic (data not shown). Panels A and B depict results from a single experiment; similar results were obtained in four other experiments. (C) The sodium resistance of the L. pneumophila cultures defined above was determined as described in the legends of Fig. 1 and 2. Shown are the means and standard errors for data from five experiments performed in duplicate.
DISCUSSION
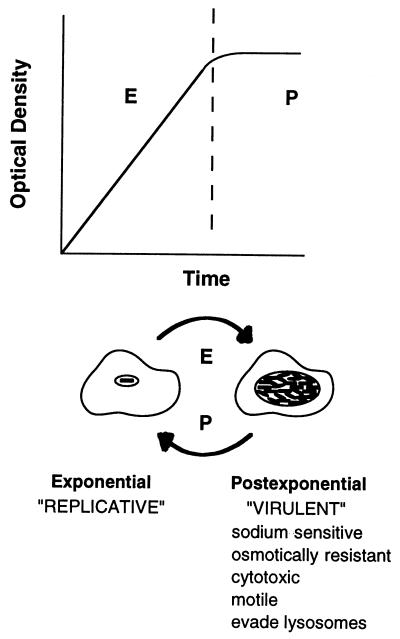
L. pneumophila is an opportunistic human pathogen whose natural reservoir is freshwater amoebae. To begin to understand how this microbe recognizes and responds to distinct extracellular and intracellular environments, the effect of culture conditions on expression of L. pneumophila sodium sensitivity, cytotoxicity, osmotic resistance, evasion of phagosome-lysosome fusion, intracellular survival, and motility was examined. The results of a series of quantitative microbiological and cell biological assays revealed the occurrence of a dramatic phenotypic switch as L. pneumophila exited the exponential growth phase. Taken together, these observations suggest the following model for the regulation of L. pneumophila virulence (Fig. 4). When nutrient levels and other conditions are favorable in host cells, L. pneumophila expresses functions to replicate maximally. When amino acids become limiting, intracellular bacteria produce factors to lyse the spent host cell, to survive osmotic stress, to disperse in the environment, and to reestablish an intracellular niche protected from lysosomal degradation. Arrival in a rich, intracellular environment stimulates a return to the replicative phenotype.
FIG. 4.
Model for L. pneumophila phenotypic regulation in response to growth conditions as described in the text. E, exponential growth phase; P, post-exponential phase of growth.
The present model is consistent with previous studies of L. pneumophila virulence. In particular, L. pneumophila flagellum expression and virulence are correlated genetically, even though neither motility nor flagella are required for intracellular growth (26, 29). Compared to bacteria grown in a microbiological medium, L. pneumophila organisms released from eukaryotic cells are short, thick, and highly motile; they have a smooth, thick cell wall, a higher β-hydroxybutyrate content, and different staining properties, and they express a different array of proteins (1, 9, 30). In addition, temperature can affect the morphology of extracellularly grown L. pneumophila (14, 24, 28). Whether the corresponding genetic determinants of all or some of these L. pneumophila traits constitute a regulon which responds to amino acid levels remains to be determined.
The pattern of expression for a particular virulence trait may provide clues to its function or biochemical basis. For example, the role of the putative L. pneumophila cytotoxin (Fig. 1C and 3B) has not been established. Our observation that maximal cytotoxicity is expressed for a limited period in the stationary phase suggests that a L. pneumophila cytotoxin mediates escape from the host cell. However, our experiments have not ruled out an alternative model in which this apparent cytotoxic activity is required by L. pneumophila during uptake to direct formation of its novel replication vacuole.
Genetic and kinetic studies (5, 31, 36) (Fig. 1) have correlated L. pneumophila sodium sensitivity with virulence; however, the biological basis for this trait is not understood. It has been proposed that the assembly and/or activity of a virulence factor translocation apparatus, such as the putative Icm-Dot protein complex (32, 35), may allow inhibitory levels of sodium to diffuse into the cytoplasm of post-exponential-phase cells (36). The coincident induction of L. pneumophila sodium sensitivity, cytotoxicity, and infectivity in response to nutrient limitation is consistent with such a mechanism.
L. pneumophila shares with several other bacterial pathogens the ability to respond to changing conditions to ensure its survival in the environment (27). For example, the virulence of Mycobacterium avium is enhanced by growth in macrophages or amoebae or by exposure to low oxygen tension or high osmolarity (3, 4, 8). Similarly, the ability of Salmonella spp. to enter mammalian cells is induced when oxygen is limiting (23). Finally, expression of streptococcal erythrogenic toxin B and the Salmonella spv genes is regulated in response to nutrient depletion (6, 13). A detailed understanding of the regulation of L. pneumophila virulence will likely facilitate the identification of the effector functions which enable this pathogen to parasitize eukaryotic cells. Knowledge of the L. pneumophila virulence regulation machinery may also suggest methods to eradicate this pathogen from the environment or the infected lung.
ACKNOWLEDGMENTS
We thank Victor DiRita, David Friedman, and Joel Swanson for critical review of the manuscript.
This work was supported by the National Institutes of Health (R29 AI 40694-01 BM) and by the Rackham Faculty Research Fund and the Department of Microbiology and Immunology at the University of Michigan.
REFERENCES
- 1.Abu Kwaik Y, Eisenstein B I, Engleberg N C. Phenotypic modulation by Legionella pneumophila upon infection of macrophages. Infect Immun. 1993;61:1320–1329. doi: 10.1128/iai.61.4.1320-1329.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Berger K H, Isberg R R. Two distinct defects in intracellular growth complemented by a single genetic locus in Legionella pneumophila. Mol Microbiol. 1993;7:7–19. doi: 10.1111/j.1365-2958.1993.tb01092.x. [DOI] [PubMed] [Google Scholar]
- 3.Bermudez L E, Petrofsky M, Goodman J. Exposure to low oxygen tension and increased osmolarity enhance the ability of Mycobacterium avium to enter intestinal epithelial (HT-29) cells. Infect Immun. 1997;65:3768–3773. doi: 10.1128/iai.65.9.3768-3773.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bermudez L E, Parker A, Goodman J R. Growth within macrophages increases the efficiency of Mycobacterium avium in invading other macrophages by a complement receptor-independent pathway. Infect Immun. 1997;65:1916–1925. doi: 10.1128/iai.65.5.1916-1925.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Catrenich C E, Johnson W. Characterization of the selective inhibition of growth of virulent Legionella pneumophila by supplemented Mueller-Hinton medium. Infect Immun. 1989;57:1862–1864. doi: 10.1128/iai.57.6.1862-1864.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chaussee M S, Phillips E R, Ferretti J J. Temporal production of streptococcal erythrogenic toxin B (streptococcal cysteine proteinase) in response to nutrient depletion. Infect Immun. 1997;65:1956–1959. doi: 10.1128/iai.65.5.1956-1959.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cianciotto N P, Fields B S. Legionella pneumophila mip gene potentiates intracellular infection of protozoa and human macrophages. Proc Natl Acad Sci USA. 1992;89:5188–5191. doi: 10.1073/pnas.89.11.5188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cirillo J D, Falkow S, Tompkins L S, Bermudez L E. Interaction of Mycobacterium avium with environmental amoebae enhances virulence. Infect Immun. 1997;65:3759–3767. doi: 10.1128/iai.65.9.3759-3767.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cirillo J D, Falkow S, Tompkins L S. Growth of Legionella pneumophila in Acanthamoeba castellanii enhances invasion. Infect Immun. 1994;62:3254–3261. doi: 10.1128/iai.62.8.3254-3261.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Feeley J C, Gibson R J, Gorman G W, Langford N C, Rasheed J K, Mackel D C, Baine W B. Charcoal-yeast extract agar: primary isolation medium for Legionella pneumophila. J Clin Microbiol. 1979;10:437–441. doi: 10.1128/jcm.10.4.437-441.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gabay J E, Horwitz M A. Isolation and characterization of the cytoplasmic and outer membrane of the Legionnaires’ disease bacterium (Legionella pneumophila) J Exp Med. 1985;161:409–422. doi: 10.1084/jem.161.2.409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gao L-Y, Harb O S, Abu Kwaik Y. Utilization of similar mechanisms by Legionella pneumophila to parasitize two evolutionarily distant host cells, mammalian macrophages and protozoa. Infect Immun. 1997;65:4738–4746. doi: 10.1128/iai.65.11.4738-4746.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Guiney D G, Libby S, Fang F C, Krause M, Fierer J. Growth-phase regulation of plasmid virulence genes in Salmonella. Trends Microbiol. 1995;3:275–279. doi: 10.1016/s0966-842x(00)88944-1. [DOI] [PubMed] [Google Scholar]
- 14.Heuner K, Bender-Beck L, Brand B C, Lück P C, Mann K-H, Marre R, Ott M, Hacker J. Cloning and genetic characterization of the flagellum subunit gene (flaA) of Legionella pneumophila serogroup 1. Infect Immun. 1995;63:2499–2507. doi: 10.1128/iai.63.7.2499-2507.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Horwitz M A. Characterization of avirulent mutant Legionella pneumophila that survive but do not multiply within human monocytes. J Exp Med. 1987;166:1310–1328. doi: 10.1084/jem.166.5.1310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Horwitz M A. Formation of a novel phagosome by the Legionnaires’ disease bacterium (Legionella pneumophila) in human monocytes. J Exp Med. 1983;158:1319–1331. doi: 10.1084/jem.158.4.1319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Horwitz M A. The Legionnaires’ disease bacterium (Legionella pneumophila) inhibits phagosome-lysosome fusion in human monocytes. J Exp Med. 1983;158:2108–2126. doi: 10.1084/jem.158.6.2108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Horwitz M A. Phagocytosis of the Legionnaires’ disease bacterium (Legionella pneumophila) occurs by a novel mechanism: engulfment within a pseudopod coil. Cell. 1984;36:27–33. doi: 10.1016/0092-8674(84)90070-9. [DOI] [PubMed] [Google Scholar]
- 19.Horwitz M A, Silverstein S C. Legionnaires’ disease bacterium (Legionella pneumophila) multiplies intracellularly in human monocytes. J Clin Invest. 1980;66:441–450. doi: 10.1172/JCI109874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Husmann L K, Johnson W. Cytotoxicity of extracellular Legionella pneumophila. Infect Immun. 1994;62:2111–2114. doi: 10.1128/iai.62.5.2111-2114.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Jenkins D E, Chaisson S A, Matin A. Starvation-induced cross protection against osmotic challenge in Escherichia coli. J Bacteriol. 1990;172:2779–2781. doi: 10.1128/jb.172.5.2779-2781.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kaiser D. Bacteria also vote. Science. 1996;272:1598–1599. doi: 10.1126/science.272.5268.1598. [DOI] [PubMed] [Google Scholar]
- 23.Lee C A, Falkow S. The ability of Salmonella to enter mammalian cells is affected by bacterial growth state. Proc Natl Acad Sci USA. 1990;87:4304–4308. doi: 10.1073/pnas.87.11.4304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mauchline W S, Araujo R, Wait R, Dowsett A B, Dennis P J, Keevil C W. Physiology and morphology of Legionella pneumophila in continuous culture at low oxygen concentration. J Gen Microbiol. 1992;138:2371–2380. doi: 10.1099/00221287-138-11-2371. [DOI] [PubMed] [Google Scholar]
- 25.McClain M S, Hurley M C, Brieland J K, Engleberg N C. The Legionella pneumophila hel locus encodes intracellularly induced homologs of heavy-metal ion transporters of Alcaligenes spp. Infect Immun. 1996;64:1532–1540. doi: 10.1128/iai.64.5.1532-1540.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Merriam J J, Mathur R, Maxfield-Boumil R, Isberg R R. Analysis of the Legionella pneumophila fliI gene: intracellular growth of a defined mutant defective for flagellum biosynthesis. Infect Immun. 1997;65:2497–2501. doi: 10.1128/iai.65.6.2497-2501.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Miller J F, Mekalanos J J, Falkow S. Coordinate regulation and sensory transduction in the control of bacterial virulence. Science. 1989;243:916–922. doi: 10.1126/science.2537530. [DOI] [PubMed] [Google Scholar]
- 28.Ott M, Messner P, Heesemann J, Marre R, Hacker J. Temperature-dependent expression of flagella in Legionella. J Gen Microbiol. 1991;137:1955–1961. doi: 10.1099/00221287-137-8-1955. [DOI] [PubMed] [Google Scholar]
- 29.Pruckler J M, Benson R F, Moyenuddin M, Martin W T, Fields B S. Association of flagellum expression and intracellular growth of Legionella pneumophila. Infect Immun. 1995;63:4928–4932. doi: 10.1128/iai.63.12.4928-4932.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Rowbotham T J. Current views on the relationships between amoebae, legionellae and man. Isr J Med Sci. 1986;22:678–689. [PubMed] [Google Scholar]
- 31.Sadosky A B, Wiater L A, Shuman H A. Identification of Legionella pneumophila genes required for growth within and killing of human macrophages. Infect Immun. 1993;61:5361–5373. doi: 10.1128/iai.61.12.5361-5373.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Segal G, Shuman H A. Characterization of a new region required for macrophage killing by Legionella pneumophila. Infect Immun. 1997;65:5057–5066. doi: 10.1128/iai.65.12.5057-5066.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Swanson M S, Isberg R R. Association of Legionella pneumophila with the macrophage endoplasmic reticulum. Infect Immun. 1995;63:3609–3620. doi: 10.1128/iai.63.9.3609-3620.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Swanson M S, Isberg R R. Identification of Legionella pneumophila mutants that have aberrant intracellular fates. Infect Immun. 1996;64:2585–2594. doi: 10.1128/iai.64.7.2585-2594.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Vogel J P, Andrews H L, Wong S K, Isberg R R. Conjugative transfer by the virulence system of Legionella pneumophila. Science. 1998;279:873–876. doi: 10.1126/science.279.5352.873. [DOI] [PubMed] [Google Scholar]
- 36.Vogel J P, Roy C, Isberg R R. Use of salt to isolate Legionella pneumophila mutants unable to replicate in macrophages. Ann N Y Acad Sci. 1996;797:271–272. doi: 10.1111/j.1749-6632.1996.tb52975.x. [DOI] [PubMed] [Google Scholar]
- 37.Wang W L L, Blaser M J, Cravens J, Johnson M A. Growth, survival, and resistance of the Legionnaires’ disease bacterium. Ann Intern Med. 1979;90:614–618. doi: 10.7326/0003-4819-90-4-614. [DOI] [PubMed] [Google Scholar]
- 38.Zambrano M M, Kolter R. GASPing for life in stationary phase. Cell. 1996;86:181–184. doi: 10.1016/s0092-8674(00)80089-6. [DOI] [PubMed] [Google Scholar]