Abstract
Purpose
Antimicrobial cement spacer (ACS) placement has been a cornerstone of two-stage management of prosthetic hip and knee infection. Pharmacokinetic modelling has described peak systemic antibiotic concentrations within the first 24–48 h post-operatively, followed by rapid clearance. A few studies have, however, identified detectable tobramycin levels in patients with a post-operative decline in creatinine clearance. Our study sought to determine how frequently detectable serum tobramycin levels occurred within the first 72 h following ACS placement in all patients regardless of baseline or subsequent changes in renal function, whether these levels correlated with tobramycin spacer dosage, creatinine clearance, or potential nephrotoxicity risk factors, and whether any patients developed acute kidney injury within the 14-day post-operative period.
Methods
We prospectively enrolled patients with prosthetic hip or knee infections and subsequent ACS placement from October 2017 to February 2020. Patient comorbidities (chronic kidney disease, diabetes mellitus, chronic liver disease, chronic obstructive pulmonary disease, and atrial fibrillation), Charleston Comorbidity Index score, risk factors for post-operative nephrotoxicity (perioperative hypotension and nephrotoxic agent receipt), total tobramycin dosage, post-operative days 1 and 3 serum tobramycin concentrations, and serum creatinine and creatinine clearance throughout a 14-day post-operative period were recorded.
Results
A total of 20 patients were enrolled, comprising 20 spacers with a median total tobramycin dosage of 4.80 g with an interquartile range (IQR) of 4.13–7.20 g. Thirteen patients had a median detectable post-operative day 1 serum tobramycin concentration of 0.80 (IQR 0.50–1.60) mcg/mL. Five of these 13 patients had a median detectable post-operative day 3 serum tobramycin concentration of 0.80 (IQR 0.50–1.10) mcg/mL. A correlation was not found between serum tobramycin drug levels and patient comorbidities, receipt of nephrotoxic medications, or baseline and subsequent post-operative creatinine clearance up to day 14.
Conclusion
The majority of patients who underwent tobramycin ACS placement had detectable serum tobramycin levels in the immediate post-operative period, but most reached undetectable levels within 72 h. There were no reliable perioperative predictors of detectable drug levels.
Keywords: Prosthetic joint infection, Antimicrobial cement spacer, Tobramycin, Pharmacokinetics
Introduction
Prosthetic joint infection is one of the most feared complications following prosthetic joint replacement, estimated to occur in approximately 0.5–1.0% of cases over the lifetime of a prosthesis [1]. As these procedures have become more common, the number of prosthetic joint infections per year has accordingly increased [2, 3]. Two-stage arthroplasty revision, which entails index prosthesis removal, placement of a temporary antimicrobial cement spacer (ACS) in combination with systemic antibiotic therapy, and eventual prosthesis reimplantation is a commonly employed strategy. These serial interventions result in success rates–defined as achieving an infection-free, functional prosthesis over the period of patient follow-up–ranging from 73 to 87% [4]. The antibiotics commonly utilized in ACS include vancomycin and aminoglycosides, agents historically believed to remain either localized to the periprosthetic tissues, or rapidly dissipated systemically within the first 1–2 days following ACS placement [5–7].
Greater systemic tobramycin exposure and risk of acute kidney events have, however, also been previously described [8]. An observational study of 50 patients reported median tobramycin serum levels exceeding 2 mcg/mL in 22% of patients, along with an acute kidney injury rate of 20% within the first week of antimicrobial cement spacer placement [9]. Noto et al. reported detectable serum tobramycin levels in all ten observed patients receiving an ACS with a 50% or greater rise in serum creatinine from baseline [10].
While the majority of prior case reports and studies focused on patients with suspected or documented acute kidney injury, our study aimed to assess the rate, duration, and associated factors that would correlate with detectable serum tobramycin concentrations in all patients within the first 3 days following ACS placement. We additionally measured weekly serum creatinine and creatinine clearance over a 14-day post-operative period to assess the rate of acute kidney injury within the first 2 weeks following surgery, and whether this rate might correlate with detectable serum tobramycin levels.
Materials and Methods
Study Design and Setting
All research was conducted at a 661-bed academic medical center in Western Pennsylvania, USA.
Participants/Study Subjects
Following obtainment of informed consent, consecutive patients ages 18 years or older, diagnosed with an index total hip or knee prosthetic joint infection, and who had undergone complete prosthesis explantation with antimicrobial cement spacer (ACS) placement were prospectively enrolled. Patients were only included if their ACS contained vancomycin and tobramycin as their exclusive antibiotics. We excluded patients who received any other antibiotics in their spacers, underwent hemiarthroplasty or exchange of a previously placed ACS, had received intravenous aminoglycoside agents, had a listed aminoglycoside allergy, were pregnant or lactating, or were incarcerated at the time of ACS placement.
Description of Experiment, Treatment, or Surgery
Antibiotic-free orthopedic cement was initially prepared in 40-g units, to which tobramycin and vancomycin were added in all cases. Both articulating and static spacers were utilized, at the discretion of the operating surgeon. The total tobramycin dose was similarly chosen at the surgeon’s discretion, but was determined by the total dose of antibiotic-free cement incorporated into each spacer. Antibiotic-free cement, tobramycin, and vancomycin dosages were recorded.
Serum tobramycin concentrations were obtained on post-operative days 1 and 3, as measured by the Cobas™ c111 analyzer (Roche), a commercially available benchtop system routinely used at our hospital to calculate serum peak and trough aminoglycoside concentrations in hospitalized patients. The minimum level of measurement for this device was 0.4 mcg/mL, while levels < 0.4 mcg/mL were considered undetectable. Time of concentration was recorded as the documented incisional closure time at conclusion of surgery; these values were rounded to the nearest one-tenth of 1 h. Cases with detectable post-operative day 1 and 3 tobramycin concentrations were tallied and expressed as a median value with the nearest interquartile range; a value of 0.00 mcg/mL represented undetectable tobramycin concentrations for median calculations in the entire 20-patient group. Operative time was measured from the documented time of procedure start to time of incision closing.
Variables, Outcome Measures, Data Sources, and Bias
Data were collected and tabulated in a descriptive fashion. Detectable post-operative day 1 and 3 tobramycin concentrations were analyzed as a function of total antimicrobial cement spacer tobramycin dosage, as well as serum creatinine, and assessed by scatter plot with a determination of the Pearson Product-Moment correlation coefficient (r2). Given the smaller population size of 20 cases, mean values were not calculated.
Demographics, Description of Study Population
Patient age, sex, BMI, noteworthy comorbidities (chronic kidney disease, diabetes mellitus, chronic liver disease, chronic obstructive pulmonary disease, and atrial fibrillation) were recorded. A median Charleston Comorbidity Index Score was additionally calculated. Baseline serum creatinine was defined as any single measurement within the 30 days prior to ACS placement. Serum creatinine was also measured on post-operative days 1 and 3 to correspond with tobramycin levels, as well as on weekly intervals over a 14-day post-operative period. Creatinine clearance was calculated using the Cockcroft-Gault equation. Acute kidney injury was defined as a two-fold increase from baseline serum creatinine, which aligns with the “Injury” category of the RIFLE (Risk, Injury, Failure, Loss of kidney function, and End-stage kidney disease) criteria classification [11]. Receipt of commonly implicated nephrotoxic medications such as non-steroidal anti-inflammatory drugs, angiotensin-converting enzyme inhibitors, angiotensin receptor blockers, intravenous contrast, and diuretics was noted. Additionally, episodes of perioperative hypotension, defined as at least one documented blood pressure measurement < 90 mm Hg systolic and/or < 60 mm Hg diastolic within 24 h before, or after, initial incision time were recorded, as well as the total antibiotic-free cement, tobramycin, and vancomycin dosage used within each spacer (Table 1). As a majority of patients received intravenous vancomycin in the peri-operative period, post-operative vancomycin levels were not measured, as the relative contribution of vancomycin secreted by the ACS could not reliably be determined.
Table 1.
Demographic and perioperative patient information
| Patient characteristic | n = 20 |
|---|---|
| Age, years, mean (SD) | 68 (11) |
| Weight, kg, mean (SD) | 85.1 (26.7) |
| Gender, female, n (%) | 13 (65%) |
| ACS procedure, n (%) | |
| Hip | 7 (35%) |
| Knee | 13 (65%) |
| Comorbidities, n (%) | |
| CKD–stage 2 | 3 (15%) |
| CKD–stage 3 | 7 (35%) |
| CKD–stage 4 | 1 (5%) |
| Diabetes* | 2 (10%) |
| Liver disease* | 2 (10%) |
| COPD* | 2 (10%) |
| Atrial fibrillation* | 3 (15%) |
| Charleston comorbidity index score | 4 (1–9)a |
| Nephrotoxic agent use*, n (%) | |
| NSAIDs | 11 (55%) |
| IV contrast | 3 (15%) |
| Diuretics | 3 (15%) |
| ACE-inhibitor | 5 (25%) |
| Perioperative hypotension, n (%) | 4 (20%) |
| Total operative time, min | 122 (93.2–190.0)a |
| Total tobramycin cement dose, grams | 4.80 (4.13–7.20)a |
| Total vancomycin cement dose, grams | 4.00 (4.00–6.00)a |
| Total antibiotic-free cement dose, grams | 90 (80–120)a |
| Pre-operative (with 30 days) | |
| Scr, mg/dL | 0.72 (0.62–0.88)a |
| CrCl, mL/min | 83.63 (59.47–94.97)a |
| Post-operative day 1 | |
| Scr, mg/dL | 1.00 (0.64–1.10)a |
| CrCl, mL/min | 75.1 (53.5–105.9)a |
| Detectable tobramycin level (n = 13), mcg/mL | 0.80 (0.50–1.60)a |
| Tobramycin level for total group (n = 20), mcg/mL | 0.45 (0.00–0.80)a,b |
| Post-op level time, hours | 16.8 (16.1–18.4)a |
| Post-operative day 3 | |
| Scr, mg/dL | 0.60 (0.53–0.96)a |
| CrCl, mL/min | 72.9 (76.7–83.2)a |
| Detectable tobramycin level (n = 5), mcg/mL | 0.80 (0.50–1.10)a |
| Tobramycin level for total group (n = 20), mcg/mL | 0.00 (0.00–0.10)a,b |
| Post-op level time, hours | 61.3 (58.5–63.1)a |
| Post-operative day 7 | |
| Scr, mg/dL | 0.69 (0.64–0.81)a |
| CrCl, mL/min | 77.4 (57.2–95.9)a |
| Post-operative day 14 | |
| Scr, mg/dL | 0.70 (0.61–0.90)a |
| CrCl, mL/min | 77.5 (57.2–90.7)a |
ACS Antibiotic-impregnated cement spacer; CKD chronic kidney disease; COPD chronic obstructive pulmonary disease; NSAID nonsteroidal anti-inflammatory drug; ACE angiotensin-converting enzyme; SCr serum creatinine; CrCl creatinine clearance
*Risk factors co-occurred within some of the same patients
aExpressed as median with interquartile range
bAll undetectable tobramycin concentrations (< 0.4 mcg/mL) were represented by a value of 0.00 mcg/mL for calculation of median concentrations
Results
A total of 20 patients were enrolled into this study, comprising 13 prosthetic knee and 7 prosthetic hip infections. The median operative antibiotic-free cement dose and spacer antibiotic dosages for both hip and knee spacers are listed in Table 1. Overall, 13 patients (65%) had a post-operative day 1 median detectable tobramycin serum concentration of 0.80 (IQR 0.50–1.60) mcg/mL. The median post-operative day 1 tobramycin concentration for the entire 20-patient group was 0.45 (IQR 0.00–0.80) mcg/mL. Five of the 13 post-operative day 1 patients with detectable tobramycin concentrations subsequently had a post-operative day 3 median detectable serum tobramycin concentration of 0.80 (IQR 0.50–1.10) mcg/mL. The median post-operative day 3 tobramycin concentration for the entire 20-patient group was 0.00 (IQR 0.00–0.10) mcg/mL (Table 1). The individual values for each patient are summarized accordingly (Table 2).
Table 2.
Individual patient characteristics and outcomes
| Patient | Tobramycin ACS dose (g) | Pre-operative Scr/CrCl/CKD staging (by baseline CrCl) | POD 1 | POD 3 | POD 14 | Antibiotic treatment/duration (weeks)/time to reimplantation (weeks) | ||
|---|---|---|---|---|---|---|---|---|
| Scr/CrCl | Tobra draw timec/leveld | Scr/CrCl | Tobra draw timec/leveld | Scr/CrCl | ||||
| 1 | 4.80 | 1.88/29.25 (4)* | 2.22/24.7 | 16.6/1.6 | 2.20/25.0 | 60.7/0.80 | 2.23/24.3 | amp/6/dest |
| 2 | 3.00 | 0.70a/54.4 (2) | 0.62a/54.4 | 15.3/2.7 | 0.53a/54.4 | 63.0/UND | 0.70/54.4 | vanc/6/12 |
| 3 | 9.60 | 0.53/120.0b | 0.72/120.0b | 16.5/UND | 0.64/120.0b | 63.8/UND | 0.79/120.0b | vanc/6/21 |
| 4 | 4.80 | 0.83a/37.1 (3b) | 0.83a/37.1 | 16.1/0.6 | 0.83a/37.1 | 62.0/UND | 0.80a/37.1 | ctx, vanc/6/55 |
| 5 | 7.20 | 0.59/118.16 | 0.47/120.0b | 16.2/0.4 | 0.55/116.7 | 58.6/UND | 0.79/81.3 | vanc, rif/6/23 |
| 6 | 12.0 | 0.68/120.0b | 0.64/120.0b | 11.5/UND | 0.60/120.0b | 58.4/UND | 0.68/120.0b | cfz/6/15 |
| 7 | 2.40 | 1.00/81.80 (2) | 0.63/88.6 | 17.2/UND | 0.49/113.9 | 56.2/UND | 0.54/118.5 | ctx/6/8 |
| 8 | 4.50 | 0.72a/40.6 (3b) | 0.70a/40.6 | 17.0/0.7 | 0.48a/40.6 | 43.0/UND | 0.67a/40.6 | vanc/6/32 |
| 9 | 4.80 | 0.56/120.0b | 0.53/120.0b | 17.6/UND | 0.57/120.0b | 61.8/UND | 0.50/120.0b | cfz/6/32 |
| 10 | 9.60 | 1.00/42.39 (3b) | 1.33/33.2 | 19.5/5.3 | 1.40/31.5 | 61.1/1.10 | 1.30/34.0 | cfz/6/24 |
| 11 | 4.80 | 0.84a/37.6 (3b) | 0.71a/37.6 | 18.2/0.8 | 0.57a/37.6 | 60.4/UND | 0.61a/37.6 | cefp, vanc/6/19 |
| 12 | 4.80 | 1.75/41.2 (3b)* | 1.75/41.2 | 16.5/0.8 | 1.65/52.8 | 70.4/0.40 | 1.23/63.6 | cfz/6/nonee |
| 13 | 4.80 | 0.72a/53.0 (3a) | 0.72a/53.0 | 8.7/0.5 | 0.61a/53.0 | 58.1/UND | 0.65a/53.0 | vanc/6/dest |
| 14 | 4.80 | 0.52/117.3 | 0.63/96.82 | 0.52/UND | 0.52/117.3 | 70.4/UND | 0.83/81.2 | vanc/8/57 |
| 15 | 7.20 | 0.84/91.39 | 0.76/94.6 | 18.8/0.4 | 0.65/110.6 | 63.3/UND | 0.70/102.7 | ctx, vanc/6/nonef |
| 16 | 7.20 | 0.79a/43.8 (3b) | 0.72a/43.8 | 21.0/0.8 | 0.54a/43.8 | 61.6/UND | 0.68/43.8 | cfp/6/dest |
| 17 | 4.80 | 1.05/90.57 | 1.83/54.5 | 27.8/UND | 1.09/91.5 | 60.0/UND | 0.69a/98.6 | vanc/6/dest |
| 18 | 4.80 | 0.63/61.67 (2) | 0.66/71.9 | 17.9/0.4 | 0.48/98.8 | 37.3/0.50 | 0.59/78.4 | vanc/6/dest |
| 19 | 7.20 | 0.60/105.72 | 0.50/99.4 | 13.3/3.1 | 0.45/110.5 | 62.2/1.10 | 0.85/52.7 | cfp, vanc/6/23 |
| 20 | 4.80 | 0.53/120.0b | 0.51/91.3 | 28.7/UND | 0.53/87.8 | 65.4/UND | 0.58/81.25 | cfp/6/dest |
ACS Antibiotic-impregnated cement spacer; CrCl creatinine clearance (mL/min); Dest long-term (‘destination’) spacer; POD post-operative day; SCr serum creatinine (mg/dL); Tobra tobramycin; UND undetectable (i.e., < 0.4 mcg/mL); amp ampicillin, vanc vancomycin, ctx ceftriaxone, rif rifampin, cfz cefazolin, cfp cefepime
*Patients with a documented diagnosis of CKD prior to procedure
aFor patients ≥ 65 years of age with a baseline Scr < 1 mg/dl, Scr values are rounded/adjusted to 1 mg/dl when calculating CrCl
bFor patients with a calculated CrCl > 120 ml/min, estimated CrCl reduced to 120 ml/min to match maximum physiological renal function
cTobramycin draw time (hours)
dTobramycin level (mcg/mL)
ePatient expired from complications related to end-stage liver disease prior to reimplantation
fPatient lost to follow-up
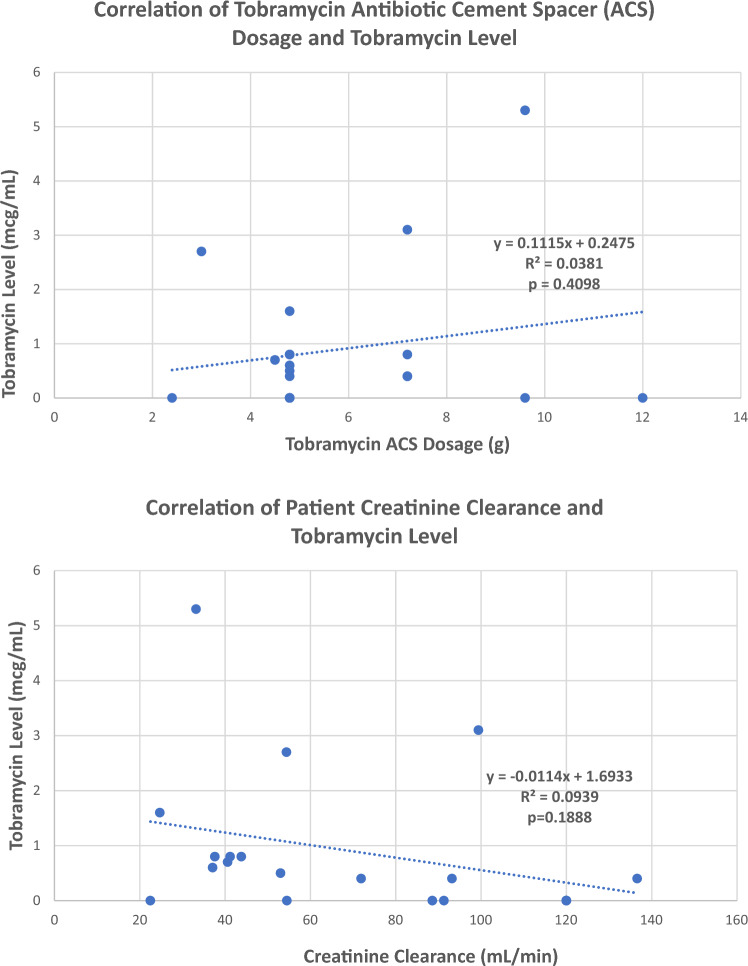
Neither total tobramycin ACS dosage, baseline creatinine clearance, nor the presence of peri-operative nephrotoxicity risk factors correlated with the frequency or duration of detectable, post-operative tobramycin levels (Fig. 1). Similarly, no correlation was found between the static or articulating spacer subgroup tobramycin dosage and post-operative day 1 and 3 tobramycin levels. Thirteen patients had a comorbid condition identified (Table 1). All patients had post-operative day 1, 3, and 14 serum creatinine and creatinine clearance values which were not significantly different from a pre-operative baseline. Sixteen patients had received a potentially nephrotoxic agent in the perioperative period, while 4 patients had at least 1 documented episode of peri-operative hypotension (Table 1). All 20 cases received general anesthesia, of which 1 case additionally received a neuraxial block. Of the 13 ACS cases with detectable post-operative day 1 tobramycin concentrations, 10 had a creatinine clearance < 65 mL/min; 4 of these patients also had detectable post-operative day 3 concentrations. All five patients with detectable post-operative day 1 and 3 tobramycin concentrations had received a total tobramycin dosage of at least 4.80 g (range 4.80–9.60). The seven antimicrobial cement spacer patients with undetectable post-operative day 1 tobramycin concentrations also had undetectable post-operative day 3 concentrations, and all had a creatinine clearance > 65 mL/min. No patients developed acute kidney injury within 14 days following ACS placement.
Fig. 1.
Scatter plot correlation of tobramycin antibiotic cement spacer (ACS) dose and creatinine clearance (CrCl) to post-operative day 1 (POD1) tobramycin levels
Discussion
Background and Rationale
Previous studies have described a varying frequency, magnitude, and duration of detectable serum antibiotic levels following ACS placement, mostly in the context of documented kidney events, but these results may have been impacted by confounding factors. First, limiting measurement of serum aminoglycoside levels to patients with documented post-operative kidney events may not capture every patient with detectable, ACS-mediated systemic exposure. Noto et al. published a prospective, nonrandomized study of 46 patients who underwent hip or knee ACS placement and found that ten patients had detectable tobramycin concentrations, with a mean value of 3.3 mcg/mL (range, 0.1–19.8 mcg/mL), however, their study only included patients with post-operative increases in serum creatinine > 50%. [10]. Within a retrospective cohort study by Menge et al. of 84 patients who underwent placement of a knee or hip ACS, 14 (17%) developed a minimum 50% increase in baseline serum creatinine within the first 90 post-operative days. A positive association was reported between increasing risk of an acute kidney event and every one-gram increment increase in ACS tobramycin dosage [12], but post-operative serum tobramycin levels were not measured, thus rendering the etiology of the renal event unclear. Our study measured short-term post-operative tobramycin levels in all patients, regardless of their baseline creatinine clearance, to help better elucidate the contribution of pre- and post-operative creatinine clearance to systemic tobramycin exposure.
Additionally, more than one aminoglycoside agent is often incorporated into ACS, wherein free tobramycin powder is added to premixed gentamicin cement in order to achieve the surgeon’s desired total dosage. Aeng et al. reported in a 50-patient prospective study detectable median tobramycin concentrations throughout their post-operative period, however, 16 patients in their study population had received premixed gentamicin cement in addition to tobramycin [9]. A similarly designed prospective study by Edelstein et al. with 21 patients followed weekly tobramycin, gentamicin, and vancomycin troughs over an eight-week period [2], but also utilized ACS with both gentamicin and tobramycin intermixed. It is currently unknown whether the presence of concomitant aminoglycosides may interfere with the accuracy of serum aminoglycoside concentration measurements, but remains a theoretical concern. For the purposes of our study, we solely utilized ACS made from antibiotic-free cement, to which tobramycin powder was added; no gentamicin was present.
Finally, the rates of nephrotoxicity reported in studies may differ on the basis of the applied definition for the same. Aeng [9], Menge [12], and Noto [10] each defined acute kidney injury as a 50% or more rise in pre-operative serum creatinine. For the current study, we instead chose to use a definition of a 100% increase (doubling) of baseline creatinine as this aligns with ‘Injury’ on the RIFLE criteria scale, as opposed to ‘Risk’ utilized by these other authors. One patient (Number 17) in our study population did have a greater than 50% rise in pre-operative serum creatinine on the first post-operative day, but had returned to its baseline values on post-operative day 3 and thereafter, suggesting a cause more related to the surgical procedure than tobramycin-mediated nephrotoxicity. The stricter definitions applied in these previous studies may thus potentially have inflated ACS antibiotic-mediated nephrotoxicity rates as compared to the results we have described herein.
Despite the fairly low tobramycin levels observed in our study (median, 0.80 mcg/mL), drug elution can result in consistent exposure with relatively high area-under-the-curve values, potentially putting a patient at higher risk due to aminoglycoside-mediated toxicity [13]. While we were unable to identify specific factors that might predict systemic aminoglycoside exposure, these considerations taken in our study provide a more standardized approach and represent a valuable contribution to this burgeoning area of prosthetic joint infection treatment research. Larger trials utilizing our methods may better identify such associations and inform strategies to mitigate adverse ACS antibiotic-mediated effects.
Limitations
We report several shortcomings with our current study. First, we were unable to capture serum tobramycin levels following patient discharge, which typically occurred within 4 days after ACS placement. Second, our small sample size likely limited our ability to identify potential correlations between total tobramycin ACS dosage, creatinine clearance, and serum tobramycin concentrations. Third, while our post-operative surveillance of serum creatinine extended for up to 14 days following surgery, we cannot exclude the possibility of subsequent acute kidney injury beyond this point in time. Finally, as opposed to Edelstein et al., we were unable to measure accurately post-operative vancomycin levels as we could not distinguish ACS-derived from intravenously administered vancomycin in the post-operative period.
Conclusion
We feel that this study is an important contribution to the prosthetic joint infection field of research.
While we were fortunate to observe no occurrences of post-ACS kidney injury within 14 days of spacer placement, suggesting that nephrotoxicity may be a relatively uncommon occurrence in this population, our data set is still limited. A prospective study featuring a larger patient population with individual tobramycin levels measured over a longer period may help further delineate the rate and duration of systemic tobramycin exposure, as well as post-operative nephrotoxicity risk. This information could, in turn, be developed into a model to predict which patients would develop systemic tobramycin exposure, their resultant risk for nephrotoxicity, and whether ACS aminoglycoside dose reduction might lessen this attendant risk without sacrificing anti-infective efficacy. We hope that our study, along with future publications, will ultimately form the basis for more robust practice guidelines with respect to determining both the safe and effective ACS antibiotic dosages for the management of prosthetic joint infection.
Funding
The Allegheny Science and Research Institute at Allegheny Health Network provided a research grant (19010109 AHN Research Initiatives) which reimbursed the sole expense of this study—the cost of drawing and processing serum tobramycin levels.
Code Availability
Not applicable.
Data Availability
Not applicable.
Declarations
Conflict of Interest
Each author certifies that he or she has no commercial associations (e.g., consultancies, stock ownership, equity interest, patent/licensing arrangements, etc.) that might pose a conflict of interest in connection with the submitted article.
Ethical Approval
This study was approved by the Allegheny Health Network IRB under a “full review” process, prior to subject enrollment, and was renewed annually for the duration of the study.
Informed Consent
For this type of study, informed consent is not required.
Consent to Participate
All subjects provided informed consent prior to study enrollment.
Consent for Publication
Not applicable.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Phillips J, Crane T, Noy M, Elliott T, Grimer R. The incidence of deep prosthetic infections in a specialist orthopaedic hospital. The Journal of Bone and Joint Surgery British. 2006;88-B:943–948. doi: 10.1302/0301-620X.88B7.17150. [DOI] [PubMed] [Google Scholar]
- 2.Edelstein A, Okroj K, Rogers T, Della Valle C, Sporer M. Systemic absorption of antibiotics from antibiotic-loaded cement spacers for the treatment. Journal of Arthroplasty. 2017;33(3):835–839. doi: 10.1016/j.arth.2017.09.043. [DOI] [PubMed] [Google Scholar]
- 3.Kendoff DO, Gehrke T, Stangenberg P, Frommelt L, Bosebeck H. Bioavailability of gentamicin and vancomycin released from an antibiotic containing bone cement in patients undergoing a septic one-stage total hip arthroplasty (THA) revision: A monocentric open clinical trial. Hip International. 2016;26(1):90–96. doi: 10.5301/hipint.5000307. [DOI] [PubMed] [Google Scholar]
- 4.Bejon P, Berendt A, Atkins B, Green N, Parry H, Masters S, Mclardy-Smith P, Gundle R, Byren I. Two-stage revision for prosthetic joint infection: Predictors of outcome and the role for preimplantation microbiology. Journal of Antimicrobial Chemotherapy. 2010;65:569–575. doi: 10.1093/jac/dkp469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bertazzoni ME, Benini A, Samaila E, Bondi M, Magnan B. Antimicrobial activity of gentamicin and vancomycin combination in joint fluids after antibiotic-loaded cement spacer implantation in two-stage revision surgery. Journal of Chemotherapy. 2015;27(1):17–24. doi: 10.1179/1973947813Y.0000000157. [DOI] [PubMed] [Google Scholar]
- 6.Hsieh PH, Huang KC, Tai CL. Liquid gentamicin in bone cement spacers: In vivo antibiotic release and systemic safety in two-stage revision of infected hip arthroplasty. Journal of Trauma. 2009;66(3):804–808. doi: 10.1097/TA.0b013e31818896cc. [DOI] [PubMed] [Google Scholar]
- 7.Sterling GJ, Crawford S, Potter JH, Koerbin G, Crawford R. The pharmacokinetics of Simplex-tobramycin bone cement. The Journal of Bone and Joint Surgery. 2003;85-B:646–649. [PubMed] [Google Scholar]
- 8.Luu A, Syed F, Raman G, et al. Two-stage arthroplasty for prosthetic joint infection: A systematic review of acute kidney injury, systemic toxicities and infection control. Journal of Arthroplasty. 2013;28(9):1490–1498. doi: 10.1016/j.arth.2013.02.035. [DOI] [PubMed] [Google Scholar]
- 9.Aeng ES, Shalansky KF, Lau TT, et al. Acute kidney injury with tobramycin-impregnated bone cement spacers in prosthetic joint infections. Annals of Pharmacotherapy. 2015;49(11):1207–1213. doi: 10.1177/1060028015600176. [DOI] [PubMed] [Google Scholar]
- 10.Noto MJ, et al. Detectable serum tobramycin levels in patients with renal dysfunction and recent placement of antibiotic-impregnated cement knee or hip spacers. Clinical Infectious Diseases. 2014;58:1783–1784. doi: 10.1093/cid/ciu159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bellomo R, Ronco C, Kellum JA, Mehta RL, Palevsky P. Acute dialysis quality initiative workgroup. Acute renal failure - definition, outcome measures, animal models, fluid therapy and information technology needs: The second international consensus conference of the acute dialysis quality initiative (ADQI) group. Critical Care. 2004;8(4):R204–R212. doi: 10.1186/cc2872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Menge TJ, et al. Acute kidney injury after placement of an antibiotic-impregnated cement spacer during revision total knee arthroplasty. The Journal of Arthroplasty. 2012;27:1221–1229. doi: 10.1016/j.arth.2011.12.005. [DOI] [PubMed] [Google Scholar]
- 13.Wu I, Marin E, Kashgarian M, Ian M, Brewster UC. A case of an acute kidney injury secondary to an implanted aminoglycoside. Kidney International. 2009;75(10):1109–1112. doi: 10.1038/ki.2008.386. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Not applicable.
Not applicable.