Abstract
Aims
To evaluate the effectiveness of toluidine blue for obtaining safe margins in oral squamous cell carcinoma.
Materials and methods
Intra-operatively irrigation of the lesion and its surrounding areas was done with toluidine blue solution for 20 s. Followed by irrigation with 1% acetic acid to remove all the mechanically retained stain. The unstained margins were demarcated using incision placed by no 15 BP blade. The lesion was resected with a safe margin of 1 cm away from the stained tissue. Neck dissection was done according to the nodal status. The tumor along with the resected margins was sent for histopathological examination. Statistical analysis was performed to calculate specificity of the vital stain. The cross tabulation between epithelium of the stained and unstained margins was done and subjected to Chi-square test to calculate the significance.
Results
The toluidine blue vital stain has a sensitivity of 93.33%. Out of 15 cases, 2 patients recorded positive excision margins leading to recurrence at primary site; 1 patients recorded positive excision margins leading to recurrence at secondary site; 1 patient recorded free excision margins but had recurrence at secondary site; remaining 11 patients recorded free excision margins and did not have recurrence.
Conclusion
Vital staining with toluidine blue is concluded to be specific in demarcating the dysplastic tissue adjacent to the carcinomatous lesion, which when excised along with the adjacent dysplastic tissue leads to a decrease in the recurrence in oral squamous cell carcinoma cases. Furthermore, it is inexpensive, easily available and does not add significantly to the operating time. Moreover, it provides a gross visualization of dysplasia surrounding the lesion especially in cases where in the margins are not well defined. Hence, toluidine blue can be a useful and inexpensive adjunct to identify margins intra-operatively in the current scenario where intra-operative frozen sections are not available.
Keywords: Toluidine blue, Squamous cell carcinoma
Introduction
Oral cancer is one of the common cancers in the Indian subcontinent due to rampant use of tobacco. Oral cancer is the sixth most common cancer in the world. It accounts for about 4% of all cancers and 2% of cancer deaths worldwide [1, 2].
It has been found that the presence of dysplasia at the surgical margin is a predictor of local recurrence. It also increases the chances of developing local recurrences by 5 times [3]. The difficulty of identifying a clear margin may be one of the most common reasons to have a wide range of safe margin. Leaving this unresected can result in local recurrence of second primary tumors [4]. The need for complete resection of dysplastic tissues surrounding the primary lesion of OSCC has been emphasized [3]. To ensure this, it is imperative to know the microscopic extent of the tumor invasion which is not possible using conventional clinical examination methods. To ensure tumor removal, surgeon excises a “safe” margin around the tumor [5]. Techniques to identify these dysplastic margins are still not clear, and new techniques are still being researched [4].
Toluidine blue has been known for various medical applications since its discovery by William Henry Perkin in 1856. Toluidine blue has been extensively used as a vital stain for mucosal lesions and also has found applications in tissue sections to specifically stain certain components owing to its acidophilic metachromatic property, as it is taken up easily by the cancerous dysplastic tissue [6]. It also provides the surgical team with straight forward method for precisely identifying the advancing edge of premalignant lesions [7].
The present study was conducted to evaluate the effectiveness of toluidine blue for obtaining safe margins in oral squamous cell carcinoma.
Materials and Methods
Criteria for selection of subjects: Patients who were clinically and histopathologically diagnosed with squamous cell carcinoma of the oral region.
Inclusion criteria
Patients who were diagnosed with oral squamous cell carcinoma with TNM staging of T1, T2, T3, N0, N1, N2 lesions were included in the present study.
Exclusion criteria
- Patients who were diagnosed with oral squamous cell carcinoma with TNM staging of
- T4 stage lesions,
- M1 OR M2 stage lesions
Patients undergoing chemotherapy or radiotherapy,
Patients who were diagnosed with oral squamous cell carcinoma of the floor of the mouth were excluded from the study.
Study design
15 patients who were diagnosed histopathologically with oral squamous cell carcinoma at the Al-Badar Dental college and Hospital, Gulbarga were taken up for study.
Investigations
Routine blood investigations
Contrast CT scan was done of the maxilla, mandible and the neck. Axial, coronal and sagittal sections of the scan were then studied to know the extent of the lesion and lymph node involvement. OPG was also done to know the involvement of the bone.
Chest X-ray and ECG were taken.
USG abdomen was done to rule out any organ metastasis
Intra-operative after examination of lesion followed photograph, irrigation of the oral cavity with saline was done to clean the area and then allow to dry. Dry area was then irrigated with toluidine blue solution for 20 s. Then, the stained areas were irrigated with 1% acetic acid to remove all the mechanically retained stain. Stained margins were demarcated using incision placed by no 15 BP blade. The lesion was resected with a safe margin of 1 cm away from the stained tissue. Neck dissection was done according to the nodal status. The specimen tagging was done using un-absorbable sutures. The tumor along with the resected margins was sent for histopathological examination. The stained epithelium was then examined for dysplastic changes. Standard histopathological analysis protocols were followed for the operated patients. Histopathological margins of the excised specimen were examined for the presence or absence of invasion by lesion or dysplasia.
Followed by neck dissection reconstruction was carried out using different types of myocutaneous flaps and buccal pad of fat.
Six months follow-up study with post-operative histopathological analysis was performed. Patients with positive margins were sent for post-operative radiotherapy.
The results obtained were subjected to statistical analysis. Statistical analysis was performed to calculate specificity of the vital stain. The cross tabulation between epithelium of the stained and unstained margins was done and subjected to Chi-square test to calculate the significance.
Results
Buccal mucosa emerged as the most common site of occurrence for oral squamous cell carcinoma (27%), followed by gingivobuccal sulcus (20%) and lateral aspect of tongue as the commonly occurring site (20%), maxilla involved (13%) one each at posterior and anterior maxilla, followed by retro-molar area (13%) and soft palate was the least site of occurrence for oral squamous cell carcinoma (7%). In our study, out of the 15 cases operated for oral squamous cell carcinoma, there were 11 cases with no recurrence, 2 cases with recurrence at primary site of lesion and 2 cases with recurrence at secondary sites. The Chi-square value for different surgical margins was found to be 0. The p value showed no statistically significant difference of staining between different surgical margins. The Chi-square value for different margins of the excised lesion was found to be 0.188. The p value showed no statistically significant difference of free and positive margins among different margins of the excised lesion. (Fig. 1, 2, 3)
Fig 1.

Squamous cell carcinoma of left lateral tongue
Fig 2.
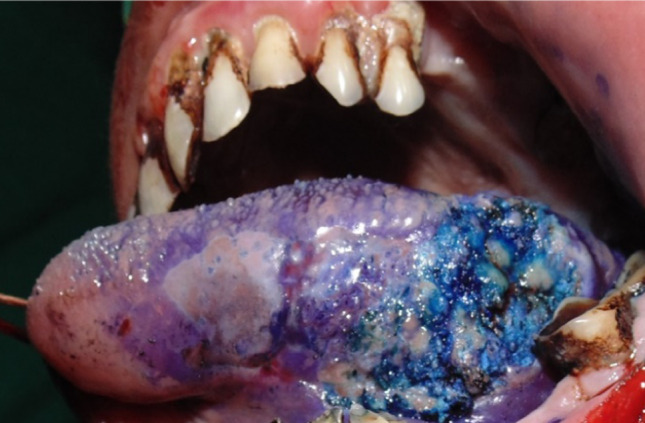
Stained dysplastic tissue using toluidine blue
Fig 3.
Histopathological procedure with microscopical view of margins
The Chi-square value between the criteria of this study was found to be 2.19. The p value showed no statistically significant difference of percentages among the three criteria (intra-operative staining, post-operative histopathological reports and recurrence of the disease). (Table 1, 2, 3)
Table 1.
Post-operative margins
| CASES | MARGINS | |||
|---|---|---|---|---|
| ANTERIOR | POSTERIOR | SUPERIOR | INFERIOR | |
| 1 | F M | F M | F M | F M |
| 2 | F M | F M | F M | F M |
| 3 | F M | F M | F M | F M |
| 4 | F M | F M | F M | F M |
| 5 | C i S | F M | F M | F M |
| 6 | I C | F M | F M | I C |
| 7 | F M | F M | F M | F M |
| 8 | F M | F M | F M | F M |
| 9 | M O D | F M | F M | F M |
| 10 | F M | I C | F M | F M |
| 11 | M O D | F M | M O D | F M |
| 12 | F M | F M | F M | F M |
| 13 | F M | F M | F M | F M |
| 14 | M O D | F M | F M | F M |
| 15 | I C | F M | F M | F M |
F M—Free margins: MOD—Moderate dysplasia: CIS—Carcinoma in situ: IC—Invasive carcinoma
Table 2.
Histopathological type of SSC at specimen margins
| ANTERIOR MARGINS |
POSTERIOR MARGINS |
SUPERIOR MARGINS |
INFERIOR MARGINS |
|||||
|---|---|---|---|---|---|---|---|---|
| NUMBER | % | NUMBER | % | NUMBER | % | NUMBER | % | |
| FREE MARGINS | 09 | 60.00 | 14 | 93.33 | 14 | 93.33 | 14 | 93.33 |
| CARCINOMA IN SITU | 01 | 6.67 | 00 | 00 | 00 | 00 | 00 | 00 |
| INVASIVE CARCINOMA | 02 | 13.33 | 01 | 6.67 | 00 | 00 | 01 | 6.67 |
| MODERATE | ||||||||
| DYSPLASIA | 03 | 20.00 | 00 | 00 | 01 | 6.67 | 00 | 00 |
| TOTAL | 15 | 100 | 15 | 100 | 15 | 100 | 15 | 100 |
CHI SQUARE VALUE = 12.5(P = 0.188 > 0.05), Shows no significant difference of free and positive margins among different margins of the excised lesion
Table 3.
6 months follow-up for recurrence
| Cases without any recurrence | Cases with recurrence at primary site | Cases with recurrence at secondary site |
|---|---|---|
| 11 | 02 | 02 |
The χ2 value for different margins of the excised lesion was found to be 0.188. The p value showed no statistically significant difference of free and positive margins among different margins of the excised lesion. Hence indicating a direct Co-Relation between Successful Intra-Operative Staining with Toluidine Blue Solution, Post-Operative Free Margin Histo-Pathological Reports and absence of recurrence of oral squamous cell carcinoma. In our study, the sensitivity of toluidine blue was found to be 0.933. Thus, it shows that TB has a higher sensitivity when compared to other stains.
Discussion
Many factors are linked with the prognosis of treatment of oral squamous cell carcinoma such as the tumor site, stage, thickness, histopathologic grade, microscopic invasion, cervical metastasis, number and anatomic level of positive nodes, presence and extent of extra-capsular spread. The status of resected margins has also been linked to prognosis [8]. Visual distinction between dysplasia and normal mucosa is based on differences between their color and texture. This makes visual clinical differentiation difficult. Studies have found a significant association between positive resection margins with development of loco-regional recurrence and also with a reduction in the disease free survival of the patient [9].
It was found that direct inspection of the lesion will always lead to varied results among examiners. Hence, direct inspection is not reliable enough to delineate the range of dysplastic epithelium, especially by novice surgeons [10].
Vital stains have long been considered as a possible answer to this problem. The stains that have been tried are toluidine blue, Lugol's iodine, Dental iodine glycerin 5% acetic acid, 0.4% indigo carmine and 0.5% Congo red [11–14].
The use of TB as a vital stain was first proposed by Richart to disclose dysplasia and carcinoma in situ of the uterine cervix. Neibel and Chomet and Shedd and co-workers were the first to report vital application of TB for the detection of premalignant and malignant lesions of the oral cavity [6].
In the present study, we used the toluidine blue to identify the squamous cell carcinoma margins intra-operatively to reduce errors in judgment made by clinical assessment. Toluidine blue was chosen as the material of choice due to its low cost, wide availability and ease of use.
According to Umeda M et al., 5 year disease specific survival rate was 93.8% with the use of Lugol’s iodine [14] and 100% with the use of Dental iodine as assessed by Kurita H et al. [10], while toluidine blue was found to be but without the use of any vital staining the survival rate declined to 75% [10].
The χ2 value between the criteria of this study was found to be 2.19. The p value showed no statistically significant difference of percentages among the criteria.
Conclusion
Toluidine blue is specific in demarcating the dysplastic tissue adjacent to carcinomatous lesion & increase the precision of the operating team in excising the dysplastic tissue surrounding the lesion leading to the decrease in recurrence at the primary site of the lesion. The stain was limited only to the upper layers of the epithelium; hence, depth evaluation by this stain is not possible. Sensitivity of toluidine blue was found to be 93.33%. The stain is constituted from materials which are easily available and inexpensive. Also, gives a gross visualization of dysplasia surrounding the lesion especially in cases where in the margins are not well defined. Hence, vital staining using toluidine blue is currently a useful and inexpensive adjunct to identify margins intra-operatively.
Declaration
Conflict of interest
The authors declare that there is no conflict of interest.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Byakodi R, Byakodi S, Hiremath S, Byakodi J, Adaki S, Marathe K, Mahind P. Oral cancer in India: an epidemiologic and clinical review. J Community Health. 2012;37:316–319. doi: 10.1007/s10900-011-9447-6. [DOI] [PubMed] [Google Scholar]
- 2.Warnakulasuriya S. Global epidemiology of oral and orophararyngeal cancer. Oral Oncol. 2009;45:309–316. doi: 10.1016/j.oraloncology.2008.06.002. [DOI] [PubMed] [Google Scholar]
- 3.Kurita H, Nakanishi Y, Nishizawa R, Xiao T, Kamata T, Koike T, et al. Impact of different surgical margin conditions on local recurrence of oral squamous cell carcinoma. Oral Oncol. 2010;46:814–817. doi: 10.1016/j.oraloncology.2010.08.014. [DOI] [PubMed] [Google Scholar]
- 4.Nomura T, Shibahara T. Detection of field alterations using useful tools for oral squamous cell carcinoma. Japanese Dental Sci Rev. 2013;49:106–115. doi: 10.1016/j.jdsr.2013.04.001. [DOI] [Google Scholar]
- 5.Ord RA, Aisner S. Accuracy of Frozen sections in assessing margins in oral cancer resection. J Oral Maxillofac Surg. 1997;55(7):663–669. doi: 10.1016/S0278-2391(97)90570-X. [DOI] [PubMed] [Google Scholar]
- 6.Sridharan G, Shankat AA. Toluidine blue: a review of its chemistry and clinical utility. J Oral Maxillofac Pathol. 2012;16(2):251–255. doi: 10.4103/0973-029X.99081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Onofre MA, Sposto MR, Navarro CM. Reliability of toluidine blue application in the detection of oral epithelial dysplasia and in situ and invasive squamous cell Carcinomas. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2001;91:535–540. doi: 10.1067/moe.2001.112949. [DOI] [PubMed] [Google Scholar]
- 8.Kerawala CJ, Beale V, Reed M, Martin IC. The role of vital tissue staining in the marginal control of oral squamous cell carcinoma. Int J Oral Maxillofac Surg. 2000;29:32. doi: 10.1016/S0901-5027(00)80121-6. [DOI] [PubMed] [Google Scholar]
- 9.Morgan JP, Haug RH, Kosman JW. Antimicrobial skin preparations for the maxillofacial region. J Oral Maxillofac Surg. 1996;54:89–94. doi: 10.1016/S0278-2391(96)90312-2. [DOI] [PubMed] [Google Scholar]
- 10.Kurita H, Kamata T, Xiangjun Li, Nakanishi Y, Shimane T, Koike T. Effectiveness of vital staining with iodine solution in reducing local recurrence after resection of dysplastic or malignant oral mucosa. Br J Oral Maxillofac surg. 2012;50:109–112. doi: 10.1016/j.bjoms.2011.03.006. [DOI] [PubMed] [Google Scholar]
- 11.Epstein JB, Scully C, Spinelli JJ. Toluidine blue and Lugol's iodine application in the assessment of oral malignant disease and lesions at risk of malignancy. J Oral Pathol Med. 1992;21:160–163. doi: 10.1111/j.1600-0714.1992.tb00094.x. [DOI] [PubMed] [Google Scholar]
- 12.Martin IC, Kerawala CJ, Reed M. The application of toluidine blue as a diagnostic adjunct in detection of epithelial dysplasia. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 1998;850:444–446. doi: 10.1016/S1079-2104(98)90071-3. [DOI] [PubMed] [Google Scholar]
- 13.Ravi SB, Annavajjula S. Surgical margins and its evaluation in oral cancer: a review. J Clin Diagn Res. 2014;8(9):1–5. doi: 10.7860/JCDR/2014/9755.4836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Umeda M, Shigeta T, Takahashi H, Minamikawa T, Komatsubara H, Oguni A, Shibuya Y, Komori T. Clinical evaluation of Lugol’s iodine staining in the treatment of stage I-II squamous cell carcinoma of the tongue. Int J Oral Maxillofac Surg. 2011;40:593–596. doi: 10.1016/j.ijom.2010.11.026. [DOI] [PubMed] [Google Scholar]



