Abstract
Introduction
The proponents of local route of Dexamethasone (DXM) administration for impacted mandibular 3rd molar (MM3) surgeries claim advantages over the traditional systemic routes. This systematic review and meta-analysis were aimed to determine whether the route of perioperative administration of DXM influences the inflammatory outcomes of MM3 surgeries.
Methodology
An electronic database search over a 25 year period of randomised trials of DXM in MM3 surgeries was conducted. The mean differences or standardised mean differences were extracted and pooled using the fixed or random-effects model.
Results
Of the sixteen selected trials, four were considered for a meta-analysis. There were no statistically significant differences in the inflammatory outcomes between the local and systemic routes of DXM.
Conclusion
The claimed advantages of the local route of DXM do not appear to be scientifically valid. Clinical trials supported with DXM plasma measurements are needed to confirm the absence of a systemic effect when DXM is administered locally.
Keywords: Third molar, Impacted, Dexamethasone, Oedema, Trismus, Placebo effect
Introduction
Corticosteroids (CS) are often used to minimise the post-operative inflammatory complications following impacted mandibular third molar (MM3) removal. Dexamethasone (DXM) with its long half-life, potency, and almost pure glucocorticoid activity makes it a safe and effective anti-inflammatory drug [1]. DXM is usually administered via the systemic routes viz; Intravenous, Intramuscular and Oral. With factors such as needle phobia and patient compliance, there have been a considerable number of trials that have investigated the efficacy of local injections of DXM for the control of inflammatory response following MM3 surgeries [2–6].
The local administration of DXM is claimed to have advantages such as localised action with minimal systemic effects, faster onset of action, need for a lower dose, and operator and patient comfort. Nevertheless, published systematic reviews and meta-analyses show conflicting conclusions on the efficacy of submucosal DXM compared to placebo/intramuscular routes in MM3 surgeries [7–10].
This article is aimed to examine the best available scientific evidence to determine if the route of administration of DXM (systemic or local routes) influenced the inflammatory complications of MM3 surgeries. Further, we also believe that the purported advantages of local administration of DXM may not be scientifically valid and need to be subject to critical scrutiny.
Materials and Methods
Search Strategy for Identification of Studies
The research question examined was “Is there a difference in inflammatory outcomes following the surgical removal of impacted MM3s when DXM is administered through a local or systemic route?”
The search strategy followed the PICOS format (Table 1).
Table 1.
Search strategy
| Search strategy | ||
|---|---|---|
| Population | #1 | "Molar, Third"[Mesh] OR “third molar surgery*”[tw] OR “mandibular third molar*”[tw] OR “third molar*”[tw] OR “wisdom teeth”[tw] OR “wisdom tooth”[tw] OR “impacted tooth”[tw] OR “impacted teeth”[tw]"Dexamethasone"[Mesh] OR "dexamethasone 21-phosphate" [Supplementary Concept] OR "Placebo Effect"[Mesh] |
| Intervention | #2 | submucosal[tw] OR submucosal[tw] OR “pterygomandibular space”[tw] |
| Comparison | #3 | oral[tw] OR intramuscular[tw] OR intramuscular[tw] OR intravenous[tw] OR intravenous[tw] OR intraosseous[tw] OR intraosseous[tw] OR “sublingual space”[tw] |
| Outcome | #4 | "Oedema"[Mesh] OR "Trismus"[Mesh] OR “Post-operative" OR “inflammatory outcome*”[tw] OR “post-operative sequel*”[tw] OR “restricted mouth opening”[tw] OR swelling[tw] OR oedema[tw] OR oedema[tw] |
| Study design | Randomised control trial & Quasi Randomised control trial | |
| Search combination | #1 AND #2 AND #3 AND #4 | |
| Language | English only | |
| Electronic database | The Cochrane Register of Controlled Trials (CENTRAL), MEDLINE (PubMed), Semantic Scholar & Google Scholar |
Study Eligibility and Selection Criteria (Table 2)
Table 2.
Inclusion and exclusion criteria
| Inclusion criteria | Exclusion criteria |
|---|---|
| Healthy adult patients (> 18 years) undergoing transalveolar extraction of impacted MM3 | Perioperative administration of a CS other than DXM in any patient group |
| Only randomised or quasi randomised clinical trials including parallel-group or split-mouth studies with not less than ten patients in each group | MM3 clinical trials comparing DXM with other pharmacological agents such as NSAIDS, etc. |
| Studies in which DXM has been peri-operatively administered in at least two patient groups by a systemic route [IV/IM/PO/IO/SL] and a local route [EA/SM/Loc IM/PMS] | Clinical Trials where the comparisons of post-operative outcome are only amongst either the local or systemic DXM routes |
| 72 h after surgery (Pain- VAS, Oedema- facial swelling measured by the distance between at least two facial points, Trismus- Reduced interincisal distance) | Clinical Trials where the post-operative outcome of local/systemic route of DXM are compared against a control/placebo |
| Restricted to publications in the English language over 25 years, viz. 1 January 1996—31 December 2020 |
Title and Abstract screening were performed independently by authors (RS and BK) for potential inclusion in the full-text review. Full-text articles were obtained for studies that seemed to satisfy the eligibility criteria from the initial screening or if there was inadequate information from the abstract alone that the inclusion or exclusion conditions had been met. Any disagreements were resolved by discussions between the two examiners. Also, if multiple publications from the same trial data were identified; and then, the selection of a single publication was again made through a consensus by discussion.
Data Extraction
The data collection was performed using a standardised form that was designed specifically for this systematic review. The data extraction form included authors country, year of publication, mean age, gender, nature of comparison group (parallel groups or split-mouth), number of patient groups, number of participants per group, DXM dose, DXM perioperative timing, DXM route of administration, outcomes measures (Pain, Trismus and swelling) at 72 h after surgery.
Risk of Bias and Quality of the Included Studies
The methodological quality of each included study was assessed using the Risk of Bias (ROB2) in randomised studies of interventions quality assessment tool [11].
Statistical Analysis
Meta-analysis was performed using RevMan Software (Version 5.3; Cochrane Collaboration). The effect size considered in the study was the difference in means (MD) for all the three outcomes; Oedema (facial swelling measured by the distance between at least two facial points in mm), Trismus (interincisal distance in mm), and VAS score for pain measured at third post-operative day. Meta-analysis was used to pool the difference in means between the systemic versus Local routes obtained from different studies separately for each outcome. Presence for heterogeneity was identified using Cochrane’s Q test and quantified using the I2 statistic. The pooled effect sizes along with their 95% confidence interval (CI) were reported.
Results
Systematic Search
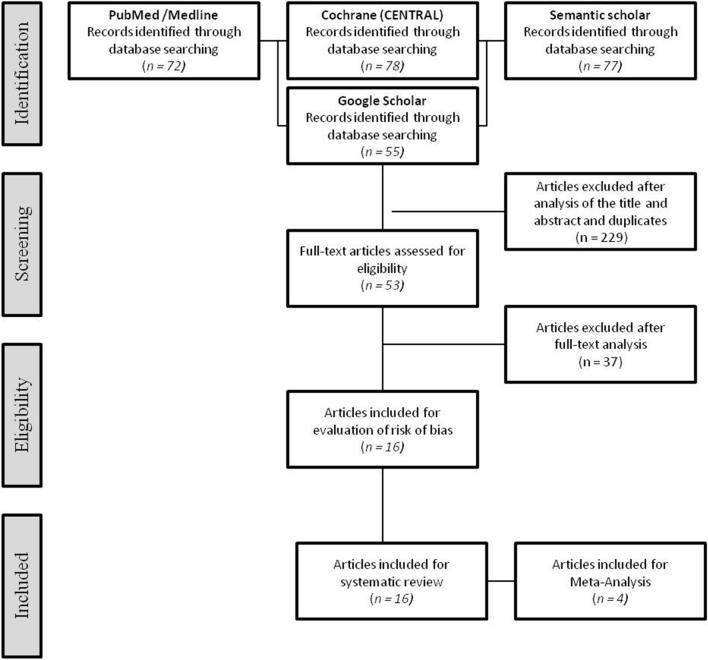
The search strategy resulted in 282 articles. Following the screening of title and abstracts and removal of duplicates, 229 articles were rejected. Of the 53 articles that underwent full-text assessment, 16 articles fulfilled the criteria for the qualitative and risk of bias assessment. Four articles were included for the meta-analysis (Fig. 1).
Fig. 1.
Study flow diagram (PRISMA format)
The characteristics of the 16 studies included in this systematic review are summarised in Table 3. The trials by Kaewkumnert et al. [21] and Moranon et al. [22] were the only crossover (split-mouth) RCTs. The sample sizes of the trials ranged from 20 to 80 participants, for a total of 631 randomised participants, excluding split-mouth studies. The age range of the pooled population was 18–50 years, with a mean age of 25.9 years. Gender distribution data were available for 13 studies, out of which only 6 provided the gender details amongst the patient groups. There was a skewed gender distribution towards male participants (M302; F208). Twelve trials reported the class and position of impacted third molars using the Pell and Gregory classification [4–6, 12–15, 17, 19–21, 24] and a single trial used the Pedersen difficulty index to select the participants. [18] Dosages of DXM ranged from 4 mg, [4, 6, 14–19, 21] 8 mg [5, 13, 20, 22, 24] combinations of different doses in different routes [12] and no dosage details in 1 trial [23]. A control/placebo group was included in 8 trials, with a majority of the control groups having no DXM administration [4, 6, 12–15, 17, 18].
Table 3.
Data extraction of the selected randomised trials
| Sl. No | Author | Year | Country | Mean age (SD) | Type of study | Gender | No of groups | No of patients per group | Administration time | Administration route | Dose of dexamethasone | Pell & Gregory Classification |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | Pappalardo et al. [12] | 2007 | Italy | 26.45 (4.2) | Parallel | 52M;48F | A | 20 | Before suturing | EA | 10 mg | Class I B |
| B | 20 | (unclear) | SM | 10 mg | ||||||||
| C | 20 | Intra-op | IV | 4 mg | ||||||||
| D | 20 | Intra-op | IM (Unclear) | 8 mg | ||||||||
| E | 20 | unclear | unclear | NA | ||||||||
| 2 | Antunes et al. [13] | 2011 | Brazil | 21 (5.4) | Parallel | 13M;5F | A | 18 | Pre-op | IM (masseter) | 8 mg | Class II B |
| 16M;4F | B | 20 | Pre-op | Oral | 8 mg | |||||||
| 8M;14F | C | 22 | NA(Control) | NA(Control) | NA(Control) | |||||||
| 3 | Majid et al [6] | 2011 | Iraq | 26.7 (6.3) | Parallel | 5M;5F | A | 10 | Post-op | SM | 4 mg | Class II/III A/B/C |
| 7M;3F | B | 10 | Post-op | IM (Unclear) | 4 mg | |||||||
| 4M;6F | C | 10 | NA(Control) | NA(Control) | NA(Control) | |||||||
| 4 | Majid et al [14] | 2013 | Iraq | 25.6(5.9) | Parallel | 8M;4F | A | 12 | Post-op | IM (deltoid) | 4 mg | Class II/III A/B/C |
| 8M;4F | B | 12 | Post-op | IV | 4 mg | |||||||
| 4M;8F | C | 12 | Post-op | PO | 4 mg | |||||||
| 4M;7F | D | 12 | Post-op | SM | 4 mg | |||||||
| 3M;9F | E | 12 | Post-op | EA | 4 mg (powder) | |||||||
| 4M;8F | F | 12 | NA(Control) | NA(Control) | NA(Control) | |||||||
| 5 | Bhargava et al. [4] | 2014 | India | 24.1(4.3) | Parallel | 34M;26F | A | 10 | NA(Control) | NA(Control) | NA(Control) | Class II B |
| B | 10 | Pre-op | PTM(Twin mix) | 4 mg | ||||||||
| C | 10 | Pre-op | SM | 4 mg | ||||||||
| D | 10 | Pre-op | IM | 4 mg | ||||||||
| E | 10 | Pre-op | IV | 4 mg | ||||||||
| F | 10 | Pre-op | PO | 4 mg | ||||||||
| 6 | Sablhok et al [15] | 2015 | India | NR | Parallel | A | 20 | NA(Control) | NA(Control) | NA(Control) | Class II B | |
| B | 20 | Post-op | PO | 4 mg | ||||||||
| C | 20 | Post-op | IM (Local) | 4 mg | ||||||||
| 7 | Gopalakrishnan et al. [16] | 2015 | India | 30 (NR) | Parallel | 22M;8F | A | 30 | Post-op | SM | 4 mg | NR |
| 20M;10F | B | 30 | NR | IM (unclear) | 4 mg | |||||||
| 8 | Saravanan et al. [17] | 2016 | India | NR | Parallel | NR | A | 20 | Pre-op | IM (unclear) | 4 mg | Class II A/B |
| B | 20 | Pre-op | SM | 4 mg | ||||||||
| C | 20 | NA(Control) | NA(Control) | NA(Control) | ||||||||
| 9 | Gopinath KA et al. [18] | 2017 | India | NR | Parallel | NR | A | 40 | Pre-op | SM | 4 mg | Pederson’s difficulty score of 5 to 10 were included |
| B | 40 | Pre-op | IV | 4 mg | ||||||||
| C | 40 | NA(Control) | NA(Control) | NA(Control) | ||||||||
| 10 | Kumar [19] | 2017 | India | 29 (NR) | Parallel | 9M;10F | A | 19 | Pre-op | SM | 4 mg | Class II/III A/B/C |
| 11M;8F | B | 19 | Pre-op | IV | 4 mg | |||||||
| 11 | Vivek et al. [20] | 2017 | India | 27 (NR) | Parallel | 28M;17F | A | 15 | Post-op | IV | 8 mg | Class II B |
| B | 15 | Post-op | SM | 8 mg | ||||||||
| C | 15 | Post-op | IM (unclear) | 8 mg | ||||||||
| 12 | Kaewkumnert S et al. [21] | 2019 | Thailand | 21.7(2.43) | Split-mouth | M13;F14 | A | 27 | Post-op | IO | 4 mg | Mesioangular/Horizontal |
| B | 27 | Post-op | SM | 4 mg | ||||||||
| 13 | Moranon et al. [22] | 2019 | Thailand | 21 (NR) | Split-mouth | M13;F17 | A | 30 | Pre-op | SLS | 8 mg | NR |
| B | 30 | Pre-op | PMS | 8 mg | ||||||||
| 14 | Mahmoud [23] | 2019 | Egypt | 27.6(4.8) | Parallel | M10;F5 | A | 15 | Pre-op | SM | NR | NR |
| 29.8(5.2) | 9M;6F | B | 15 | Pre-op | IM(Masseter) | NR | ||||||
| 28.5(5.9) | 9M;6F | C | 15 | Pre-op | IM(Systemic) | NR | ||||||
| 15 | Vivek et al. [5] | 2020 | India | 27 (NR) | Parallel | 38M;22F | A | 15 | Post-op | IV | 8 mg | Class II B |
| B | 15 | Post-op | SM | 8 mg | ||||||||
| C | 15 | Post-op | IM(Masseter) | 8 mg | ||||||||
| D | 15 | Post-op | PO | 8 mg | ||||||||
| 16 | Hiriyanna et al. [24] | 2020 | India | 28.67 (5.88) | Parallel | 20M;10F | A | 15 | Pre-op | SM | 8 mg | Class II/III A/B |
| 29.06 (5.73) | B | 15 | Pre-op | IV | 8 mg |
IV Intravenous; IM Intramuscular; PO Oral; IO Intraosseous; SL Sublingual; EA Endo-alveolar; SM Submucosal; Loc IM Local intramuscular (intramassetric); PMS Pterygomandibular space; VAS Visual analogue scale; M Male; F Female; SD Standard deviation; EA Endo-alveolar; IV Intravenous; IM Intramuscular; SM Submucosal; PO Peroral; PMS Pterygomandibular space; SLS Sublingual space; IO Intraosseous; NR Not reported; NA Not available
Quality Assessment
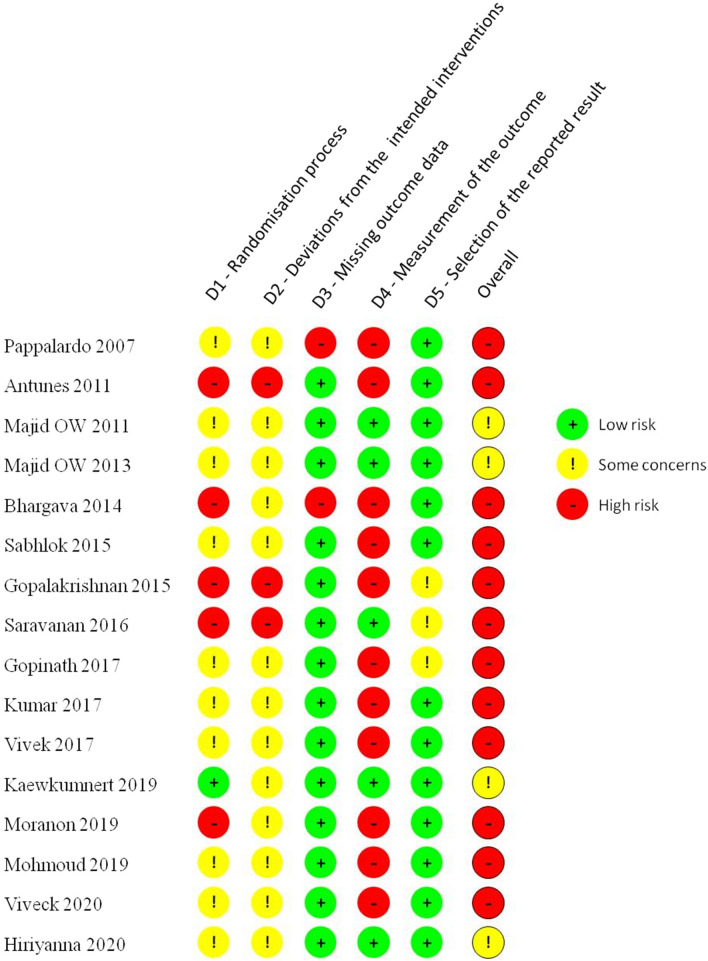
None of the trials had a low risk of bias across all domains. Following the exclusion of studies with a high risk of overall bias, there remained four studies with “some concern” of bias [6, 14, 21, 24] on whom a meta-analysis was performed (Fig. 2). Since there were fewer than ten studies, publication bias was not assessed.
Fig. 2.
Risk of Bias (ROB) assessment
Outcomes
Oedema
While 8 articles [4, 6, 14, 15, 17, 21, 22, 24] documented an equal response to post-surgical oedema following systemic or local dexamethasone administration, 3 articles [16, 18, 23] recorded oedema reduction with a systemic route and 1 article [19] with a local route. Pappalardo et al. [12] found that the IV and IM (systemic route) and EA (local) routes exerted better oedema control. Vivek et al. [5, 20] recorded comparable oedema control on day 1 with systemic and local routes, while on Day 3, IV and SM routes were better than the IM route.
Pain
Five articles [4, 6, 17, 21, 22, 24] documented an equal response to post-surgical pain following systemic or local dexamethasone administration. 4 articles [5, 19, 20, 23] recorded post-surgical pain reduction with a systemic route and 2 articles [16, 18] with a local route. However, an important point noticed in the study by Gopinath et al. [18] was that, while the local route was found superior in pain control compared to a control placebo group, no comparison between the systemic and local routes of DXM had been analysed. Likewise, Shablok et al. [15] provided no information on pain control achieved despite pain assessment being one of the outcome parameters in the methodology. Pappalardo et al. [12] found that the IV and IM (systemic route) and EA (local) routes exerted better pain control. Majid et al. [14] found that the IV route provided maximal pain relief, but the EA and SM routes exerted better pain control than the IM route.
Mouth Opening
Three articles [15, 18, 19] documented an equal response to post-surgical trismus following systemic or local DXM administration. Six articles [14, 16, 17, 21, 23, 24] recorded significant reductions on post-surgical trismus with a local route. Four articles [5, 6, 12, 20] recorded improved mouth opening with a systemic route of DXM.
As there were differences in mouth opening values on Day 1 and Day 3, certain additional points are given below for a better understanding.
-
I.
The study by Majid et al. [14] indicated that while all routes of DXM produced lesser trismus on D1 and D3 when compared to a control, on Day 1, the SM route of DXM was found to exert superior action on mouth opening, but on Day 3 the EA route had the least amount of trismus.
-
II.
The SM route in the study by Papillardo et al. [12] was found to exert the least effect on post-surgical trismus on Day 1. Further, on Day 1, the EA route had a similar impact to the IV and IM routes.
-
III.
Vivek et al. [20] on day 1 observed maximum benefit on mouth opening with the IM route compared to IV and SM routes, but the effect on day 3 was a reversal of Day 1.
-
IV.
Vivek et al. [5] on day 1 recorded a superior effect with the PO route, but on day 3 IV and SM routes exerted better outcomes than the Loc IM route.
-
V.
Gopinath et al. [18] found that SM and IV routes were better than control on day 2, but no comparison was done between the SM and IV groups.
Results of Meta-analysis
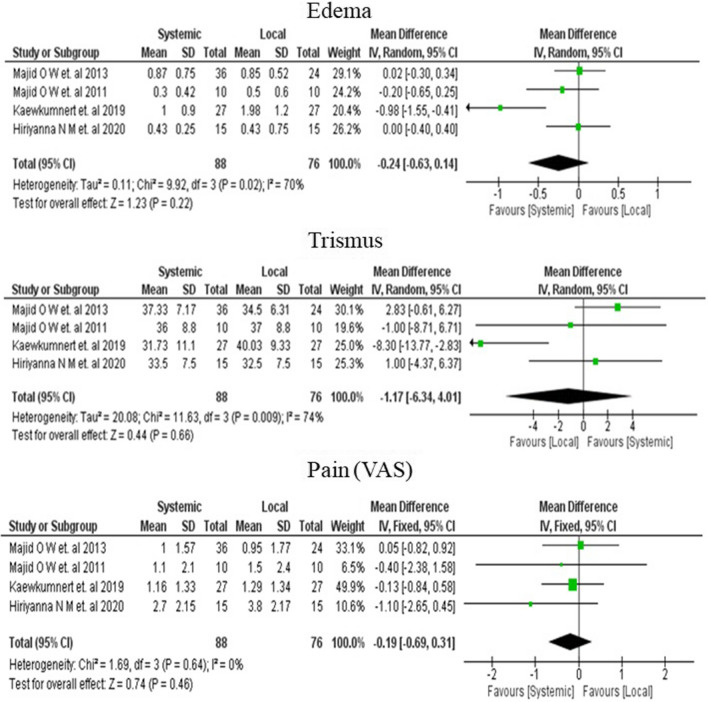
For the meta-analysis, all the routes of DXM administration in the four studies were clubbed into local or systemic routes, as explained in the study eligibility criteria. A random-effect (I2 > 50%) / Fixed effect model (I2 < 50%) was employed for outcome data of the 3rd post-operative day.
Oedema
Meta-analysis showed a pooled effect size of − 0.24 mm [95% CI; − 0.63, 0.14], indicating a reduction in oedema with the systemic route, albeit statistically insignificant (p − 0.22) (Fig. 3).
Fig. 3.
Forest plot showing the random-effect meta-analysis of mean difference in Oedema (mm), Trismus (mm) and fixed-effect meta-analysis of mean difference in Pain (VAS score) comparing systemic versus local routes of DXM on the third post-operative day
Trismus
Meta-analysis showed a pooled effect size of − 1.17 mm [95% CI; − 6.34, 4.01], suggesting that the local route is better. However, the result was not statistically significant (p − 0.66) (Fig. 3).
Pain
Meta-analysis showed a pooled effect size of − 0.19[95% CI; − 0.69,0.31], indicating a slight reduction in pain with the systemic route, although the effect was not significant statistically (p − 0.46) (Fig. 3).
Discussion
A critical examination of the conclusions of studies that have claimed a better efficacy with local routes of DXM seem to be derived from comparison to the placebo/control groups and not against systemic routes [4, 6, 12–15, 17, 18]. Earlier published meta-analyses had concluded on the effectiveness of the local route from the information provided by these studies. There is enough evidence in the literature confirming the positive influence of DXM on inflammatory outcomes after MM3 surgery and it is but natural to observe that DXM shows better results than a placebo. Conversely, as our systematic review examines the results of only systemic and local DXM routes and excludes placebos/control groups, the findings are more likely to accurately reflect the influence of the route of DXM on the inflammatory outcomes after MM3 surgery. The results of our meta-analysis indicate that the effect of local routes of DXM administration on MM3 surgical inflammatory outcomes are not statistically significant when compared to the systemic routes. Further, the systemic routes provide better outcomes for oedema and pain following MM3 surgeries.
The claimed advantages of local routes of DXM in M3 surgeries are a rapid onset of action, minimal systemic absorption, thereby ensuring a prolonged localised action with minimal side effects, and comfort for both patient and operator. It is necessary to ascertain if the above assumptions are scientifically valid.
a) Does the DXM injected directly into the site of action have a local action as intended with minimal systemic absorption and side effects?
Local delivery via injections is reserved for highly specialised therapies wherein the ability to accomplish systemic administration is hampered by poor perfusion or difficult-to-reach tissues [25]. Suppose a tissue site is either isolated or distinct from other nearby structures, then it may be possible to inject the drug directly into the tissue allowing a local therapeutic concentration to be reached. Since these sites have limited vascularity, only small amounts of the drug are transferred and diluted into the systemic volume of distribution [26]. However, the operative area in MM3 surgeries does not suffer from any of the above limitations. Moreover, the apprehension that a systemic route of DXM in MM3 surgery will cause side effects is unfounded as it is administered as a single dose. There are established guidelines that recommend intraosseous infusion in adults when intravenous access is unavailable. As the bioequivalence of intraosseous and intravenous routes are comparable, the presumption that intraosseous route exerts a purely local action is inaccurate [27].
b) What would be the appropriate dosage of DXM when it is administered locally?
If a drug could be deposited directly into the site of action, a significant advantage would be that a lower dose can be used to achieve the clinical effect. Further, since the process of distribution throughout the entire body can be avoided, it would imply that clinically effective levels at the site of action could be obtained more rapidly [26]. Yet, in all the studies that have recommended the effectiveness of a localised administration of DXM in MM3 surgeries, dosages have ranged from the usual 4–8 mg (Table 2). While such dosages are necessary for systemic routes to overcome the expected dilution in blood volume, the continued use of similar dosages for local routes is inexplicable. In fact, Moraschini et al. [7], in their meta-analysis, indicate that there is no scientific evidence demonstrating the advantage of administering SM DXM in doses in excess of 4 mg. Nonetheless, they still recommend that when preoperative corticosteroids are used, more concentrated doses may be needed since the use of a flap and tissue displacement can affect the concentration of the drug and its absorption into the bloodstream. This recommendation by Moraschini et al. contradicts the evidence from their meta-analysis.
c) Does the formulation of DXM matter?
Local drug administration requires specific formulations to allow the drug to remain at the application site for a sufficient length of time. The water solubility of glucocorticoids is very low, thus requiring their administration as a prodrug in the form of water-soluble esters such as DXM Sodium phosphate (DXMSP) [28]. Being a prodrug, the DXMSP must first undergo biotransformation by metabolic processes before becoming an active pharmacological agent for which entry into the systemic circulation is critical. Further, if a drug is intended to exert a local effect, absorption into the systemic vasculature would represent the loss of the drug from its primary site of activity [25]. Bhargava et al. compared the DXM plasma concentration using liquid chromatography following systemic gluteal IM and local PMS injections of 4 mg dexamethasone [29]. The mean plasma DXM concentration in the IM group was 226.41 + /48.67 ng/ml, and for the PMS group was 209.67 + /88.13 ng/ml and determined to be statistically insignificant. The interpretation that proximity administration of DXM is a “local route” is therefore erroneous from a pharmacological viewpoint. A purely localised action is not possible unless the DXM formulation is specifically altered such as DXM Acetate. In no study has there been a mention of altered formulations of DXM for local administration at the M3 surgical site. Despite a recommendation to evaluate the efficacy and safety of the acetate formulation of DXM in dentistry, there seems to be no published research on the use of DXM acetate in MM3 or dentoalveolar surgery even after two decades [1].
d) Is injecting IM DXM close to MM3 have a more rapid action than distant IM injections?
Messer et al. [30] were amongst the earliest advocates for using the masseter muscle as a route of administration of DXM for MM3 surgeries. They opined that the masseter muscle was in the immediate area, anaesthetised, and injection into the muscle could be considered part of the operative procedure. They claimed to have used the local DXM in over 5000 patients with a considerable decrease in all inflammatory outcomes. However, their study did not provide any objective data on inflammatory outcomes and their conclusions appear to be derived from clinical observations. Likewise, their assertion that the masseter muscle was anaesthetised is incorrect as its innervation is not addressed in the local anaesthesia blocks for MM3 surgeries.
When a drug is injected through the IM route, the injected volume spreads through the interstitial spaces and comes into close proximity with the muscle capillary network [26]. This would mean that the absorption of IM drug is determined by the bulk of the muscle and its vascularity [31]. It is often assumed that injecting the drug close to the site of surgery will result in better and faster efficacy of the injected drug. The increased blood flow in the maxillofacial region can possibly contribute to the faster entry of DXM into the systemic vasculature when injected into the masseter muscle. Selvaraj et al. [32] assessed the efficacy of IM steroid injections administered through the gluteal and masseter muscles and found no significant differences in the inflammatory complications. Similarly, other studies have reported that administration of high dose steroids into the masseter muscle did not improve anti-inflammatory action over injections at a distant site [13, 33]. Additionally, iatrogenic muscle injury resultant from such direct injections can contribute to post-operative trismus confounding the effectiveness of DXM.
Studies have thrown up varied results with findings ranging from no differences between IM and Oral DXM [13, 34], more effectiveness with IM [12], lesser effectiveness with IM DXM than SM DXM [6]. A meta-analysis examining the efficacy of IM DXM vs a placebo/control in MM3 surgeries suggested that since the rate of absorption of DXM is highly related to blood flow in the muscle of administration, there was a need to select a standardised muscle for future clinical trials of IM DXM in MM3 surgeries [35]. The assumption that injecting the DXM into a muscle close to MM3 would have an enhanced activity than other muscle sites is not borne out by available evidence.
e) Is the local route of DXM comfortable for operator and patient?
Dental surgeons who are not comfortable with performing IV/IM injections would find the local oral route more convenient. However, DXM injections, either SM or PMS, without a prior local anaesthesia injection can be quite painful and distressing to the patient. While Bhargava et al. [4] employed the use of Twin Mix injections into the PMS space before MM3 surgery, it is still likely to be causing some discomfort to the patient due to the time lag taken for the local anaesthesia to start acting. Another concern would be the effect of DXM on the disassociation constant (PKa and pH) of Lignocaine hydrochloride.
Conclusion
These results of the systematic review and meta-analysis cast doubt on the purported advantages of local routes of DXM in MM3 surgery. We hypothesise that the outcomes observed after local injections of DXM appears to be primarily due to systemic uptake and biotransformation rather than proximity to the MM3. An accurate picture can be obtained only with well-designed randomised clinical trials supported with DXM plasma measurements to confirm the absence of a systemic effect when DXM is administered locally.
Acknowledgements
We sincerely thank Drs Sreekumaran Nair (Dept. of Biostatistics, JIPMER) and Vishnuvardhan (Dept. of Biostatistics, Pondicherry University) for their valuable advice and inputs.
Funding
This manuscript received no funding.
Declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical Approval
The pre-specified protocol of this systematic review was registered with the International Prospective Register of Systematic Reviews (PROSPERO): (Registration No. CRD 42020149687).
Consent for Publication
Authors have presented the findings of this review as Oral paper in Annual conference of AOMSI, Mangaluru, India.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Alexander RE, Throndson RR. A review of perioperative corticosteroid use in dentoalveolar surgery. Oral Surg Oral Med Oral Pathol. 2000;90:406–415. doi: 10.1067/moe.2000.109778. [DOI] [PubMed] [Google Scholar]
- 2.Warraich R, Faisal M, Rana M, Shaheen A, Gellrich NC, Rana M. Evaluation of post-operative discomfort following third molar surgery using submucosal dexamethasone - a randomised observer blind prospective study. Oral Surg Oral Med Oral Pathol Oral Radiol. 2013;116:16–22. doi: 10.1016/j.oooo.2012.12.007. [DOI] [PubMed] [Google Scholar]
- 3.Grossi GB, Maiorana C, Garramone RA, Borgonovo A, Beretta M, Farronato D, Santoro F. Effect of submucosal injection of dexamethasone on post-operative discomfort after third molar surgery: a prospective study. J Oral Maxillofac Surg. 2007;65:2218–2226. doi: 10.1016/j.joms.2006.11.036. [DOI] [PubMed] [Google Scholar]
- 4.Bhargava D, Sreekumar K, Deshpande A. Effects of intra-space injection of twin mix versus intraoral-submucosal, intramuscular, intravenous and per-oral administration of dexamethasone on post-operative sequelae after mandibular impacted third molar surgery: a preliminary clinical comparative study. Oral Maxillofac Surg. 2014;18:293–296. doi: 10.1007/s10006-013-0412-7. [DOI] [PubMed] [Google Scholar]
- 5.Vivek GK, Vaibhav N, Shetty A, Mohammad I, Ahmed N, Umeshappa H. Efficacy of various routes of dexamethasone administration in reducing post-operative sequelae following impacted third molar surgery. Ann Maxillofac Surg. 2020;10:61–65. doi: 10.4103/ams.ams_66_19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Majid OW, Mahmood WK. Effect of submucosal and intramuscular dexamethasone on post-operative sequelae after third molar surgery: comparative study. Br J Oral Maxillofac Surg. 2011;49:647–652. doi: 10.1016/j.bjoms.2010.09.021. [DOI] [PubMed] [Google Scholar]
- 7.Moraschini V, Hidalgo R, Porto Barboza ED. Effect of submucosal injection of dexamethasone after third molar surgery: a meta-analysis of randomised controlled trials. Int J Oral Maxillofac Surg. 2016;45:232–240. doi: 10.1016/j.ijom.2015.09.008. [DOI] [PubMed] [Google Scholar]
- 8.Troiano G, Laino L, Cicciù M, Cervino G, Fiorillo L, D'amico C, Zhurakivska K, Lo ML. Comparison of two routes of administration of dexamethasone to reduce the postoperative sequelae after third molar surgery: a systematic review and meta-analysis. Open Dent J. 2018;12:181–188. doi: 10.2174/1874210601812010181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.O'Hare PE, Wilson BJ, Loga MG, Ariyawardana A. Effect of submucosal dexamethasone injections in the prevention of post-operative pain, trismus, and oedema associated with mandibular third molar surgery: a systematic review and meta-analysis. Int J Oral Maxillofac Surg. 2019;48:1456–1469. doi: 10.1016/j.ijom.2019.04.010. [DOI] [PubMed] [Google Scholar]
- 10.Larsen MK, Kofod T, Christiansen AE, Starch-Jensen T. Different dosages of corticosteroid and routes of administration in mandibular third molar surgery: a systematic review. J Oral Maxillofac Res. 2018;9(2):e1. doi: 10.5037/jomr.2018.9201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sterne JAC, Savović J, Page MJ, Elbers RG, Blencowe NS, Boutron I, Cates CJ, Cheng H-Y, Corbett MS, Eldridge SM, Hernán MA, Hopewell S, Hróbjartsson A, Junqueira DR, Jüni P, Kirkham JJ, Lasserson T, Li T, McAleenan A, Reeves BC, Shepperd S, Shrier I, Stewart LA, Tilling K, White IR, Whiting PF, Higgins JPT. RoB 2: a revised tool for assessing risk of bias in randomised trials. BMJ. 2019;366:l4898. doi: 10.1136/bmj.l4898. [DOI] [PubMed] [Google Scholar]
- 12.Pappalardo S, Puzzo S, Cappello V, Mastrangelo F, Adamo G, Caraffa A, Tete S. The efficacy of four ways of administrating dexamethasone during surgical extraction of partially impacted lower third molars. Eur J Inflamm. 2007;5:151–158. doi: 10.1177/1721727X0700500306. [DOI] [Google Scholar]
- 13.Antunes AA, Avelar RL, Martins Neto EC, Frota R, Dias E. Effect of two routes of administration of dexamethasone on pain, edema, and trismus in impacted lower third molar surgery. Oral Maxillofac Surg. 2011;15:217–223. doi: 10.1007/s10006-011-0290-9. [DOI] [PubMed] [Google Scholar]
- 14.Majid O, Mahmood W. Dexamethasone in oral surgery. Oral Surg. 2013;6:200–208. doi: 10.1111/ors.12049. [DOI] [Google Scholar]
- 15.Sabhlok S, Kenjale P, Mony D, Khatri I, Kumar P. Randomized controlled trial to evaluate the efficacy of oral dexamethasone and intramuscular dexamethasone in mandibular third molar surgeries. J Clin Diagn Res. 2015;9:48–51. doi: 10.7860/JCDR/2015/13930.6813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gopalakrishnan V, Darekar HS, Sahoo NK. Effectiveness of submucosal V/S intramuscular dexamethasone in mandibular third molar surgeries. Int J Med Sci Clin Inv. 2015;2:648–655. [Google Scholar]
- 17.Saravanan K, Kannan R, John RR, Nantha KC. A single pre operative dose of sub mucosal dexamethasone is effective in improving post operative quality of life in the surgical management of impacted third molars: a comparative randomised prospective study. J Maxillofac Oral Surg. 2016;15:67–71. doi: 10.1007/s12663-015-0795-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gopinath KA, Chakraborty M, Arun V. Comparative evaluation of submucosal and intravenous dexamethasone on post-operative sequelae following third molar surgery: a prospective randomised control study. Int J Oral Care Res. 2017;5:191–195. doi: 10.5005/jp-journals-10051-0095. [DOI] [Google Scholar]
- 19.Kumar HHR. Comparison of intravenous and submucosal dexamethasone on post-operative sequale following third molar surgery. J Adv Med Dent Scie Res. 2017;5:20–23. [Google Scholar]
- 20.Vivek GK, Vaibhav N, Shafath A, Imran M. Efficacy of intravenous, intramassetric, and submucosal routes of dexamethasone administration after impacted third molar surgery: a randomised, comparative clinical study. J Adv Clin Res Insights. 2017;4:3–7. doi: 10.15713/ins.jcri.146. [DOI] [Google Scholar]
- 21.Kaewkumnert S, Phithaksinsuk K, Changpoo C, et al. comparison of intraosseous and submucosal dexamethasone injection in mandibular third molar surgery: a split-mouth randomised clinical trial. Int J Oral Maxillofac Surg. 2020;49:529–535. doi: 10.1016/j.ijom.2019.10.006. [DOI] [PubMed] [Google Scholar]
- 22.Moranon P, Chaiyasamut T, Sakdajeyont W, Vorakulpipat C, Klongnoi B, Kiattavornchareon S, Wongsirichat N. Dexamethasone injection into pterygomandibular space versus sublingual space on post-operative sequalae of lower third molar intervention. J Clin Med Res. 2019;11:501–508. doi: 10.14740/jocmr3844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mahmoud NR. Efficacy of sub-mucosal, intra-masseteric and intra-muscular routes of Dexamethasone administration on post-operative complications following impacted mandibular third molar surgeries, comparative clinical trial. Egy Dent J. 2019;65:113–133. doi: 10.21608/edj.2015.71254. [DOI] [Google Scholar]
- 24.Hiriyanna NM, Degala S. Objective and subjective comparison of submucosal and intravenous routes of single-dose preoperative dexamethasone for mandibular third molar surgery-a prospective randomised observer-blind study. Oral Maxillofac Surg. 2021;25:207–213. doi: 10.1007/s10006-020-00904-0. [DOI] [PubMed] [Google Scholar]
- 25.Benet LZ. Effect of route of administration and distribution on drug action. J Pharmacokinet Biopharm. 1987;6:559–585. doi: 10.1007/BF01062110. [DOI] [PubMed] [Google Scholar]
- 26.Donavan MD (2009) Effect of route of administration and distribution on drug action. In: Florence AT, Siepmann J (Eds). Modern Pharmaceutics. Vol 1, 5th Edn, Basic Principles and Systems: Informa Healthcare USA, Inc, 155–79
- 27.Von Hoff DD, Kuhn JG, Burris HA, 3rd, Miller LJ. Does intraosseous equal intravenous? A pharmacokinetic study. Am J Emerg Med. 2008;26(1):31–38. doi: 10.1016/j.ajem.2007.03.024. [DOI] [PubMed] [Google Scholar]
- 28.Rohdewald P, Möllmann H, Barth J, Rehder J, Derendorf H. Pharmacokinetics of dexamethasone and its phosphate ester. Biopharm Drug Dispos. 1987;8:205–212. doi: 10.1002/bdd.2510080302. [DOI] [PubMed] [Google Scholar]
- 29.Bhargava D, Ahirwal R, Koneru G, Srikanth BR, Beena S. A preliminary study on plasma concentration achieved following intrapterygomandibular space injection of dexamethasone as a route of drug delivery with lignocaine inferior alveolar nerve block-correlation of clinical effects. Oral Maxillofac Surg. 2018;22:457–461. doi: 10.1007/s10006-018-0733-7. [DOI] [PubMed] [Google Scholar]
- 30.Messer E, Keller J. The use of intraoral dexamethasone after extraction of mandibular third molars. Oral Surg Oral Med Oral Pathol. 1975;40:594–598. doi: 10.1016/0030-4220(75)90369-2. [DOI] [PubMed] [Google Scholar]
- 31.Evans EF, Proctor JD, Fratkin MJ, Velandia J, Wasserman AJ. Blood flow in muscle groups and drug absorption. Clin Pharmacol Therapeut. 1975 doi: 10.1002/cpt197517144. [DOI] [PubMed] [Google Scholar]
- 32.Selvaraj L, Hanumantha Rao S, Lankupalli AS. Comparison of efficacy of methylprednisolone injection into masseter muscle versus gluteal muscle for surgical removal of impacted lower third molar. J Maxillofac Oral Surg. 2014;13:495–498. doi: 10.1007/s12663-013-0562-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Montgomery MT, Hogg JP, Roberts DL, Redding SW. The use of glucocorticosteroids to lessen the inflammatory sequelae following third molar surgery. J Oral Maxillofac Surg. 1990;48:179–187. doi: 10.1016/S0278-2391(10)80207-1. [DOI] [PubMed] [Google Scholar]
- 34.Boonsiriseth K, Klongnoi B, Sirintawat N, Saengsirinavin C, Wongsirichat N. Comparative study of the effect of dexamethasone injection and consumption in lower third molar surgery. Int J Oral Maxillofac Surg. 2012;41:244–247. doi: 10.1016/j.ijom.2011.12.011. [DOI] [PubMed] [Google Scholar]
- 35.Fernandes IA, de Souza GM, Pinheiro MLP, Falci SGM. Intramuscular injection of dexamethasone for the control of pain, swelling, and trismus after third molar surgery: a systematic review and meta-analysis. Int J Oral Maxillofac Surg. 2019;48:659–668. doi: 10.1016/j.ijom.2018.09.014. [DOI] [PubMed] [Google Scholar]