Abstract
Iron deficiency anemia (IDA) forms a major share of global burden of anemia. Frequent blood donation is a common iatrogenic cause of iron insufficiency in healthy adults. Serum iron and hemoglobin levels are normal despite low serum ferritin levels, referred to as latent iron deficiency (LID). Aim of the present study was to evaluate the role of novel RBC parameters–percentage of hypochromic RBCs (%HPO), percentage of microcytic RBCs (%MIC), and haemoglobin content of reticulocytes (MCHr) of Abbott Alinity autoanalyzer as indicators of latent iron deficiency in blood donors. 260 consenting and eligible blood donors were included in the study. Complete blood counts including new RBC parameters on Abbott Alinity autoanalyzer and serum iron profile were measured for all donors. Donors were categorized into LID and No LID based on Ferritin and Transferrin saturation (TSAT). Serum transferrin receptors (sTfR) were studied in a subset of samples [LID (n = 46), No LID (n = 18) and IDA (n = 27)]. Statistical analyses was done on IBM SPSS version 22. Among 260 donors, 56 (21.5%) were found to have LID. The difference in mean values for % HPO, % MIC, and MCHr were not found to be statistically significant in LID and No LID groups. sTfR results between LID, No LID and IDA sub-groups revealed significant difference. This study does not support the role of % HPO, % MIC and MCHr measured on Abott Alinity analyzer, as potential screening parameters for LID amongst blood donors. STfr was more informative in this regard. Further research on much larger sample size is required to confirm these findings.
Supplementary Information
The online version contains supplementary material available at 10.1007/s12288-023-01683-w.
Keywords: Latent iron deficiency, Blood donors, Iron deficiency, % HPO, % MIC, % MCHr
Introduction
Iron plays an important role in numerous cellular activities in body. Disruption of Iron balance leads to severe iron deficiency (ID), which is usually associated with anemia. Typically, iron deficiency anemia (IDA) is linked with a low iron saturation of accessible transferrin [1]. According to an Indian study, 9.5% of male and 26.7% of female regular voluntary donors developed anemia [2]. Hemoglobin (Hb) < 12 g/dL in females and < 13 g/dL in males, as well as serum ferritin < 15 mcg/L and low transferrin saturation (TSAT) < 15%, are used to establish laboratory diagnosis [3, 4]. IDA is preceded by a period of latent iron deficiency (LID), during which serum ferritin levels are decreased but hemoglobin and red cell indices remain normal. Individuals with latent iron deficiency usually have serum ferritin levels less than 20–30 mcg/L and haemoglobin levels > 12 g/dL. LID may be three times as prevalent as IDA. In the first stage, iron stores are depleted, resulting in low ferritin levels despite normal serum iron and hemoglobin levels. Persistent iron depletion in the absence of mobilizable iron results in low hemoglobin levels, referred to as IDA. Various hematological parameters namely Hb, hematocrit (Hct), and red blood cell (RBC) indices like mean corpuscular hemoglobin concentration (MCHC), mean corpuscular hemoglobin (MCH), mean corpuscular volume (MCV), and Red cell distribution width (RDW) and biochemical parameters like Serum iron, serum ferritin, total iron binding capacity (TIBC), TSAT, soluble transferrin receptors (sTfR), and zinc erythrocyte protoporphyrin (ZEP) have been used in detection of IDA. Red cells become microcytic and hypochromic in overt iron deficiency resulting in low MCV and MCH, however these changes do not manifest yet in LID [5–7]. ID is more prevalent in young infants [8, 9], females of reproductive age [10, 11], patients with CRF, and habitual blood donors. Since, donors are screened for overt iron deficiency anemia, donors with normal hemoglobin levels but low iron stores may end up donating blood, despite LID. As a result, this subgroup of donors are at risk of developing frank IDA. The purpose of this study was to assess the diagnostic efficacy of novel RBC parameters, specifically the percentage of hypochromic RBCs (% HPO), the percentage of microcytic RBCs (% MIC), and the hemoglobin content of reticulocytes (%MCHr), as predictors of latent iron deficiency in blood donors.
Materials & Methods
Study Design
A cross-sectional study on whole-blood donors was performed after taking informed consent. Donors who attended the blood bank attached to a tertiary care hospital in North India (RMLIMS, Lucknow, Uttar Pradesh) from Februaury 2021 to May 2022 were included in the study. The study was approved by the institutional ethics committee prior to commencement (IEC-144/20) and partially funded by the Student fund, RMLIMS.
Donor Selection Criteria
A total of 260 voluntary blood donors were included and their respective peripheral blood samples were tested.
Inclusion Criteria
Consenting blood donors who had Hb level of ≥ 12.5gm/dl and meeting the selection criteria for whole blood donation mentioned in Drugs and Cosmetic Act,1940 [12]/ criteria mentioned in Directorate General of Health Services (DGHS) Technical Manual, 2003 [6] were included in study.
Exclusion Criteria
All donors who did not fulfill the selection criteria for blood donation or did not provide written consent for the study. Additionally, those donors were excluded in whom, after performing complete blood counts, thalassemia trait was suspected based on red cell indices or in whom MCV > 101 fl was detected.
Study Methodology
Blood samples from the study subjects were collected for hematological and biochemical tests. Hematological tests included Complete blood counts (CBC). % HPO, % MIC and MCHr were performed on Abbott Alinity Hq autoanalyzer, available in the Hematology laboratory of the Department of Pathology. CBC was carried out within 3 h of blood collection. Samples with red cell indices suggestive of thalassemia trait or with MCV > 101 fl were excluded. Additional donors were then recruited to attain the desired sample size. Biochemical tests, including Serum ferritin, Serum Iron, TIBC and TSAT (calculated) were performed on the cobas e601 and c501 analyzer (Roche diagnostic Switzerland). A total of 5 ml whole blood sample was collected, divided into plain vial (3 ml) and EDTA vial (2 ml). The donors were divided into two categories: LID and No LID (no deficiency). Gold standard criteria for diagnosis of latent iron deficiency included serum ferritin (15–30 mcg/L), Transferrin saturation (< 15%) and Hemoglobin (normal for age and sex) [7].
Additional testing for Serum transferrin receptors (sTfR) was performed in a subgroup of LID (n = 46), No LID (n = 18) (blood donor cohort) and IDA (n = 27) subjects (known cases enrolled from medicine department after taking consent) on Cobas e501 analyzer (Roche diagnostic Switzerland) to validate the correctness and authenticity of subgroups and additionally study the utility of sTfR.
Statistical Analysis
Data collected were entered in MS Excel and analyzed using IBM SPSS, version 22.0, licensed to Dr RMLIMS. Descriptive summary such as mean, standard deviation, median, IQR, range etc., was calculated for various blood parameters for LID and No LID. Statistical difference in blood parameters was determined using the Unpaired T-test at 95% confidence interval. ROC analysis was done to determine the test's validity and appropriate cut-off values. Spearman correlation analysis was performed among the various parameters. P-values < 0.05 were considered statistically significant.
Results
A total of 260 blood donors were enrolled for the study, including 205 males (78.5%) and 55 females (21.5%). 56 of these 260 donors (21.5%) were found to have LID and 204 (78.5%) had No LID, based on the diagnostic criteria. Mean ± SD, Median and age range of LID and No LID groups was 34.14 ± 5.15, 32, 26–45.12 years and 33.71 ± 4.85, 31, 26–44.12 years respectively. A statistically non-significant difference (p = 0.7374) was observed in mean age among the groups suggesting similarity of the study groups with respect to age. 16.5% males and 40% female donors were found to have LID.
On comparison of different parameters between the LID and No LID groups, separately for male and female donors, the difference between mean ± SD values were found to be statistically significant (P < 0.05) for two parameters, namely serum Ferritin and TSAT, as expected. For other parameters, namely Hb (g/dl), RBC(106/ul), HCT(%), MCV(fl), MCH(pg), MCHC(g/dl), RDW(%), %MIC(fl), %HPO(g/dl) and MCHr(pg), the differences between the two groups were not statistically significant (P > 0.05) (Table 1).
Table 1.
Comparison of results for all the measured parameters between the LID and No LID groups in both sexes
| Parameter | Total enrolled donors | Enrolled donor males | Enrolled donor females | ||||||
|---|---|---|---|---|---|---|---|---|---|
| LID [N = 56] |
NO LID [N = 204] |
P-Value | LID [N = 34] |
NO LID [N = 171] |
P-Value | LID [N = 22 |
NO LID [N = 33] |
P-Value | |
| HB(g/dl) | 13.86 ± 0.73 | 13.86 ± 1.02 | p > 0.9999 | 14.20 ± 0.79 | 14.01 ± 0.89 | p = 0.2486 | 13.10 ± 1.16 | 12.87 ± 0.38 | p = 0.2935 |
| RBC(106/ul) | 4.39 ± 0.57 | 4.57 ± 0.48 | p = 0.0628 | 4.65 ± 0.36 | 4.63 ± 0.44 | p = 0.8037 | 3.96 ± 0.61 | 4.28 ± 0.57 | p = 0.0525 |
| HCT(%) | 41.09 ± 4.27 | 42.51 ± 3.31 | p = 0.0730 | 42.55 ± 2.62 | 43.13 ± 2.97 | p = 0.2907 | 37.49 ± 4.01 | 39.73 ± 4.32 | p = 0.2135 |
| MCV(fl) | 92.74 ± 5.91 | 92.65 ± 7.61 | p = 0.9252 | 92.47 ± 5.98 | 92.56 ± 7.72 | p = 0.9488 | 92.36 ± 8.03 | 93.51 ± 6.70 | p = 0.5672 |
| MCH(pg) | 30.31 ± 2.66 | 30.54 ± 2.72 | p = 0.5714 | 30.20 ± 1.63 | 31.19 ± 3.28 | p = 0.3235 | 30.68 ± 2.73 | 29.96 ± 2.97 | p = 0.1579 |
| MCHC(g/dl) | 32.40 ± 1.21 | 32.49 ± 0.95 | p = 0.6099 | 32.67 ± 1.22 | 32.65 ± 0.89 | p = 0.9109 | 32.27 ± 0.81 | 31.72 ± 0.97 | p = 0.0525 |
| RDW(%) | 14.01 ± 1.56 | 13.93 ± 1.74 | p = 0.7421 | 13.84 ± 1.17 | 13.92 ± 1.60 | p = 0.7821 | 15.24 ± 2.32 | 14.18 ± 2.15 | p = 0.0884 |
| %MIC(fl) | 2.41 ± 2.63 | 2.64 ± 4.25 | p = 0.6189 | 2.32 ± 1.98 | 2.64 ± 4.40 | p = 0.6785 | 3.74 ± 4.74 | 2.42 ± 3.13 | p = 0.2183 |
| %HPO(g/dl) | 6.70 ± 7.03 | 5.37 ± 6.58 | p = 0.2080 | 5.73 ± 5.74 | 5.12 ± 6.47 | p = 0.6099 | 6.45 ± 6.56 | 7.24 ± 8.17 | p = 0.7062 |
| MCHr(pg) | 28.73 ± 2.48 | 28.72 ± 2.41 | p = 0.9787 | 28.27 ± 2.14 | 28.63 ± 2.29 | p = 0.3986 | 28.73 ± 2.47 | 29.37 ± 2.97 | p = 0.4071 |
| S. FERRITIN (mcg/L) | 18.20 ± 7.08 | 105.80 ± 79.44 | p< 0.0001* | 16.90 ± 7.68 | 103.92 ± 76.20 | p< 0.0001* | 20.31 ± 5.74 | 116.28 ± 97.11 | p< 0.0001* |
| TSAT(%) | 9.98 ± 10.80 | 22.58 ± 13.50 | p< 0.0001* | 16.08 ± 9.71 | 26.94 ± 10.17 | p< 0.0001* | 12.14 + 3.35 | 23.84 ± 6.49 | p< 0.0001* |
Values expressed as mean + − SD. P value < 0.05 significant *
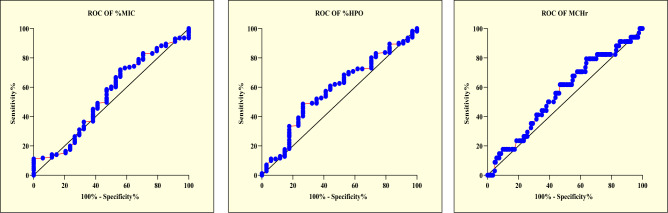
The ROC curve analysis was done for the three potential diagnostic parameters under study- namely the %MIC; %HPO and MCHr separately for male and female donors. The area under the curve and p-value were calculated. Also, the most appropriate cut-off value was determined from the curve and sensitivity and specificity was calculated with this cut-off value for all three parameters (Figs. 1 & 2) (Supplementary Table 1).
Fig. 1.
Graphical representation of ROC analysis for the novel RBC parameters among male donors. a Graphical representation of ROC analysis of %MIC: male donors. Cut off <1.56, AUC 0.54, p 0.41. b Graphical representation of ROC analysis of %HPO: male donors. Cut off <3.63, AUC 0.58, p 0.12. c Graphical representation of ROC analysis of MCHr: male donors. Cut off <27.81, AUC 0.55, p 0.27
Fig. 2.
Graphical representation of ROC analysis for the novel RBC parameters among female donors. a Graphical representation of ROC analysis of %MIC: female donors. Cut off <1.67, AUC 0.52, p 0.77. b Graphical representation of ROC analysis of %HPO: female donors. Cut off <4.39, AUC 0.54, p 0.53. c Graphical representation of ROC analysis of MCHr: female donors. Cut off <28.78, AUC 0.54, p 0.55
While analyzing spearman correlation between the measured parameters %MIC, %HPO and MCHr with MCV, MCH and MCHC, all parameters showed a significant correlation with % MIC except MCHC (p = 0.8348) and MCH (p = 0.0519); with % HPO except MCHC (p = 0.1764), MCH (p = 0.3841) and MCHr (p = 0.0979); with MCHr except MCH (P = 0.2456) and %HPO (p = 0.0979. (Supplementary Table 2; Supplementary Fig. 1).
As the novel RBC Parameters %MIC, %HPO and MCHr did not perform well for detection of LID in blood donors, we conducted an additional analysis based on sTfR on a subgroup of donors and known patients of Iron deficiency anaemia. This was done to confirm the correctness and authenticity of the subgroups and additionally study the utility of sTfR. This included LID = 46 (25 males, 21 females); No LID/control donors = 18 (9 males, 9 females) and IDA = 27 (10 males, 17 females). sTfR values (nmol/L) in No LID, LID, and IDA were 2.45 + − 0.43, 3.29 + − 0.59, 4.7 + − 1.16 with the difference being statistically significant (p < 0.05). (Table 2). This pattern was maintained in further analysis on male and female donors separately. (data not shown).
Table 2.
Comparison of soluble transferrin receptor, serum ferritin and TSAT values in LID, IDA and No LID donors/controls
| Parameters | LID donors n = 46 (both sexes) (mean + − SD) |
IDA n = 27 (both sexes) (mean + − SD) |
No LID donors/Control n = 18 (both sexes) (mean + −SD) |
Min value | Max value | P value |
|---|---|---|---|---|---|---|
| sTfR (nmol/L) | 3.29 + − 0.59 | 4.7 + − 1.16 | 2.45 + − 0.43 | 2.13 | 5.6 | < 0.05 |
| S.FERRITIN (mcg/L) | 30.69 + − 74.55 | 10.34 + − 4.92 | 123.15 + − 102.15 | 5.3 | 193.3 | < 0.05 |
| TSAT (%) | 14.19 + − 17.32 | 22.4 + − 17.95 | 27.64 + − 17.19 | 4.3 | 50.6 | < 0.05 |
Discussion
Latent iron deficiency (LID) is defined as a hematological condition in which iron stores, i.e. ferritin levels, are found to be low, but hemoglobin levels and often the hematocrit and red blood indices are still found within normal reference ranges [13] or there is evidence of iron deficiency without anaemia (normal hemoglobin level) [14]. It is also named as iron-deficient erythropoiesis [15], non-anemic iron deficiency (NAID), iron deficiency without anaemia (IDA) and depleted iron stores (DIS) [16]. LID if not checked timely, leads to iron deficiency anaemia. LID is characterized in individuals with serum ferritin ≤ 20 mcg/L and hemoglobin > 12 g/dL [17, 18]. LID was detected in 21.5% donors (16.5% males, 40% females) emphasizing the need for actively screening voluntary donor pool for iron deficiency.
The age of the blood donors included in the study, ranged from 26 years to a maximum of 45 years with a median age of 32 years for both the groups of LID and No LID. The study groups were comparable and show a similar trend as previously reported literature [19–21].
We found no significant differences in the mean values of hematocrit (%), MCV, MCHC, MCH, RDW, %MIC, %HPO, and MCHr in both the LID and No LID groups, when analysed separately for both the gender groups (p = 0. > 05) This implies that none of these parameters have proven to be suitable diagnostic parameters for latent iron deficiency in blood donors. S. ferritin and TSAT (%) showed significant difference in both LID and No-LID groups for each gender (p = < 0.05). As indicated by Mayoclinic and others, the normal hematocrit range is 35.5% to 44.9% for women [22], while for men it ranges from 41 to 50%. According to literature, the normal range for MCV in healthy individuals is 80–100 fl [23, 24]. Hasan A. Al-Jafar reported that in his study population, the LID males with S. ferritin between 12.5 and 21 had an MCV range of 85–90 fl and the LID females with serum ferritin between 5.7 and 10 had an MCV range of 85–92 fl [25].
Furthermore, the cut off value of %MIC set for males and females from ROC analysis were < 1.560 and < 1.670, respectively which are very close to median values of %MIC count and hence resulted in 54.97% sensitivity and 52.94% specificity for males and 54.55% sensitivity and 54.55% specificity for females. Abbott Alinity analyser is based on MAPSS Technology and %MIC is derived from the RBC volume distribution histogram. It includes RBCs with a volume below 60 fl. This cut-off appears too low to detect LID as in LID, RBCs are not expected to become so small. Probably, this explains the insignificant p value for this parameter. The measuring principle for %micro-R (percentage of microcytic RBCs) in Sysmex auto analyzer is based on Impedence and hydrodynamic focusing where % micro-R is derived from RBC volume distribution histogram and samples with microcytic RBCs are shifted to left of histogram curve. Literature reports %micro-R as a useful parameter for detecting LID. Threshold used for defining %micro-R and %MIC might be different in the two systems resulting in different sensitivities for detecting LID.
Norani Amir et.al. reported that %Hypo-He (percentage of RBC with cellular hemoglobin content) at a cut-off of 0.6% (Sysmex) had a sensitivity of 88.24% sensitivity and specificity of 88.24% in detecting LID in blood donors, thereby supporting its role as a potential screening parameter [5]. Malczewska-Lenczowska J et al., worked on the use of erythrocyte hypochromia markers in the detection of iron deficiency in adolescent female athletes and found that the %HPOm had a mean value of 1.20 ± 2.1 with a range of 0.04–13.4. They concluded that of all the parameters, female subjects with ‘latent iron deficiency’ had a significantly increased value of %HPOm, that was 3.82 ± 3.94 compared to females with normal S ferritin [26].
However, in our study, the ROC cut-off values of % HPO set for males and females were < 3.630 and < 4.390, respectively, close to median values of % HPO count, resulting in 60.82% sensitivity and 55.88% specificity of %HPO for males and 60.61% sensitivity and 59.09% specificity of %HPO for females. Thus, %HPO also could not stand out as a potential screening parameter for latent iron deficiency in blood donors. For measurement of RBC-He, Sysmex Autoanalyzer uses reticulocyte (RET) channel where this parameter is derived from a proprietary algorithm. Abott Alinityhq analyzer is based on MAPSS technology for calculation of % HPO. Probably the different measuring principles in both analysers have resulted in discordance of findings between the two.
Reticulocyte haemoglobin content (MCHr) is usually reported with the reticulocyte count, with a reference range of 30–38 pg. A value below 30 pg indicates iron deficient erythropoiesis. Cai et al. reported a mean cut-off value of reticulocyte haemoglobin content for diagnosing iron deficiency anaemia as 27.2 pg, with a sensitivity of 87.5% and a specificity of 92.9% [27]. Urrechagaet et. al. worked on the predictive value of MCHr for diagnosis of LID and reported that MChr in LID group was 26.9 pg, which was statistically significantly different from MCHr value (30.9 pg) of normal population (P < 0.0001). ROC analysis (gold standard Ferritin < 30 µg/L) revealed a cut-off value of 30.0 pg, with a sensitivity of 84.1% and specificity of 71.1%. They finally stated that MCHr is a reliable test for investigating ID and could improve the detection of iron deficient adults [28].
However, the ROC curve analysis for MCHr in our study population, based on 55.88% sensitivity and 56.14% specificity for males and 57.58% sensitivity and 59.09% specificity for females highlighted the lack of usefulness of %MCHr as a potential screening parameter for latent iron deficiency in blood donors.
The additional analysis done on Serum Transferrin receptors on a subgroup of our study population of donors as well as known patients of Iron deficiency anaemia, revealed that the mean + / − SD values were significantly different in the three study groups. Similar pattern was observed for both males and females, analysed separately. Osman Yokus et al., reported that Mean ± SD of sTfR was lower in Non-Iron deficiency group [1.90 ± 1.15] than in Iron Deficiency group [5.99 ± 2.98]. A significant difference (p < 0.05) was observed between the groups [29]. Leonard et al., reported that Mean + / − SD of sTfR was lower in LID group [0.99 + − 0.20] than in Iron deficiency group [1.26 + − 0.36]. A statistically significant difference (p < 0.01) was observed between the groups [30] similar to the results in our study population. Hence, sTfR was able to discriminate LID from No LID and also highlighted that our case definitions and categorization of donors into LID and No LID sub-groups was accurate.
Conclusion
In our study, the ROC based sensitivity and specificity for the parameters analysed i.e. percentage of hypochromic RBCs (% HPO), percentage of microcytic RBCs (% MIC), and hemoglobin content of reticulocytes (%MCHr) on Abotth Alinity Hq autoanalyzer, do not support their role as potential screening parameters to detect latent iron deficiency amongst habitual/non-habitual blood donors. More elaborate research on much larger sample size with comparable number of female subjects is required to confirm these findings. To the best of our knowledge, this is the first study evaluating the role of novel RBC parameters, provided by Abbott Alinity Hq hematology analyser, for detection of LID. Similar parameters have been studied on Sysmex series of analysers and have been found to have acceptable sensitivity and specificity. Studies should be conducted including more than one analyzer, that are based on different measurement principles, to compare their performance (e.g., Sysmex and Abbott). Study population of cases and controls (LID and No LID) should be kept similar in size to avoid subject number-based bias and study findings should also be validated on a geographically different study population.
Supplementary Information
Below is the link to the electronic supplementary material.
Funding
The study was partially funded by the Student fund, RMLIMS.
Declarations
Conflict of interest
The authors have no relevant financial or non-financial interests to disclose.
Ethical Approval
Approval was obtained from the Institutional Ethics Committee. The procedures used in this study adhere to the tenets of the Declaration of Helsinki.
Consent to Participate
Informed consent was obtained from all individual participants included in the study.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Zhu YP, Liao QK (2004) Collaborative study group for “The epidemiological survey of iron deficiency in children in China”. [Prevalence of iron deficiency in children aged 7 months to 7 years in China]. Zhonghua Er Ke Za Zhi 42(12):886–91. Chinese. PMID: 15733354 [PubMed]
- 2.Mahida VI, Bhatti A, Gupte SC. Iron status of regular voluntary blood donors. Asian J Transfuse Sci. 2008;2(1):9–12. doi: 10.4103/0973-6247.39504.PMID:20041071;PMCID:PMC2798760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Camaschella C (2019) Iron deficiency. Blood. 133(1):30–39. 10.1182/blood-2018-05-815944. Epub 2018 Nov 6. Erratum in: Blood. 2023; 141(6):682. PMID: 30401704 [DOI] [PubMed]
- 4.Lopez A, Cacoub P, Macdougall IC, Peyrin-Biroulet L. Iron deficiency anemia. Lancet. 2016;387(10021):907–916. doi: 10.1016/S0140-6736(15)60865-0. [DOI] [PubMed] [Google Scholar]
- 5.Amir N, Md Noor S, Subbiah I, Osman M, Seman Z. Percentage of hypochromic red cells as a potential screening test to evaluate iron status in blood donors. Int J Lab Hematol. 2019;41(3):418–423. doi: 10.1111/ijlh.13009. [DOI] [PubMed] [Google Scholar]
- 6.WHO (2011) Hemoglobin concentrations for the diagnosis of anemia and assessment of severity. Vitamin and mineral nutrition information system. Geneva, World Health Organization, (WHO/NMH/NHD/MNM/11.1) (http://www.who.int/vmnis/indicators/haemoglobin.pdf)
- 7.Milman N, Kirchhoff M. Influence of blood donation on iron stores assessed by serum ferritin and hemoglobin in a population survey of 1433 Danish males. Eur J Haematol. 1991;47(2):134–139. doi: 10.1111/j.1600-0609.1991.tb00136.x. [DOI] [PubMed] [Google Scholar]
- 8.Sikosana PL, Bhebhe S, Katuli S. A prevalence survey of iron deficiency and iron deficiency anemia in pregnant and lactating women, adult males and pre-school children in Zimbabwe. Cent Afr J Med. 1998;44(12):297–305. [PubMed] [Google Scholar]
- 9.Milman N, Taylor CL, Merkel J, Brannon PM. Iron status in pregnant women and women of reproductive age in Europe. Am J Clin Nutr. 2017;106(Suppl 6):1655S–1662S. doi: 10.3945/ajcn.117.156000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Nissenson AR, Strobos J. Iron deficiency in patients with renal failure. Kidney Int Suppl. 1999;69:S18–21. doi: 10.1046/j.1523-1755.1999.055suppl.69018.x. [DOI] [PubMed] [Google Scholar]
- 11.Jain A, Chowdhury N, Jain S, Uttam N, Meinia SK. Altered red cell indices in repeat blood donors: experience of a North Indian blood bank. Indian J Hematol Blood Transfuse. 2018;34(4):666–670. doi: 10.1007/s12288-018-0954-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Okoroiwu HU, Asemota EA. Blood donors deferral prevalence and causes in a tertiary health care hospital, southern Nigeria. BMC Health Serv Res. 2019;19(1):510. doi: 10.1186/s12913-019-4352-2.PMID:31331326;PMCID:PMC6647304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Arcanjo FP, Arcanjo CP, Santos PR. Schoolchildren with learning difficulties have low iron status and high anemia prevalence. J Nutr Metab. 2016;2016:7357136. doi: 10.1155/2016/7357136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Yehuda S, Mostofsky DI (2010) Iron deficiency and overload: from basic biology to clinical medicine. Humana Press, New York, vol 307, pp P165–167
- 15.Suominen P, Punnonen K, Rajamäki A, Irjala K. Serum transferrin receptor and transferrin receptor-ferritin index identify healthy subjects with subclinical iron deficits. Blood. 1998;92(8):2934–2939. doi: 10.1182/blood.V92.8.2934.420k07_2934_2939. [DOI] [PubMed] [Google Scholar]
- 16.Iron deficiency, Sydpath (2006) The pathology service of St Vincent’s hospital, Sydney, Australia, useful summary of blood tests for iron status
- 17.Alkhaldy HY, Hadi RA, Alghamdi KA, Alqahtani SM, Al Jabbar ISH, Al Ghamdi IS, Bakheet OSE, Saleh RAM, Shehata SF, Aziz S. The pattern of iron deficiency with and without anemia among medical college girl students in high altitude southern Saudi Arabia. J Family Med Prim Care. 2020;9(9):5018–5025. doi: 10.4103/jfmpc.jfmpc_730_20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.(WHO) (2001) Iron deficiency anemia assessment prevention and control: a guide for programme managers
- 19.Misje AH, Bosnes V, Heier HE. Gender differences in presentation rates, deferrals and return behavior among Norwegian blood donors. Vox Sang. 2010;98(3 Pt 1):e241–e248. doi: 10.1111/j.1423-0410.2009.01267.x. [DOI] [PubMed] [Google Scholar]
- 20.Erhabor O, Adias TC, Mainasara A. Provision of safe blood transfusion services in a low income setting in West Africa. Case study of Nigeria. In: Berhardt LV, editor. Blood transfusions: procedures, risks and role in disease treatment. 1. New York: Nova Science Publishers; 2013. pp. 1–58. [Google Scholar]
- 21.Patel RM, Lukemire J, Shenvi N, Arthur C, Stowell SR, Sola-Visner M, Easley K, Roback JD, Guo Y, Josephson CD. Association of blood donor sex and age with outcomes in very low-birth-weight infants receiving blood transfusion. JAMA Netw Open. 2021;4(9):e2123942. doi: 10.1001/jamanetworkopen.2021.23942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hematocrit test [Internet]. Mayoclinic.org. 2021. Available from: https://www.mayoclinic.org/tests-procedures/hematocrit/about/pac-20384728
- 23.Martin L (2021) Mean corpuscular volume (MCV) levels: what is normal? [Internet]. Medical news today.com.. Available from: https://www.Medicalnnews today.com/articles/mcv-levels
- 24.Maner BS, Moosavi L (2022) Mean corpuscular volume. [Updated 2022 Jul 4]. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing. Available from: https://www.ncbi.nlm.nih.gov/books/NBK545275/ [PubMed]
- 25.Al-Jafar HA. HWA: hypoferritinemia without anemia a hidden hematology disorder. J Family Med Prim Care. 2017;6(1):69–72. doi: 10.4103/2249-4863.214986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Malczewska-Lenczowska J, Orysiak J, Szczepańska B, Turowski D, Burkhard-Jagodzińska K, Gajewski J. Reticulocyte and erythrocyte hypochromia markers in detection of iron deficiency in adolescent female athletes. Biol Sport. 2017;34(2):111–118. doi: 10.5114/biolsport.2017.64584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Cai J, Wu M, Ren J, Du Y, Long Z, Li G, Han B, Yang L. Evaluation of the efficiency of the reticulocyte hemoglobin content on diagnosis for iron deficiency anemia in Chinese adults. Nutrients. 2017;9(5):450. doi: 10.3390/nu9050450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Urrechaga Igartua E, Hoffmann JJML, Izquierdo-Álvarez S, Escanero JF. Reticulocyte hemoglobin content (MCHr) in the detection of iron deficiency. J Trace Elem Med Biol. 2017;43:29–32. doi: 10.1016/j.jtemb.2016.11.001. [DOI] [PubMed] [Google Scholar]
- 29.Yokus O, Yilmaz B, Albayrak M, Balcik OS, Helvaci MR, Sennaroglu E. The significance of serum transferrin receptor levels in the diagnosis of the coexistence of anemia of chronic disease and iron deficiency anemia. Eurasian J Med. 2011;43(1):9–12. doi: 10.5152/eajm.2011.02. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Leonard AJ, Chalmers KA, Collins CE, Patterson AJ. A study of the effects of latent iron deficiency on measures of cognition: a pilot randomized controlled trial of iron supplementation in young women. Nutrients. 2014;6(6):2419–2435. doi: 10.3390/nu6062419. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.