Abstract
To identify the role in periodontal inflammatory diseases of human gingival fibroblasts (HGF), the major constituents of gingival tissue, the expression of CD14, a possible lipopolysaccharide (LPS) receptor, and the release of soluble CD14 (sCD14) by HGF were examined. Among the HGF samples from the nine donors tested, more than 50% of the HGF from five donors expressed CD14 but less than 20% of HGF from the other four donors did so, as determined by flow cytometric analysis. The CD14 expression on the cell surface was correlated with the expression of CD14 mRNA. The HGF and skin and lung fibroblasts tested expressed no CD18, which indicates that fibroblasts do not possess other LPS receptors, such as CD11b/CD18 and CD11c/CD18. The CD14 expression by the HGF was decreased after subculturing and was highest at the confluent stage of culture. The treatment of high-CD14-expressing (CD14high) HGF with phosphatidylinositol-phospholipase C reduced CD14 expression; this result and the increase in a 55-kDa CD14 indicate that the membrane CD14 (mCD14) on the HGF may be a 55-kDa glycosylphosphatidylinositol-anchored protein. CD14high HGF spontaneously released 48- and 57-kDa sCD14. The total release of sCD14 by the HGF was augmented by gamma interferon and Escherichia coli LPS in accordance with the increased expression of mCD14. The CD14high HGF secreted interleukin-8 in response to LPS, and the secretion was completely inhibited by anti-CD14 antibody. These results suggest that (i) HGF consist of populations that are heterogeneous on the basis of different levels of expression of CD14 and (ii) CD14high HGF secrete inflammatory cytokines in response to LPS via CD14.
CD14 is a myeloid cell differentiation molecule expressed strongly on monocytes and weakly on neutrophils, and it functions as a receptor for lipopolysaccharide (LPS) combined with LPS-binding protein (36, 38, 41). CD14 exists in two forms: a glycosylphosphatidylinositol (GPI)-anchored protein on the cell surface (membrane CD14 [mCD14]) (16), and a soluble form (soluble CD14 [sCD14]) found in serum and urine (3). Although the origin of sCD14 is unclear, sCD14 is shed from the surfaces of monocytes upon activation by gamma interferon (IFN-γ), LPS, and other stimuli, suggesting that sCD14 is derived from GPI-anchored mCD14 (4, 8, 12). sCD14 is required for the activation of CD14-deficient endothelial and epithelial cells by LPS (13, 28). Recent reports have provided evidence that two members of the leukocyte integrin family, CD11b/CD18 and CD11c/CD18, are involved in LPS-induced cell activation (19, 25, 39, 40).
Human gingival fibroblasts (HGF) are the major constituents of gingival tissue, producing various inflammatory cytokines such as interleukin-1 (IL-1), IL-6, and IL-8 upon stimulation with bacteria and their components, including LPS from oral black-pigmented bacteria such as Porphyromonas gingivalis and Prevotella intermedia, which may in turn initiate and exacerbate periodontal inflammatory diseases (33–35). Human dermal fibroblasts have been reported to not produce IL-1 or IL-8 in response to common LPS from members of the family Enterobacteriaceae such as Escherichia coli and Salmonella species (21, 30, 31). It is still unclear whether HGF are able to respond to common LPS from enterobacterial species in addition to that from black-pigmented bacteria. In this connection, it should also be resolved whether LPS-induced HGF activation is mediated by CD14. The findings of recent studies regarding the expression of CD14 by HGF are contradictory. Watanabe et al. (37) reported that HGF express mCD14 and that the LPS-induced signal-transducing pathway is mediated by mCD14. In contrast, Hayashi et al. (15) found that HGF expressed CD14 neither on the surface nor in an mRNA analysis but were activated with LPS in a serum-derived sCD14-dependent manner.
It is suggested that fibroblasts in periodontal and other tissues consist of heterogeneous populations (27). We therefore investigated whether HGF express mCD14 in several HGF cultures from different individuals. We found heterogeneous mCD14 expression in the HGF subjects. We then examined the possible release of CD14 by HGF, characterized the CD14 molecule, and analyzed the biological function of CD14-expressing HGF in response to E. coli LPS.
MATERIALS AND METHODS
Reagents.
LPS of E. coli O55:B5 was obtained from Sigma Chemical Co. (St. Louis, Mo.). Bacillus cereus phosphatidylinositol-specific phospholipase C (PI-PLC) was purchased from Boehringer Mannheim (Tokyo, Japan). Human natural IFN-γ (antiviral activity, 8.0 × 106 IU/mg of protein) was kindly provided by the Hayashibara Bioscience Institute (Okayama, Japan). Alpha minimal essential medium (α-MEM) and fetal calf serum (FCS) were obtained from Flow Laboratories (McLean, Va.) and Gibco BRL Life Technologies (Auckland, New Zealand), respectively. All other reagents were obtained from Sigma unless otherwise indicated.
Cells and cell culture.
HGF were prepared from the explants of normal gingival tissues of 8- to 18-year-old patients under informed consent (34). The explants were cut into pieces and cultured in 100-mm-diameter tissue culture dishes (Falcon; Becton Dickinson Labware, Lincoln Park, N.J.) in α-MEM supplemented with 10% FCS with a medium change every 3 days for 10 to 15 days until confluent cell monolayers were formed. After three to four subcultures by trypsinization, homogeneous, slim, spindle-shaped cells grown in characteristic swirls were obtained. The cells were used as confluent monolayers at subculture levels 5 through 15.
Human lung fibroblasts (IMR-90, WI-38, and MRC-5) and human skin fibroblasts (SF-MA) were obtained from the Japanese Cancer Research Resources Bank (Tokyo, Japan) and cultured in α-MEM with 10% FCS. Another human skin fibroblast line, FS-4, was generously supplied by M. Kohase, National Institute of Infectious Diseases (Tokyo, Japan).
Human blood mononuclear cells (MNC) were isolated from heparinized blood of healthy adult donors by Ficoll-Isopaque density gradient centrifugation (1). The isolated MNC were washed three times with phosphate-buffered saline (PBS) and suspended in RPMI 1640 medium.
For the treatment with PI-PLC, HGF were collected by trypsinization, and HGF (105 cells) and MNC (106 cells) were treated with medium (20 μl) containing 0.2 to 5.0 U of PI-PLC per ml for 1 h at 37°C. The cellular viability of the HGF after this PI-PLC treatment was more than 95%, as assessed by a 0.2% trypan blue exclusion test. After the treatment, the cell suspensions were centrifuged. The cells were analyzed for the expression of CD14, and the amount of CD14 in the supernatants was determined by an sCD14 enzyme-linked immunosorbent assay (ELISA). For the time kinetics of CD14 expression, confluent HGF were subcultured at 105 cells/well in 5 ml of α-MEM with 10% FCS in a six-well Falcon dish for up to 7 days.
Flow cytometry.
Flow cytometric analyses were performed with a fluorescence-activated cell sorter (FACS) (FACScan; Becton Dickinson, Mountain View, Calif.). For immunofluorescent staining, fibroblasts were collected by trypsinization, washed in PBS, and used for staining. We determined that trypsinization did not affect the amounts of CD14 and other surface markers detected by the FACS. The following directly conjugated monoclonal antibodies (MAbs) were used: anti-CD14 (Leu-M3, mouse immunoglobulin G2b [IgG2b]) (conjugated to phycoerythrin [PE]), anti-CD18 (mouse IgG1) (conjugated to fluorescein isothiocyanate [FITC]), anti-CD54 (intercellular adhesion molecule-1 [ICAM-1], mouse IgG2b) (PE) (obtained from Becton Dickinson), and anti-human leukocyte antigen (HLA)-DR (FITC) (mouse IgG1) (obtained from Nichirei, Tokyo, Japan). Cells were also stained with anti-CD14 (MEM-18, mouse IgG1 [Monosan, Uden, The Netherlands]; MY4, mouse IgG2b [Coulter, Miami, Fla.]) and anti-HLA-ABC (W6/32, mouse IgG2a [Dako, Glostrup, Denmark]) at 4°C for 30 min, followed by incubation with FITC-conjugated goat anti-mouse IgG (Biosource, Camarillo, Calif.) at 4°C for a further 30 min. Preliminary experiments revealed that the staining pattern of the isotype-matched control MAb and second Ab and that of the second Ab only showed no difference. Anti-CD14 (MEM-18), anti-CD18, and anti-HLA-DR were isotype-matched murine IgG1 MAbs, and anti-CD14 (Leu-M3 and MY4) and anti-CD54 (ICAM-1) were isotype-matched murine IgG2b MAbs. The use of isotype-matched MAbs also excludes the possibility of the nonspecific binding of anti-CD14 MAbs (MEM-18 and MY4). To calculate the percentage of positive cells, the baseline cursor was set at a channel that yielded less than 2% of the events positive with a second Ab control. Fluorescence to the right was counted as specific binding.
Reverse transcriptase PCR (RT-PCR) assay.
Several HGF were incubated with or without IFN-γ (200 IU/ml) for various periods in a humidified atmosphere of 95% air and 5% CO2 at 37°C. Total cellular RNA was extracted from 2.5 × 106 cells (one 100-mm-diameter plate) by Isogen (Nippon Gene, Tokyo, Japan) according to the manufacturer’s instructions. Reverse transcription of the RNA samples to cDNA was done with Moloney murine leukemia virus reverse transcriptase (GIBCO BRL, Grand Island, N.Y.) and oligo(dT)12-18 primer (GIBCO BRL). To transcribe the total RNA into cDNA, 2.5 μg of RNA, 0.25 μg of oligo(dT)12-18 primer, First-Strand Buffer (GIBCO BRL), 0.2 mM deoxynucleoside triphosphates, 500 U of Moloney murine leukemia virus reverse transcriptase, and 10 mM dithiothreitol were added to a total volume of 50 μl. The reaction mixture was incubated for 1 h at 37°C followed by 5 min at 95°C. The primers used for PCR had the following sequences: CD14, forward 5′-CTCAACCTAGAGCCGTTTCT-3′ and reverse 5′-CAGGATTGTCAGACAGGTCT-3′; and human glyceraldehyde-3-phosphate dehydrogenase (GAPDH), forward 5′-TGAAGGTCGGAGTCAACGGATTTGGT-3′ and reverse 5′-CATGTGGGCCATGAGGTCCACCAC-3′. The primers for CD14 and GAPDH were constructed to generate fragments of 426 and 983 bp, respectively. The PCR mixture contained 5 μl of the cDNA mixture, 2 μl of 10× PCR buffer, 0.2 mM deoxynucleoside triphosphates, 50 pmol of each primer, and 0.1 μl of Ex Taq DNA polymerase (Takara, Tokyo, Japan) in a total volume of 20 μl. Amplification was performed in a model MP TP3000 PCR thermal cycler (Takara) as follows: with CD14, 25 cycles of denaturation at 94°C for 1 min, annealing at 65°C for 1 min, and extension at 72°C for 1 min and then a final extension at 72°C for 3 min; and with GAPDH, 25 cycles of denaturation at 94°C for 1 min, annealing at 60°C for 1 min, and extension at 72°C for 1 min and then a final extension at 72°C for 3 min. Amplified samples were visualized on 2.0% agarose gels stained with ethidium bromide and photographed under UV light.
Detection of sCD14 and IL-8 by ELISA.
For the detection of sCD14 in the HGF culture supernatant, confluent HGF in six-well plates in 5 ml of α-MEM with 10% FCS were cultured in the absence or presence of LPS (100 ng/ml) or IFN-γ (200 IU/ml) for 3 days. At the time indicated, the supernatants were collected. The level of sCD14 in the supernatants was determined by using a human sCD14 ELISA kit (Biosource).
For the determination of IL-8 produced by HGF in response to LPS, confluent HGF in 96-well flat-bottomed plates in 200 μl of α-MEM with 1% FCS were stimulated with various doses of LPS for 24 h. The HGF in the 96-well plates in the medium were incubated with dialyzed anti-CD14 MAb (MY4) at 1:100 and 1:1,000 dilutions or with dialyzed isotype control mouse IgG2b (Becton Dickinson) at a 1:100 dilution at 37°C for 30 min. Antibody-treated cells were stimulated with 10 ng of LPS per ml for 24 h. After the incubation, the supernatants were collected and the level of IL-8 in the supernatants was determined with a human IL-8 ELISA kit (Biotrak, Amersham Life Science, Little Chalfont, Buckinghamshire, England).
The sCD14 and IL-8 assays were performed exactly as instructed by the ELISA manufacturers. The concentrations of sCD14 and IL-8 in the supernatants were determined by using the Softmax data analysis program (Molecular Devices Corp., Menlo Park, Calif.). Each sample was assayed in duplicate.
Preparation of membrane and cytoplasm fractions.
HGF were collected by trypsinization, and the crude cell membrane fraction was prepared from HGF by hypotonic lysis in 10 mM Tris-HCl–1 mM MgCl2 (pH 7.4) at 4°C (20 min on ice followed by Dounce homogenization). The homogenate was mixed with a 0.25 M sucrose solution and centrifuged twice at 500 × g for 5 min at 4°C. A crude membrane fraction was recovered after centrifugation of the nucleus-free supernatant at 15,000 × g for 30 min at 4°C. A suspension of HGF (106 cells/40 μl) in the same buffer as that used for the membrane preparation was frozen-thawed three times in liquid nitrogen and centrifuged at 15,000 × g for 10 min at 4°C to remove nuclei and debris. Samples were suspended in Laemmli sample buffer (22), and samples from 0.25 × 106 cells were used for a Western blot analysis.
sCD14 detection by Western blotting.
Sodium dodecyl sulfate-polyacrylamide gel electrophoresis analyses were performed in 10% polyacrylamide slab gels containing 0.1% sodium dodecyl sulfate under reducing conditions, according to the method of Laemmli (22). Proteins were transferred to a polyvinylidene difluoride membrane (ATTO Co., Tokyo, Japan) by a semidry transblot system (ATTO). The blots were blocked for 4 h with 3% bovine serum albumin–PBS followed by incubation with anti-CD14 MAb (MEM-18, 1:100) in 3% bovine serum albumin–PBS with 0.1% NaN3 overnight at 4°C. The blots were washed three times with 0.02% Tween 20–PBS and then incubated for 2 h with biotinylated anti-mouse IgG (Vector Laboratories, Burlingame, Calif.) and 1 h with the Vectastain ABC system (Vector). After being washed, the CD14 glycoproteins were visualized with diaminobenzidine. The molecular weights of the proteins were estimated by comparison with the positions of standards (Pharmacia Biotech, Uppsala, Sweden). Bands were analyzed using a LabScan and ImageMaster 1D software (Pharmacia Biotech).
Data analysis.
All experiments in this study were performed at least three times to test the reproducibility of the results, and representative results are shown. In some experiments, experimental values are given as means ± standard deviations. The statistical significance of differences between two means was evaluated by Student’s unpaired t test, and P values less than 0.05 were considered significant.
RESULTS
Heterogeneous expression of CD14 by HGF.
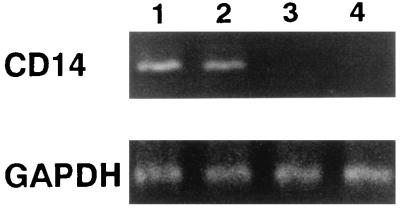
To examine the expression of mCD14 on HGF, we tested HGF from different donors. Fibroblasts from lung and skin were also tested as a control. Among the HGF samples from the nine donors, five showed weak reactivity with the Leu-M3 anti-CD14 MAb (range, 6.3 to 15.6% positive) but reacted at higher intensity with the MEM-18 anti-CD14 MAb (range, more than 50% positive) (Table 1). The HGF from the other four donors showed only weak reactivity with MEM-18 (less than 20%). The fibroblasts from lung and skin showed no reactivity with Leu-M3 or MEM-18. CD54 (ICAM-1) was expressed on all of the fibroblasts to some extent, but CD18 and HLA-DR were not expressed on any of the fibroblasts tested. MEM-18, anti-CD18, and anti-HLA-DR MAbs are isotype-matched MAbs (mouse IgG1), which excludes the possibility of nonspecific binding of MEM-18 to the cell surface. To test the evidence obtained by flow cytometry, the high-CD14-expressing (CD14high) and the low-CD14-expressing (CD14low) HGF were analyzed for the constitutive expression of CD14 mRNA by RT-PCR (Fig. 1). The CD14high HGF from two donors expressed CD14 mRNA, but CD14 mRNA from CD14low HGF from two other donors was not visible by this method. These results showed that (i) HGF consist of heterogeneous populations on the basis of their expression of mCD14 and CD14 mRNA and (ii) MEM-18 reacted more strongly than did Leu-M3 with the CD14 molecules, as reported previously (24, 41).
TABLE 1.
Expression of CD14, CD18, CD54, and HLA-DR on the surfaces of fibroblasts of different originsa
| Fibroblasts (name or donor) | Origin | % Positive cellsb
|
||||
|---|---|---|---|---|---|---|
| CD14 (Leu-M3) | CD14 (MEM-18) | CD18 | CD54 | HLA-DR | ||
| IMR-90 | Lung | 0.4 | 1.1 | 0.2 | 91.4 | 0.5 |
| WI-38 | Lung | 0.4 | 3.9 | 1.1 | 76.9 | 0.9 |
| MRC-5 | Lung | 1.1 | 0.5 | 1.3 | 80.9 | 1.2 |
| FS-4 | Skin | 0.5 | 0.7 | 0.4 | 70.1 | 0.2 |
| SF-MA | Skin | 1.4 | 1.9 | 1.2 | 41.5 | 1.6 |
| HN | Gingiva | 1.4 | 17.2 | 0.7 | 50.2 | 0.7 |
| KT | Gingiva | 1.5 | 19.1 | 0.9 | 46.4 | 1.2 |
| MH | Gingiva | 6.3 | 56.1 | 0.8 | 51.2 | 0.6 |
| MM | Gingiva | 15.5 | 52.9 | 0.5 | 55.1 | 0.7 |
| MY | Gingiva | 14.7 | 51.9 | 0.9 | 50.6 | 1.5 |
| NK | Gingiva | 1.9 | 10.6 | 0.7 | 40.4 | 0.7 |
| SM | Gingiva | 15.6 | 66.3 | 1.1 | 19.5 | 1.2 |
| TT | Gingiva | 15.1 | 53.2 | 0.6 | 78.1 | 0.8 |
| YN | Gingiva | 1.5 | 9.6 | 0.8 | 87.8 | 0.6 |
Fibroblasts from confluent cultures were collected and stained with the MAbs indicated.
The percentage of positive cells was analyzed by flow cytometry.
FIG. 1.
Heterogeneous expression of CD14 mRNA by CD14high and CD14low HGF. CD14high HGF (lane 1, donor MH; lane 2, donor SM) and CD14low HGF (lane 3, donor KT; lane 4, donor NK) from confluent cultures were collected by trypsinization. RNA was extracted from the cells, and its cDNA was prepared and analyzed by RT-PCR. The results are representative of three different experiments.
High expression of mCD14 on HGF at the confluent stage of culture.
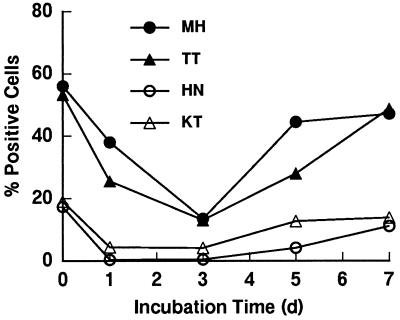
Preliminary experiments demonstrated that the expression of mCD14 on HGF varied among the experiments, even though HGF cultures from the same donor were used. This finding suggests that mCD14 expression was modulated at different stages of the culture. It was therefore of interest to examine the time kinetics of mCD14 expression by HGF. The CD14high HGF of two donors and CD14low HGF of two other donors were examined. As shown in Fig. 2, the CD14high HGF from confluent cultures highly expressed CD14 (more than 50% on day 0). Upon reculturing, the expression of CD14 by these HGF was gradually decreased until day 3 and then increased to a plateau level at day 5 or 7, the time when the cells became confluent. The CD14low HGF showed the same time kinetics, although CD14 expression by the CD14low HGF was less than 20%. These results suggest that the CD14 expression was increased at the confluent stage of HGF culture and also confirmed the finding that the expression of CD14 on HGF was heterogeneous.
FIG. 2.
Time kinetics of mCD14 expression by HGF from four individuals. After HGF were harvested from confluent cultures from 100-mm-diameter dishes (day [d] 0), they were recultured in six-well plates at 105 cells/well in 5 ml of culture medium. At the times indicated, the cells were collected and stained with anti-CD14 MAb (MEM-18) and analyzed by FACS. The results are representative of four different experiments.
CD14 expression on the HGF as a GPI-anchored protein.
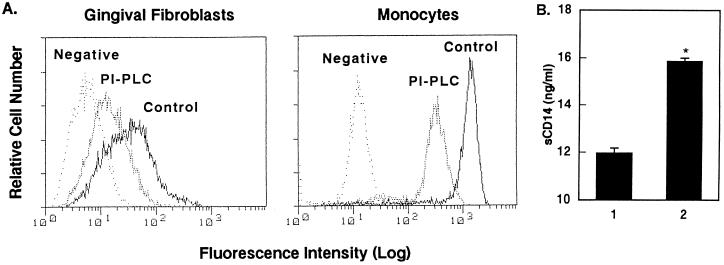
The above observation that CD14 is expressed by HGF at different levels raised the question of whether CD14 on the surfaces of HGF is anchored via GPI as in monocytes and neutrophils (16, 17). HGF and monocytes as a positive control were treated with PI-PLC, which specifically leaves GPI-anchored protein (18), and analyzed for the expression of CD14 by flow cytometry. The treatment of HGF with PI-PLC resulted in a marked decrease in the level of expression of CD14 on the HGF as well as monocytes compared with that of the untreated cells (Fig. 3A). CD14 expression by the monocytes was higher than that of the HGF. The relative mean fluorescence intensity values of CD14 expression by the HGF and monocytes were 45.22 and 1,157.91 for the untreated cells and 16.77 and 305.75 for the PI-PLC-treated cells, with negative control values of 7.75 and 15.42, respectively, showing an about fourfold decrease in the expression of CD14 after PI-PLC treatment. The treatment had no effect on the expression of HLA-A, -B, or -C (32). The amount of CD14 released in the supernatants of PI-PLC-treated HGF was determined by ELISA, and the level of CD14 released by HGF was increased after the PI-PLC treatment (Fig. 3B). These results indicate that the efficacy of the PI-PLC treatment of HGF was the same as that of the monocytes and that mCD14 on the surfaces of HGF is a GPI-anchored protein, as it is in monocytes.
FIG. 3.
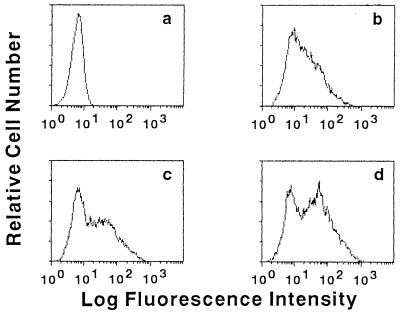
Decrease of HGF and monocyte CD14 expression by PI-PLC treatment. HGF (donor MH) from confluent cultures and MNC were suspended at 105 cells/20 μl and 106 cells/20 μl in the medium, respectively, and cultured in the presence or absence of PI-PLC (1 U/ml) for 1 h at 37°C. (A) The cells were then stained with anti-CD14 MAb (MEM-18) and analyzed by FACS analysis. The fluorescence of the monocyte population was analyzed by gating on the basis of forward/side scatter characteristics. Negative, fluorescence of negative control cells incubated with the second Ab only. (B) The amount of CD14 released in the supernatant of HGF in the absence (lane 1) or presence (lane 2) of PI-PLC was analyzed by ELISA. ∗, P < 0.05 versus lane 1. The results are representative of three different experiments.
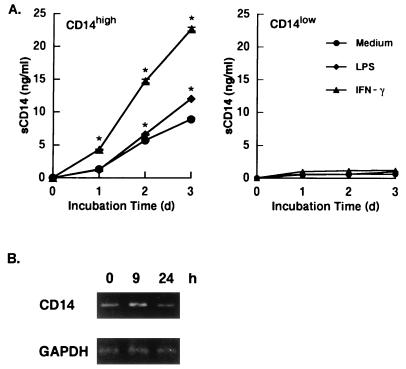
Release of sCD14 by HGF, and augmentation of the release and expression by IFN-γ and LPS.
We next examined whether mCD14-expressing HGF release sCD14 in the culture supernatant. Confluent CD14high and CD14low HGF were cultured in medium alone or in the presence of IFN-γ (200 IU/ml) and LPS (100 ng/ml) for 3 days, and the amount of sCD14 in the supernatants was determined at 24-h intervals. Figure 4A shows that the CD14high HGF released 1.3 ng of sCD14 per ml after 1 day of culture, and an increase in sCD14 release was observed according to the incubation time. In the cultures with LPS, no effect on sCD14 release was observed after 1 day of culture, whereas a significant increase was observed after 2 and 3 days of culture. Of interest, the incubation with IFN-γ resulted in a more than twofold increase in sCD14 secretion compared to the level in medium alone. In contrast, CD14low HGF did not release sCD14 at any time point, even when the cells were incubated with LPS or IFN-γ. The analysis of the CD14 mRNA expression by the RT-PCR method revealed that the CD14 mRNA expression by CD14high HGF was increased after 9 h of IFN-γ treatment and decreased thereafter (Fig. 4B). The treatment of CD14low HGF with IFN-γ had no effect on the expression of CD14 mRNA (data not shown).
FIG. 4.
The release of sCD14 by CD14high HGF was augmented by IFN-γ and LPS. (A) Confluent CD14high HGF (donor MH) and CD14low HGF (donor KT) were incubated with medium alone, with IFN-γ (200 IU/ml), or with LPS (100 ng/ml). Supernatants were collected at days (d) 1, 2, and 3 and analyzed for the presence of sCD14 by ELISA. Results are the means ± standard deviations of duplicate determinations from one representative experiment. ∗, P < 0.05 versus medium alone. (B) Confluent CD14high HGF (donor MH) were incubated with IFN-γ (200 IU/ml) for the times indicated and were collected by trypsinization. RNA was extracted from the cells, and its cDNA was prepared and analyzed by RT-PCR. The results are representative of three different experiments.
After 3 days of culture, the mean fluorescence intensity of the CD14 expression by CD14high HGF was 37.91 for the untreated cells, 51.08 for the LPS-stimulated cells, and 75.24 for the IFN-γ-stimulated cells, with a negative control value of 7.28 (Fig. 5). These results indicate that the sCD14 release was correlated with the expression of mCD14.
FIG. 5.
Up-regulation of mCD14 expression by LPS and IFN-γ. Confluent HGF (donor MH) were cultured with medium alone (a and b), with 100 ng of LPS per ml (c), or with 200 IU of IFN-γ per ml (d) for 3 days. Cells were then collected and stained with the second Ab (a) or anti-CD14 MAb (MEM-18) (b to d) and analyzed with a FACS. The results are representative of three different experiments.
Identification of the CD14 molecule expressed by HGF.
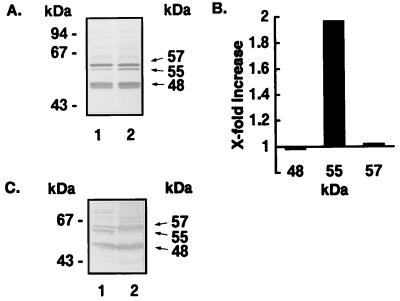
The observation that HGF spontaneously release sCD14 (Fig. 4) prompted the question of the size of the CD14 glycoprotein expressed by HGF. CD14high HGF were cultured in the presence or absence of 5 U of PI-PLC per ml for 1 h at 37°C, and the cultured supernatants were analyzed by Western blotting with anti-CD14 MAb MEM-18. The results in Fig. 6A show that the CD14high HGF released mainly 48- and 57-kDa sCD14 bands and a small 55-kDa band. The PI-PLC treatment resulted in an about twofold increase in the amount of the 55-kDa band, whereas the amounts of the 48- and 57-kDa bands were unchanged by the treatment (Fig. 6B). The membrane fraction of the CD14high HGF possessed a large amount of the 55-kDa band, but the 55-kDa band was scarcely detected in the cytoplasms of the cells (Fig. 6C). These results suggest that HGF release 57- and 48-kDa sCD14 and that the GPI-anchored mCD14 is a 55-kDa glycoprotein.
FIG. 6.
Western blot analysis of CD14 released by HGF. (A) HGF (donor MH) from confluent cultures were suspended at 106 cells/20 μl in medium without FCS and incubated in the absence (lane 1) or presence (lane 2) of PI-PLC (5 U/ml) for 1 h at 37°C. The supernatants were subjected to a Western blot analysis under reducing conditions. (B) The bands in panel A were analyzed by using a LabScan and ImageMaster ID software, and the results are expressed as fold increases (lane 2/lane 1). (C) Crude membrane (lane 1) and cytoplasm (lane 2) fractions were prepared from HGF (donor MH), and the fractions (0.25 × 106 cells each) were subjected to Western blot analysis under reducing conditions. The results are representative of three different experiments.
CD14-mediated IL-8 secretion by HGF in response to LPS.
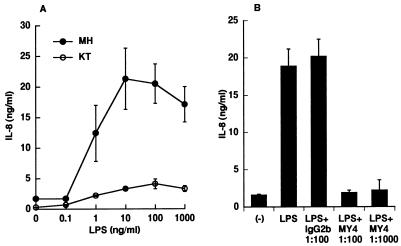
It has been shown that HGF respond to LPS from P. gingivalis and P. intermedia, which are implicated in periodontal diseases and secrete several inflammatory cytokines such as IL-1, IL-6, and IL-8 (33–35). However, HGF secrete IL-6 but not IL-1 and IL-8 in response to LPS from E. coli and Salmonella species. There is a strong possibility that CD14high and CD14low HGF respond to LPS in different manners. To examine this possibility, CD14high and CD14low HGF were stimulated with various doses of E. coli LPS, and the IL-8 concentration in the culture supernatants was analyzed. As shown in Fig. 7A, the IL-8 secretion by the CD14high HGF in response to LPS began at 1 ng/ml and reached a maximum at 10 ng/ml. In contrast, the CD14low HGF showed only a marginal response which peaked at 100 ng of LPS per ml. The E. coli LPS-induced IL-8 secretion by the CD14high HGF was completely inhibited by anti-CD14 MAb (MY4) at 1:100 and 1:1,000 dilutions (Fig. 7B). The control mouse IgG2b had no effect on this response.
FIG. 7.
LPS induced IL-8 production of CD14high HGF via mCD14. (A) Confluent CD14high (•) and CD14low (○) HGF were stimulated with various doses of E. coli LPS for 24 h in medium with 1% FCS. The culture supernatants were collected and analyzed for the presence of IL-8 by ELISA. (B) Confluent CD14high HGF (donor MH) were stimulated with 10 ng of LPS per ml in the absence or presence of isotype control mouse IgG2b (1:100) or anti-CD14 MAb MY4 (1:100 and 1:1,000) for 24 h. (−), medium alone. The amounts of IL-8 in the culture supernatants were analyzed by ELISA. The results are representative of three different experiments.
DISCUSSION
The HGF and the fibroblasts from lung and skin did not express CD18 or HLA-DR but were distinct in their expressions of CD14 and CD54 (Table 1). Recent studies have obtained inconsistent evidence pertaining to the CD14 expression by HGF. One study showed that HGF express CD14 and that the LPS-induced signal transducing pathway is mediated by mCD14 (37). Another study found that HGF express CD14 neither on the surface nor in the mRNA and that ICAM-1 expression by HGF is up-regulated by serum-derived sCD14 (15). Our data support both sets of observations. It has been shown that fibroblasts from many tissues such as lung, skin, and periodontium are heterogeneous and that these cells can be separated into subsets on the basis of morphology, size, and function (27). It is also reported that papillary and reticular gingival fibroblasts of the lamina propria of an attached human gingiva differ in morphology and in the production of migration-stimulating factor (20) and that CD40 expression by HGF is correlated with phenotype and function (11). Identification of the expression of cell surface markers such as Thy-1, collagen receptors, C1q receptors, CD4, and CD40 has been used to separate subsets of fibroblasts, as have morphology and function (6, 7, 9, 10, 14, 26). These observations support our findings that HGF heterogeneously express CD14 and can be separated into two populations, i.e., CD14high and CD14low subsets. However, we cannot distinguish these two populations on the basis of morphology and proliferation rate at present, and a precise analysis of their characteristics remains to be conducted. HGF used in this study were grown from clinically normal gingival tissue, but it is unclear whether the tissues were normal histologically. Gingival tissues easily give rise to inflammation due to bacteria existing in oral floras and their products. Another possible explanation with regard to the heterogeneous expression of CD14 by HGF is the extent of inflammation of the gingival tissue obtained; i.e., HGF from inflamed tissue may express levels of CD14 higher than those from noninflamed tissue.
In our examination of the CD14 expression by fibroblasts, Leu-M3 showed weaker reactivity than MEM-18. It has been shown that Leu-M3 stains cells with narrow reactivity and that MY4 has broad reactivity and stains more cells with higher intensity, even when the cells are Leu-M3 negative (24, 41). Our preliminary experiments showed that the reactivity of MY4 was the same as that of MEM-18 and that MEM-18 reacted slightly more strongly than did MY4 (32).
Two forms of the leukocyte integrin family, CD11b/CD18 and CD11c/CD18, were recently shown to be involved in LPS-induced cell activation (19, 25, 39, 40). Endothelial and epithelial cells which do not express mCD14 require sCD14 for activation by LPS (13, 28). In the present study, however, no fibroblasts from gingiva, lung, or skin expressed CD18 on their surfaces, indicating that fibroblasts do not use the integrin LPS receptors and use mCD14 and/or sCD14 for LPS-mediated cell activation.
The treatment of HGF with PI-PLC, an enzyme from B. cereus which specifically cleaves the GPI anchor (18), resulted in a decrease in the expression of mCD14 with a concomitant increase in released CD14 (Fig. 3). The PI-PLC digestion is CD14 specific, because the expression of HLA-A, -B, and -C was unchanged after PI-PLC treatment (32). The efficacy of the PI-PLC treatment of both HGF and MNC reached a plateau at 1 U/ml, and increasing the concentration of PI-PLC to more than 10 U/ml leads to a decrease in cellular viability (32). One of the reasons for the inefficiency of this enzyme treatment might be that a portion of the CD14 pool of HGF is structurally modified in the lipid region, and therefore resistant to the action of this enzyme, as suggested previously (24). This possibility is supported by the finding that the enzyme treatment of monocytes resulted in the incomplete removal of CD14, although no CD14 was detected on the monocytes from a patient with paroxysmal nocturnal hemoglobinuria, a disease characterized by a lack of the cell surface expression of GPI-anchored proteins (16, 24). These results support the possibility that mCD14 expressed by HGF is a GPI-anchored protein, as it is in monocytes and neutrophils as shown previously (16, 17).
CD14 is a differentiation antigen, and its expression increases with monocytes and neutrophil maturation (2, 29). It is suggested that the expression of CD14 is governed by tissue-specific nuclear factors or tissue-specific combinations of common nuclear factor, although little is known about the transcription factors involved in CD14 expression (41). The data shown in Fig. 2 revealed that CD14 expression by HGF decreased after subculturing, increased thereafter and reached a plateau at the confluent stage, indicating that mCD14 expression by HGF during the course of cell culture represents the regulation of the de novo synthesis of CD14.
The CD14high HGF examined in this study spontaneously released sCD14; the release was augmented markedly by IFN-γ and weakly by LPS, and the expression of CD14 mRNA by the cells was increased after IFN-γ treatment. Furthermore, the augmented release was correlated with an increased expression of mCD14 (Fig. 4 and 5). These results indicate that the up-regulation of the CD14 de novo synthesis described above may be responsible for the IFN-γ- and LPS-induced augmentations of CD14 expression and release. This interpretation is supported by the evidence that human monocyte and microglia CD14 are transcriptionally up-regulated by LPS and IFN-γ, respectively, after 24 to 48 h of incubation (5, 23).
The CD14 expressed by monocytes is a 55-kDa GPI-anchored membrane protein (16) and is cleaved by an endogenous cellular enzyme which is inhibitable by serine protease inhibitor, as is 48-kDa sCD14 (4, 8). Neutrophil CD14 has the same characteristics as those of monocyte CD14 (17). It has also been shown that monocyte sCD14 is released via two different mechanisms, protease dependent and protease independent (8, 12). Taking these observations into account, we consider the possibility indicated in Fig. 6 that (i) the mCD14 expressed by HGF is a 55-kDa GPI-anchored protein, (ii) a 48-kDa sCD14 is derived from the 55-kDa mCD14, which is cleaved by endogenous protease, and (iii) a 57-kDa sCD14 is released in a protease-independent manner. It is apparently unique to HGF that the 57-kDa sCD14 (which is larger than mCD14) is released by HGF, unlike monocytes and neutrophils; and unlike monocytes, the release of 48-kDa sCD14 by HGF is not inhibited by several protease inhibitors, including phenylmethylsulfonyl fluoride (32). Investigations are under way to clarify these properties of CD14 expressed by HGF.
The expression of CD14 by HGF raises the question of whether the CD14 on HGF functionally mediates LPS-induced cell activation. As shown in Fig. 7, the CD14high HGF produced IL-8 which peaked at 10 ng of LPS per ml in the medium with 1% FCS, and this production was completely inhibited by the anti-CD14 MAb, MY4. The CD14low HGF showed only a marginal response to LPS. These data indicate that the CD14 expressed by HGF binds to LPS and mediates the signal to produce IL-8. Previous reports indicated that human dermal fibroblasts do not produce IL-1 or IL-8 following a challenge with enterobacterial LPS in the absence of FCS (21, 30, 31). A possible reason for this unresponsiveness to the LPS is that the fibroblasts used did not express CD14 or CD18 on the cell surface, since the skin fibroblasts used in the present study were not shown to express CD14 or CD18 (Table 1). Our previous studies (34, 35) also showed that HGF did not respond to enterobacterial LPS. The HGF used in our previous studies may belong to the CD14low HGF group. It is also possible that serum-derived sCD14 or LPS-binding protein was not sufficient to support LPS-induced cell activation via an sCD14- and/or mCD14-dependent pathway, as indicated previously (13, 28). When HGF were stimulated with E. coli LPS in the presence of 10% FCS, the CD14high and CD14low HGF each secreted more than three times as much IL-8 as that in the presence of 1% FCS (32).
Finally, the present results suggest the possibility that during the inflammatory response, (i) CD14high HGF secrete inflammatory cytokines in response to LPS and produce an increased release of sCD14 in response to IFN-γ from other cells and LPS and (ii) the released sCD14 may in turn function as an LPS-receptor for CD14low HGF to induce inflammatory cytokines at the site of inflammation to exacerbate the inflammatory response. By these mechanisms, HGF might have important roles in the development of chronic inflammatory lesions in periodontal tissues.
ACKNOWLEDGMENTS
We thank S. Hanazawa, Meikai University School of Dentistry, for providing one of the HGF preparations. We also thank Yuri Togashi for expert editorial assistance and D. Mrozek (Medical English Service, Kyoto, Japan) for reviewing the paper.
This work was supported in part by Grants-in-Aid for Scientific Research 09671843 and 10470378 from the Ministry of Education, Sports, Science and Culture, Japan.
REFERENCES
- 1.Abo T, Sugawara S, Amenomori A, Itoh H, Rikiishi H, Moro I, Kumagai K. Selective phagocytosis of gram-positive bacteria and interleukin 1-like factor production by a subpopulation of large granular lymphocytes. J Immunol. 1986;136:3189–3197. [PubMed] [Google Scholar]
- 2.Ball E D, Guyre P M, Shen L, Glynn J M, Maliszewski C R, Baker P E, Franger M W. Gamma interferon induces monocytoid differentiation in the HL-60 cell line. J Clin Invest. 1984;73:1072–1077. doi: 10.1172/JCI111292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bažil V, Heřejší V, Baudyš M, Krištofová H, Strominger J L, Kostka W, Hilgert I. Biochemical characterization of a soluble form of the 53-kDa monocyte surface antigen. Eur J Immunol. 1986;16:1583–1589. doi: 10.1002/eji.1830161218. [DOI] [PubMed] [Google Scholar]
- 4.Bažil V, Strominger J L. Shedding as a mechanism of down-modulation of CD14 on stimulated human monocytes. J Immunol. 1991;147:1567–1574. [PubMed] [Google Scholar]
- 5.Becher B, Fedorowicz V, Antel J P. Regulation of CD14 expression on human adult central nervous system-derived microglia. J Neurosci Res. 1996;45:375–381. doi: 10.1002/(SICI)1097-4547(19960815)45:4<375::AID-JNR6>3.0.CO;2-6. [DOI] [PubMed] [Google Scholar]
- 6.Bordin S, Kolb W P, Page R C. C1q receptors on cultured human gingival fibroblasts: analysis of binding properties. J Immunol. 1983;130:1871–1875. [PubMed] [Google Scholar]
- 7.Breen E, Falco V M, Absher M, Cutroneo K R. Subpopulations of rat lung fibroblasts with different amounts of type I and type III collagen mRNAs. J Biol Chem. 1990;265:6286–6290. [PubMed] [Google Scholar]
- 8.Bufler P, Stiegler G, Schuchmann M, Hess S, Krüger C, Stelter F, Eckerskorn C, Schütt C, Engelmann H. Soluble lipopolysaccharide receptor (CD14) is released via two different mechanisms from human monocytes and CD14 transfectants. Eur J Immunol. 1995;25:604–610. doi: 10.1002/eji.1830250244. [DOI] [PubMed] [Google Scholar]
- 9.Derdak S, Dixon P, Watts H, Penney P, Phipps R P. CD4 expression in lung fibroblasts. Lancet. 1991;337:374. doi: 10.1016/0140-6736(91)91015-m. [DOI] [PubMed] [Google Scholar]
- 10.Dolei A, Serra C, Arca M V, Toniolo A. Acute HIV-1 infection of CD4+ human lung fibroblasts. AIDS. 1992;6:232–243. [PubMed] [Google Scholar]
- 11.Dongari-Bagtzoglou A I, Warren W D, Berton M T, Ebersole J L. CD40 expression by gingival fibroblasts: correlation of phenotype with function. Int Immunol. 1997;9:1233–1241. doi: 10.1093/intimm/9.9.1233. [DOI] [PubMed] [Google Scholar]
- 12.Durieux J-J, Vita N, Popescu O, Guette F, Calzada-Wach J, Munker R, Schmidt R E, Lupker J, Ferrara P, Ziegler-Heitbrock H W L, Labeta M O. The two soluble forms of the lipopolysaccharide receptor, CD14: characterization and release by normal human monocytes. Eur J Immunol. 1994;24:2006–2012. doi: 10.1002/eji.1830240911. [DOI] [PubMed] [Google Scholar]
- 13.Frey E A, Miller D S, Jahr T G, Sundan A, Bažil V, Espevik T, Finlay B B, Wright S D. Soluble CD14 participates in the response of cells to lipopolysaccharide. J Exp Med. 1992;176:1665–1671. doi: 10.1084/jem.176.6.1665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fries K M, Sempowski G D, Gaspari A A, Blieden T, Looney R J, Phipps R P. CD40 expression by human fibroblasts. Clin Immunol Immunopathol. 1995;77:42–51. doi: 10.1016/0090-1229(95)90135-3. [DOI] [PubMed] [Google Scholar]
- 15.Hayashi J, Masaka T, Saito I, Ishikawa I. Soluble CD14 mediates lipopolysaccharide-induced intercellular adhesion molecule 1 expression in cultured human gingival fibroblasts. Infect Immun. 1996;64:4946–4951. doi: 10.1128/iai.64.12.4946-4951.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Haziot A, Chen S, Ferrero E, Low M G, Silber R, Goyert S M. The monocyte differentiation antigen, CD14, is anchored to the cell membrane by a phosphatidylinositol linkage. J Immunol. 1988;141:547–552. [PubMed] [Google Scholar]
- 17.Haziot A, Tsuberi B, Goyert S M. Neutrophil CD14: biochemical properties and role in the secretion of tumor necrosis factor-α in response to lipopolysaccharide. J Immunol. 1993;150:5556–5565. [PubMed] [Google Scholar]
- 18.Ikezawa H, Taguchi R. Phosphatidylinositol-specific phospholipase C from Bacillus cereus and Bacillus thuringiensis. Methods Enzymol. 1981;71:731–741. [Google Scholar]
- 19.Ingalls R R, Golenbock D T. CD11c/CD18, a transmembrane signaling receptor for lipopolysaccharide. J Exp Med. 1995;181:1473–1479. doi: 10.1084/jem.181.4.1473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Irwin C R, Picardo M, Ellis I, Sloan P, Grey A M, McGurk M, Schor S L. Inter- and intra-site heterogeneity in the expression of fetal-like phenotypic characteristics by gingival fibroblasts: potential significance for wound healing. J Cell Sci. 1994;107:1333–1346. doi: 10.1242/jcs.107.5.1333. [DOI] [PubMed] [Google Scholar]
- 21.Kurt-Jones E A, Fiers W, Pober J S. Membrane interleukin 1 induction on human endothelial cells and dermal fibroblasts. J Immunol. 1987;139:2317–2324. [PubMed] [Google Scholar]
- 22.Laemmli U K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970;227:680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- 23.Landmann R, Knopf H, Link S, Sansano S, Schumann R, Zimmerli W. Human monocyte CD14 is upregulated by lipopolysaccharide. Infect Immun. 1996;64:1762–1769. doi: 10.1128/iai.64.5.1762-1769.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Pedron T, Girard R, Chaby R. Variation of LPS-binding capacity, epitope expression, and shedding of membrane-bound CD14 during differentiation of human monocytes. J Immunol. 1995;155:1460–1471. [PubMed] [Google Scholar]
- 25.Petty H R, Todd R F., III Integrins as promiscuous signal transduction devices. Immunol Today. 1996;17:209–212. doi: 10.1016/0167-5699(96)30013-3. [DOI] [PubMed] [Google Scholar]
- 26.Phipps R P, Baecher C, Frelinger J G, Penney D P, Keng P, Brown D. Differential expression of interleukin 1α by Thy-1+ and Thy-1− lung fibroblast subpopulations: enhancement of interleukin 1α by tumor necrosis factor α. Eur J Immunol. 1990;20:1723–1727. doi: 10.1002/eji.1830200815. [DOI] [PubMed] [Google Scholar]
- 27.Phipps R P, Borrello M A, Blieden T M. Fibroblast heterogeneity in the periodontium and other tissues. J Periodont Res. 1997;32:159–165. doi: 10.1111/j.1600-0765.1997.tb01398.x. [DOI] [PubMed] [Google Scholar]
- 28.Pugin J, Schürer-Maly C-C, Leturcq D, Moriarty A, Ulevitch R J, Tobias P S. Lipopolysaccharide activation of human endothelial and epithelial cells is mediated by lipopolysaccharide-binding protein and soluble CD14. Proc Natl Acad Sci USA. 1993;90:2744–2748. doi: 10.1073/pnas.90.7.2744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Rigby W F C, Shen L, Ball E D, Guyre P M, Fanger M W. Differentiation of a human monocytic cell line by 1,25-dihydroxyvitamin D3 (calcitriol): a morphologic, phenotypic, and functional analysis. Blood. 1984;64:1110–1115. [PubMed] [Google Scholar]
- 30.Schröder J-M, Sticherling M, Henneicke H H, Preissner W C, Christophers E. IL-1α or tumor necrosis factor-α stimulate release of three NAP-1/IL-8-related neutrophil chemotactic proteins in human dermal fibroblasts. J Immunol. 1990;144:2223–2232. [PubMed] [Google Scholar]
- 31.Strieter R M, Phan S H, Showell H J, Remick D G, Lynch J P, Genord M, Raiford C, Eskandari M, Marks R M, Kunkel S L. Monokine-induced neutrophil chemotactic factor gene expression in human fibroblasts. J Biol Chem. 1989;264:10621–10626. [PubMed] [Google Scholar]
- 32.Sugawara, S., and H. Takada. Unpublished data.
- 33.Takada H, Mihara J, Morisaki I, Hamada S. Production of cytokines by human gingival fibroblasts. In: Hamada S, Holt S C, McGhee J R, editors. Periodontal disease: pathogens and host immune responses. Tokyo, Japan: Quintessence Publishing Co.; 1991. pp. 265–276. [Google Scholar]
- 34.Takada H, Mihara J, Morisaki I, Hamada S. Induction of interleukin-1 and -6 in human gingival fibroblast cultures stimulated with Bacteroides lipopolysaccharides. Infect Immun. 1991;59:295–301. doi: 10.1128/iai.59.1.295-301.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Tamura M, Tokuda M, Nagaoka S, Takada H. Lipopolysaccharides of Bacteroides intermedius (Prevotella intermedia) and Bacteroides (Porphyromonas) gingivalis induce interleukin-8 gene expression in human gingival fibroblast cultures. Infect Immun. 1992;60:4932–4937. doi: 10.1128/iai.60.11.4932-4937.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ulevitch R J, Tobias P S. Receptor-dependent mechanisms of cell stimulation by bacterial endotoxin. Annu Rev Immunol. 1995;13:437–457. doi: 10.1146/annurev.iy.13.040195.002253. [DOI] [PubMed] [Google Scholar]
- 37.Watanabe A, Takeshita A, Kitano S, Hanazawa S. CD14-mediated signal pathway of Porphyromonas gingivalis lipopolysaccharide in human gingival fibroblasts. Infect Immun. 1996;64:4488–4494. doi: 10.1128/iai.64.11.4488-4494.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Wright S D, Ramos R A, Tobias P S, Ulevitch R J, Mathison J C. CD14, a receptor for complexes of lipopolysaccharide (LPS) and LPS binding protein. Science. 1990;249:1431–1433. doi: 10.1126/science.1698311. [DOI] [PubMed] [Google Scholar]
- 39.Wright S D, Ramos R A, Hermanowski-Vosatka A, Rockwell P, Detmers P A. Activation of the adhesive capacity of CR3 on neutrophils by endotoxin: dependence on lipopolysaccharide binding protein and CD14. J Exp Med. 1991;173:1281–1286. doi: 10.1084/jem.173.5.1281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Zarewych D M, Kindzelskii A L, Todd III R F, Petty H R. LPS induces CD14 association with complement receptor type 3, which is reversed by neutrophil adhesion. J Immunol. 1996;156:430–433. [PubMed] [Google Scholar]
- 41.Ziegler-Heitbrock H W L, Ulevitch R J. CD14: cell surface receptor and differentiation marker. Immunol Today. 1993;14:121–125. doi: 10.1016/0167-5699(93)90212-4. [DOI] [PubMed] [Google Scholar]