Abstract
We present a rare and unusual case of thrombotic microangiopathy (TMA) in a patient who ingested chafing fuel containing diethylene glycol. The patient showed a typical clinical course of initial gastrointestinal symptoms followed by acute kidney injury (AKI) and peripheral sensorimotor neuropathy. A kidney biopsy showed TMA and diffuse acute tubular injury. Diethylene glycol is widely used as a solvent in numerous consumer products, including brake fluid, antifreeze, chafing fuel, and artificial fog solutions. Diethylene glycol has been implemented in mass poisonings, and the incidence of AKI in diethylene glycol poisonings is linked to high-mortality rates. TMA, a pathologic lesion observed in a wide spectrum of diseases, is triggered by endothelial injury. Our case shows that TMA should be considered as a possible life-threatening complication in the setting of acute diethylene glycol poisoning. Direct toxic injury to endothelial cells by diethylene glycol is a possible mechanism. It is therefore plausible that patients with a genetic predisposition to endothelial injury may develop TMA following diethylene glycol exposure.
Index Words: AKI, diethylene glycol, toxicity, kidney biopsy, thrombotic microangiopathy
Diethylene glycol is a clear liquid that is employed as an industrial diluent and is highly toxic to the kidney and brain.1 Ingesting diethylene glycol can cause acute kidney injury (AKI) as well as neurologic symptoms, including acute flaccid paralysis, cranial nerve palsies, and encephalopathy.1 Most cases of diethylene glycol poisoning are due to pharmaceutical products formulated with diethylene glycol as a diluent instead of a safe agent, such as pharmaceutical-grade glycerin or propylene glycol. Most diethylene glycol mass poisonings occur in children and are associated with variable case fatalities that may be as high as 98%.2, 3, 4 Similar to ethylene glycol, diethylene glycol is initially metabolized by alcohol dehydrogenase, and treatment with its inhibitor fomepizole may have a protective effect.5 However, diethylene glycol poisoning is often not recognized early due to its enigmatic clinical course or because diethylene glycol poisoning is not suspected.6
The predominant kidney tissue lesions reported in cases of diethylene glycol poisoning are extensive necrosis of the proximal tubule, severe interstitial hemorrhage, and hyaline casts.7,8 Thrombotic microangiopathy (TMA) is a pathologic lesion triggered by endothelial injury. Diethylene glycol poisoning injures the kidney most frequently, but other systems can also be affected, such as the central nervous system and cardiovascular and pulmonary systems.9 Endothelial injury, which is associated with platelet activation and consumption, leads to the formation of microvascular thrombosis, tissue ischemia, and subsequent tissue necrosis. Although lactate dehydrogenase (LDH) elevation is related to the destruction of red blood cells (RBCs), highly elevated LDH also reflects ischemic tissue injury. Systemic findings of TMA are not required for the diagnosis, and kidney-limited TMA is seen more often in solid organ transplantation and drug-induced TMA.9 For a list of toxicology conditions associated with TMA, see Box 1.
Box 1. Drugs and Toxins Associated with Thrombotic Microangiopathy.
Calcineurin inhibitors
Proteasome inhibitors
Antineoplastic agents
Tyrosine kinase inhibitors
Vascular endothelial growth factor inhibitors
Type I interferon
Valproic acid
Quinine
Snake venom
Shiga toxin
Case Report
Clinical History
A man in his 50s with a history of alcohol use disorder and chronic pancreatitis presented to the emergency room with 5 days of nausea, vomiting and diarrhea, with subsequent worsening altered mental status. The patient’s wife reported he had a relapse with severe alcohol cravings and found small amounts of hand sanitizer (isopropyl alcohol) and chafing fuel (diethylene glycol) in the basement. His medical history was also significant for hypertension and hemochromatosis. At the time of presentation, his vitals were significant for tachypnea of 38 breaths per minute, and his physical examination demonstrated disorientation to time. He was admitted to the medical intensive care unit, and emergency hemodialysis was initiated after fluid resuscitation with isotonic fluids, bicarbonate, and fomepizole. He developed uncontrolled hypertension and worsening thrombocytopenia. Thrombocytopenia was present from day 1 of hospitalization, and platelet numbers decreased continuously until day 3, correlating with peak blood pressure levels on the same day. Following hemodialysis, he had resolution of metabolic acidosis, anion gap, and osmolal gap along with improvement in mentation. Intermittent hemodialysis was continued as he remained anuric with no evidence of kidney function recovery.
Laboratory Data
Venous blood gas revealed severe metabolic acidosis with a pH value of 6.87, a pCO2 of 13 mm Hg, and a bicarbonate concentration of 2 mmol/L. His serum creatinine concentration was 8.26 mg/dL, and LDH was 4138 U/L. He had an elevated anion gap of 44 and an elevated osmolal gap of 45, whereas lactate and β-hydroxybutyrate were within normal range. Ethanol, toxic alcohols, acetaminophen, and salicylate were undetectable. A peripheral smear lacked schistocytes. LDH levels remained elevated. Haptoglobin levels decreased to less than 10,and serum C3 levels were low at 79 mg/dL (90-180 mg/dL). His C4 level and CH50 were normal. He was negative for heparin-induced thrombocytopenia antibody, and a negative direct antiglobulin test was obtained. ADAMTS13 activity was mildly low at 50%. Hemoglobin levels decreased from 14.4 g/dL on admission to 7.8 g/dL on hospital day 5. Prothrombin time, partial thromboplastin time, and international normalized ratio values were normal throughout admission. Serum antinuclear antibody and antineutrophil autoantibodies levels were within normal limits. The remaining the laboratory values and their trends during the hospitalization period are summarized in Table 1. A kidney biopsy was performed on hospital day 5.
Table 1.
Laboratory Values During Clinical Course
| Laboratory Parameters (Reference Range and Units) | At Presentation | Day 3 | Day 5 | Day 10 |
|---|---|---|---|---|
| Hemoglobin (14-18 g/dL) | 14.4 | - | 7.8 | - |
| Bicarbonate (20-30 mmol/L) | 2 | 21 | 20 | 21 |
| BUN (6-20 mg/dL) | 42 | 38 | 59 | 90 |
| Creatinine (0.4-1.3 mg/dL) | 8.26 | 6.30 | 6.78 | 7.41 |
| Measured serum osmolarity (275-300 mOsm/kg) | 331 | - | - | - |
| Platelet count (150-420 × 1,000/μL) | 158 | 28 | 60 | 137 |
| LDH (122-241 U/L) | 4138 | 3522 | 1610 | - |
| Haptoglobin (30-200 mg/dL) | 24 | <10 | <10 | - |
Abbreviations: BUN, blood urea nitrogen; LDH, lactate dehydrogenase.
Kidney Biopsy
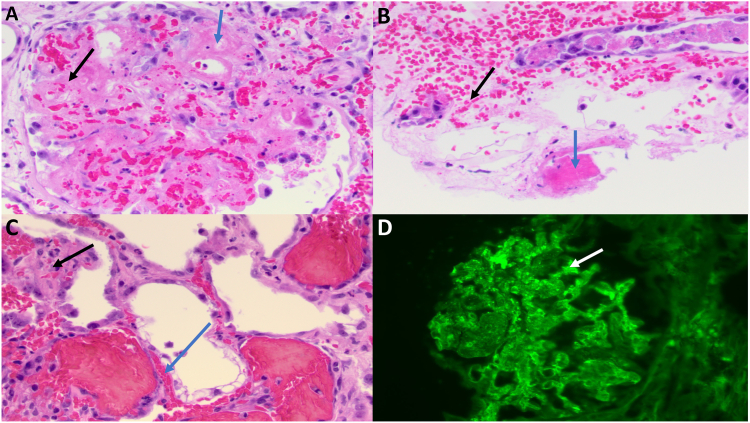
A kidney biopsy was performed and showed 2 tissue cores, which were stained with hematoxylin and eosin, periodic acid–Schiff, trichrome, Jones silver, and hematoxylin phloxine saffron stains. Review of all stained sections revealed 11 glomeruli, 2 of which showed endothelial swelling, RBC fragments and fibrin strands. The interstitium showed diffuse edema, congestion in peritubular capillaries, and a patchy, focal interstitial infiltrate composed of lymphocytes, occasional neutrophils, and rare plasma cells. Proximal tubules showed severe acute tubular injury with markedly dilated lumen, cytoplasmic vacuolization, loss of brush border, and nuclear drop out. Hyaline casts, fragmented RBC casts, and cellular casts were present. One glomerulus showed endothelial and mesangial cell nuclear dropout, fragmented RBCs in mesangial areas and capillary loops, and fibrinoid necrosis of the vascular pole (see Fig 1A). Another glomerulus showed mesangiolysis with RBC fragments and segmental endothelial swelling in capillary loops. Several profiles of arterioles with fibrinoid necrosis were seen, including a partially necrotic vessel containing an adherent fibrin thrombus (Fig 1B). Peritubular capillaries were markedly congested with RBC fragments and small thrombi (Fig 1C).
Figure 1.
(A) Glomerulus with fibrin thrombus (blue arrow) and red blood cell fragments (black arrow). (B) Arteriole with fibrinoid necrosis and adherent fibrin thrombus (blue arrow). Numerous red blood cell fragments (black arrow) are seen in peritubular capillaries. (C) Peritubular capillaries with fibrin thrombus (blue arrow) and red blood cell fragments (black arrow). (D) Immunofluorescence stain for fibrinogen shows strong capillary wall staining (white arrow). All light microscopy images represent hematoxylin and eosin stains. Original magnification, ×400.
Frozen sections were stained for IgA, IgG, IgM, C3, C1q, kappa, lambda, and fibrinogen. The glomeruli showed granular staining of mesangial areas and peripheral capillary walls with fibrinogen (2+) (Fig 1 D). All other immunofluorescence stains were negative.
An additional section of the biopsy was prepared for electron microscopy. The glomerular architecture showed segmentally thickened basement membranes with corrugation. There was global effacement of podocyte foot processes (>90%). There was severe swelling of endothelial cells, and the mesangium showed a minimal increase in matrix. Electron dense deposits were not identified.
The biopsy diagnosis was TMA and diffuse acute tubular injury.
Clinical Follow-up
On day 6, the patient developed slurred speech, right sided facial droop, and bilateral hearing loss. Brain computed tomography was negative for intracranial hemorrhage, and brain magnetic resonance imaging only showed nonspecific focal enhancement at the fundus of the left internal auditory canal. Over the next 24-48 hours, he began to develop significant progression of cranial neuropathies, including dysarthria, facial diplegia, lagophthalmos, and peripheral neuropathies, including loss of pain, temperature, and vibration sense in both lower extremities. He then required intubation and mechanical ventilation for airway protection. An empiric trial of intravenous methylprednisolone was attempted for neuropathies without any improvement. He had a prolonged hospital course and was managed conservatively with intermittent hemodialysis. He failed multiple spontaneous breathing trials, and his family opted to pursue comfort measures. On hospital day 19, he was compassionately extubated and died shortly thereafter.
Discussion
Diethylene glycol is widely used as a solvent in numerous consumer products, including wallpaper strippers, brake fluid, antifreeze, chafing fuel, and artificial fog solutions.6 Diethylene glycol has been implemented in mass poisonings when used as a diluent in pharmaceutical preparations.4,10 The case fatality in mass poisonings ranges from 76%-98%.3,11,12 The clinical symptoms in patients with diethylene glycol poisoning depend on the amount and duration of exposure. During the initial period post exposure, patients often present with gastrointestinal discomfort that begins with nausea, vomiting, and diarrhea. Later in the course, oliguria, metabolic acidosis, liver failure, and AKI develop.13 Peripheral sensorimotor neuropathy is another key finding in the diagnosis of diethylene glycol poisoning.8 The AKI is characterized by marked acute tubular injury.8 AKI caused by diethylene glycol poisonings is associated with high-mortality rates, and patients who survive AKI are more likely to develop chronic kidney disease.14 The molecular mechanisms of diethylene glycol poisoning are still poorly understood. When diethylene glycol is metabolized, it yields 2 primary metabolites, diglycolic acid and 2-hydroxyethoxyacetic acid.5 Several studies have identified diglycolic acid as the toxic metabolite.15, 16, 17 Diglycolic acid accumulates 100-fold in the kidney, indicative of concentrative uptake and retention.18 Recent studies have shown that diglycolic acid accumulates in human proximal tubule cells through transport by the sodium-dependent dicarboxylate transporters NaDC-1 and NaDC-3.19 However, the efflux mechanism for diglycolic acid from proximal tubule cells remains enigmatic because it does not involve the SLC family of organic anion transporters.20
A previous case of diethylene glycol poisoning reported coagulative necrosis of kidney cortical structures accompanied by severe interstitial hemorrhage, similar to the findings in this case.7 Another study, reporting autopsy findings of mass poisoning victims, described similar findings of severe vacuolization of proximal tubule and acute tubular epithelial cell necrosis.8 An animal study of diethylene glycol toxicity in rats showed severe, diffuse acute tubular necrosis affecting proximal convoluted tubules of the kidney cortex.21 In our case, the kidney biopsy showed 2 glomeruli with numerous RBC fragments, swollen endothelial cells with loss of nuclei, and fibrin strands or fibrinoid necrosis. The glomerular findings were consistent with TMA. There was also extensive acute tubular injury involving predominantly the proximal tubule.
TMA is a pathologic lesion that has been observed in a wide variety of diseases and is triggered by endothelial injury. TMA lesions are often associated with the clinical features of hemolytic anemia, thrombocytopenia, and ischemic end-organ injury. Underlying etiologies of the endothelial injury span a wide range of conditions, including infection, pregnancy, drugs, autoimmune diseases, transplantation, and congenital ADAMTS13-mediated and complement-mediated TMA. Our patient showed a TMA lesion on kidney biopsy with glomerular RBC fragments, mesangiolysis, endothelial swelling, and fibrinoid necrosis at the vascular pole. Given that the patient showed no evidence of malignant hypertension, autoimmune disease, ADAMTS13 deficiency, or C3 nephritic factor, the likely underlying etiology is diethylene glycol poisoning. Although no widespread cortical necrosis was seen on biopsy examination, it is possible that the vascular injury associated with the necrotic glomerulus triggered widespread endothelial injury. Another possible mechanism is direct toxic injury to endothelial cells by diethylene glycol. An animal study of rabbit carotid artery injury showed that ethylene glycol, the single molecule from which diethylene glycol is synthesized through hydrolysis, is toxic to mammalian endothelial cells.22 It is therefore plausible that patients with a genetic predisposition to endothelial injury may develop TMA following diethylene glycol exposure.
Article Information
Authors’ Full Names and Academic Degrees
Grace Malvar, MD, Deepthi Gunasekaran, MD, Nazanin Vaghari Mehr, MD, Shuta Ishibe, MD, PhD and Gilbert Moeckel, MD, PhD.
Support
None.
Financial Disclosure
The authors declare that they have no relevant financial interests.
Patient Protections
The authors declare that they have obtained consent from a relative with appropriate authority for publication of the information about the patient reported in this article and any associated supplementary material.
Peer Review
Received May 26, 2023. Evaluated by 1 external peer reviewer, with direct editorial input from an Associate Editor and the Editor-in-Chief. Accepted in revised form September 4, 2023.
Footnotes
Complete author and article information provided before references.
References
- 1.Schep L.J., Slaughter R.J., Temple W.A., Beasley D.M. Diethylene glycol poisoning. Clin Toxicol (Phila) 2009;47(6):525–535. doi: 10.1080/15563650903086444. [DOI] [PubMed] [Google Scholar]
- 2.Bowie M.D., McKenzie D. Diethylene glycol poisoning in children. S Afr Med J. 1972;46(27):931–934. [PubMed] [Google Scholar]
- 3.Okuonghae H.O., Ighogboja I.S., Lawson J.O., Nwana E.J. Diethylene glycol poisoning in Nigerian children. Ann Trop Paediatr. 1992;12(3):235–238. doi: 10.1080/02724936.1992.11747577. [DOI] [PubMed] [Google Scholar]
- 4.O'Brien K.L., Selanikio J.D., Hecdivert C., et al. Epidemic of pediatric deaths from acute renal failure caused by diethylene glycol poisoning. Acute Renal Failure Investigation Team. JAMA. 1998;279(15):1175–1180. doi: 10.1001/jama.279.15.1175. [DOI] [PubMed] [Google Scholar]
- 5.Besenhofer L.M., Adegboyega P.A., Bartels M., et al. Inhibition of metabolism of diethylene glycol prevents target organ toxicity in rats. Toxicol Sci. 2010;117(1):25–35. doi: 10.1093/toxsci/kfq167. [DOI] [PubMed] [Google Scholar]
- 6.Marraffa J.M., Holland M.G., Stork C.M., Hoy C.D., Hodgman M.J. Diethylene glycol: widely used solvent presents serious poisoning potential. J Emerg Med. 2008;35(4):401–406. doi: 10.1016/j.jemermed.2007.06.025. [DOI] [PubMed] [Google Scholar]
- 7.Wittschieber D., Heuberger K., Schulz R., Köhler H., Varchmin-Schultheiß K. Fatal poisoning with diethylene glycol in an unusual setting. Forensic Sci Med Pathol. 2019;15(4):649–652. doi: 10.1007/s12024-019-00123-4. [DOI] [PubMed] [Google Scholar]
- 8.Sosa N.R., Rodriguez G.M., Schier J.G., Sejvar J.J. Clinical, laboratory, diagnostic, and histopathologic features of diethylene glycol poisoning--Panama, 2006. Ann Emerg Med. 2014;64(1):38–47. doi: 10.1016/j.annemergmed.2013.12.011. [DOI] [PubMed] [Google Scholar]
- 9.Schaefer F., Ardissino G., Ariceta G., et al. Clinical and genetic predictors of atypical hemolytic uremic syndrome phenotype and outcome. Kidney Int. 2018;94(2):408–418. doi: 10.1016/j.kint.2018.02.029. [DOI] [PubMed] [Google Scholar]
- 10.Wax P.M. Elixirs, diluents, and the passage of the 1938 Federal Food, Drug and Cosmetic Act. Ann Intern Med. 1995;122(6):456–461. doi: 10.7326/0003-4819-122-6-199503150-00009. [DOI] [PubMed] [Google Scholar]
- 11.Hanif M., Mobarak M.R., Ronan A., et al. Fatal renal failure caused by diethylene glycol in paracetamol elixir: the Bangladesh epidemic. BMJ. 1995;311(6997):88–91. doi: 10.1136/bmj.311.6997.88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Singh J., Dutta A.K., Khare S., et al. Diethylene glycol poisoning in Gurgaon, India, 1998. Bull World Health Organ. 2001;79(2):88–95. [PMC free article] [PubMed] [Google Scholar]
- 13.Alkahtani S., Sammons H., Choonara I. Epidemics of acute renal failure in children (diethylene glycol toxicity) Arch Dis Child. 2010;95(12):1062–1064. doi: 10.1136/adc.2010.183392. [DOI] [PubMed] [Google Scholar]
- 14.Amdur R.L., Chawla L.S., Amodeo S., Kimmel P.L., Palant C.E. Outcomes following diagnosis of acute renal failure in U.S. veterans: focus on acute tubular necrosis. Kidney Int. 2009;76(10):1089–1097. doi: 10.1038/ki.2009.332. [DOI] [PubMed] [Google Scholar]
- 15.Landry G.M., Martin S., McMartin K.E. Diglycolic acid is the nephrotoxic metabolite in diethylene glycol poisoning inducing necrosis in human proximal tubule cells in vitro. Toxicol Sci. 2011;124(1):35–44. doi: 10.1093/toxsci/kfr204. [DOI] [PubMed] [Google Scholar]
- 16.Robinson C.N., Latimer B., Abreo F., Broussard K., McMartin K.E. In-vivo evidence of nephrotoxicity and altered hepatic function in rats following administration of diglycolic acid, a metabolite of diethylene glycol. Clin Toxicol (Phila) 2017;55(3):196–205. doi: 10.1080/15563650.2016.1271128. [DOI] [PubMed] [Google Scholar]
- 17.Sprando R.L., Mossoba M.E., Black T., et al. 28-day repeated dose response study of diglycolic acid: renal and hepatic effects. Food Chem Toxicol. 2017;106(A):558–567. doi: 10.1016/j.fct.2017.03.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Besenhofer L.M., McLaren M.C., Latimer B., et al. Role of tissue metabolite accumulation in the renal toxicity of diethylene glycol. Toxicol Sci. 2011;123(2):374–383. doi: 10.1093/toxsci/kfr197. [DOI] [PubMed] [Google Scholar]
- 19.Tobin J.D., Robinson C.N., Luttrell-Williams E.S., et al. Role of plasma membrane dicarboxylate transporters in the uptake and toxicity of diglycolic acid, a metabolite of diethylene glycol, in human proximal tubule cells. Toxicol Sci. 2022;190(1):1–12. doi: 10.1093/toxsci/kfac091. [DOI] [PubMed] [Google Scholar]
- 20.Tobin J.D., Robinson C.N., Luttrell-Williams E.S., Landry G.M., McMartin K.E. Lack of efflux of diglycolic acid from proximal tubule cells leads to its accumulation and to toxicity of diethylene glycol. Toxicol Lett. 2023;379:48–55. doi: 10.1016/j.toxlet.2023.03.007. [DOI] [PubMed] [Google Scholar]
- 21.Jamison Courtney N., Cuevas-Ocampo Areli K., Flowers Ashley B., et al. Histopathological evidence that diethylene glycol produces kidney and nervous system damage in rats. Neurotoxicology. 2022;91:200–210. doi: 10.1016/j.neuro.2022.05.015. [DOI] [PubMed] [Google Scholar]
- 22.Wusteman M., Boylan S., Pegg D.E. The effect of cooling rate and temperature on the toxicity of ethylene glycol in the rabbit internal carotid artery. Cryobiology. 1996;33(4):423–429. doi: 10.1006/cryo.1996.0042. [DOI] [PubMed] [Google Scholar]