Abstract
Background:
Vaccination against Ebolavirus is an emerging public health tool during Ebola Virus Disease outbreaks. We examined demand issues related to deployment of Ebolavirus vaccine during the 2014– 2015 outbreak in Sierra Leone.
Methods:
A cluster survey was administered to a population-based sample in December 2014 (N = 3540), before any Ebola vaccine was available to the general public in Sierra Leone. Ebola vaccine demand was captured in this survey by three Likert-scale items that were used to develop a composite score and dichotomized into a binary outcome to define high demand. A multilevel logistic regression model was fitted to assess the associations between perceptions of who should be first to receive an Ebola vaccine and the expression of high demand for an Ebola vaccine.
Results:
The largest proportion of respondents reported that health workers (35.1%) or their own families (29.5%) should receive the vaccine first if it became available, rather than politicians (13.8%), vaccination teams (9.8%), or people in high risk areas (8.2%). High demand for an Ebola vaccine was expressed by 74.2% of respondents nationally. The odds of expressing high demand were 13 times greater among those who said they or their families should be the first to take the vaccine compared to those who said politicians should be the first recipients (adjusted odds ratio [aOR] 13.0 [95% confidence interval [CI] 7.8–21.6]). The ultra-brief measure of the Ebola vaccine demand demonstrated acceptable scale reliability (Cronbach’s α = 0.79) and construct validity (single-factor loadings > 0.50).
Conclusion:
Perceptions of who should be the first to get the vaccine was associated with high demand for Ebola vaccine around the peak of the outbreak in Sierra Leone. Using an ultra-brief measure of Ebola vaccine demand is a feasible solution in outbreak settings and can help inform development of future rapid assessment tools.
Keywords: Ebola, Vaccine, Demand, Acceptance, Measure, Scale, Reliability, Validity, Sierra Leone
1. Background
Outbreaks of Ebola virus disease (Ebola) continue to occur in both previous and new outbreak locations in sub-Saharan Africa [1]. Strengthening health systems capacity to effectively prevent, detect, and respond to Ebola is an important global health security priority [2–4]. Vaccination strategies against Ebola are an increasingly important part of the public health response to prevent the spread of Ebola, as seen during the 2014–2015 outbreak in West Africa and more recently during the 2018–2019 outbreaks in the Democratic Republic of Congo (DRC) [5–10]. Numerous vaccine candidates have successfully gone through phase II/III clinical trials implemented in Liberia [11,12], Guinea [13] and Sierra Leone [14–16] to examine the safety, immunogenicity, and efficacy of these Ebola vaccines. Results from the Guinea Ebola Ça Suffit! trial revealed 100% (95% confidence interval [CI]: 74–100%) vaccine efficacy of the recombinant, replication-competent, vesicular stomatitis virus-based vaccine expressing the glycoprotein of a Zaire Ebolavirus (rVSV-ZEBOV-GP) [13]. The unlicensed rVSV-ZEBOV-GP vaccine has been administered under compassionate use protocols during outbreaks or as part of outbreak preparedness [5–10]. In October 2019, the European Medicines Agency recommended a conditional marketing authorization for rVSV-ZEBOBV-GP vaccine [17]. The United States Food and Drug Administration approved the first live, attenuated single-dose vaccine against the Zaire strain of Ebolavirus in December 2019 [18].
Perceptions of Ebola vaccines have been studied across different settings [19–21], and the results consistently demonstrated high acceptability of the vaccine but with slight differences based on demographic, economic, and social factors. A survey among general public participants in one district in Sierra Leone revealed that over 70% of respondents intended to accept an Ebola vaccine if provided free-of-charge and nearly 30% intended to do so even if a fee was levied. In that study, income was identified as a predictor of willingness-to-pay for an Ebola vaccine [19]. Another survey in Sierra Leone found that nearly eight out of every ten health care workers in two districts held positive attitudes towards an Ebola vaccine [20]. A national household survey in Guinea during the West Africa outbreak revealed that nearly 90% of respondents perceived that a vaccine against Ebola was needed to help end the outbreak, and over 80% of them intended to accept an Ebola vaccine if it became available [21]. In the Guinea study, the intention to accept an Ebola vaccine was significantly higher among those who were aware of new Ebola transmissions in the community, knew someone affected by Ebola, perceived that it was possible to recover from Ebola, and were willing to seek medical care if Ebola was suspected. Living in a household with children under the age of five years was significantly associated with hypothetical Ebola vaccine acceptability [21]. Implementation of Ebola ring vaccination during outbreaks has faced complex challenges, including issues related to institutional mistrust and misinformation about the vaccine as seen in DRC [22]. Outright refusals of Ebola vaccines have been documented in some instances [9,23]. Therefore, as part of global health security efforts, it is important to be able to rapidly measure and quantify demand for Ebola vaccines during outbreaks or as part of emergency preparedness.
The concepts of vaccine acceptability and vaccine demand have been used interchangeably, however, the relationship between the two has not been clarified in the published literature to date. An article by Dudley et al. draws attention to this issue and calls for the harmonization of these concepts by focusing on specific vaccination intentions and behaviors [24]. Hickler et al. have defined vaccine demand as “the actions of individuals and communities to seek, support, and/or advocate for vaccines and immunization services [25].” They further elaborated that vaccine demand is “dynamic and varies by context, vaccine, immunization services provided, time, and place. Demand is fostered by governments, immunization program managers, public and private sector providers, local leadership, and civil society organizations hearing and acting on the voices of individuals and communities.” We use the Hickler et al. definition when referring to vaccine demand in this paper with a focus on the (i) perceived need for an Ebola vaccine, (ii) intention to accept an Ebola vaccine, and (iii) normative belief about the acceptability of an Ebola vaccine. According to the Theory of Planned Behavior, normative belief is an important predictor of behavioral intention [26]; meaning that intention to accept an Ebola vaccine may be influenced by the belief that family members or other important people in the community also want the vaccine. Based on the Health Belief Model [26], the perceived need for an Ebola vaccine may also be driven by the perceived susceptibility of being exposed to Ebola, the perceived severity of Ebola, and the perceived benefit of the vaccine in preventing Ebola in the context of an outbreak. These factors taken together with other socio-behavioral determinants may ultimately impact community level demand for an Ebola vaccine.
In this assessment, we aimed to describe Ebola vaccine demand among the general public right after the peak of new Ebola cases during the 2014–2015 outbreak in Sierra Leone. The assessment was conducted before the implementation of any Ebola vaccine trial in the country. Therefore, we specifically aimed to test the hypothesis of Ebola vaccine demand being associated with perceptions of who the public thinks should be the first to get an Ebola vaccine if it became available during the outbreak. Secondarily, we wanted to understand the reliability and construct validity of a brief measure of Ebola vaccine demand to inform future developments of rapid assessment tools given the lack of validated and field-tested quantitative measures of Ebola vaccine demand. As use of experimental vaccines increasingly becomes an integral part of outbreak preparedness and outbreak responses, our assessment may help to inform how to rapidly evaluate community level demand for new vaccines for outbreak-prone pathogens.
2. Methods
2.1. Design and sampling
A multi-stage cluster survey was conducted in December 2014 (N = 3540) as part of serialized surveys to measure changes in the general public’s knowledge, attitudes, and practices (KAP) during the outbreak in Sierra Leone [27,28]—about four months before the implementation of any Ebola vaccine trial started in the country. A total target sample of 3600 respondents was initially estimated based on sample size calculation that aimed to obtain national and regional level estimates for the KAP outcomes. Primary sampling units for the survey design were defined as the national census-defined enumeration areas (EA). The primary sampling units were randomly selected across all the 14 districts in Sierra Leone, using the EA list from the 2004 Sierra Leone Census as the sampling frame [29]. Systematic sampling was used to randomly select households within EAs. On average, 20 households within each EA were randomly selected for interviews. Two categories of individuals were selected for interview within each household: 1) the head of the household and 2) a young person aged 15–24 years, or an adult woman aged 25 and above. For the second category, if more than two eligible persons were available in the household, simple random sampling was used to select one respondent. Therefore, up to 40 interviews were conducted in each EA on average. The interviews were administered face-to-face within the premises of the household by trained data collectors from FOCUS 1000, a local non-governmental organization in Sierra Leone. All data collectors were trained on the proper administration of the questionnaire and were supervised by the senior staff. We trained data collectors to conduct the two interviews separately to reduce the chance of household heads biasing subsequent interviews.
2.2. Data collection instrument
Data were collected using a structured questionnaire programmed in the Open Data Kit (version 1.0; www.opendatakit.org) application installed on computer-tablets. Closed-ended and open-ended items were included in the survey to measure various domains of Ebola-related KAP; and those results have been reported elsewhere [27]. This paper focuses on items related to Ebola vaccine demand. All items were pilot tested and refined to reflect ease of translation to Krio, the primary local language, and other local languages in Sierra Leone. The survey questionnaire was written in English. The English version of the Ebola vaccine items had a readability score ranging from 9th grade (The SMOG Index) to 11th grade (Flesch-Kincaid Grade Level) reading level.1 All data collectors had post-secondary school education with more than half of them having at least a bachelor’s degree. During the one-week training of data collectors, each questionnaire item was translated to the predominant local language (Krio) and other local languages (Mende, Temne, Susu, Fullah, and Limba). Because these are predominantly oral languages, substantial amount of time was dedicated to orally translate the items under the supervision of local linguist experts. A standardized oral translation for each item was practiced by all data collectors during the training. Language proficiency was assessed by the supervisory team before matching data collectors to field assignments.
2.3. Main outcome
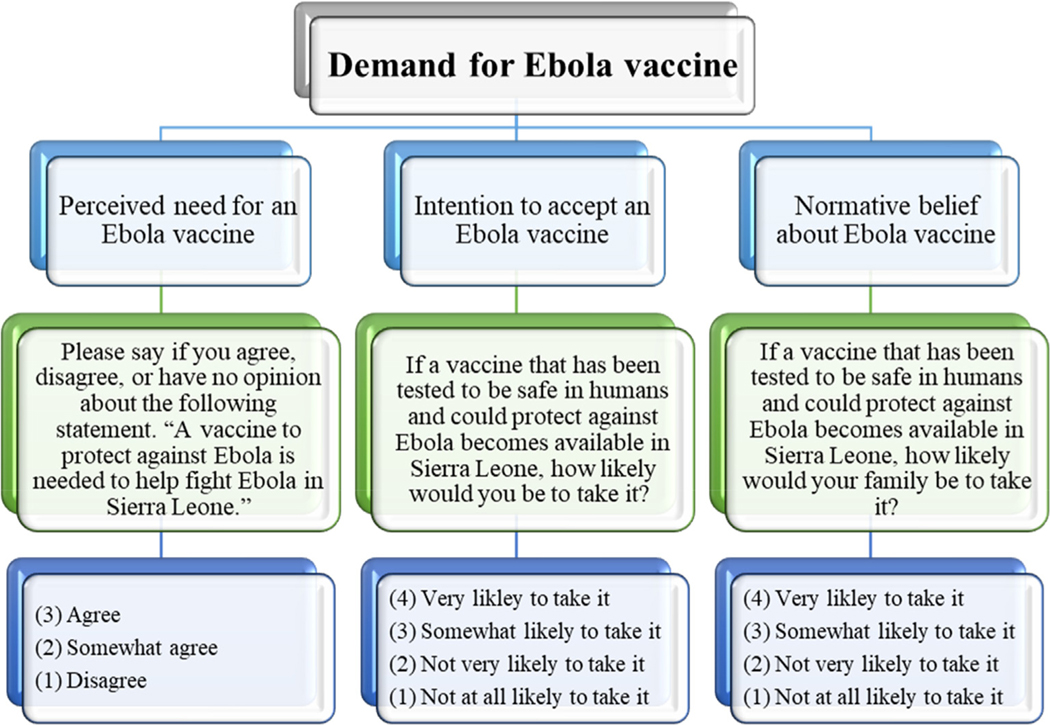
Ebola vaccine demand was the main outcome of interest in the assessment, which was measured by three items on: 1) the perceived need for an Ebola vaccine, 2) intention to accept an Ebola vaccine, and 3) normative belief about acceptability of an Ebola vaccine (Fig. 1). The first item aimed to capture the perceived need for an Ebola vaccine by asking respondents to say if they agree, somewhat agree, or disagree with the statement that “a vaccine to protect against Ebola is needed to help fight Ebola in Sierra Leone.” The second item aimed to measure intention to accept Ebola vaccine by asking respondents, “if a vaccine that has been tested to be safe in humans, and could protect against Ebola becomes available in Sierra Leone, how likely would you be to take it?” Response options were: very likely to take it, somewhat likely to take it, not very likely to take it, not at all likely to take it. The third item aimed to capture normative belief about the acceptability of Ebola vaccine, which was measured by asking respondents, “if a vaccine has been tested to be safe in humans and could protect against Ebola becomes available in Sierra Leone, how likely would your family be to take it?” Response options were the same as those for the second item.
Fig. 1.
Measurement of Ebola vaccine demand among respondents in a national household survey, Sierra Leone, December 2014.
2.4. Explanatory variables
Perceptions of who should be the first recipient of an Ebola vaccine were measured by a categorical item that asked, “if a vaccine has been tested to be safe in humans and could protect against Ebola becomes available in Sierra Leone, who do you think should take it first?” Participants could choose one of the following response options: me/my family, health care workers, burial teams, political leaders, pregnant women, children, the team that is offering the Ebola vaccine to others, people who live in the worst affected areas, or other (open-ended response not captured in the previous categories).
Sociodemographic characteristics of the respondents were measured by closed-ended items for the region of residence (East, West, North, South) gender (male, female), age (in years), education (none, primary, secondary, or higher), and religious affiliation (Islam, Christianity, other).
2.5. Data analysis
The survey dataset was imported into Stata version 15SE (Stata-Corp. 2017. Stata Statistical Software: Release 15. College Station, TX: Stata-Corp LLC) for analysis. We adjusted for the multistage sampling approach in the statistical analysis by using Stata’s SVY command to account for the effect of the sampling design on the calculation of point estimates and their standard errors.
All variables were recoded with numerical values. “Don’t know” and declined responses were excluded from the recoded variables. For the variable on perceived Ebola vaccine need, “agree” was coded 3, “somewhat agree” was coded 2, and “disagree” was coded 1. For the two variables on hypothetical acceptability of an Ebola vaccine for oneself and by family members respectively, “very likely” was coded 4, “somewhat likely” was coded 3, “not very likely” was coded 2, and “not at all likely” was coded 1. A new variable was generated to calculate the composite score for Ebola vaccine demand, with possible scores ranging from 3 to 11. A binary outcome variable was generated for the level of Ebola vaccine demand based on the score. Low demand (coded 0) was defined as a score less than or equal to the sample mean score while high demand (coded 1) was defined as a score greater than the mean.
We fitted a multilevel mixed-effects logistic regression model, with a random intercept accounting for the clustering of respondents within EAs, to assess associations between perceptions of who should be the first recipient of an Ebola vaccine and expressing a high demand for an Ebola vaccine. We set “politicians” as the reference category because we hypothesized that those who wanted politicians to be the first to take an Ebola vaccine may have a low demand for the vaccine. The model was adjusted for sociodemographic characteristics (sex, age, education, occupation, and religion). Sociodemographic characteristics were included in the model as covariates because they have shown to be associated with vaccine uptake [30–32]. All statistical tests were based on two-sided Wald-type tests and the level of significance was set to α = 0.05.
Exploratory Factor Analysis (EFA) was conducted using the principal-component factors extraction method to examine construct validity because the relationship among the three items aiming to measure the Ebola vaccine demand was not established in any prior research [33]. We examined factor loadings, the proportion of variance explained by extracted factors, and generated a Scree plot of eigenvalues. Kaiser-Meyer-Olkin measure was used to determine sampling adequacy [33]. We assessed reliability based on the internal consistency of the three items with a Cronbach’s alpha value greater than 0.7 indicating acceptable scale reliability [34].
2.6. Ethical approval
The assessment was approved by the Office of the Sierra Leone Ethics and Scientific Review Committee, and it also received non-research determination from the U.S. Centers of Disease Control and Prevention. All participants provided written or thumb-printed consent prior to enrollment into the assessment. No incentives were provided to participants. An Ebola prevention flyer was handed out to each participating household at the end of the interviews.
3. Results
Out of the 1800 households approached by data collection teams, 1770 households agreed to participate in the survey (98% response rate). Two interviews were conducted in each household, resulting in 3540 respondents. On average, households had seven members (interquartile range of 3). Of the total sample that consented, 1731 (48.9%) were females, 1177 (33.3%) were aged 15–24 years, 1194 (33.8%) had no education, and 2335 (66.0%) identified Islam as their religion (Table 1). Overall, 92.5% of respondents agreed that an Ebola vaccine was necessary to fight the outbreak, 77.9% said that they were very likely to accept an Ebola vaccine for themselves, and similarly, 77.2% said that their family would be very likely to accept an Ebola vaccine. When asked who should be the first to receive an Ebola vaccine if it were to become available, the leading responses were health workers (36.9%), me or my family (25.5%), politicians (13.8%), team that is offering the vaccine to others (9.8%), and people who live in worst affected areas (8.2%) (Table 2). We compared responses across the three participant categories (heads of households, adult women, and young people) and found no statistically significant differences for any of three Ebola vaccine demand items (data not shown).
Table 1.
Demographic characteristics of respondents# in a national household survey, Sierra Leone, December 2014.
| Characteristic* | N | % |
|---|---|---|
| Region | ||
| Western Area | 812 | 22.9% |
| Northern Province | 1247 | 35.2% |
| Eastern Province | 919 | 26.0% |
| Southern Province | 562 | 15.9% |
| Sex | ||
| Male | 1809 | 51.1% |
| Female | 1731 | 48.9% |
| Age category | ||
| 15–24 years | 1177 | 33.3% |
| 25 + years | 2363 | 66.7% |
| Education | ||
| None | 1194 | 33.8% |
| Some primary | 677 | 19.1% |
| Secondary or higher | 1668 | 47.1% |
| Religion | ||
| Islam | 2335 | 66.0% |
| Christianity | 1200 | 33.9% |
| Other/no religion | 2 | 0.1% |
N = 3.540 respondents.
1 missing value for education; 2 missing values for religion.
Table 2.
Attitudes towards Ebola vaccine and Ebola risk perception among respondents# in a national household survey, Sierra Leone, December 2014.
| # | Item& | N | % | 95% CI |
|---|---|---|---|---|
| 1 | Please say if you agree, disagree, or have no opinion about the following statement. “A vaccine to protect against Ebola is needed to help fight Ebola in Sierra Leone.” | |||
| Agree | 3217 | 92.5 | 90.6–94.0 | |
| Somewhat agree | 143 | 4.1 | 3.0–5.6 | |
| Disagree | 119 | 3.4 | 2.6–4.5 | |
| 2 | If a vaccine that has been tested to be safe in humans and could protect against Ebola becomes available in Sierra Leone, how likely would you be to take it? | |||
| Very likely to take it | 2701 | 77.9 | 74.1–81.3 | |
| Somewhat likely to take it | 590 | 17.0 | 14.3–20.1 | |
| Not very likely to take it | 114 | 3.3 | 2.5–4.3 | |
| Not at all likely to take it | 62 | 1.8 | 1.2–2.6 | |
| 3 | If a vaccine that has been tested to be safe in humans and could protect against Ebola becomes available in Sierra Leone, how likely would your family be to take it? | |||
| Very likely to take it | 2653 | 77.2 | 73.3–80.7 | |
| Somewhat likely to take it | 595 | 17.3 | 14.6–20.4 | |
| Not very likely to take it | 129 | 3.8 | 2.9–4.9 | |
| Not at all likely to take it | 58 | 1.7 | 1.2–2.5 | |
| 4 | If a vaccine that has been tested to be safe in humans and could protect against Ebola becomes available in Sierra Leone, who do you think should take it first? | |||
| Me or my family | 869 | 25.5 | 22.2–29.0 | |
| Healthcare workers or burial teams | 1262 | 36.9 | 40.0–40.9 | |
| Political leaders | 471 | 13.8 | 11.8–16.0 | |
| Pregnant women | 41 | 1.2 | 0.7–2.1 | |
| Children | 98 | 2.9 | 2.0–4.1 | |
| Team that is offering the Ebola vaccine to others | 336 | 9.8 | 8.0–12.0 | |
| People who live in worst affected areas | 281 | 8.2 | 6.6–10.2 | |
| Other | 57 | 1.7 | 1.0–2.7 | |
CI = confidence interval.
N = 3540 respondents.
Don’t know and declined responses excluded: 61 missing for item-1; 73 missing for item-2; 105 missing for item-3; 125 missing for item-4.
3.1. Ebola vaccine demand
The mean for the aggregated Ebola vaccine demand score was 10, and the score ranged from 3 to 11. After dichotomizing the score at the mean value, 74.2% of respondents met the criteria for expressing a high demand for an Ebola vaccine (score of 11). Therefore, respondents classified as expressing high Ebola vaccine demand scored the maximum number of points on each of the three items by (i) agreeing that a vaccine was needed to protect against Ebola, (ii) saying that they were very likely to take the vaccine if it became available, and (iii) also saying that they would accept the vaccine for their family.
3.2. Predictors of high Ebola vaccine demand
Compared to those who said politicians should be the first to receive an Ebola vaccine, the odds of expressing high demand for an Ebola vaccine was: 13 times greater among those who said “me or my family” (adjusted odds ratio [aOR] 13.0 [95% confidence interval 7.8–21.6]), six times greater among those who said “pregnant women” (aOR 5.7 [1.9–17.5]), five times greater among those who said “children” (aOR 4.7 [2.4–9.1]), three times greater among those who said “people who live in worst affected areas” (aOR 2.9 [1.7–5.1]), and two times greater among those who said “health care workers or burial teams” (aOR 2.0 [1.4–2.8]) (Table 3). The odds of expressing high demand were not different between respondents who said the team offering Ebola vaccine should be the first to take the vaccine and those who said politicians should be the first to take the vaccine (aOR 1.4 [0.9–2.1]). Respondents with secondary school education had 60% increased odds of expressing high demand compared to those with no education (aOR 1.6 [1.2–2.1]). Besides education, all other sociodemographic covariates (i.e., region, sex, age, religion) were not significantly associated with high demand (Table 3).
Table 3.
Multilevel logistic regression model for expressing high demand for Ebola vaccine among respondents# in a national household survey, Sierra Leone, December 2014.
| Multivariable model |
||
|---|---|---|
| aOR‡ (95%CI) | P value† | |
| Perceived first recipient | ||
| Politicians | Reference | |
| Me/my family | 13.0(7.8–21.6) | 0.000 |
| Pregnant women | 5.7 (1.9–17.5) | 0.003 |
| Children | 4.7 (2.4–9.1) | 0.000 |
| People who live in worst affected areas | 2.9 (1.7–5.1) | 0.000 |
| Healthcare workers/burial teams | 2.0 (1.4–2.8) | 0.000 |
| Other | 2.0 (0.9–4.2) | 0.051 |
| The team offering an Ebola vaccine | 1.4 (0.9–2.1) | 0.157 |
| Geographic region | ||
| Western Area | Reference | |
| North Province | 1.4 (0.8–2.3) | 0.188 |
| Eastern Province | 1.8 (0.9–3.4) | 0.057 |
| Southern Province | 1.1 (0.5–2.5) | 0.891 |
| Gender | ||
| Male | Reference | |
| Female | 0.9 (0.8–1.1) | 0.426 |
| Age | 1.0 (0.9–1.0) | 0.242 |
| Education | ||
| None | Reference | |
| Primary | 1.1 (0.8–1.5) | 0.633 |
| Secondary or higher | 1.6 (1.2–2.1) | 0.001 |
| Religion | ||
| Islam | Reference | |
| Christianity | 1.0 (0.6–1.7) | 0.881 |
CI = confidence interval.
N = 3290 respondents; 250 (7%) had one or more missing responses that were excluded.
Adjusted odds ratio (aOR) is adjusted for region of residence, sex, age, education, and religion.
Wald statistical p value from multiple logistic regression model.
3.3. Reliability and construct validity
The three items demonstrated acceptable internal consistency with a Cronbach’s alpha value of 0.79. Results from the EFA indicated one retained factor for the three items, which explained 71% of the variance and had an eigenvalue of 2.14 (Supplemental Material). The last two items on Table 4 regarding the hypothetical acceptability of an Ebola vaccine had very high loadings onto the retained factor (0.95 respectively) and the item on perceived need for an Ebola vaccine also had an acceptable loading (0.57) (Table 4). Kaiser-Meyer-Olkin value for sampling adequacy was 0.56.
Table 4.
Exploratory factor analysis for the brief measure of Ebola vaccine demand among respondents# in a national household survey, Sierra Leone, December 2014.
| Item | Range | Mean | SD* | Loadings | Uniqueness |
|---|---|---|---|---|---|
| “A vaccine to protect against Ebola is needed to help fight Ebola in Sierra Leone. | 1–3 | 2.90 | 0.37 | 0.57 | 0.67 |
| If a vaccine that has been tested to be safe in humans and could protect against Ebola becomes available in Sierra Leone, how likely would you be to take it? | 1–4 | 3.72 | 0.59 | 0.95 | 0.09 |
| If a vaccine that has been tested to be safe in humans and could protect against Ebola becomes available in Sierra Leone, how likely would your family be to take it? | 1–4 | 3.70 | 0.61 | 0.95 | 0.10 |
N = 3540 respondents.
SD = standard deviation.
4. Discussion
Our findings revealed a high demand for the Ebola vaccine among the general public during the peak of the largest ever Ebola outbreak, which occurred in Sierra Leone. About seven out of every ten respondents expressed high demand for the vaccine. Our findings also demonstrated the feasibility of using a brief three-item questionnaire to measure demand for the Ebola vaccine among the general public in an outbreak setting. Perception of who should be the first to take the vaccine if it became available was a strong predictor of a high demand for an Ebola vaccine. The odds of having high demand for an Ebola vaccine were much greater among those who expressed that they or their families should be the first to take the vaccine compared to those who wanted politicians to be the first to do so. On the other hand, demand was not different between those who said that the team offering the Ebola vaccine should be the first to take it and those who said politicians should be the first. It is possible that those who wanted to see politicians, or the Ebola vaccine team be the first to take the vaccine had lower institutional trust [35]. For instance, a survey conducted in 2018 during the Ebola outbreak in DRC found that institutional trust was a strong predictor of Ebola vaccine acceptability among the general public [22]. These results in the context of prior evidence point to the importance of investigating and addressing underlying institutional mistrust when planning to implement Ebola vaccination during an outbreak.
We acknowledge that there could be alternative explanations for why some respondents said that politicians should be the first to receive the vaccine in our survey. For instance, some respondents may have said so out of reverence of their political leaders, but this notion is not supported by the data we have presented. Our results demonstrate that those who expressed high demand for the vaccine wanted themselves or their families to be the first to get the vaccine. In our view, this is one form of validation of the Ebola vaccine demand measure used in the assessment because it shows that people with high demand for the vaccine would prioritize themselves or their families for the vaccine if it became available to the public. Conversely, those with low demand for the vaccine were more likely to say that politicians should be the first to receive the vaccine. We suspect that low demand for the vaccine may have be confounded by mistrust of politicians, which we did not measure in the study. Additional qualitative research may be needed to further explore the underlying reasons for low demand and how mistrust of authority may play a role in influencing Ebola vaccine demand. Additionally, respondents who wanted health workers (outside of the team offering the Ebola vaccine) should be the first to get the vaccine may have said so because they perceived health workers to be at higher risk of getting infected with Ebola. At the time of the survey, participants may have been aware of a planned Ebola vaccine clinical trial aimed at health workers, which may have influenced perceptions that health workers should be prioritized for the getting the vaccine if it became available.
The level of Ebola vaccine demand found in our assessment is consistent with other studies in both outbreak and non-outbreak settings. In 2018, among community members in DRC who received an Ebola vaccine as part of ring vaccination, 84% expressed willingness to promote the vaccine to others while 82% said they would accept the vaccine for their families [9]. Among community controls who were not offered the vaccine in the DRC study, 72% expressed some intention to accept the vaccine if it became available and they were offered to take it. In a mixed-methods study in Nigeria, 80% of respondents expressed intention to accept an Ebola vaccine; however, most of them only intended to accept the vaccine if they could first have an opportunity to observe its effects on others who have taken it [36].
Our brief measure of Ebola vaccine demand demonstrated acceptable scale reliability and construct validity. Items measuring Ebola vaccine demand in our assessment were framed around the hypothetical Ebola vaccine “that has been tested to be safe in humans and could protect against Ebola.” It is possible that respondents may have understood such framing as referring to a licensed Ebola vaccine, similar to those offered as part of the routine childhood immunization. In a prior qualitative assessment, most respondents could not distinguish a licensed Ebola vaccine from an experimental Ebola vaccine, and positive perceptions of vaccines offered though the childhood immunization programs served as a facilitator for intending to accept an Ebola vaccine [35]. Progress made in building demand for childhood vaccines [37] may have contributed to the high Ebola vaccine demand among the general public. Therefore, emergence of misinformation about the Ebola vaccines may likewise hinder childhood immunization if not adequately addressed.
Qualitative assessments have highlighted the complexity of factors that may influence intentions to accept Ebola vaccines [35,38,39], including safety perceptions, subjective evaluations of the need for an Ebola vaccine, altruistic desires to prevent Ebola transmission, Ebola risk perception, mistrust of authorities, confidence in the team offering the vaccine to others, and the notion of power, fairness, and trust—issues that will remain relevant even with a licensed Ebola vaccine. Adapting some of these qualitative themes into quantitative items that could be further examined for reliability and validity should be explored in future adaptations and refinement of the current items to measure Ebola vaccine demand.
5. Limitations
Our assessment has limitations. The data used in our analysis were collected near the peak of the largest Ebola outbreak to date, which poses limitations in interpreting and generalizing the results to non-outbreak contexts. However, a brief measure of Ebola vaccine demand is most likely to be of use in an outbreak situation, when time-efficiency might be essential. Another possible limitation is that our measure of Ebola vaccine demand only contained three items that loaded onto a single factor. Therefore, other meaningful domains of Ebola vaccine demand were likely missed. Nevertheless, having few items to measure the Ebola vaccine demand increases the feasibility of including such items into other planned data collection efforts during outbreak response and preparedness. Finally, our study was not designed to allow us to assess the extent to which the three vaccine demand items may predict the actual uptake of an Ebola vaccine because our sample did not comprise individuals who had been offered an Ebola vaccine. Therefore, we cannot ascertain if behavioral intentions to accept an Ebola vaccine would translate into actual uptake of the vaccine based on the available data. However, our results are consistent with findings from a community-level survey of Ebola vaccine recipients in DRC in that institutional trust was an important predictor of intention to accept an Ebola vaccine [9].
6. Conclusions
Demand for an Ebola vaccine was high among the general public around the peak of the outbreak in Sierra Leone. The Ebola vaccine landscape has continued to evolve since the end of the 2014– 2016 West Africa outbreak. During the 2018–2019 outbreak in DRC, rVSV-ZEBOV-GP (Merck) vaccine has been offered under a compassionate use protocol to persons at risk for Ebola (contact, contacts of contacts and healthcare and frontline workers) using a ring vaccination or geographically targeted strategy [40]. Successful implementation of Ebola ring vaccination depends on adequate community demand for the vaccine. As the World Health Organization and global partners prioritize the scalability of a Research and Development Blueprint for action to prevent epidemics [41], we believe there is an urgent need to adapt brief measures of community demand for new vaccines and therapeutics during emerging outbreaks. Information on vaccine demand will provide important information for social mobilization and community engagement to address rumors, mistrust to improve demand. Information on who the community feels should be first to get vaccine can be used, where feasible, for a strong start to vaccine implementation. Experimental vaccines can help save many lives during outbreaks but only if there is enough demand for the vaccines being offered.
Supplementary Material
Footnotes
Disclaimer
The findings and conclusions in this report are those of the authors, and do not necessarily represent the official position of the U.S. Centers for Disease Control and Prevention.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Appendix A. Supplementary material
Supplementary data to this article can be found online at https://doi.org/10.1016/j.vaccine.2020.03.044.
Readability formulas. https://readabilityformulas.com/freetests/six-readability-formulas.php
References
- [1].U.S. Centers Disease Control and Prevention. Years of Ebola Virus Disease outbreaks; 2018. Available from: https://www.cdc.gov/vhf/ebola/history/chronology.html. [Google Scholar]
- [2].Fitzmaurice AG, Mahar M, Moriarty LF, Bartee M, Hirai M, Li W, et al. Contributions of the US centers for disease control and prevention in implementing the global health security Agenda in 17 partner countries. Emerg Infect Dis 2017;23(13). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Marston BJ, Dokubo EK, van Steelandt A, Martel L, Williams D, Hersey S, et al. Ebola response impact on public health programs, West Africa, 2014–2017. Emerg Infect Dis 2017;23(13). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Heymann DL, Chen L, Takemi K, Fidler DP, Tappero JW, Thomas MJ, et al. Global health security: the wider lessons from the west African Ebola virus disease epidemic. Lancet 2015;385(9980):1884–901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].World Health Organization. Ebola vaccines, therapies, and diagnostics; 2015. Available from: https://www.who.int/medicines/emp_ebola_q_as/en/. [Google Scholar]
- [6].Walldorf JA, Cloessner EA, Hyde TB, MacNeil A. Considerations for use of Ebola vaccine during an emergency response. Vaccine 2019;37(48):7190–200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].World Health Organization. Guinea Ring Vaccination trial extended to Sierra Leone to vaccinate contacts of new Ebola case; 2015. Available from: https://www.who.int/news-room/detail/31-08-2015-guinea-ring-vaccination-trial-extended-to-sierra-leone-to-vaccinate-contacts-of-new-ebola-case. [Google Scholar]
- [8].Merler S, Ajelli M, Fumanelli L, Parlamento S, Pastore YPA, Dean NE, et al. Containing Ebola at the source with ring vaccination. PLoS NeglTrop Dis 2016;10(11):e0005093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Kasereka MC, Sawatzky J, Hawkes MT. Ebola epidemic in war-torn Democratic Republic of Congo, 2018: acceptability and patient satisfaction of the recombinant Vesicular Stomatitis Virus - Zaire Ebolavirus Vaccine. Vaccine 2019;37(16):2174–8. [DOI] [PubMed] [Google Scholar]
- [10].Gsell PS, Camacho A, Kucharski AJ, Watson CH, Bagayoko A, Nadlaou SD, et al. Ring vaccination with rVSV-ZEBOV under expanded access in response to an outbreak of Ebola virus disease in Guinea, 2016: an operational and vaccine safety report. Lancet Infect Dis 2017;17(12):1276–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Doe-Anderson J, Baseler B, Driscoll P, Johnson M, Lysander J, McNay L, et al. Beating the odds: successful establishment of a phase II/III clinical research trial in resource-poor liberia during the largest-ever ebola outbreak. Contemp Clin Trials Commun 2016;4:68–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Kennedy SB, Neaton JD, Lane HC, Kieh MW, Massaquoi MB, Touchette NA, et al. Implementation of an Ebola virus disease vaccine clinical trial during the Ebola epidemic in Liberia: design, procedures, and challenges. Clin Trials 2016;13 (1):49–56. [DOI] [PubMed] [Google Scholar]
- [13].Henao-Restrepo AM, Camacho A, Longini IM, Watson CH, Edmunds WJ, Egger M, et al. Efficacy and effectiveness of an rVSV-vectored vaccine in preventing Ebola virus disease: final results from the Guinea ring vaccination, open-label, cluster-randomised trial (Ebola Ca Suffit!). Lancet 2017;389 (10068):505–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Widdowson MA, Schrag SJ, Carter RJ, Carr W, Legardy-Williams J, Gibson L, et al. Implementing an Ebola vaccine study - Sierra Leone. MMWR Suppl 2016;65(3):98–106. [DOI] [PubMed] [Google Scholar]
- [15].Zhu FC, Wurie AH, Hou LH, Liang Q, Li YH, Russell JB, et al. Safety and immunogenicity of a recombinant adenovirus type-5 vector-based Ebola vaccine in healthy adults in Sierra Leone: a single-centre, randomised, double-blind, placebo-controlled, phase 2 trial. Lancet 2017;389(10069):621–8. [DOI] [PubMed] [Google Scholar]
- [16].Samai M, Seward JF, Goldstein ST, Mahon BE, Lisk DR, Widdowson MA, et al. The Sierra Leone trial to introduce a vaccine against Ebola: an evaluation of rVSVG-ZEBOV-GP vaccine tolerability and safety during the West Africa Ebola outbreak. J Infect Dis 2018;217(suppl_1):S6–S15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].World Health Organization. Major milestone for WHO-supported Ebola vaccine; 2019. [cited 2019 November]. Available from: https://www.who.int/news-room/detail/18-10-2019-major-milestone-for-who-supported-ebola-vaccine. [Google Scholar]
- [18].U.S. Food and Drug Administration. First FDA-approved vaccine for the prevention of Ebola virus disease, marking a critical milestone in public health preparedness and response; 2019. Available from: https://www.fda.gov/news-events/press-announcements/first-fda-approved-vaccine-prevention-ebola-virus-disease-marking-critical-milestone-public-health. [Google Scholar]
- [19].Huo X, Shi G, Li X, Lai X, Deng L, Xu F, et al. Knowledge and attitudes about Ebola vaccine among the general population in Sierra Leone. Vaccine 2016;34 (15):1767–72. [DOI] [PubMed] [Google Scholar]
- [20].Jendrossek M, Edmunds WJ, Rohan H, Clifford S, Mooney TA, Eggo RM. Health care worker vaccination against Ebola: Vaccine acceptance and employment duration in Sierra Leone. Vaccine. 2019;37(8):1101–8. [DOI] [PubMed] [Google Scholar]
- [21].Irwin KL, Jalloh MF, Corker J, Alpha Mahmoud B, Robinson SJ, Li W, et al. Attitudes about vaccines to prevent Ebola virus disease in Guinea at the end of a large Ebola epidemic: results of a national household survey. Vaccine 2017;35(49 Pt B):6915–23. [DOI] [PubMed] [Google Scholar]
- [22].Vinck P, Pham PN, Bindu KK, Bedford J, Nilles EJ. Institutional trust and misinformation in the response to the 2018–19 Ebola outbreak in North Kivu, DR Congo: a population-based survey. Lancet Infect Dis 2019;19(5):529–36. [DOI] [PubMed] [Google Scholar]
- [23].Kardas-Nelson M. Ebola in Sierra Leone and DRC: lessons learnt. BMJ 2019;366:l5012. [DOI] [PubMed] [Google Scholar]
- [24].Dudley MZ, Privor-Dumm L, Dube E, MacDonald NE. Words matter: vaccine hesitancy, vaccine demand, vaccine confidence, herd immunity and mandatory vaccination. Vaccine 2020;38(4):709–11. [DOI] [PubMed] [Google Scholar]
- [25].Hickler B, MacDonald NE, Senouci K, Schuh HB. Efforts to monitor Global progress on individual and community demand for immunization: development of definitions and indicators for the Global Vaccine Action Plan Strategic Objective 2. Vaccine 2017;35(28):3515–9. [DOI] [PubMed] [Google Scholar]
- [26].Glanz KR, Barbara KR, Viswanath K. Health beahvior: theory, research, and practice, 5th ed., San Francisco, California: Jossey-Bass; 2015. July 2015. 512 p. [Google Scholar]
- [27].FOCUS1000. Ebola KAP-3 Preliminary Findings; 2014. Available from: http://focus1000.org/index.php/downloads-resources/download/4-ebola-kap-study/25-ebola-kap-3-presentation. [Google Scholar]
- [28].Jalloh MF, Sengeh P, Monasch R, Jalloh MB, DeLuca N, Dyson M, et al. National survey of Ebola-related knowledge, attitudes and practices before the outbreak peak in Sierra Leone: August 2014. BMJ Glob Health 2017;2(4): e000285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Government of Sierra Leone. Sierra Leone 2004 population and housing census. Freetown: Statistics Sierra Leone; 2004. [Google Scholar]
- [30].Mvula H, Heinsbroek E, Chihana M, Crampin AC, Kabuluzi S, Chirwa G, et al. Predictors of uptake and timeliness of newly introduced pneumococcal and rotavirus vaccines, and of measles vaccine in rural Malawi: a population cohort study. PLoS ONE 2016;11(5):e0154997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Han YK, Michie S, Potts HW, Rubin GJ. Predictors of influenza vaccine uptake during the 2009/10 influenza A H1N1v (‘swine flu’) pandemic: results from five national surveys in the United Kingdom. Prev Med 2016;84:57–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Ochu CL, Beynon CM. Hepatitis B vaccination coverage, knowledge and sociodemographic determinants of uptake in high risk public safety workers in Kaduna State, Nigeria: a cross sectional survey. BMJ Open 2017;7(5):e015845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Exploratory Thompson B. and confirmatory factor analysis: understanding concepts and applications. Washington DC: American Psychological Association; 2004. [Google Scholar]
- [34].de Vet HCW, Mokkink LB, Mosmuller DG, Terwee CB. Spearman-Brown prophecy formula and Cronbach’s alpha: different faces of reliability and opportunities for new applications. J Clin Epidemiol 2017;85:45–9. [DOI] [PubMed] [Google Scholar]
- [35].Jalloh MF, Jalloh MB, Albert A, Wolff B, Callis A, Ramakrishnan A, et al. Perceptions and acceptability of an experimental Ebola vaccine among health care workers, frontline staff, and the general public during the 2014–2015 Ebola outbreak in Sierra Leone. Vaccine 2019;37(11):1495–502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Ughasoro MD, Esangbedo DO, Tagbo BN, Mejeha IC. Acceptability and willingness-to-pay for a hypothetical Ebola virus vaccine in Nigeria. PLoS NeglTrop Dis 2015;9(6):e0003838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].World Health Organization. Global vaccine action plan 2011– 2020. Geneva: WHO; 2013. [Google Scholar]
- [38].Tengbeh AF, Enria L, Smout E, Mooney T, Callaghan M, Ishola D, et al. “We are the heroes because we are ready to die for this country”: participants’ decision-making and grounded ethics in an Ebola vaccine clinical trial. Soc Sci Med 1982;2018(203):35–42. [DOI] [PubMed] [Google Scholar]
- [39].Enria L, Lees S, Smout E, Mooney T, Tengbeh AF, Leigh B, et al. Power, fairness and trust: understanding and engaging with vaccine trial participants and communities in the setting up the EBOVAC-Salone vaccine trial in Sierra Leone. BMC Public Health 2016;16(1):1140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].World Health Organization. Preliminary results on the efficacy of rVSV-ZEBOV-GP Ebola vaccine using the ring vaccination strategy in the control of an Ebola outbreak in the Democratic Republic of the Congo: an example of integration of research into epidemic response; 2019. [cited 2019 November]. Available from: https://www.who.int/csr/resources/publications/ebola/ebola-ring-vaccination-results-12-april-2019.pdf?ua=1. [Google Scholar]
- [41].World Health Organization. A research and development Blueprint for action to prevent epidemics; 2020. Available from: https://www.who.int/blueprint/about/en/. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.