Summary
The incidence of cancer has shown a great increase during the past decades and poses tough challenges to cancer treatment. Anti-tumour immunotherapy, represented by immune checkpoint inhibitors (ICIs), possesses favorable remission in unrestricted spectrum of cancer types. However, its efficacy seems to be heterogeneous among accumulating studies. Emerging evidences suggest that gut microbiota can modulate anti-tumour immuno-response and predict clinical prognosis. Therefore, remodeling microbiota characteristics with fecal microbiota transplantation (FMT) may be capable of reinforcing host ICIs performance by regulating immune-tumour cell interactions and altering microbial metabolites, thereby imperceptibly shifting the tumour microenvironment. However, the long-term safety of FMT is under concern, which calls for more rigorous screening. In this review, we examine current experimental and clinical evidences supporting the FMT efficacy in boosting anti-tumour immuno-response and lessening tumour-related complications. Moreover, we discuss the challenges in FMT and propose feasible resolutions, which may offer crucial guidance for future clinical operations.
Keywords: Fecal microbiota transplantation, Tumour immunotherapy, Immune checkpoint inhibitors, Gut microbiota, Tumour microenvironment
Searching strategy and selection criteria.
We searched the databases of Pubmed, MEDLINE and ClinicalTrials.gov for articles and trials published only in English, using the terms “fecal microbiota transplantation”, “gut microbiome and cancer”, “tumour immune and immunotherapy”, “immune checkpoint inhibitors and microbiome”, “FMT and cancer” from 2005 to 2023.
Introduction
Worldwide, cancer has evolved into a major public health problem, further drawing on a heavy and ever-increasing social and economic burden. Mechanisms of tumour development, progression and metastasis have been stepwise revealed during past decades, which constructs a intricate net between host immune status and external environment. Interfering the downstream of immune evasion and co-inhibitory signal of T cell activation has consistently shown notable effects in anti-tumour immunotherapy. Blocking immune checkpoint pathway, through which cancer cells can disguise themselves as normal components of the human body, is one of the most common approaches to establish anti-tumour immunity. With the penetrating investigation of immuno-blocking therapy, immune checkpoint inhibitors (ICIs) gradually come to front and obtain positive clinical evidences.1 Interestingly, it is worth noting that the therapeutic efficacy of ICIs seems to have a relationship with host microbiome environment.
Among host organisms, gut microbiota constituted by trillions of symbiotic microorganisms is constantly described as a “super organ” as a whole. Gut microbial communities keep a subtle balance of suppressing-promoting tumourigenesis with its metabolites among mass factors. Previous studies highlighted that microbial alternations, characterized by a marked boost in the numbers of pathogens and a relative decrease in beneficial bacteria, are interrelated with the development of gastrointestinal and extra-gastrointestinal cancers.2,3 It is a well-established statement that gut microbiota act a distinct role in regulating host immuno-modulation, maintaining cancer immune homeostasis and sustaining tumour microenvironment (TME).4,5 Some bacteria help fight tumours by activating immunity, while some bacteria mediate immunosuppression to help cancer cells escape from immune surveillance.6 Studies have reported that the gut microbiota is related to anti-tumour immune factors, as commensal bacteria Bacteroidetes is positively correlated with anti-tumour immune factors, while pathogenic subset Proteobacteria has opposite correlations.6 Furthermore, compelling evidences suggest that modulating gut microbiota can enhance the efficacy of cancer therapies, especially immunotherapy.2,7, 8, 9 Hence modulating immune response to anti-tumour immunotherapy by shifting microbial combination are of immense feasibility and bright prospect. Under these circumstances, FMT as an intervention to manipulate gut microbiota as a whole, shows a promising foreground.10 This tool of modulating gut microbiota has great advantages in restoring healthy functioning intestinal microbiota especially after conventional antibiotic therapy disturbs the normal gut microbial balance.11 Nevertheless, concerns about the safety, efficacy and precision of FMT still exist.12 Due to the proportion of ineffectiveness and the potential risk underlying, FMT application needs discreet screening of both donors and recipients before administration.
Our review aims to jointly underline the complicated association between gut microbiota and anti-tumour immunotherapy, and highlight the clinical applications of FMT in up-regulating therapeutic efficacy. We will provide an overview of FMT administration in promoting anti-tumour immuno-response on specific histopathological tumour types and finally discuss its cons of current challenges and prospects.
ICIs application in anti-tumour clinical practices
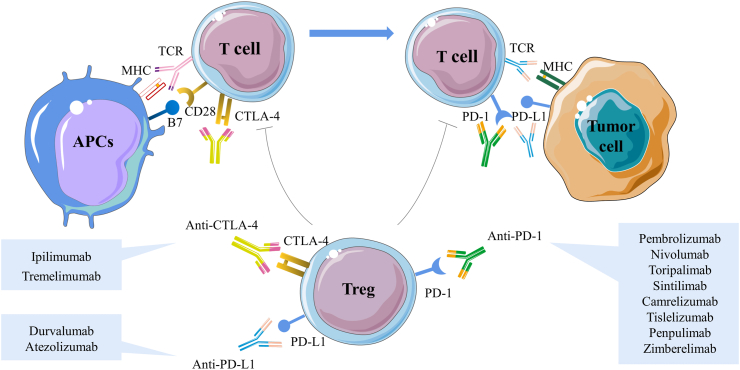
Tumour immunotherapy has expanded dramatically over the past few decades. The immunological checkpoint molecules involved in immune process of tumourigenesis as co-inhibitory receptors of T-cell activation, which pave the way for their antibodies, commonly referred to as ICIs, to be applied in anti-tumour therapy. Among these checkpoints, programmed cell death 1 (PD-1) and its ligands PD-L1, as well as cytotoxic T-lymphocyte associated antigen-4 (CTLA-4), play a decisive role in maintaining T cell activation and tolerance. PD-1 is expressed on activated T cells, B cells, natural killer (NK) cells, and myeloid cells. Once PD-1 interacts with its ligands, it decreases the immune response. Tumour cells are able to activate CTLA-4, which silences activated T cells by competitively binding to CD80/86 ligand to generate inhibitory signals, thus moderating the activation of CD4+ helper T cells while promoting the proliferation of Tregs13 (Fig. 1). PD-1 and CTLA-4, along with other negative immuno-modulatory molecules like lymphocyte activation gene 3 (LAG-3), T cell immunoglobulin and mucin-containing molecule 3 (TIM-3), T cell immunoglobulin and ITIM domain (TIGIT) and V-domain immunoglobulin suppressor of T cell activation (VISTA) produce an immuno-suppressive phenotype of tumour progression.14 Therefore, by blocking PD-1/PD-L1 and CTLA-4, ICIs provide novel targets for reactivating the function of immune cells and restoring the anti-tumour activity of immune cells.
Fig. 1.
Immune checkpoint inhibitors mediate negative co-stimulation and modulate tumour antigens to inhibit T cell activation and differentiation. The expression and function of CTLA-4 are intrinsically linked to T cell activation. Normally, with T cell receptor (TCR) engagement, CTLA-4 is immediately up-regulated. CTLA-4 inhibits TCR signaling by competing with the co-stimulatory molecules CD28 for the B7 ligands, and CTLA-4 has a higher affinity and binding strength, thus causing simultaneous competitive inhibition of both molecules and effectively attenuating T cell activation. In the peripheral TME, PD-1 is expressed mainly on activated T cells. Once PD-1 interacts with its ligand PD-L1, it decreases the immune response, which is thought to be the primary mechanism of tumour immune escape. The extracellular suppressive effects of ICIs are mainly mediated by Tregs, which are necessary for the maintenance of immune tolerance. Currently, ICIs mainly include anti-PD-1 antibodies pembrolizumab, nivolumab, toripalimab, sintilimab, camrelizumab, tislelizumab, penpulimab, zimberelimab, anti-PD-L1 antibodies durvalumab, atezolizumab and anti-CTLA-4 antibodies ipilimumab, tremelimumab and are therefore very attractive therapeutic targets. CTLA-4, cytotoxic T-lymphocyte associated antigen-4; ICIs, immune checkpoint inhibitors; PD-1, programmed cell death 1; PD-L1, programmed cell death ligand 1; TCR, T cell receptor; TME, tumour microenvironment; Tregs, regulatory T cells.
ICIs are gradually considered as vanguard and luminary in immunotherapy due primarily to their wide bio-activity among several metastatic tumour types, prominently represented by metastatic melanoma, non-small-cell lung cancer (NSCLC).15,16 However, even among these tumours the outcomes of ICIs sometimes turn out to be regrettably unfavourable. Despite the substantial progress in the application of ICIs, heterogeneous efficiency exists among individuals with cancer. Some cancer patients are resistant to ICIs or only show a transient response, concurrently multiple complications are of possibility to occur, thus the safety of ICIs application is hard to be assured. Extended exposure time and increased administered dose may improve the immune response, but in the meantime accompanied by higher frequency of immune-related adverse events (irAEs).17 Various mechanisms have been proposed to be ICI non-response-related, including low mutational burden, poor antigen presentation, low tumour antigen load, immune checkpoint-independent immune suppression and tumour-specific T cells depletion.18 Accumulating studies have shown that the generation of this heterogeneity may be related to gut microbiota.14
Gut microbiota modulation in anti-tumour immunotherapy
During past decades, intestinal dysbiosis is reported to be epidemiologically related to autoimmune diseases and tumour development.19 Intestinal dysbiosis underlies tumourigenesis through several pathways: transformation of host genomes, virulence factors damaging DNA stability, metabolic dysregulation, inappropriate immune system initiation and barrier impairment.20,21 Meanwhile, it has become a clearer recognition that gut microbiota can exert an impact on both tumour development and tumour immuno-response fanned by the quick development of RNA and DNA sequencing, metabolic function analysis, bacterial identification and culture techniques, and specialized animal models. Piles of studies have demonstrated that specific microbiota is representative in tumour progression and anti-tumour process through the analysis of fecal samples. Helicobacter pylori is widely recognized to be associated with gastric carcinoma. Escherichia coli,22 Bacteroides fragilis23 or Fusobacterium nucleatum19,24 are considered as associated with colonic neoplasia. Streptococcus bovis may induce a suppressive immunity that is conducive to colorectal cancer by recruiting CD11b⁺TLR-4⁺ cells.25
Apart from tumour development, microbiota is also reported to affect the response to anti-tumour immunotherapy. Using 16S rRNA gene screening, metagenomic shotgun sequencing and unbiased metabolomic profiling, researchers identified the gut microbiome in ICIs-differentially responding patients with tumour. Notably, associations between certain bacterial species and response to ICIs have been demonstrated across different cancer types, suggesting the presence of “responder” and “non-responder” gut microbiome profiles. We summarized the characteristic of gut microbiota associated with better ICIs clinical benefits in cancer patients26, 27, 28, 29, 30, 31, 32, 33 (Table 1). Among the varying identified microbiota composition, Akkermansia muciniphila was found to be the only consistent microbiome-based signature across cohorts of melanoma, NSCLC and RCC. Confounding factors may contribute to this lack of consensus, such as collection and DNA extraction protocols, inadequate sample size,34 short duration of study, biases of genetics and geography, dietary and medication-use differences, thus microbial signatures of responders and non-responders are functionally related but intrinsic to each cohort. Still, host microbiota signature shows its potential in predicting the prognosis, as well as modulating immunotherapy response potency. Further studies should evaluate the optimal formula of favorable gut microbiota characteristics in larger cohorts and standardize research methods to facilitate comparison across clinical trials.
Table 1.
Characteristics of gut microbiota associated with better ICIs clinical benefits in cancer patients.
| Cancer type | ICIs therapy | Study cohorts | Methods | Major shifts in bacteria species |
|---|---|---|---|---|
| Hepatocellular carcinoma | Anti-PD-1 | Stool samples from 8 patients treated with anti-PD-1 after progression on sorafenib, and antibiotics were not applied | Metagenomic sequencing | Akkermansia muciniphila and Ruminococcaceae spp.26 |
| Melanoma | Ipilimumab | Stool samples from 26 patients at baseline and before each ipilimumab infusion | 16S rRNA gene sequencing | Faecalibacterium prausnitzii27 |
| Anti-PD-1 or anti-CTLA-4 | Stool samples from 42 patients before ICIs treatment | 16S rRNA gene sequencing, metagenomic sequencing and qPCR |
Bifidobacterium longum, Collinsella aerofaciens and Enterococcus faecium28 |
|
| Combined anti-CTLA-4 and anti-PD-1 | Stool samples from 77 patients treated with combined ICIs | Whole-exome sequencing, 16S rRNA gene sequencing and whole metagenomic shotgun sequencing | Bacteroides stercoris and Parabacteroides distasonis29 | |
| Ipilimumab, nivolumab, pembrolizumab or ipilimumab plus nivolumab | Stool samples from 39 patients treated with ICIs | Metagenomic shotgun sequencing and unbiased metabolomic profiling | Bacteroides caccae30 | |
| Non-small-cell lung cancer | Pembrolizumab | Stool samples from 16 NSCLC patients treated with pembrolizumab | 16S rRNA gene sequencing | Parabacteroides distasonis and Bacteroides vulgatus31 |
| Renal cell carcinoma | Nivolumab or nivolumab plus ipilimumab | Stool samples from 31 patients before initiation of ICIs | Metagenomic shotgun sequencing | Akkermansia muciniphila32 |
| Epithelial tumour (NSCLC and RCC) | Anti-PD-1 | Stool samples from 60 NSCLC patients and 40 RCC patients | Metagenomic shotgun sequencing | Akkermansia muciniphila33 |
CTLA-4, cytotoxic T-lymphocyte associated antigen-4; irAEs, immune-related adverse events; ICIs, immune checkpoint inhibitors; NSCLC, non-small-cell lung cancer; PD-1, programmed cell death 1; qPCR, quantitative polymerase chain reaction; RCC, renal cell carcinoma.
Paramount importance has been attached to the stable and functional commensal microbiota community, subsequently the rectification of gut microbiota to mitigate tumour progression has aroused extensive attention. Diet control is one of the modification strategies, as ketogenic diet induced T cell-dependent tumour growth retardation in aggressive tumour models.35 Supplementation of specific bacteria may produce positive effects. Enterococcus modulates response to anti-PD-1 anti-tumour immunotherapy in mice models.36 Next generation probiotics (NGPs), namely Faecalibacterium prausnitzii and B. fragilis as bioactive medications have gained increasing attention.37 Notably, nontoxigenic B. fragilis strains could inhibit the growth or translocation of Clostridium difficile38 and Salmonella Heidelberg39 to obtain competitive protection, thus exert probiotic-like properties. Regrettably, there is still no consensus on whether specific strains can enhance the effect of anti-tumour immunotherapy. One probable reason is that supplementation of a single probiotic disrupts the diversity of the gut microbiota as a balanced whole.40 Meanwhile, the complexity of the human gut microbiota makes it difficult to identify specific species, much less stable culturing favorable strains.40 Further, the possible adverse effect that probiotic strains can cause bacteremia may outweigh their potential benefits. This major limitation impels researchers to transplant gut microbiota as a whole, among which FMT seizes the most attention.
FMT can modulate the intestinal microbial homeostasis and immune balance through microorganisms and their active products to treat diseases. This cutting-edge technological advance shows secure efficacy in C. difficile infection (CDI).41 Lots of clinical trials are now under conducted to ascertain the effectiveness of FMT in diverse cancerous disorders.7,8,33 FMT directly shapes the gut microbiota and selectively alters microbial composition and abundance, thereby indirectly affecting ICIs. FMT assisting anti-tumour immunotherapy has been long eye-catching and several clinical trials are under process (Table 2). By transplanting healthy and balanced microbial population as a whole to an imbalanced ecosystem, FMT might reestablish the microbial equilibrium of the GIT and homeostasis of the entire body.42 FMT therapy exerts its immune-boosting effects mainly by improving heterogeneous response rate, reversing ICI immunotherapy resistance and weakening latent irAEs,43 while with a notable advantage of less requirement for frequent interventions.40 Two milestone FMT clinical trials carried on metastatic melanoma patients demonstrated FMT's efficacy and safety in boosting anti-PD-1 response, illustrating its huge application prospects in anti-tumour combination treatment.7,8 These results certified the effectiveness and safety of FMT in terms of re-induction of anti-PD-1 therapy, and together supported the concept of boosting tumour immunotherapy through modulating gut microbiota.
Table 2.
Summary of clinical trials investigating fecal microbiota transplantation in immune checkpoint blockade therapy.
| Cancer type | Identifier | Phase | Donors | Recipients | ICIs therapy | Pre-treatment regimen | Route | FMT treatment time frame | Outcome measures |
|---|---|---|---|---|---|---|---|---|---|
| Gastrointestinal cancer | NCT04130763 | Phase I, from 2019 | Healthy people | Unresectable or metastatic solid tumours of the GIT, failed at least 2-dose anti-PD-1/PD-L1 | Anti-PD-1 | Not stated | Capsule | Capsules for 3 days + maintenance dose Q2W for up to 6 times | ORR |
| NCT04729322 | Phase II, from 2021 | Anti-PD-1 responders | MSI-H or dMMR CRC; failed at least 2-dose anti-PD-1/PD-L1 | Nivolumab and Pembrolizumab | Metronidazole for a week, then vancomycin + neomycinon for a week | Colonoscopy followed by capsule | Colonoscopic FMT once + capsules once a week per cycle for up to 6 months | ORR | |
| Melanoma | NCT03341143 | Phase II, from 2017 | Anti-PD-1 responders | Unresectable stage III or IV melanoma; failed at least 2-dose pembrolizumab or nivolumab | Pembrolizumab | Not stated | Colonoscopy | Single dose administration | ORR |
| NCT03353402 | Phase I, from 2017 | Anti-PD-1 responders | Unresectable stage III or IV melanoma; failed at least one line of PD-1 blockade | Anti-PD-1 | Not stated | Colonoscopy followed by capsules | Single dose colonoscopic FMT infusion + capsules | Adverse events incidence | |
| NCT03772899 | Phase I, from 2018 | Healthy people | Unresectable or metastatic cutaneous melanoma (BRAF wild type or mutant) | Pembrolizumab or nivolumab | Not stated | Capsule | Single dose administration | Measure of safety | |
| NCT04577729 | Not applicable, from 2020 | ICIs responders or autologous donors | Unresectable stage III or IV melanoma; with disease progression or recurrence during previous anti-PD-1 | Any ICIs | Not stated | Not stated | Not stated | Progression free survival | |
| NCT04988841 | Phase II, From 2021 |
MaaT013 (full-ecosystem gut microbiome drug) | Unresectable or metastatic melanoma; unexposed to ipilimumab and anti PD-1/PD-L1 | Ipilimumab, Nivolumab |
Osmotic laxative solution before first administration | Enema | Q3W between baseline and week 9 then Q4W from week 15 to week 23 | Measure of safety | |
| NCT05251389 | Phase I &II, from 2022 | ICIs responders or non-responders | Unresectable stage III or IV melanoma | Anti-PD-1 | Vancomycin for 4 days, then MoviPrep for bowel clearance | Colonoscopy | Single dose colonoscopic FMT infusion | ORR | |
| Non-small-cell lung cancer | NCT04924374 | Not applicable, from 2021 | High-fiber-diet individuals | Unresectable stage III non-small cell cancer | Pembrolizumab, Nivolizumab, Atezolizumab | Not stated | Capsule | Not stated | Measure of safety |
| NCT05008861 | Phase I, from 2021 | Not stated | Locally advanced/metastatic non-small cell lung cancer; received at least 2-dose anti-PD-1/PD-L1 | Anti-PD-1/PD-L1 | Not stated | Capsule | Not stated | Adverse events incidence | |
| Melanoma or non-small-cell lung cancer |
NCT04521075 | Phase I, from 2021 | ICIs responders | Advanced non-small cell lung cancer or unresectable or metastatic melanoma; at most 1-dose therapy after failure of anti-PD-1/PDL1 | Nivolumab | Not stated | Capsule | 30 capsules for 2 days + 12 capsules maintenance Q2W for 6 combined cycles |
ORR and adverse events incidence |
| NCT04951583 | Phase II, from 2021 | ICIs responders | Unresectable or metastatic melanoma/uveal melanoma/non-small cell lung cancer; no prior anti-PD-1 therapy | Pembrolizumab, Nivolumab+ Ipilimumab |
Not stated | Capsule | Full FMT prior to first cycle of ICIs + supportive FMT within 7 days of the second and third cycle | ORR | |
| Renal cell carcinoma | NCT04163289 | Phase I, from 2019 | Healthy people | Advanced or metastatic (AJCC Stage IV) renal cell carcinoma | Nivolumab, Ipilimumab |
Not stated | Capsule | 7 days before first ICIs cycle + 1–3 days prior to the next two ICIs cycle | Adverse events incidence |
| NCT04758507 | Phase I & II, from 2021 | ICIs responders | Advanced or metastatic renal cell carcinoma | Any immune checkpoint inhibitor | Not stated | Colonoscopy followed by capsules | Single dose colonoscopic infusion + 8 capsules t.i.d. at 3 and 6 months | Progression free survival | |
| Prostate cancer | NCT04116775 | Phase II, from 2019 | Pembrolizumab-Enzalutamide treatment responders | Metastatic castration resistant prostate cancer with castrate-level testosterone (<50 ng/dL) | Pembrolizumab and Enzalutamide (antiandrogen) | Not stated | Endoscopy | Daily dose Enzalutamide +4 cycles of pembrolizumab + FMT once |
PSA decline |
| Mesothelioma | NCT04056026 | Phase I, from 2019 | Healthy people | Metastatic mesothelioma | Pembrolizumab | Not stated | Colonoscopy | Single dose administration | Progression free survival |
| Advanced solid cancer | NCT04264975 | Not applicable, from 2020 | Immunotherapy responders | Advanced solid cancer resistant to immuno-oncology | Any Immunotherapy |
Not stated | Colonoscopy | Not stated | ORR |
| NCT05533983 | Not applicable, from 2022 | Not stated | Advanced, unresectable, or metastatic solid cancer; with progression during anti-PD-1/PD-L1 | Nivolumab | Not stated | Not stated | 2 times administration in a 2-week interval following by Nivolumab | ORR |
dMMR, deficient in mismatch repair; FMT, fecal microbiota transplantation; ICIs, immune checkpoint inhibitors; MSI-H, microsatellite instability high; ORR, objective response rate; PD, progressive disease; PD-1, programmed cell death 1; PSA, prostate specific antigen.
Mechanisms of FMT enhancing the efficacy of tumour immunotherapy
FMT modulates gut microbiota for long persistence
Altering gut microbiota diversity and composition by antibiotics negatively affects the host response to anti-PD-1 in patients with NSCLC or RCC, which indicates microbiota as a key factor modulating immunotherapy outcomes.9 FMT significantly increases the diversity of gut microbiota populations in cancer-bearing recipients.8 It is the most direct method to modulate the gut microbiota by transferring the entire donor microbial ecosystem, which is more likely to establish ecological homeostasis than a single putative bacterium. Therefore, FMT is expected to increase the diversity and composition of gut microorganism populations in cancer patients who are resistant to immunotherapy.44 Moreover, this perturbation seems to be in persistent force. Davar et al. conducted a long observation on melanoma patients who received response or non-response to anti-PD-1 treatment after FMT and evaluated the microbiota signature pre- and post-FMT by shotgun metagenomic sequencing. The microbiota in all post-FMT feces exhibited higher alpha diversity than in pre-FMT feces in a long period, and the differences were more significant in response recipients (Rs) compared with non-response recipients (NRs).8
Microbial metabolites mediated anti-tumour responses to ICIs
Notably, multiple metabolites synthesized and transformed by gut microbiota, which could be transformed by FMT, can spread and impact anti-tumour immune response in immunotherapy.45 Intestinal Bifidobacterium pseudolongum derived metabolite inosine translocated and stimulated T-cell-specific adenosine A2A receptor (A2AR) to promote Th1 cell differentiation in the presence of exogenous interferon-gamma (IFN-γ) for the systemic effect during anti–CTLA-4 and anti–PD-L1 therapy.46 Short-chain fatty acids (SCFAs) were found to promote the anti-tumour cytotoxicity of CD8+ T cells and provide energy for immune cells. Combined with recent researches which manifested that SCFAs promoted CD8+ T cell long-term survival as memory cells,47,48 F. prausnitzii may enhance the antitumour immune response by increasing SCFAs-mediated CD8+ T cells' memory potential. These investigations jointly demonstrate that FMT may restore the anti-tumour performance by modifying microbiota and tumour metabolism.
FMT modulates the anti-tumour immune response regulated by gut microbiota
Microbiota profiling suggests that the composition of gut microbiota is associated with tumour-infiltrating immune cells in TME, which modulates the efficacy of immunotherapy. Multiple studies have identified some specific bacterial species, such as A. muciniphila,33,49 Faecalibacterium, Ruminococcaceae15 Bifidobacterium breve, Bifidobacterium adolescentis,50 Bifidobacterium longum, Collinsella aerofaciens, and Enterococcus faecium,28 which were found to promote the efficacy of anti-PD-1 immunotherapy by increased antigen presentation and improved effector T cell function in systematic and TME (Fig. 2). In contrast, a greater abundance of Bacteroidales exhibited higher levels of Treg cells and myeloid-derived suppressor cells (MDSCs), limited infiltration of intratumoural lymphoid, and weakened capacity of antigen presentation, which led to a poor prognosis in NRs.28,51 Notably, treatment with FMT has also shown promising results in clinical immunotherapy-refractory melanoma models, which was associated with favorable changes in immune cell infiltration and gene expression in gut lamina propria and the increase of CD8+ T cell, DC activation and enhanced IFN-γ signaling in TME.7,8
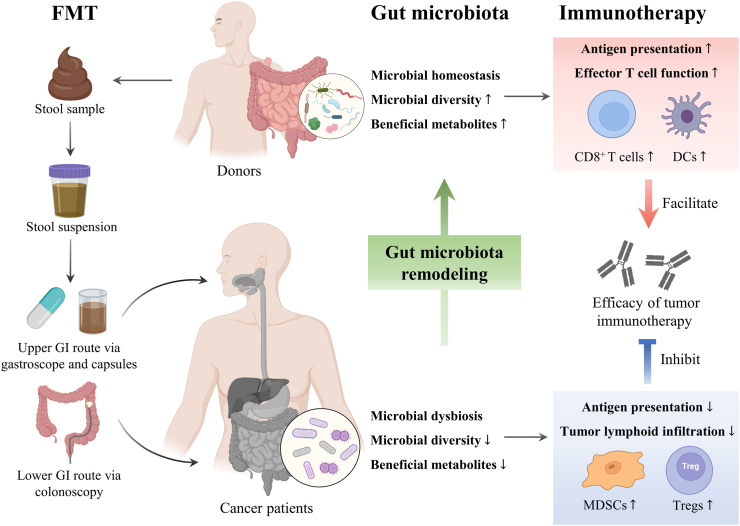
Fig. 2.
Fecal microbiota transplantation strengthens the anti-tumour immune response by altering the gut microbiota. FMT is the method to transfer the gut microbiota from a donor to a recipient in the form of diluted fecal material via the upper or lower digestive tract to restore microbial diversity. The higher microbial diversity, more beneficial metabolites and re-establishment of gut microbiota homeostasis were found to promote the efficacy of immunotherapy by increasing the DCs and CD8+ T cells in ICIs-responding cancer patients after FMT. Higher abundance of Akkermansia muciniphila, Ruminococcaceae spp., Faecalibacterium prausnitzii, Bifidobacterium longum, Collinsella aerofaciens and Enterococcus faecium was observed in ICIs responders. In contrast, lower microbial adversity, less beneficial metabolites and gut dysbiosis exhibited higher levels of Tregs and MDSCs leading to the poor prognosis in non-responding cancer patients after FMT. Higher abundance of Bacteroidales, Escherichia coli, Roseburia intestinalis, Ruminococcus obeum, Anaerotruncus colihominis and Blautia producta was observed in ICIs non-responders. DCs, dendritic cells; FMT, fecal microbiota transplantation; ICIs, immune checkpoint inhibitors; MDSCs, myeloid-derived suppressor cells.
These clinical observations were further confirmed by patient-derived FMT in germ-free or antibiotic-treated mice. Mice receiving feces from Rs exhibited improved anti-PD-L1 responses, but FMT from nonresponding patients were failed. Mechanically, mice getting FMT from Rs had enriched effector cells, such as CD8+ T cells and CD45+CD11b+Ly6G+ cells, and decreased suppressive CD11b+CD11c+ myeloid cells. Mice getting FMT from NRs had higher frequencies of regulatory RORγT+ T helper 17 cells, CD4+FoxP3+ and CD4+IL-17+ T cells, suggesting the impaired host immune responses.15 Likewise, another study indicated that NRs-derived FMT in mice is resistant to PD-1 blockade, and the relative abundance of A.muciniphila was significantly decreased. The efficacy of PD-1 blockade could be restored with A. muciniphila by increasing the recruitment of CCR9+CXCR3+CD4+ T lymphocytes into TME, promoting the IFN-γ release, and inducing DCs to secrete IL-12, a Th1 cytokine involved in the immunogenicity of PD-1 blockade.33 In addition, a notable change in the relative abundance of B. fragilis, Bacteroides thetaiotaomicron and Burkholderia was found to enhance the inhibitory effect of CTLA-4 blockade on tumour growth in mice by boosting the IL-12-dependent Th1 immune response in the tumour-draining lymph nodes and promoting the maturation of intratumoural DCs.52 Gut commensal Bifidobacterium could augment DC function and improve CD8+ T cell to facilitate the anti-PD-L1 efficacy. Surprisingly, oral administration of Bifidobacterium was found to have the capacity to accumulate within TME and facilitate the local anti-CD47 immunotherapy via stimulator of interferon genes (STING) signaling in DCs, which increases the type I IFN to activate CD8+ T-dependent anti-tumour immunity53(Fig. 3).
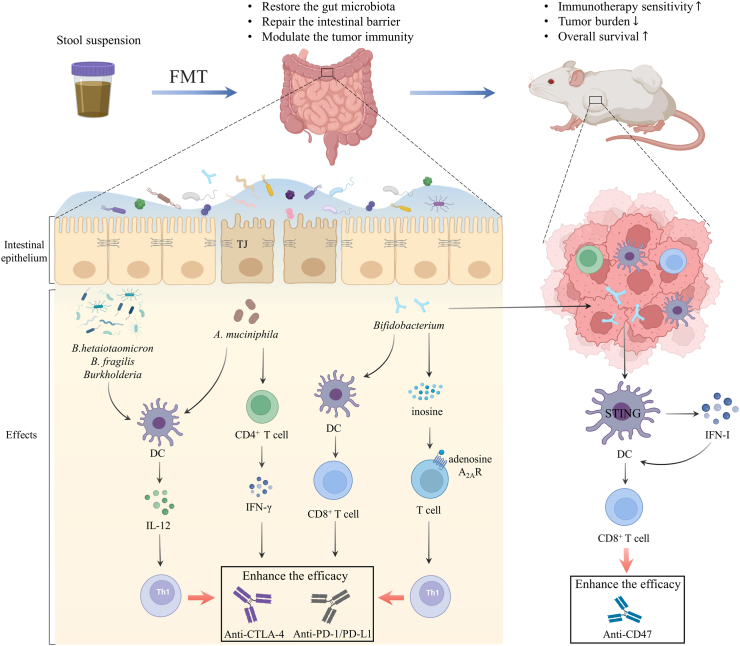
Fig. 3.
Fecal microbiota transplantation reshapes the tumour microenvironment, thus boosting the efficacy of cancer immunotherapy. FMT is a potential therapeutic strategy to restore the gut microbiota, reinforce the intestinal barrier and modulate tumour immunity. FMT modulates gut microbiome and promotes the integrity of tight junction proteins and the intestinal barrier. The abundance of B. fragilis, B. thetaiotaomicron, and Burkholderia enhanced the effect of CTLA-4 blockade by boosting the IL-12-dependent Th1 immune response. A. muciniphila promoted the IFN-γ release and induced DCs to secrete IL-12 to improve the immunogenicity of PD-1 blockade. Gut Bifidobacterium augmented the function of DCs and CD8+ T cells to facilitate the anti-PD-L1 efficacy. Intestinal Bifidobacterium pseudolongum derived metabolite inosine translocated and stimulated T cell-specific adenosine A2AR to promote Th1 cell differentiation in the presence of exogenous IFN-γ for the systemic effect during anti-CTLA-4 and anti-PD-L1 therapy. Bifidobacterium also colonized tumour sites and facilitated local anti-CD47 immunotherapy via STING signaling in DCs which increased the type I IFN to stimulate the tumour-associated DCs in turn and activate CD8+ T-dependent anti-tumour immunity; A2AR, A2A receptor; B. fragilis, Bacteroides fragilis; B. thetaiotaomicron, Bacteroides thetaiotaomicron; DCs, Dendritic cells; FMT, fecal microbiota transplantation; IFN-γ, interferon-gamma; IL, interleukin; STING, stimulator of interferon genes; TJ, tight junction.
FMT as a promising clinical therapy in facilitating anti-tumour immuno-therapeutic strategies
FMT on digestive system tumours
Colorectal cancer
Immunotherapy based on ICIs has proved to be a therapeutic option for several cancers, but only a handful proportion of colorectal cancer (CRC) patients obtain definite therapeutic benefits. A recent study revealed that the anti-PD-1 mAb efficacy was largely impaired in the mice received feces from CRC patients compared to those from healthy controls. The up-regulation of butyrate-producing bacteria, increased T cell infiltration and activation were observed in FMT-combinational therapy.54 Another research reported that FMT combined with anti-PD-1 showed synergistic effect in colorectal tumor bearing mice compared with mice received anti-PD-1 alone, probably owing to the upregulating metabolites including punicic acid.55 Several clinical trials precisely measure the objective response rate (ORR) of ICIs combining FMT treatment, thus laying out a blueprint of potential synergetic combination therapy.
Pancreatic cancer
In pancreatic cancer, alterations of gut microbiota composition are noticed both in patients and mice models. Compared with healthy individuals, pancreatic cancer patients possess an increasing abundance of Malassezia spp., Pseudomonas aeruginosa, Fusobacterium spp.56 as well as a decreasing abundance of butyrate-producing bacteria and Lactobacillus.56 The ablation of the microbiota might protect against pancreatic ductal adenocarcinoma by reducing myeloid-derived suppressor cells and increasing M1 macrophage differentiation, promoting Th1 differentiation of CD4+ T cells and CD8+ T cell activation. Microbiota ablation also enhanced ICIs efficacy by upregulating PD-1 expression.57 A clinical trial in the progress in the U.S. implemented FMT in pancreatic cancer patients to assess the safety, tolerability, and feasibility of FMT in resectable patients with pancreatic ductal adenocarcinoma (NCT04975217).
FMT on extra digestive system tumours
Melanoma
During the past years, ICIs immunotherapy has got booming applications in advanced melanoma patients and has markedly improved patients’ general survival rates. ICIs currently applied in melanoma immunotherapy include anti-CTLA-4 monoclonal antibodies (ipilimumab and tremelimumab), anti-PD-1 agents (nivolumab, pembrolizumab and lambrolizumab), and BRAF and MEK inhibitors (dabrafenib plus trametinib, vemurafenib plus cobimetinib, and encorafenib plus binimetinib), the vast majority of which show positive facilitating anti-tumour efficacy. Nevertheless, heterogeneity in response to immunotherapy persists, and this variation may be due to the diverse gut microbiota and metabolite composition.
In preclinical studies, it has been proved that certain gut microbiota could facilitate tumour burden remission in mice with melanoma.58 Further clinical studies focus on the gut microbiota variances among melanoma patients. Bacteroides caccae is enriched in Rs for all types of ICIs including ipilimumab, nivolumab and pembrolizumab, as well as a higher level of anacardic acid. In a prospective study profiled 103 trial patients with metastatic melanoma treated with neoadjuvant ICIs from Australia and the Netherlands, researchers observed higher response rates in Ruminococcaceae-dominated microbiomes than in Bacteroidaceae-dominated microbiomes.59 Notably, this microbial signature might be affected by external disturbances (diet, lifestyle and geography). In another study, researchers identified Bacteroides vulgatus and Bacteroides dorei to be able to predict immune-related adverse effects in advanced melanoma patients treated with ipilimumab and nivolumab.60 However, manipulation of the entire host gut microbiota still stays in the preclinical stage.
Two concomitant clinical trials published in Science complemented these outcomes on pre-clinical models and firstly vindicated the proof-of-concept that altering whole gut microbiota through FMT may rectify immunotherapy resistance in refractory cancer patients. In the first phase I trial (NCT03353402), researchers treated 10 patients of anti-PD-1 refractory metastatic melanoma with sequential FMT from two donors who attained complete response (CR) for over one year after receiving nivolumab monotherapy, and evaluated the safety and feasibility of nivolumab re-induction. Three recipients obtained the progression-free survival for six months, among them two partial responses (PR) and one CR. Then the researchers went a step further in microbiota analysis and biopsies. On the whole, the gut microbiota composition shifted among all FMT recipients; the Rs had a notable expansion of immunotherapy-favorable features, with a higher relative abundance of Enterococcaceae, Enterococcus and Streptococcus australis, and lower relative abundance of Veillonella atypica. Favorable changes in immune cell infiltration and gene expression profiles were observed in both the gut lamina propria and TME.7
In another concurrent phase II clinical trial (NCT03341143), patients resistant to anti-PD-1 alone or combined with anti-CTLA-4 or investigational agents were enrolled in a single donor-derived FMT administered endoscopically along with pembrolizumab. Donors with metastatic melanoma were undergoing durable PR or CR after being treated with (nivolumab or pembrolizumab. Unlike the previous study, only patients who met the criteria of primary ICIs resistance (no prior response and confirmed progressive disease) were eligible. Results turned out that 6 out of 15 patients got clinical benefits, including one CR, 2 PR as well as three recipients presenting stable disease (SD) for more than one year. Similarly, FMT successfully re-colonized gut microbiota toward favoring anti-PD-1 responding composition. In post-FMT responders, the phylum Firmicutes (Lachnospiraceae and Ruminococcaceae families) and Actinobacteria (Bifidobacteriaceae and Criobacteriaceae families) showed significant enrichment, whereas phylum Bacteroidetes decreased. Furthermore, host immune responses and metabolism were modulated among responders as described before, enhancing the activation of mucosal-associated invariant cells in peripheral blood and CD8+ T cell in both periphery and TME, thus counteracting myeloid-induced immunosuppression.8
Ultimately, these two weighty clinical breakthroughs conjointly verify that FMT combining PD-1 blockade strategy can ameliorate gut microbiota and reprogram the TME to reverse the resistance against anti-PD-1 therapy to treat refractory melanoma. It is noteworthy that both studies stress an association between the phylum Firmicutes and ICIs clinical responding efficacy; nonetheless, the relationship between host taxa and clinical response was controversial. In a recent study, the researchers analyzed metagenomes from 316 FMTs, sampled pre- and post-intervention, for the treatment of ten different disease indications. They suggested that recipient rather than the donor determined the “mixed” status of the gut microbiota after FMT. In-depth studies did not find strong evidence that any strain was inherently more aggressive/resilient than others. On the contrary, colony structure and diversity, and donor-recipient colony complementarity determined resilience, coexistence, and colonization of gut microbiota after FMT.61 In another integrated shotgun metagenomic systematic meta-analysis, higher donor strain engraftment was reported to be more likely to experience clinical success after FMT.62 Robust randomized controlled trials are warranted to outline the linkage more unambiguously.
Non-small-cell lung cancer
Numerous previous evidences have laid the foundation for anti-PD-1/PD-L1 to take effect in metastatic NSCLC lacking sensitizing EGFR or ALK mutations.63 However, the response rate still hovers under 25%.63 Gut microbiota has fueled great enthusiasm in extensive research on anti-tumour response. Recent research revealed that upregulated abundance of Prevotella, Gemmiger, and Roseburia was observed in NSCLC patients. Further FMT on mice from NSCLC patients led to intestinal inflammation and immune dysregulation.3 In another study, Huang et al. enrolled 16 Chinese patients with NSCLC, and observed that pembrolizumab Rs and NRs exhibited distinct gut microbiota diversity, among which Parabacteroides distasonis and B. vulgatus showed differential abundance. The researchers then induced conjoint ginseng polysaccharides, FMT and αPD-1 monoclonal antibody (mAb) to reshape gut microbiota composition and considered them as novel prebiotics to enhance the response to anti-PD-1 immunotherapy in NSCLC patients.31 Intriguingly, this study can provide an additional valuable insight that gut microbiota features, such as alpha diversity or more specific species can act as a biomarker to predict ICIs efficacy.
Renal cell carcinoma
Introduction of ICIs to RCC has brought out revolutionary transformation in clinical outcomes, yet the heterogeneous responses remain unsatisfied with stable medical needs. Ample evidences have uncovered the role of gut microbiota in RCC development and patient prognosis.9 In a phase II trial (NCT03013335), 69 RCC patients treated with nivolumab were enrolled. According to their result, recent antibiotic use silenced response rates (from 28% to 9%) to nivolumab, but notably enhanced Clostritidium hathewayi dominance. This alteration in microbiota composition was also observed in RCC patients compared to healthy donors. Parallel pre-clinical study of FMT on RCC-bearing mice from Rs showed compensatory responsiveness in the resistant group. Similar compensation was also observed in beneficial commensals (A. muciniphila and Bacteroides salyersiae) transplantation,2 accordingly established causation between gut microbiota and ICIs clinical efficacy.
FMT enhances anti-tumour immunotherapy efficacy: doubts and hopes
Despite the alluring and promising clinical data of FMT reducing infectious complications and enhancing tumour immunotherapy, apprehension of the long-term safety remains. First of all, since FMT is the transplantation of the donor's entire live gut microbiota as a whole, it may pose a risk of importation of multi-drug-resistant bacteria and transmission of unidentified causative agents. Several cases and researches have reported Norovirus enteritis, E. coli bacteremia, cytomegalovirus infection, fungi and parasite contamination and resulting complications after FMT.64,65 According to a case report published on NEJM, after undergoing FMT in two independent clinical trials, two patients endured extended spectrum of β-lactamase (ESBL)-producing E. coli.65 Remarkably, both patients above were linked to the same stool donor. Besides, transmission of unscreened seven Shiga toxin-producing E. coli infections and two enteropathogenic E.coli infections were reported,66,67 together stressing the need of comprehensive donor screening and careful evaluation of risks and benefits of FMT. Afterwards, although antibiotics can reduce irAEs-related bacterial taxa theoretically, several studies have shown poorer clinical efficacy of tumour ICIs therapy in antibiotic-treated patients.33,49 This inhibition could be a result of the fact that broad-spectrum antibiotics also affect the healthy microbiota essential for optimum ICIs efficacy at the same time. Notably, orally administration of DAV132, a colon-targeting adsorbent, in combination with antibiotics could prevent the antibiotics-related dysbiosis and preserve the responsiveness to anti-PD-1 after FMT.68 Collectively, it reminds clinicians to carefully balance the benefits and drawbacks of antibiotics when considering FMT administration. In addition to infection-related adverse events, some other severe sequels was presumably due to the dissemination of unknown disease-causing genes. Several researches have reported that fecal materials from donors who suffered from obesity, diabetes and other metabolic diseases could bring these conditions to recipients.69,70 Finally, the optimal microbial profiles differ between tumour types. For example, whether the optimal microbiota is same in promoting immuno-response between NSCLC patients and melanoma patients remains unclear. It is still far away from identifying several “super gut microbiota” that can best promote responsiveness to ICIs among different tumour types.
The determinants of FMT success are quite complex. Several options could be considered to promote engraftment and reduce accompanying side-effects. Firstly, a favorable screening evaluation for both donors and recipients is crucial, which consists of imaging, tumour biopsy, and serological/stool studies to confirm suitability for FMT administration.8 To prevent the transmission of pathogenic microorganisms during FMT, attention should be paid to screening donors: screening blood and feces, conducting virus PCR on feces samples before FMT, establishing a follow-up system for donors and conducting regular inspections.71 For example, during COVID-19 pandemic, all fecal samples were sequenced by RT-PCR to prevent it from spreading during FMT.72 Secondly, the donor-recipient complementarity determines the resilience, coexistence and colonization of gut microbiota post-FMT.61 Microbiota stability and species evenness are confirmed to be new metrics related to treatment response.73 Therefore, careful selection of suitable donor-recipient matching is needed to achieve targeted treatment. Thirdly, the physical conditions of individuals. In the light of growing awareness of manipulating microbiome as a synergistic therapy for cancer treatment, concerns that whether the same outcomes will apply to cancer patients or non-cancer-bearing individuals cannot be over estimated. As previously mentioned, the efficacy of ICIs requires a specific inflammation-induced TME. Therefore, the desirable clinical modulating tools of microbiome may differ between cancer patients and non-cancer individuals.74 More solid investigations and precise characterizations of what constitutes favorable or unfavorable microbiome manipulation objects are needed. Last but not least, the delivery routes of FMT must be carefully evaluated to further protect therapeutic gut microbiota and achieve optimal efficacy. Traditional delivery routes include enema, endoscopy or nasoenteric tubes, while recent applications of oral capsules show advantages of less limitation in FMT formulations,75 non-invasion and easier acception. Single route of upper GI tract administration, lower GI tract administration or capsulized FMT is of strong recommendation, and the determinants are lesion site and FMT dosage.71 Increased engraftment was observed when receiving FMT from multiple routes.62 Sequentially combined routes of FMT were also applied in several clinical trials (e.g. NCT04729322, NCT03353402 and NCT04758507), which might exert direct and non-invasion administrations. However, it still lacks adequate clinical trials when considering determinants of FMT success and clinical efficacy in patients. More large-scale cohorts and mechanism researches of FMT combining tumour immunotherapy are required for precision and personalized tumour management.
Concluding remarks and future perspectives
The tumour immunotherapy represented by ICIs has received accumulating interest during the past few years and gut microbiota is confirmed to play a crucial role in modulating anti-tumour therapeutic efficacy. FMT as an integral manipulation, has been proposed as a desirable combining synergetic therapy to boost ICIs efficacy and eliminate the heterogeneous outcomes. By remodeling gut microbiota, balancing microbiota metabolites and reshaping the TME, FMT acts as an adjuvant to cooperate with ICIs to improve anti-tumour immune response. Nonetheless, multiple exploration gaps remain in the FMT validity and its long-term consequences. Due to the intricacy of the fecal components, risks of FMT are frequently not adequately evaluated until after the procedure, and it could be more challenging to pinpoint the precise source of danger. Much progress has been made, although many questions remain unanswered. For example, how to eliminate the harness of importing pathogenic micro-organisms and disease-causing genes? How to maximize the benefits of antibiotics usage? What is the best strategy to operate FMT?What is the optimal microbiota for improving clinical outcomes in patients receiving ICIs? Optimization of combined treatment, appropriate route of FMT delivery, enhanced donor screening prior to translation and regular recipient monitoring during the whole process may help reduce the risk to some extent. Taken together, FMT provides a more effective and safe microbial treating thread to synergize anti-tumour immunotherapy. We seek a deeper investigation of the “friendly microbiota” and their underlying functions on the anti-tumour immumo-response to optimize the effectiveness of immunotherapy among unrestricted spectrum of tumour types.
Outstanding questions
Cancer immunotherapy against immune checkpoint inhibitors, showing significant efficacy in multifarious tumors, has emerged and been widely approved in recent years. However, its efficacy seems to be complex and uncertain, with adverse reactions due to the overaction of immune system.
Gut microbiota may not only contribute to carcinogenesis, but also shaping the response to immunological checkpoints. Targeting the gut microbiota hints a new strategy of tumor treatment but still needs further exploration.
Modulating the gut microbiome by fecal microbiota transplantation (FMT) may affect the efficacy of cancer therapy through remodeling the microbial composition, regulating metabolites, and activating immune response.
FMT provides a novel insight for improving the efficacy of immunotherapy but with some safety issues. The pros and cons of FMT coupled with cancer immunotherapy need a close watch in the future.
Contributors
Yunwei Yang, Yaping An and Hailong Cao searched the literature, accessed and verified the data reported in the manuscript and finished the manuscript. Yunwei Yang, Yaping An, Yue Dong, Qiao Chu and Jingge Wei wrote sections of the manuscript and prepared the figure. Bangmao Wang and Hailong Cao organised the framework. All authors contributed to manuscript revision and approved the submitted version, including the authorship list. Yunwei Yang and Yaping An contributed equally to this work.
All authors have read and approved the final version of the manuscript, and ensured it is the case.
Declaration of interests
The authors declare no conflict interests.
Acknowledgements
The paper designed, data collection and analysis are supported by the grants (82270574, 82070545 and 81970477) from the National Natural Science Foundation of China. The data interpretation is supported by the Diversified Fund Project of the Natural Science Foundation of Tianjin, China (21JCYBJC00810), Tianjin Key Medical Discipline (Specialty) Construction Project (TJYXZDXK-002A) and Tianjin Medical University General Hospital Fund for Distinguished Young Scholars (22ZYYJQ02). I have not been paid to write this review by a pharmaceutical company or other agency. The authors were not precluded from accessing data in this study, and they accept responsibility to submit for publication.
Contributor Information
Bangmao Wang, Email: mwang02@tmu.edu.cn.
Hailong Cao, Email: caohailong@tmu.edu.cn.
References
- 1.Sepich-Poore G.D., Zitvogel L., Straussman R., Hasty J., Wargo J.A., Knight R. The microbiome and human cancer. Science. 2021;371(6536) doi: 10.1126/science.abc4552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Derosa L., Routy B., Fidelle M., et al. Gut bacteria composition drives primary resistance to cancer immunotherapy in renal cell carcinoma patients. Eur Urol. 2020;78(2):195–206. doi: 10.1016/j.eururo.2020.04.044. [DOI] [PubMed] [Google Scholar]
- 3.Qian X., Zhang H.Y., Li Q.L., et al. Integrated microbiome, metabolome, and proteome analysis identifies a novel interplay among commensal bacteria, metabolites and candidate targets in non-small cell lung cancer. Clin Transl Med. 2022;12(6):e947. doi: 10.1002/ctm2.947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Park E.M., Chelvanambi M., Bhutiani N., Kroemer G., Zitvogel L., Wargo J.A. Targeting the gut and tumor microbiota in cancer. Nat Med. 2022;28(4):690–703. doi: 10.1038/s41591-022-01779-2. [DOI] [PubMed] [Google Scholar]
- 5.Yang J., Wei H., Zhou Y., et al. High-fat diet promotes colorectal tumorigenesis through modulating gut microbiota and metabolites. Gastroenterology. 2022;162(1):135–149.e2. doi: 10.1053/j.gastro.2021.08.041. [DOI] [PubMed] [Google Scholar]
- 6.Wu M., Bai J., Ma C., Wei J., Du X. The role of gut microbiota in tumor immunotherapy. J Immunol Res. 2021;2021 doi: 10.1155/2021/5061570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Baruch E.N., Youngster I., Ben-Betzalel G., et al. Fecal microbiota transplant promotes response in immunotherapy-refractory melanoma patients. Science. 2021;371(6529):602–609. doi: 10.1126/science.abb5920. [DOI] [PubMed] [Google Scholar]
- 8.Davar D., Dzutsev A.K., McCulloch J.A., et al. Fecal microbiota transplant overcomes resistance to anti-PD-1 therapy in melanoma patients. Science. 2021;371(6529):595–602. doi: 10.1126/science.abf3363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Derosa L., Hellmann M.D., Spaziano M., et al. Negative association of antibiotics on clinical activity of immune checkpoint inhibitors in patients with advanced renal cell and non-small-cell lung cancer. Ann Oncol. 2018;29(6):1437–1444. doi: 10.1093/annonc/mdy103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chen D., Wu J., Jin D., Wang B., Cao H. Fecal microbiota transplantation in cancer management: current status and perspectives. Int J Cancer. 2019;145(8):2021–2031. doi: 10.1002/ijc.32003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Suez J., Zmora N., Zilberman-Schapira G., et al. Post-antibiotic gut mucosal microbiome reconstitution is impaired by probiotics and improved by autologous FMT. Cell. 2018;174(6):1406–1423.e16. doi: 10.1016/j.cell.2018.08.047. [DOI] [PubMed] [Google Scholar]
- 12.Bibbò S., Settanni C.R., Porcari S., et al. Fecal microbiota transplantation: screening and selection to choose the optimal donor. J Clin Med. 2020;9(6):1757. doi: 10.3390/jcm9061757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zappasodi R., Serganova I., Cohen I.J., et al. CTLA-4 blockade drives loss of T(reg) stability in glycolysis-low tumours. Nature. 2021;591(7851):652–658. doi: 10.1038/s41586-021-03326-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Matson V., Chervin C.S., Gajewski T.F. Cancer and the microbiome-influence of the commensal microbiota on cancer, immune responses, and immunotherapy. Gastroenterology. 2021;160(2):600–613. doi: 10.1053/j.gastro.2020.11.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gopalakrishnan V., Spencer C.N., Nezi L., et al. Gut microbiome modulates response to anti-PD-1 immunotherapy in melanoma patients. Science. 2018;359(6371):97–103. doi: 10.1126/science.aan4236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kim E.S., Velcheti V., Mekhail T., et al. Blood-based tumor mutational burden as a biomarker for atezolizumab in non-small cell lung cancer: the phase 2 B-F1RST trial. Nat Med. 2022;28(5):939–945. doi: 10.1038/s41591-022-01754-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Conroy M., Naidoo J. Immune-related adverse events and the balancing act of immunotherapy. Nat Commun. 2022;13(1):392. doi: 10.1038/s41467-022-27960-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Andrews M.C., Vasanthakumar A. Gut microbiota - a double-edged sword in cancer immunotherapy. Trends Cancer. 2022;9(1):3–5. doi: 10.1016/j.trecan.2022.08.003. [DOI] [PubMed] [Google Scholar]
- 19.Kong C., Yan X., Zhu Y., et al. Fusobacterium nucleatum promotes the development of colorectal cancer by activating a cytochrome P450/epoxyoctadecenoic acid Axis via TLR4/keap1/NRF2 signaling. Cancer Res. 2021;81(17):4485–4498. doi: 10.1158/0008-5472.CAN-21-0453. [DOI] [PubMed] [Google Scholar]
- 20.Garrett W.S. Cancer and the microbiota. Science. 2015;348(6230):80–86. doi: 10.1126/science.aaa4972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sacks D., Baxter B., Campbell B., et al. Multisociety consensus quality improvement revised consensus statement for endovascular therapy of acute ischemic stroke. Int J Stroke. 2018;13(6):612–632. doi: 10.1177/1747493018778713. [DOI] [PubMed] [Google Scholar]
- 22.Arthur J.C., Perez-Chanona E., Mühlbauer M., et al. Intestinal inflammation targets cancer-inducing activity of the microbiota. Science. 2012;338(6103):120–123. doi: 10.1126/science.1224820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Dejea C.M., Fathi P., Craig J.M., et al. Patients with familial adenomatous polyposis harbor colonic biofilms containing tumorigenic bacteria. Science. 2018;359(6375):592–597. doi: 10.1126/science.aah3648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Jiang S.S., Xie Y.L., Xiao X.Y., et al. Fusobacterium nucleatum-derived succinic acid induces tumor resistance to immunotherapy in colorectal cancer. Cell Host Microbe. 2023;31(5):781–797.e9. doi: 10.1016/j.chom.2023.04.010. [DOI] [PubMed] [Google Scholar]
- 25.Deng Q., Wang C., Yu K., et al. Streptococcus bovis contributes to the development of colorectal cancer via recruiting CD11b⁺TLR-4⁺ cells. Med Sci Monit. 2020;26 doi: 10.12659/MSM.921886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zheng Y., Wang T., Tu X., et al. Gut microbiome affects the response to anti-PD-1 immunotherapy in patients with hepatocellular carcinoma. J Immunother Cancer. 2019;7(1):193. doi: 10.1186/s40425-019-0650-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Chaput N., Lepage P., Coutzac C., et al. Baseline gut microbiota predicts clinical response and colitis in metastatic melanoma patients treated with ipilimumab. Ann Oncol. 2017;28(6):1368–1379. doi: 10.1093/annonc/mdx108. [DOI] [PubMed] [Google Scholar]
- 28.Matson V., Fessler J., Bao R., et al. The commensal microbiome is associated with anti-PD-1 efficacy in metastatic melanoma patients. Science. 2018;359(6371):104–108. doi: 10.1126/science.aao3290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Andrews M.C., Duong C., Gopalakrishnan V., et al. Gut microbiota signatures are associated with toxicity to combined CTLA-4 and PD-1 blockade. Nat Med. 2021;27(8):1432–1441. doi: 10.1038/s41591-021-01406-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Frankel A.E., Coughlin L.A., Kim J., et al. Metagenomic shotgun sequencing and unbiased metabolomic profiling identify specific human gut microbiota and metabolites associated with immune checkpoint therapy efficacy in melanoma patients. Neoplasia. 2017;19(10):848–855. doi: 10.1016/j.neo.2017.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Huang J., Liu D., Wang Y., et al. Ginseng polysaccharides alter the gut microbiota and kynurenine/tryptophan ratio, potentiating the antitumour effect of antiprogrammed cell death 1/programmed cell death ligand 1 (anti-PD-1/PD-L1) immunotherapy. Gut. 2022;71(4):734–745. doi: 10.1136/gutjnl-2020-321031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Salgia N.J., Bergerot P.G., Maia M.C., et al. Stool microbiome profiling of patients with metastatic renal cell carcinoma receiving anti-PD-1 immune checkpoint inhibitors. Eur Urol. 2020;78(4):498–502. doi: 10.1016/j.eururo.2020.07.011. [DOI] [PubMed] [Google Scholar]
- 33.Routy B., Le Chatelier E., Derosa L., et al. Gut microbiome influences efficacy of PD-1-based immunotherapy against epithelial tumors. Science. 2018;359(6371):91–97. doi: 10.1126/science.aan3706. [DOI] [PubMed] [Google Scholar]
- 34.Lee K.A., Thomas A.M., Bolte L.A., et al. Cross-cohort gut microbiome associations with immune checkpoint inhibitor response in advanced melanoma. Nat Med. 2022;28(3):535–544. doi: 10.1038/s41591-022-01695-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ferrere G., Tidjani Alou M., Liu P., et al. Ketogenic diet and ketone bodies enhance the anticancer effects of PD-1 blockade. JCI Insight. 2021;6(2) doi: 10.1172/jci.insight.145207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Griffin M.E., Espinosa J., Becker J.L., et al. Enterococcus peptidoglycan remodeling promotes checkpoint inhibitor cancer immunotherapy. Science. 2021;373(6558):1040–1046. doi: 10.1126/science.abc9113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kaźmierczak-Siedlecka K., Skonieczna-Żydecka K., Hupp T., Duchnowska R., Marek-Trzonkowska N., Połom K. Next-generation probiotics - do they open new therapeutic strategies for cancer patients. Gut Microb. 2022;14(1) doi: 10.1080/19490976.2022.2035659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Deng H., Yang S., Zhang Y., et al. Bacteroides fragilis prevents Clostridium difficile infection in a mouse model by restoring gut barrier and microbiome regulation. Front Microbiol. 2018;9:2976. doi: 10.3389/fmicb.2018.02976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Vernay T., Cannie I., Gaboriau F., et al. Bacteroides fragilis prevents Salmonella Heidelberg translocation in co-culture model mimicking intestinal epithelium. Benef Microbes. 2020;11(4):391–401. doi: 10.3920/BM2020.0004. [DOI] [PubMed] [Google Scholar]
- 40.Ting N.L., Lau H.C., Yu J. Cancer pharmacomicrobiomics: targeting microbiota to optimise cancer therapy outcomes. Gut. 2022;71(7):1412–1425. doi: 10.1136/gutjnl-2021-326264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Hvas C.L., Dahl Jørgensen S.M., Jørgensen S.P., et al. Fecal microbiota transplantation is superior to fidaxomicin for treatment of recurrent clostridium difficile infection. Gastroenterology. 2019;156(5):1324–1332.e3. doi: 10.1053/j.gastro.2018.12.019. [DOI] [PubMed] [Google Scholar]
- 42.Zhou C.B., Zhou Y.L., Fang J.Y. Gut microbiota in cancer immune response and immunotherapy. Trends Cancer. 2021;7(7):647–660. doi: 10.1016/j.trecan.2021.01.010. [DOI] [PubMed] [Google Scholar]
- 43.Chen M., Liu M., Li C., et al. Fecal microbiota transplantation effectively cures a patient with severe bleeding immune checkpoint inhibitor-associated colitis and a short review. Front Oncol. 2022;12 doi: 10.3389/fonc.2022.913217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kouidhi S., Zidi O., Belkhiria Z., et al. Gut microbiota, an emergent target to shape the efficiency of cancer therapy. Explor Target Antitumor Ther. 2023;4(2):240–265. doi: 10.37349/etat.2023.00132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Lu Y., Yuan X., Wang M., et al. Gut microbiota influence immunotherapy responses: mechanisms and therapeutic strategies. J Hematol Oncol. 2022;15(1):47. doi: 10.1186/s13045-022-01273-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Mager L.F., Burkhard R., Pett N., et al. Microbiome-derived inosine modulates response to checkpoint inhibitor immunotherapy. Science. 2020;369(6510):1481–1489. doi: 10.1126/science.abc3421. [DOI] [PubMed] [Google Scholar]
- 47.Tanoue T., Morita S., Plichta D.R., et al. A defined commensal consortium elicits CD8 T cells and anti-cancer immunity. Nature. 2019;565(7741):600–605. doi: 10.1038/s41586-019-0878-z. [DOI] [PubMed] [Google Scholar]
- 48.Bachem A., Makhlouf C., Binger K.J., et al. Microbiota-derived short-chain fatty acids promote the memory potential of antigen-activated CD8(+) T cells. Immunity. 2019;51(2):285–297.e5. doi: 10.1016/j.immuni.2019.06.002. [DOI] [PubMed] [Google Scholar]
- 49.Derosa L., Routy B., Thomas A.M., et al. Intestinal Akkermansia muciniphila predicts clinical response to PD-1 blockade in patients with advanced non-small-cell lung cancer. Nat Med. 2022;28(2):315–324. doi: 10.1038/s41591-021-01655-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Sivan A., Corrales L., Hubert N., et al. Commensal Bifidobacterium promotes antitumor immunity and facilitates anti-PD-L1 efficacy. Science. 2015;350(6264):1084–1089. doi: 10.1126/science.aac4255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Oey O., Liu Y.Y., Sunjaya A.F., Simadibrata D.M., Khattak M.A., Gray E. Gut microbiota diversity and composition in predicting immunotherapy response and immunotherapy-related colitis in melanoma patients: a systematic review. World J Clin Oncol. 2022;13(11):929–942. doi: 10.5306/wjco.v13.i11.929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Vétizou M., Pitt J.M., Daillère R., et al. Anticancer immunotherapy by CTLA-4 blockade relies on the gut microbiota. Science. 2015;350(6264):1079–1084. doi: 10.1126/science.aad1329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Shi Y., Zheng W., Yang K., et al. Intratumoral accumulation of gut microbiota facilitates CD47-based immunotherapy via STING signaling. J Exp Med. 2020;217(5) doi: 10.1084/jem.20192282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Zhang S.L., Mao Y.Q., Zhang Z.Y., et al. Pectin supplement significantly enhanced the anti-PD-1 efficacy in tumor-bearing mice humanized with gut microbiota from patients with colorectal cancer. Theranostics. 2021;11(9):4155–4170. doi: 10.7150/thno.54476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Huang J., Zheng X., Kang W., et al. Metagenomic and metabolomic analyses reveal synergistic effects of fecal microbiota transplantation and anti-PD-1 therapy on treating colorectal cancer. Front Immunol. 2022;13 doi: 10.3389/fimmu.2022.874922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Del Castillo E., Meier R., Chung M., et al. The microbiomes of pancreatic and duodenum tissue overlap and are highly subject specific but differ between pancreatic cancer and noncancer subjects. Cancer Epidemiol Biomarkers Prev. 2019;28(2):370–383. doi: 10.1158/1055-9965.EPI-18-0542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Pushalkar S., Hundeyin M., Daley D., et al. The pancreatic cancer microbiome promotes oncogenesis by induction of innate and adaptive immune suppression. Cancer Discov. 2018;8(4):403–416. doi: 10.1158/2159-8290.CD-17-1134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Spencer C.N., McQuade J.L., Gopalakrishnan V., et al. Dietary fiber and probiotics influence the gut microbiome and melanoma immunotherapy response. Science. 2021;374(6575):1632–1640. doi: 10.1126/science.aaz7015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Simpson R.C., Shanahan E.R., Batten M., et al. Diet-driven microbial ecology underpins associations between cancer immunotherapy outcomes and the gut microbiome. Nat Med. 2022;28(11):2344–2352. doi: 10.1038/s41591-022-01965-2. [DOI] [PubMed] [Google Scholar]
- 60.Usyk M., Pandey A., Hayes R.B., et al. Bacteroides vulgatus and Bacteroides dorei predict immune-related adverse events in immune checkpoint blockade treatment of metastatic melanoma. Genome Med. 2021;13(1):160. doi: 10.1186/s13073-021-00974-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Schmidt T., Li S.S., Maistrenko O.M., et al. Drivers and determinants of strain dynamics following fecal microbiota transplantation. Nat Med. 2022;28(9):1902–1912. doi: 10.1038/s41591-022-01913-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Ianiro G., Punčochář M., Karcher N., et al. Variability of strain engraftment and predictability of microbiome composition after fecal microbiota transplantation across different diseases. Nat Med. 2022;28(9):1913–1923. doi: 10.1038/s41591-022-01964-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Kim C.G., Kim K.H., Pyo K.H., et al. Hyperprogressive disease during PD-1/PD-L1 blockade in patients with non-small-cell lung cancer. Ann Oncol. 2019;30(7):1104–1113. doi: 10.1093/annonc/mdz123. [DOI] [PubMed] [Google Scholar]
- 64.Bilinski J., Lis K., Tomaszewska A., et al. Eosinophilic gastroenteritis and graft-versus-host disease induced by transmission of Norovirus with fecal microbiota transplant. Transpl Infect Dis. 2021;23(1) doi: 10.1111/tid.13386. [DOI] [PubMed] [Google Scholar]
- 65.DeFilipp Z., Bloom P.P., Torres Soto M., et al. Drug-resistant E. coli bacteremia transmitted by fecal microbiota transplant. N Engl J Med. 2019;381(21):2043–2050. doi: 10.1056/NEJMoa1910437. [DOI] [PubMed] [Google Scholar]
- 66.Zellmer C., Sater M., Huntley M.H., Osman M., Olesen S.W., Ramakrishna B. Shiga toxin-producing Escherichia coli transmission via fecal microbiota transplant. Clin Infect Dis. 2021;72(11):e876–e880. doi: 10.1093/cid/ciaa1486. [DOI] [PubMed] [Google Scholar]
- 67.Gupta S., Mullish B.H., Allegretti J.R. Fecal microbiota transplantation: the evolving risk landscape. Am J Gastroenterol. 2021;116(4):647–656. doi: 10.14309/ajg.0000000000001075. [DOI] [PubMed] [Google Scholar]
- 68.Vehreschild M., Ducher A., Louie T., et al. An open randomized multicentre Phase 2 trial to assess the safety of DAV132 and its efficacy to protect gut microbiota diversity in hospitalized patients treated with fluoroquinolones. J Antimicrob Chemother. 2022;77(4):1155–1165. doi: 10.1093/jac/dkab474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Aron-Wisnewsky J., Warmbrunn M.V., Nieuwdorp M., Clément K. Metabolism and metabolic disorders and the microbiome: the intestinal microbiota associated with obesity, lipid metabolism, and metabolic health-pathophysiology and therapeutic strategies. Gastroenterology. 2021;160(2):573–599. doi: 10.1053/j.gastro.2020.10.057. [DOI] [PubMed] [Google Scholar]
- 70.Hanssen N., de Vos W.M., Nieuwdorp M. Fecal microbiota transplantation in human metabolic diseases: from a murky past to a bright future. Cell Metab. 2021;33(6):1098–1110. doi: 10.1016/j.cmet.2021.05.005. [DOI] [PubMed] [Google Scholar]
- 71.Mullish B.H., Quraishi M.N., Segal J.P., et al. The use of faecal microbiota transplant as treatment for recurrent or refractory Clostridium difficile infection and other potential indications: joint British Society of Gastroenterology (BSG) and Healthcare Infection Society (HIS) guidelines. Gut. 2018;67(11):1920–1941. doi: 10.1136/gutjnl-2018-316818. [DOI] [PubMed] [Google Scholar]
- 72.Kazemian N., Kao D., Pakpour S. Fecal microbiota transplantation during and post-COVID-19 pandemic. Int J Mol Sci. 2021;22(6):3004. doi: 10.3390/ijms22063004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Haifer C., Luu L., Paramsothy S., Borody T.J., Leong R.W., Kaakoush N.O. Microbial determinants of effective donors in faecal microbiota transplantation for UC. Gut. 2022;72:90–100. doi: 10.1136/gutjnl-2022-327742. [DOI] [PubMed] [Google Scholar]
- 74.Andrews M.C., Vasanthakumar A. Gut microbiota - a double-edged sword in cancer immunotherapy. Trends Cancer. 2023;9(1):3–5. doi: 10.1016/j.trecan.2022.08.003. [DOI] [PubMed] [Google Scholar]
- 75.Ng S.C., Kamm M.A., Yeoh Y.K., et al. Scientific frontiers in faecal microbiota transplantation: joint document of asia-pacific association of gastroenterology (APAGE) and asia-pacific society for digestive endoscopy (APSDE) Gut. 2020;69(1):83–91. doi: 10.1136/gutjnl-2019-319407. [DOI] [PMC free article] [PubMed] [Google Scholar]