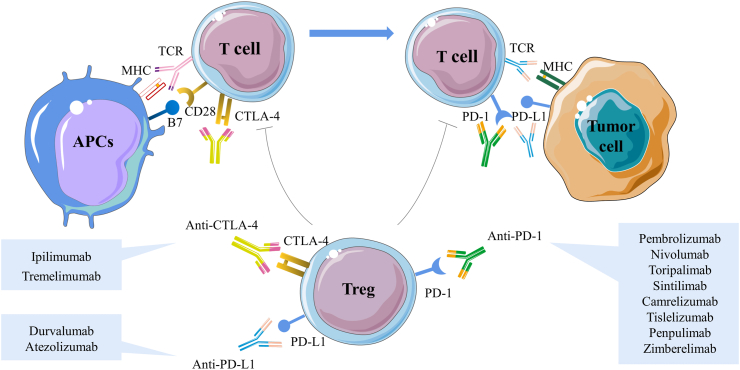
Fig. 1.
Immune checkpoint inhibitors mediate negative co-stimulation and modulate tumour antigens to inhibit T cell activation and differentiation. The expression and function of CTLA-4 are intrinsically linked to T cell activation. Normally, with T cell receptor (TCR) engagement, CTLA-4 is immediately up-regulated. CTLA-4 inhibits TCR signaling by competing with the co-stimulatory molecules CD28 for the B7 ligands, and CTLA-4 has a higher affinity and binding strength, thus causing simultaneous competitive inhibition of both molecules and effectively attenuating T cell activation. In the peripheral TME, PD-1 is expressed mainly on activated T cells. Once PD-1 interacts with its ligand PD-L1, it decreases the immune response, which is thought to be the primary mechanism of tumour immune escape. The extracellular suppressive effects of ICIs are mainly mediated by Tregs, which are necessary for the maintenance of immune tolerance. Currently, ICIs mainly include anti-PD-1 antibodies pembrolizumab, nivolumab, toripalimab, sintilimab, camrelizumab, tislelizumab, penpulimab, zimberelimab, anti-PD-L1 antibodies durvalumab, atezolizumab and anti-CTLA-4 antibodies ipilimumab, tremelimumab and are therefore very attractive therapeutic targets. CTLA-4, cytotoxic T-lymphocyte associated antigen-4; ICIs, immune checkpoint inhibitors; PD-1, programmed cell death 1; PD-L1, programmed cell death ligand 1; TCR, T cell receptor; TME, tumour microenvironment; Tregs, regulatory T cells.