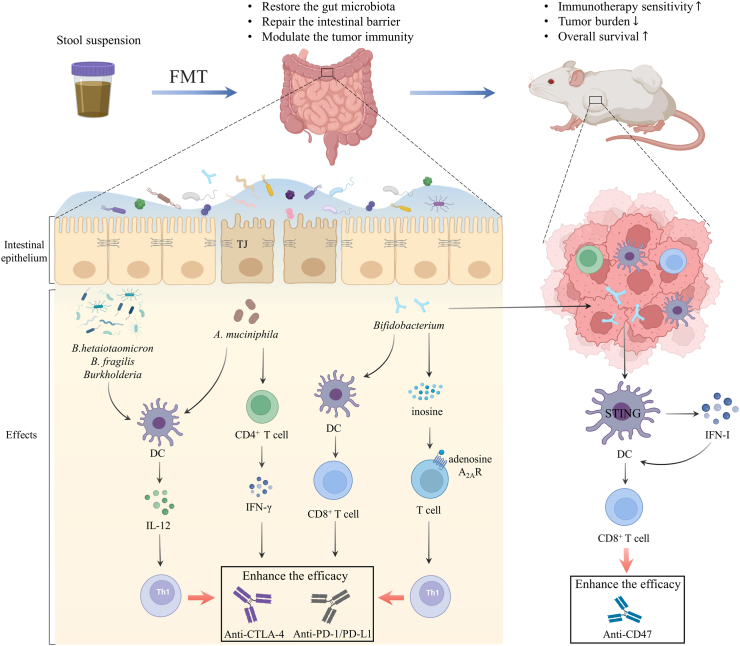
Fig. 3.
Fecal microbiota transplantation reshapes the tumour microenvironment, thus boosting the efficacy of cancer immunotherapy. FMT is a potential therapeutic strategy to restore the gut microbiota, reinforce the intestinal barrier and modulate tumour immunity. FMT modulates gut microbiome and promotes the integrity of tight junction proteins and the intestinal barrier. The abundance of B. fragilis, B. thetaiotaomicron, and Burkholderia enhanced the effect of CTLA-4 blockade by boosting the IL-12-dependent Th1 immune response. A. muciniphila promoted the IFN-γ release and induced DCs to secrete IL-12 to improve the immunogenicity of PD-1 blockade. Gut Bifidobacterium augmented the function of DCs and CD8+ T cells to facilitate the anti-PD-L1 efficacy. Intestinal Bifidobacterium pseudolongum derived metabolite inosine translocated and stimulated T cell-specific adenosine A2AR to promote Th1 cell differentiation in the presence of exogenous IFN-γ for the systemic effect during anti-CTLA-4 and anti-PD-L1 therapy. Bifidobacterium also colonized tumour sites and facilitated local anti-CD47 immunotherapy via STING signaling in DCs which increased the type I IFN to stimulate the tumour-associated DCs in turn and activate CD8+ T-dependent anti-tumour immunity; A2AR, A2A receptor; B. fragilis, Bacteroides fragilis; B. thetaiotaomicron, Bacteroides thetaiotaomicron; DCs, Dendritic cells; FMT, fecal microbiota transplantation; IFN-γ, interferon-gamma; IL, interleukin; STING, stimulator of interferon genes; TJ, tight junction.