Summary
Background
Chronic cough is a prevalent and difficult to treat condition often accompanied by cough hypersensitivity, characterised by cough triggered from exposure to low level sensory stimuli. The mechanisms underlying cough hypersensitivity may involve alterations in airway sensory nerve responsivity to tussive stimuli which would be accompanied by alterations in stimulus-induced brainstem activation, measurable with functional magnetic resonance imaging (fMRI).
Methods
We investigated brainstem responses during inhalation of capsaicin and adenosine triphosphate (ATP) in 29 participants with chronic cough and 29 age- and sex-matched controls. Psychophysical testing was performed to evaluate individual sensitivities to inhaled stimuli and fMRI was used to compare neural activation in participants with cough and control participants while inhaling stimulus concentrations that evoked equivalent levels of urge-to-cough sensation.
Findings
Participants with chronic cough were significantly more sensitive to inhaled capsaicin and ATP and showed a change in relationship between urge-to-cough perception and cough induction. When urge-to-cough levels were matched, participants with chronic cough displayed significantly less neural activation in medullary regions known to integrate airway sensory inputs. By contrast, neural activations did not differ significantly between the two groups in cortical brain regions known to encode cough sensations whereas activation in a midbrain region of participants with chronic cough was significantly increased compared to controls.
Interpretation
Cough hypersensitivity in some patients may occur in brain circuits above the level of the medulla, perhaps involving midbrain regions that amplify ascending sensory signals or change the efficacy of central inhibitory control systems that ordinarily serve to filter sensory inputs.
Funding
Supported in part by a research grant from Investigator-Initiated Studies Program of Merck Sharp & Dohme Pty Ltd. The opinions expressed in this paper are those of the authors and do not necessarily represent those of Merck Sharp & Dohme (Australia) Pty Ltd.
Keywords: Vagal sensory, Cough, Brain imaging, Purinergic, Brainstem, ATP, Sensitisation
Research in context.
Evidence before this study
Chronic cough in adults, regardless of the aetiology, is widely considered to be a hypersensitivity disorder in which coughing is triggered by relatively low levels of thermal, mechanical or chemical stimuli. The site of this hypersensitivity has been presumed to include the airway vagal sensory nerve fibres, such that they become more responsive to stimuli and therefore more readily able to evoke coughing. Although the mechanisms leading to vagal hypersensitivity have not been identified, inflammatory mediators such as adenosine triphosphate (ATP) have been implicated and this has led to major advancements of purinergic (P2X3) receptor antagonists through clinical trials for treating chronic refractory cough. Nevertheless, the precise neurobiological actions of ATP in human cough hypersensitivity are unknown but could be investigated by assessing the patterns of brainstem neural activation using functional brain imaging associated during ATP challenges in patients with chronic cough compared to healthy controls.
Added value of this study
Contrary to the prevailing hypothesis, the results observed in patients with chronic cough in the present study are inconsistent with substantive increased sensitivity of peripheral cough evoking sensory fibres as a primary mechanism contributing to cough hypersensitivity. Instead, cough hypersensitivity may involve amplification of low levels of vagal sensory input to the medullary brainstem through altered higher brain processes.
Implications of all the available evidence
These findings support and extend our previous work demonstrating that changes in how the central nervous system processes incoming vagal sensory signals occur in patients with chronic cough. The findings challenge our understanding of cough hypersensitivity and therefore may reshape future therapeutic approaches to manage this challenging clinical condition.
Introduction
Chronic cough is a prevalent clinical problem, affecting as many as 10% of the global population and commonly associated with pulmonary, gastro-oesophageal reflux (GERD) or nasal (e.g., rhinosinusitis) diseases.1,2 Individuals with chronic cough characteristically display signs of cough hypersensitivity, a condition characterised by a lowered threshold for cough initiation indicative of heightened nervous system responses to airway stimuli.2,3 For many people with chronic cough, treating their associated disease restores cough sensitivity and relieves coughing. However, for a significant percentage of people chronic cough can be insensitive to therapeutic intervention, despite effective management of their associated conditions (termed refractory chronic cough, RCC),2,4, 5, 6 or present without evidence of an associative or causative condition, despite extensive clinical investigation (termed unexplained chronic cough, UCC).2,4, 5, 6 Effective management of RCC and UCC therefore relies on therapeutic approaches that target the processes that mediate cough hypersensitivity and/or induce coughing, rather than therapies that treat diseases commonly associated with chronic cough.7 Consequently, efforts have focussed on understanding putative mechanisms that regulate peripheral and central processes involved in cough neural circuit activity as targeting these will likely prove effective in the management of RCC and UCC.2,7
Recent studies in animals and humans have identified adenosine triphosphate (ATP) as an important endogenous signalling molecule involved in the development of chronic cough, perhaps released from injured or activated airway epithelial cells during inflammation or irritant exposure.2,8, 9, 10, 11, 12, 13 ATP acts on cough-evoking sensory nerve fibres through purinergic receptors, including both the heteromeric and homomeric P2X2/3 and P2X3 receptors, respectively.9,10 In support of this, recent clinical trials have demonstrated antitussive actions of several antagonists with P2X2/3 and P2X3 activity in RCC and UCC,2,14,15 consistent with a role for ATP release in the airways leading to the subsequent activation or sensitisation of cough evoking vagal sensory nerve fibres. However, preclinical animal studies have shown that ATP reliably activates only one of the two known cough evoking sensory neural pathways in healthy airways.9,10,16 This may also be true in humans. Thus, using functional brain imaging (fMRI) in healthy humans12 we showed that inhalation of the cough-evoking stimulant capsaicin resulted in medullary brainstem activations that encompassed both the nucleus of the solitary tract and paratrigeminal nucleus, regions where capsaicin-sensitive airway sensory neurons arising from the nodose and jugular vagal ganglia, respectively, are known to project.16 By contrast, when these same participants inhaled ATP, medulla activations were largely restricted to the nucleus of the solitary tract, suggestive that ATP preferentially activated the nodose cough pathway.12,16
Although ATP may specifically activate nodose sensory neural pathways in healthy airways, it is conceivable that the pattern of neural circuitry activated by ATP may be altered in cough hypersensitivity. To investigate this, we have again employed fMRI, this time to interrogate the potential brainstem pathways involved in ATP-evoked cough responses in RCC or UCC compared to control participants without chronic cough. We set out to test the hypothesis that changes in the activation patterns in the brainstem evoked by inhaled ATP in participants with chronic cough may inform the identity of neural circuits responsible for the hypersensitive state under pathological conditions.
Methods
Ethics
The study and procedures were approved by the University of Melbourne (project number 1852642) and Monash University (registration number 17642) Human Research Ethics Committees and conform to the standards set by the Declaration of Helsinki (last modified in 2013). The study was registered with ClinicalTrials.gov (NCT03722849). All participants were initially screened via telephone interviews for inclusion and exclusion criteria and gave written, informed consent prior to study enrolment.
Participants
The study was open to both male and female participants (sex self-reported by study participants), although chronic cough is significantly more prevalent in females.17 Chronic cough was diagnosed by a respiratory or laryngology specialist or general practitioner and inclusion required cough of at least 6 months duration with no abnormalities on chest X-ray or CT scan, and 61 potential participants with chronic cough were initially identified (Fig. 1). Of these, n = 29 were enrolled into the study, along with n = 29 age- and sex-matched control participants (without chronic cough). One male participant with chronic cough did not complete MRI scans after initial psychophysical testing due to personal circumstances. One female control participant withdrew from the study during the MRI scanning due to claustrophobia. Thus, a total of 56 participants (n = 28 per group) completed the full study and were included in the final fMRI data analyses.
Fig. 1.
Summary of screening and recruitment for participants with chroniccough.
Cough challenge testing
Nebulised saline, capsaicin and ATP were used to investigate individuals’ cough psychophysical characteristics. Sterile normal saline (0.9% sodium chloride) served as a control substance. Capsaicin (Sigma, Australia) was prepared at a stock concentration of 125 μM and serially diluted to achieve a concentration range of 0.12–62.5 μM as described previously.13,18, 19, 20 ATP (Sigma, Australia) was prepared at a stock concentration of 465 mM and serially diluted to achieve a concentration range of 0.45–232.5 mM as previously described.12 All dilutions were prepared fresh on the day of testing and participants inhaled nebulised vapour of saline, capsaicin or ATP via a face mask which was connected to jet nebuliser (RapidFlo; Allersearch, Scoresby, Victoria, Australia). The nebuliser cups containing each concentration of capsaicin or ATP were connected via a tubing to the jet nebuliser which generated vapour (∼2–3 μm particle size). During the fMRI data acquisition, compressed medical air (5 L min−1) was used to generate vapour and a series of parallel shut-off valves directed the air to one of the three nebulisers (containing saline, capsaicin or ATP) during the stimulus periods, or to a vent for passive exhaust during non-stimulus periods. Each nebuliser was connected in parallel to the face mask via independent tubing, preventing direct mixing of test reagents.
Experimental design
Psychophysical assessment of sensitivity to inhaled capsaicin and ATP
All enrolled participants completed the Leicester Cough Questionnaire (LCQ),21 Newcastle Laryngeal Hypersensitivity Questionnaire (LHQ),22 and Hull Cough Hypersensitivity Questionnaire (HARQ).3 The urge-to-cough threshold (Cu), defined as the first concentration of stimulus to evoke a perceptible level of urge-to-cough, and the cough threshold (C2), defined as the first concentration of stimulus to elicit two coughs or more, were determined by single vital capacity inhalation of doubling concentrations of capsaicin and ATP via the mouth as recommended23 and described in our previous studies.12,19,20,24 The order of testing for capsaicin and ATP was randomised among the participants. Immediately after each inhalation, participants rated their level of urge-to-cough using a Borg scale ranging from 0 (no urge) to 10 (most intense urge imaginable). Participants recorded the nature and locations of evoked sensations using body map charts. To limit exposure to test substances, each test concentration was presented only once to participants to estimate Cu and C2. In preparation for fMRI scanning, the maximum suppressible stimulus concentration (Smax), defined as the highest possible concentration of stimulus participants could repeatedly inhale over 24 s of tidal breathing without coughing, was subsequently determined for both capsaicin and ATP using a method of limits.12,20 Participants were challenged with progressively higher stimulus concentrations over 24 s until breakthrough coughing was evoked, after which the final Smax concentration was refined by retesting participants using stimulus concentrations above and below that initially identified.
fMRI scanning parameters
Functional brain imaging was performed at Monash Biomedical Imaging, Clayton, Australia using a Siemens Skyra 3T scanner (Siemens Healthineers, Erlangen, Germany) and a 32-channel head coil. All but 3 participants underwent fMRI scanning on the same day as cough challenge testing. The maximum duration between cough challenge testing and scanning was 7 days. Immediately prior to scanning, confirmation of the chosen Smax stimulus concentration was performed for all participants, regardless of when their original cough challenge testing was performed, and minor adjustments were made to the delivered stimulus if necessary to limit coughing (defined as fMRI stimulus concentration). In the scanner, participants wore earplugs for ear protection from the MRI scanner noise and lay on the patient table of the MRI scanner with their heads stabilised with foam padding and their arms supported by sandbags. A nebuliser mask connected to three nebulisers was placed and secured over the participants’ nose and mouth for delivery of saline, capsaicin or ATP.
High resolution T1-weighted anatomical images were acquired at the resolution of 1 × 1 × 1 mm3 with MPRAGE sequence with the following parameters: repetition time (TR) = 1900 ms, echo time (TE) = 2.07 ms, inversion time = 900 ms, flip angle = 9°, matrix = 256 × 256. Four fMRI scans with a view localised to the brainstem (Fig. 2) were performed with an echo planar imaging (EPI) sequence with the following parameters: TR = 1930 ms, TE = 31 ms, flip angle = 90°, total number of volumes = 186, slice thickness = 2.6 mm, total number of slices = 21. The slices had 1.8 × 1.8 mm2 in-plane resolution and a matrix of 106 × 106 that constituted a field of view (FOV) of sufficient size to incorporate a portion of the brain hemispheres rostral to the brainstem (Fig. 2).
Fig. 2.
Overview of fMRI experimental design. (a) Control participants and participants with chronic cough underwent inhaled cough challenge testing during brainstem optimised functional imaging (restricted field of view highlighted by yellow shaping). (b) ATP and capsaicin challenge concentrations were individually tailored to produce behaviourally equivalent levels of urge-to-cough in all participants. Stimuli were delivered across four brain imaging runs, comprising two 24 s blocks each of saline, capsaicin, and ATP inhalation (6 blocks/run in total).
An additional image with the same slice orientation as in brainstem fMRI images with 70 slices that covered the whole brain was also acquired for registration purposes with similar parameters (TR = 6370 ms, TE = 31 ms, matrix = 106 × 192). Fieldmap images were acquired for correction of distortion with the following parameters: TR = 744 ms; TE = 4.92 ms; slice thickness = 3.3 mm; total number of slices = 70.
fMRI scanning with inhalation of tussive agents
Participants received visual instructions through a mirror attached to the head coil that allowed them to view a screen outside the scanner. Visual cues were presented by a Windows computer running Presentation software (Neurobehavioural Systems) and included the following: “prepare to inhale” 3 s prior to the onset of each inhalation period; “go” to align the first inspiratory phase to the onset of stimulus delivery, “prepare to rate” after the conclusion of the inhalation period and “rate your urge-to-cough” during which participants indicated their urge-to-cough ratings via a trackball combination mouse (Fig. 2). A visual fixation cross was displayed during the rest period (35 s) as well as during the inhalation challenges (24 s). Most participants completed 4 fMRI scans. The exceptions were one control participant who completed only 3 fMRI scans due to urgent bathroom needs, one participant with chronic cough who completed only 3 fMRI scans due to coughing bouts during the scans and one participant with chronic cough who completed only 2 fMRI scans due to issues with viewing of the visual instructions. Each fMRI scan included 6 blocks of nebulised stimuli (2 each for saline, capsaicin and ATP delivered in a pseudorandom order, Fig. 2). Any behavioural changes during the fMRI scans, such as total number of coughs, with the inhalation challenges were also noted down.
Analysis of the fMRI data was performed in FSL (FMRIB’s software library; https://fsl.fmrib.ox.ac.uk/fsl/fslwiki/) that included the FSL Expert Analysis Tool (FEAT, version 6.0.3). Non-brain voxels were stripped from the anatomical and functional MRI images using the brain extraction tool,25 motion-correction was performed using the MCFLIRT tool,26 spatial distortion was corrected using fieldmaps and FMRIB’s Utility for Geometrically Unwarping EPIs (FUGUE)27 and spatial smoothing was performed with a Gaussian kernel of 3.0 mm full width at half maximum, and the data high pass filtered with a cut-off of 0.01 Hz.
Registration of the functional MRI data to the standard Montreal Neurological Institutes (MNI) template was performed in three steps using FLIRT (FMRIB’s Linear Image Registration Tool). First, linear registration of motion-corrected and unwarped functional brainstem EPI data to the unwarped whole brain mean EPI was performed. Next, the unwarped whole brain mean EPI image was linearly registered to the high-resolution structural T1-weighted image, and the latter image to the standard MNI152 template brain (1-mm resolution). Multiplication of the three matrices was used to transform statistical parametric maps from the native to the standard space for analysis of group effects.
BOLD data series were analysed with a general linear model with prewhitening for local autocorrelation.28 Regressors representing the timing for inhalation of saline, capsaicin and ATP were included as explanatory variables (EVs) in the general linear model. The timings for presentation of other visual cues and rating tasks were also included as EVs. To account for physiological noise, confound regressors were also included in the general linear model. Having been described in previous studies by our group,12,24 these confound regressors have been adopted according to the outcomes of empirical studies that assessed the influence of respiration on BOLD signal variance, and how this variance can be modelled when the events of interest are likely to correlate with respiration,29, 30, 31 as is the case in this study. Notably, the manifestation of respiratory-related variance in BOLD signal is not reliably predicted from concurrent recordings of the respiratory cycle, being more accurately represented in recordings of the BOLD time series from carefully selected regions. These regions, including the white matter and ventricles, are likely to show respiratory and other physiological-related noise while being less likely to incorporate variance associated with neural events. Additionally, a confound regressor was included that was identified after creating a standard deviation image from each of the BOLD time series acquired from patients and control participants. This noisy cofound regressor corresponded to the time series of the voxel with the highest standard deviation in the image and was typically located near the midline at the superior margin of the brain in a region likely to include the sagittal sinus. Six motion parameters (three translations, three rotations) were also included as confound regressors. Participants with chronic cough had an increased tendency to cough upon inhalation of tussive agents during fMRI scans compared to healthy controls (for capsaicin: 5.21 ± 6.05 vs. 0.928 ± 2.12 coughs, P = 0.0054; for ATP: 2.57 ± 3.55 vs. 0.57 ± 1.91 coughs, P = 0.0072; for saline: 0.14 ± 0.59 vs. 0 ± 0, P = 0.4919). These breakthrough coughing events were not always temporally aligned with the 24 s stimulus blocks. Thus, for participants with cough vs. control participants, cough during stimulus blocks for capsaicin and ATP equalled 3.67 ± 5.16 vs. 0.89 ± 0.21 (P = 0.0868) and 1.32 ± 2.74 vs. 0.50 ± 1.67 (P = 0.3954), respectively. To identify and account for the occasional signal distortions that accompanied breakthrough coughing events, an automated algorithm for detecting cough-related distortions in the BOLD signal was developed in-house in Python (version 3.9) (unpublished) and detections were used as additional EVs in the general linear model as previously reported.19,20
An additional confound variable that represented widely distributed signal changes across the acquired partial brain volume was generated. Preliminary activation maps of the main effects of interest (ATP, ATP > Saline, Capsaicin, Capsaicin > Saline) were created. All voxels showing activation for any main effect in either group were excluded from the partial brain volume, and mean signals across the remaining voxels were used to create the large volume confound regressor. This approach was used to further characterise changes in BOLD signal due to respiratory-related noise,12,19,20,32 as advocated to model global effects while excluding the contribution of activation to this variance.33 An assessment of the level of BOLD signal variance and any associations between signals measured from confound regressors and effects of interest is provided in Supplementary Information.
Contrasts of parameter estimates (COPEs) were calculated for each EV (saline, capsaicin, ATP, rating, etc.) and for differences among the three inhalation stimuli (Capsaicin > Saline, ATP > Saline) or between cohorts (Control Participant > Cough Participant and vice versa). Higher level analyses were performed after transforming these statistical parametric maps representing COPEs to the standard MNI space. At the second level analyses, a fixed effects model was used to calculate average effects out of the four different runs for each participant. At the group level analyses, the outputs of the second level analyses were used as inputs and a mixed effects model was used. Demeaned urge-to-cough ratings for capsaicin were used as an additional regressor in the group level analyses to examine the relationship between the behaviour and the level of activations. In line with our previous brainstem fMRI study,12 the outcomes of the group level analyses were thresholded to include only the voxels with Z values >2.3 and cluster probability level of P < 0.05 corrected for multiple comparisons based on Gaussian random field theory.34
Regions of interest (ROIs), identified using whole group (n = 56) activation maps, neuroanatomical maps35 and our prior studies,12,24 were defined by manually creating masks over voxels in activated clusters from the Capsaicin > Saline and ATP > Saline contrasts. These masks included voxels likely corresponding to ROIs in the left and right nucleus of the solitary tract, left and right paratrigeminal nucleus, left midbrain, right anterior cingulate cortex and left primary somatosensory cortex, regions comprising core elements of the urge-to-cough network.12 Percentage signal changes from each of the ROIs were extracted using FEATQUERY tool from the FSL package.
Statistics
Sample size estimates were made using BOLD signal changes extracted from regions of interest reported in our previous study12 and analysed with fMRIpower.36 Inclusion of 25 participants per group afforded at least 80% power to detect a between-group difference of 0.48–0.72 standard deviation units in the regions of interest. Participants were included if their chronic cough exhibited features of RCC or UCC, including a history of unsuccessful therapy interventions or an absence of identified comorbid conditions that can cause chronic cough. Exclusion criteria included conditions that could potentially affect cough sensitivity such as chronic respiratory disease, neurological disease or recent acute respiratory infection, a history of smoking during the previous 5 years, conditions that could influence MRI safety such as recent surgery and MR-unsafe implants, use of medications that were likely to influence cough sensitivity, brain activity and blood oxygen level dependent (BOLD) responses of functional magnetic resonance imaging (fMRI). The study was single blinded as participants (but not investigators) were blinded to the identity of the challenge stimulus order used in the fMRI scanning.
All statistical analyses of demographic and behavioural data were performed in GraphPad Prism (version 9). Data sets were first tested for normality using the D'Agostino-Pearson test to determine appropriate parametric or non-parametric groupwise tests. Data are presented as mean ± standard deviation (SD) and statistical significance was defined as P < 0.05. See also statistical summary table (Supplementary Material Table S1).
Differences in group demographics were assessed using unpaired t-tests (age) or Mann–Whitney U tests (cough duration) reflecting the normality of the data. Mean (overall) and subdomain response scores to questionnaires were investigated using multiple Mann–Whitney U tests with correction for multiple comparisons with Holm-Šídák's method, as data were not normally distributed.
The stimulus response data were log-transformed37 to restore data to linearity and calculate Cu, C2, Smax and fMRI values for capsaicin and ATP. Between group differences in stimulus response measures were analysed with two-way ANOVA followed by post-hoc Šídák's multiple comparisons testing, as the transformed data were normally distributed, and independence existed among testing statistics of interest. Difference scores between urge-to-cough ratings at Cu and C2 stimulus concentrations were calculated by subtracting the urge-to-cough ratings at C2 from those at Cu (defined as C2-Cu UTC difference) and tested for significant differences between group effects using unpaired t-tests, as data were normally distributed. Sensory experiences and body maps were recorded as the proportion of respondents for each response option and differences between cohorts analysed using Fisher’s exact test. The sum of terms used to describe participant experiences during capsaicin and ATP inhalation was calculated and compared between groups using unpaired t-tests. Mean urge-to-cough ratings induced by inhalation of saline, capsaicin and ATP across four fMRI runs were calculated and compared between groups using two-way ANOVA followed by Šídák's multiple comparisons testing, as data were normally distributed, and independence existed among testing variables of interest. Total numbers of coughs during the fMRI scans were compared with multiple Mann–Whitney U tests with correction for multiple comparisons with Holm-Šídák's method given their non-normal distribution.
For fMRI data, the mean percentage BOLD signal changes for each ROI across four runs were calculated for each participant and differences between ROIs, as planned a priori, were compared between the control and the chronic cough groups using non-parametric Mann–Whitney U tests as data were not normally distributed.
Role of funders
This study was supported in part by a research grant from Investigator-Initiated Studies Program of Merck Sharp & Dohme (Australia) Pty Ltd. The funders reviewed and approved the final study design but played no role in data collection, data analyses, interpretation, or writing of report. The opinions expressed in this paper are those of the authors and do not necessarily represent those of Merck Sharp & Dohme (Australia) Pty Ltd.
Results
Participant demographics
Control participants and participants with chronic cough displayed no significant group differences between sex and age demographics. Consistent with the known demographics of chronic cough,17 65.5% of participants were female with an overall average age of 60.1 ± 12.0 years (Table 1). The mean duration of self-reported chronic cough by participants was 14.3 ± 14.6 years, with more than half of the participants having chronic cough for over 10 years and 4 participants reporting ongoing troublesome cough persisting for 3–5 decades of their life. None of the enrolled participants with chronic cough had any comorbid lung disease and based on their clinical histories assessed by respiratory or laryngology specialists or general practitioners, all were suspected to have refractory or unexplained chronic cough. Scores across the HARQ, Newcastle LHQ and LCQ, and their subdomains, for participants with chronic cough were highly significantly different (P values ranged from P = 0.0022 to P < 0.0001; Multiple Mann–Whitney U tests with correction for multiple comparison), to control participants, indicative of the burden of their chronic cough (Table 1).
Table 1.
Participant characteristics.
| Control | Cough | Statistics | |
|---|---|---|---|
| Number of participants | 29 | 29 | – |
| Sex | |||
| Male | 10 (34.5%) | 10 (34.5%) | P < 0.999 |
| Female | 19 (65.5%) | 19 (65.5%) | P < 0.999 |
| Age (years) | 59.7 ± 12.0 | 60.6 ± 12.2 | P = 0.771 |
| Male | 62.2 ± 12.1 | 60.2 ± 12.6 | P = 0.722 |
| Range | 41–77 | 40–79 | – |
| Female | 58.3 ± 12.1 | 60.8 ± 12.3 | P = 0.536 |
| Range | 36–78 | 41–78 | – |
| Cough duration (years) | |||
| Mean | 0 ± 0 | 14.3 ± 14.6 | P < 0.0001 |
| Range | – | 0.5–50 | – |
| HARQ | |||
| Mean score | 2.3 ± 2.8 | 33.1 ± 13.1 | P < 0.0001 |
| Newcastle LHQ | |||
| Mean score | 20.1 ± 1.1 | 14.8 ± 3.0 | P < 0.0001 |
| Obstruction domain | 6.7 ± 0.4 | 4.8 ± 1.1 | P < 0.0001 |
| Irritation domain | 6.5 ± 0.6 | 3.8 ± 1.4 | P < 0.0001 |
| Pain/thermal domain | 6.9 ± 0.3 | 6.2 ± 1.1 | P < 0.0001 |
| LCQ | |||
| Mean score | 20.8 ± 0.3 | 13.9 ± 3.6 | P < 0.0001 |
| Physical domain | 6.8 ± 0.2 | 5.2 ± 1.0 | P < 0.0001 |
| Psychological domain | 7.0 ± 0.2 | 4.5 ± 1.4 | P < 0.0001 |
| Social domain | 7.0 ± 0.0 | 4.2 ± 1.6 | P < 0.0001 |
Behavioural responses to inhalation of capsaicin and ATP
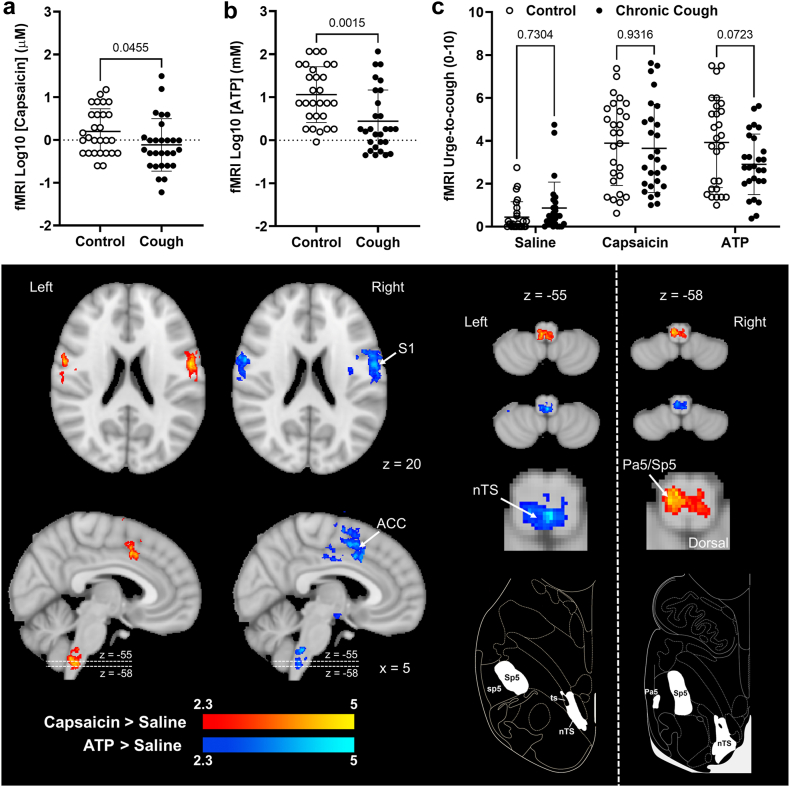
Psychophysical responses to inhalation of nebulised capsaicin and ATP showed differences between participants with chronic cough and the healthy participant cohort (Fig. 3). Saline inhalation produced no urge-to-cough sensation in any control participants (group rating = 0.0 ± 0.0) and a small urge-to-cough in 6 participants with chronic cough (group rating = 0.5 ± 1.2). A significant difference in response sensitivity between control participants and participants with chronic cough was observed for capsaicin and ATP (F(2,165) = 25.4, P < 0.0001, F(2,165) = 27.3, P < 0.0001; two-way ANOVA, respectively). Post-hoc multiple comparison testing showed that participants with chronic cough had significantly lower capsaicin thresholds to evoke two or more coughs (C2, P = 0.0018) and a lower maximum concentration that could be inhaled for 24 s without coughing (Smax, P = 0.0031). Similarly for ATP inhalation, participants with chronic cough displayed significantly lower C2 (P < 0.0001) and Smax (P < 0.0001) thresholds. Notably, even though both capsaicin and ATP urge-to-cough thresholds were lower in participants with chronic cough compared to controls, this did not reach statistical significance (Cu capsaicin, P = 0.081; Cu ATP, P = 0.282). Urge-to-cough ratings were related to stimulus concentration in the psychophysical testing session, and consequently the urge-to-cough levels reported at Cu and C2 challenge concentrations were significantly different for both capsaicin and ATP (F(3,110) = 37.8, P < 0.0001, F(3,110) = 29.8, P < 0.0001, respectively; one-way ANOVA) in heathy and cough cohorts (Fig. 3). However, participants with chronic cough consistently reached their C2 cough threshold at lower urge-to-cough ratings. Thus, the difference between urge-to-cough ratings at Cu and C2 stimulus concentrations was significantly smaller in participants with chronic cough for both capsaicin (t(56) = 4.2, P < 0.0001; unpaired t-test) and ATP (t(56) = 3.3, P = 0.002; unpaired t-test). Compared to control participants, participants with chronic cough had significantly higher number of coughs to both capsaicin and ATP (4.64 ± 2.47 vs. 2.59 ± 1.12 coughs; P = 0.0003, and 4.71 ± 2.58 vs. 2.14 ± 1.22, P = 0.0001, multiple Mann–Whitney U tests with correction for multiple comparisons) at C2 concentrations.
Fig. 3.
Comparison of capsaicin and ATP psychophysical cough indices in control participants and participants with chronic cough. Upper panels show (a) Capsaicin and (b) ATP behavioural thresholds. Lower panels show urge-to-cough ratings provided by control participants and participants with chronic cough while inhaling Cu and C2 concentrations of (c, d) capsaicin and (e, f) ATP. C2-Cu urge-to-cough rating difference scores were calculated for both capsaicin (d) and ATP (f). Cu, Urge-to-cough threshold; C2, stimulus concentration producing two spontaneous coughs; Smax, maximum stimulus concentration tolerated without coughing during 24 s of repeated inhalation. Data points represent individual participants (horizontal line denotes mean and error bars are standard deviation) of n = 29 per group. P values calculated using two-way ANOVA with Šídák's multiple comparison tests (a, b) and unpaired t-tests (c–f).
During fMRI scanning, stimulus type (saline, capsaicin, ATP) was a significant factor in variance of urge-to-cough ratings (F(2,156) = 57.01, P < 0.0001; two-way ANOVA). Group (control, chronic cough) was not a significant factor (F(1,156) = 1.17, P = 0.280), but there was an interaction between group and stimulus type (F(2,156) = 3.46, P = 0.041). Subsequent post hoc testing showed that whilst urge-to-cough ratings during saline challenges were significantly lower than urge-to-cough ratings during challenges with fMRI concentrations of capsaicin and ATP (P < 0.0001), there were no group differences between the levels of urge-to-cough evoked by capsaicin (P = 0.997) and ATP (P = 0.285). However to achieve this matched behavioural experience, significant differences in the concentration of inhaled capsaicin and ATP were required for control participants and participants with chronic cough (t(54) = 2.0, P = 0.046, t(54) = 3.4, P = 0.002; unpaired t-test; Fig. 4). Tests for sex-related differences were not significant for any of the comparisons above (P values ranged from P = 0.1006 to P > 0.9999). As we previously reported,12 stimulus order was not a factor affecting urge-to-cough ratings during scanning (Supplementary Table S2) consistent with no known interactions between capsaicin and ATP. Urge-to-cough ratings across individual scanning runs for both cohorts did not differ for either capsaicin or ATP (Supplementary Table S3), suggesting an absence of response tachyphylaxis over the course of the scanning session, again consistent with our previous study.12
Fig. 4.
Capsaicin and ATP inhalation during fMRI scanning. (a) Capsaicin and (a) ATP stimulus concentrations used for control participants and participants with chronic cough during fMRI scanning to achieve (c) equivalent urge-to-cough experiences for control and chronic cough groups for each test stimulus. Data points represent individual participants (horizontal line denotes mean and error bars are standard deviation) of n = 28 per group. P values calculated using unpaired t-tests (a, b) and two-way ANOVA with Šídák's multiple comparison tests. Images show cluster-corrected Blood Oxygen Level Dependent (BOLD) signal responses in the cerebral hemispheres and brainstem reflecting the Capsaicin > Saline (red) and ATP > Saline (blue) activation patterns common for all participants in the study (n = 56). Slices and region co-ordinates displayed were chosen based on the outcomes of our prior fMRI studies.12,20,24,32 Atlas drawings show the expected location of brainstem regions of interest (modified from35). ACC, anterior cingulate cortex; nTS, nucleus of the solitary tract; Pa5, paratrigeminal nucleus; Sp5, spinal trigeminal nucleus; sp5, spinal trigeminal tract; S1, primary somatosensory cortex; ts, solitary tract.
Participants also reported a range of sensations associated with inhalation of capsaicin and ATP (Table 2). Most participants, regardless of cough status, reported inhalation of capsaicin and ATP inhalation as irritating and associated with a tickle, while a minority experienced itchy, burning, hot, stinging, and breathless sensations during inhalation challenges. Almost all participants recorded the body map localisation of sensations over the throat area, with a minority also noting sensations in the chest, mouth, and nose. Notably, there were no significant differences between control participants and participants with chronic cough with respect to the relative frequency of each sensory experience or its location values ranged from (P = 0.18 to P = 0.78; Fisher’s exact test). However, participants with chronic cough tended to choose significantly more terms in total to describe their experience during capsaicin inhalation (t(56) = 2.3, P = 0.024; unpaired t-test), although this was not the case for ATP challenges (t(56) = 0.8, P = 0.429; unpaired t-test).
Table 2.
Summary of sensory experiences and sensation location associated with capsaicin and ATP inhalation.
| Capsaicin |
ATP |
|||||
|---|---|---|---|---|---|---|
| Control | Cough | Statistics | Control | Cough | Statistics | |
| Sensations reported (% participants) | ||||||
| Irritating | 55.2% | 72.4% | P = 0.274 | 66.5% | 72.4% | P = 0.777 |
| Tickle | 55.2% | 69.0% | P = 0.417 | 69.0% | 55.2% | P = 0.417 |
| Itchy | 17.2% | 27.6% | P = 0.530 | 10.3% | 27.6% | P = 0.179 |
| Burning | 17.2% | 17.2% | P > 0.999 | 3.5% | 17.2% | P = 0.194 |
| Hot | 6.9% | 3.5% | P > 0.999 | 0% | 0% | – |
| Breathless | 6.9% | 27.6% | P = 0.079 | 20.7% | 13.8% | P = 0.730 |
| Stinging | 0% | 10.3% | P = 0.237 | 0% | 0% | – |
| Pleasant | 0% | 3.5% | P > 0.999 | 0% | 0% | – |
| Pressure | 0% | 0% | – | 0% | 10.3% | P = 0.237 |
| Cold | 0% | 0% | – | 0% | 0% | – |
| Stabbing | 0% | 0% | – | 0% | 0% | – |
| Throbbing | 0% | 0% | – | 0% | 0% | – |
| Total sensationsa | 2.1 ± 0.94 | 2.79 ± 1.26 | P = 0.023 | 2.14 ± 0.79 | 2.32 ± 0.94 | P = 0.436 |
| Sensation location (% participants) | ||||||
| Throat | 96.6% | 93.1% | P > 0.999 | 96.6% | 93.1% | P > 0.999 |
| Chest | 10.3% | 10.3% | P > 0.999 | 10.3% | 13.8% | P > 0.999 |
| Tongue | 6.9% | 0% | P = 0.491 | 0% | 0% | – |
| Nose | 13.8% | 6.9% | P = 0.670 | 0% | 3.5% | P > 0.999 |
| Mouth | 0% | 3.5% | P > 0.999 | 0% | 0% | – |
| Head | 0% | 6.9% | P = 0.491 | 3.5% | 10.3% | P = 0.612 |
| Eyes | 0% | 0% | – | 10.3% | 0% | P = 0.237 |
Total sensations, the numerical sum of total words selected by participants to describe their sensory experience, expressed as mean ± SD, n = 29 per group.
Regional BOLD signal changes associated with capsaicin and ATP inhalation
Combined analysis of fMRI data from all participants (n = 56) revealed the locations of activations in common for control participants and participants with chronic cough (Fig. 4; Table 3). In the cerebral hemispheres, noting the restricted imaging field of view available, Capsaicin > Saline and ATP > Saline activations were distributed across overlapping territories in the somatosensory and midcingulate cortices, consistent with previous studies.12,19,20,32 In the brainstem, Capsaicin > Saline and ATP > Saline activations encompassed regions of the dorsomedial and dorsolateral medulla, extending for several millimetres above and below the level of the Obex, as previously reported.12 The Capsaicin > Saline cluster was more prominent and included medullary territories that likely contained vagal sensory processing sites in the dorsomedial brainstem (at z = −55), including the nucleus of the solitary tract, and the dorsolateral brainstem (at z = −58), including the spinal trigeminal nucleus and paratrigeminal nucleus. The ATP > Saline activation cluster in the caudal medulla (z = −55) displayed a prominent dorsomedial location, in the region of the caudal nucleus of the solitary tract, but additionally extended laterally (at z = −58) into areas that likely encompassed parts of the spinal trigeminal nucleus.
Table 3.
Regions in brain showing activation during inhalation of tussive substances for all participants.
| Region | Peak coordinates |
|||
|---|---|---|---|---|
| X | Y | z | Max Z score | |
| Capsaicin > Saline (n = 56) | ||||
| Precentral gyrus | −45 | −15 | 40 | 6.21 |
| 46 | −11 | 41 | 6.30 | |
| Paracingulate gyrus | −3 | 15 | 46 | 4.98 |
| Cingulate gyrus | 0 | −18 | 32 | 4.54 |
| Superior frontal gyrus | −13 | 12 | 66 | 4.43 |
| Putamen | −26 | −1 | −1 | 3.92 |
| Medulla | −2 | −43 | −53 | 5.33 |
| ATP > Saline (n = 56) | ||||
| Precentral gyrus | −36 | −16 | 39 | 6.57 |
| Supplementary motor cortex | 7 | 4 | 49 | 5.79 |
| Insular cortex | 35 | 8 | 10 | 5.33 |
| Middle temporal gyrus | −55 | −36 | −10 | 4.56 |
| Cerebellum | 35 | −51 | −50 | 4.30 |
| Medulla | −1 | −43 | −55 | 4.99 |
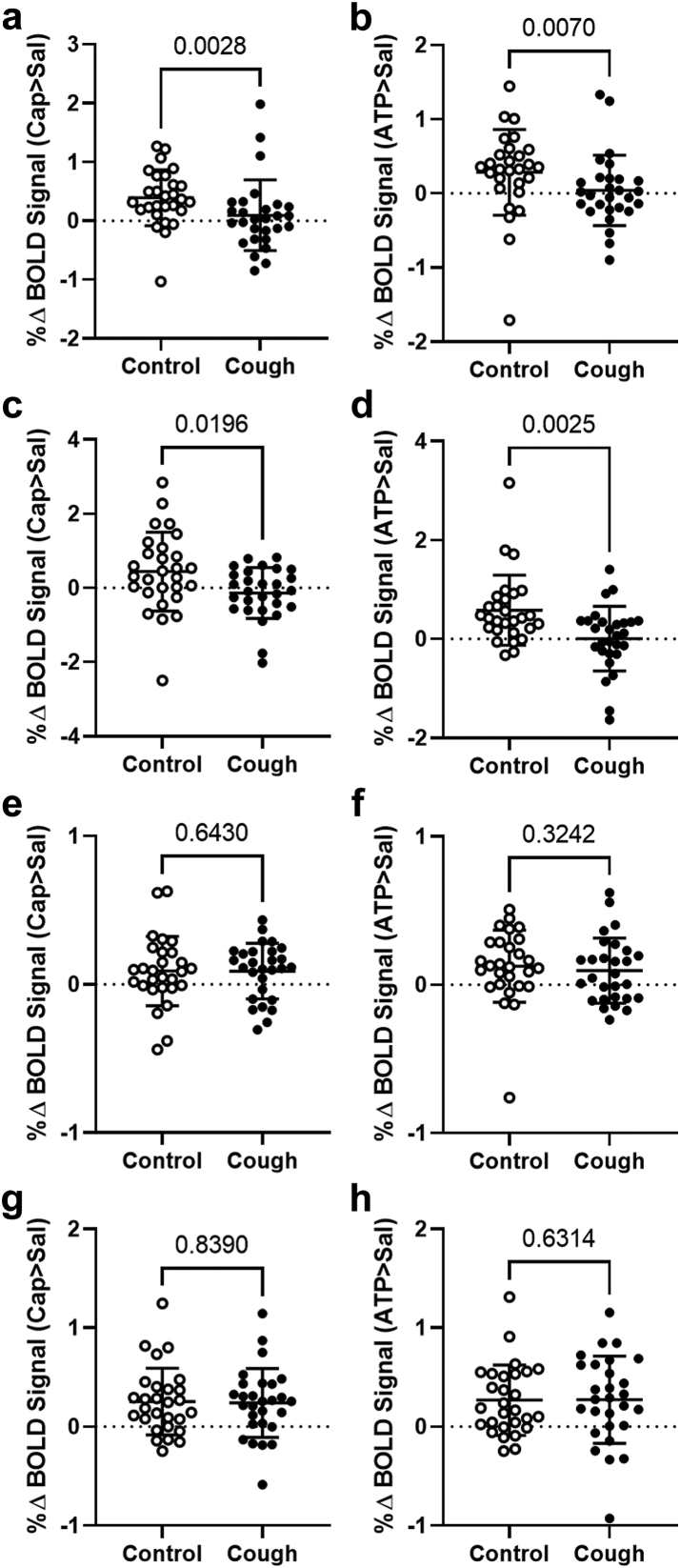
Control participants (n = 28) displayed comparable Capsaicin > Saline and ATP > Saline BOLD activation profiles in the brainstem and cortex as those observed for the whole group (n = 56), with BOLD signal magnitudes (Fig. 5) comparable to our prior studies (e.g.,12,20) and other published studies of noxious stimulus evoked BOLD responses.38,39 Surprisingly, however, participants with chronic cough failed to yield any medullary brainstem activations at a cluster corrected level for either Capsaicin > Saline or ATP > Saline contrasts, despite the cough and control cohorts demonstrating comparable cluster-corrected BOLD signal responses to both agents in cortical ROIs. Consequently, none of the medullary or cortical ROIs demonstrated Cough participant > Control participant responses for either capsaicin or ATP. The percentage BOLD signal change (Fig. 5a and b) measured from the nucleus of the solitary tract was significantly lower in participants with chronic cough compared to controls for both Capsaicin > Saline (U(54) = 212, P = 0.003) and ATP > Saline (U(54) = 229, P = 0.007; Mann–Whitney U test). Similarly, percentage BOLD signal change measured from the paratrigeminal nucleus (Fig. 5c and d) was also significantly lower in participants with chronic cough compared to controls for both Capsaicin > Saline (U(54) = 250, P = 0.001) and ATP > Saline (U(54) = 210, P = 0.003; Mann–Whitney U test). By contrast, percentage signal changes measured from both the anterior cingulate cortex and primary sensory cortex (Fig. 5e–h) were comparable for the two stimuli in both participant groups (P values ranged from P = 0.324 to P = 0.839; Mann–Whitney U test). In the midbrain, a region previously shown to demonstrate increased BOLD signal changes during capsaicin inhalation in participants with chronic cough compared to controls,20 Cough participant > Control participant contrasts revealed a cluster of activated voxels for Capsaicin > Saline in and adjacent to the periaqueductal grey (Fig. 6a). Percentage BOLD signal changes (Fig. 6b and c) extracted from this ROI were significantly higher in participants with chronic cough compared to controls for both Capsaicin > Saline (U(54) = 195, P = 0.001) and ATP > Saline (U(54) = 262, P = 0.033; Mann–Whitney U test).
Fig. 5.
Medullary and cortical BOLD signal changes during inhalation of tussive substances in control participants and participants with chronic cough. Percentage Blood Oxygen Level Dependent (BOLD) signal changes for Capsaicin > Saline (left column graphs, a, c, e, g) and ATP > Saline (right column graphs, b, d, f, h) extracted from regions of interest in the brainstem and cortex of control participants and participants with chronic cough. (a, b) Nucleus of the solitary tract; (c, d) Paratrigeminal nucleus; (e, f) Anterior cingulate cortex; (g, h) Primary sensory cortex. Data points represent individual participants (horizontal line denotes mean and error bars are standard deviation) of n = 28 per group. P values calculated using Mann–Whitney U tests. Abbreviations: ATP, adenosine triphosphate; CAP, capsaicin; SAL, saline.
Fig. 6.
Midbrain BOLD signal changes during inhalation of tussive substances in control participants and participants with chronic cough. (a) Activation map (red) for the contrast Cough Participant > Control Participant, Capsaicin > Saline. Percentage Blood Oxygen Level Dependent (BOLD) signal changes extracted from the activated region (arrow) show that participants with chronic cough have significantly higher signal levels compared to controls for (b) Capsaicin > Saline and (c) ATP > Saline contrasts. Data points represent individual participants (horizontal line denotes mean and error bars are standard deviation) of n = 28 per group. P values calculated using Mann–Whitney U tests. Abbreviations: ATP, adenosine triphosphate; CAP, capsaicin; SAL, saline.
Discussion
Participants with chronic cough in the present study displayed hypersensitivity to inhaled cough challenge stimuli. Thus, lower stimulus levels were needed to evoke equivalent cough behaviours in participants with chronic cough compared to control participants. However, this hypersensitivity was not represented at the level of the medullary brainstem in regions that receive incoming primary sensory inputs from the airways. Instead, BOLD signal responses in the nucleus of the solitary tract and paratrigeminal nucleus were significantly lower in participants with chronic cough compared to controls, commensurate with the lower level tussive stimuli needed to evoke equivalent behavioural responses. By contrast, despite the lower activation levels in the medulla, BOLD signal responses in higher brain cortical regions that have been shown to encode urge-to-cough sensations19,32 were not different between chronic cough and control participants, while a region in the midbrain displayed significantly greater activation in the participants with chronic cough compared to controls. These data are therefore at odds with the prevailing hypothesis of heightened responsivity of peripheral sensory neural pathways as a mechanism underlying cough hypersensitivity. Rather, hypersensitivity in cough neural pathways may involve processes that amplify incoming cough signals at more rostral levels in the central nervous system.
Cough-evoking vagal sensory neurons arise from both the nodose and jugular ganglia40 and, via their medullary termination sites, provide input to cough reflex circuits in the brainstem and urge-to-cough sensation circuits in the higher brain.12,16 Some cough-evoking sensory nerve fibres are sensitive to chemical stimuli, including a range of noxious compounds entering the respiratory passages via inspired air or aspiration and/or produced during airway inflammation.2 Preclinical studies in animals, have shown that capsaicin-sensitive airway sensory neurons exist in both the nodose and jugular vagal ganglia, the former terminating in the nucleus of the solitary tract and the latter in the paratrigeminal nucleus.10,16,40, 41, 42 With respect to ATP, complexity exists in the likely mechanism of action due to differential expression of purinergic receptor subtypes on subsets of sensory nerve fibres regulating cough. For example, in animal studies most nodose neurons respond robustly to ATP due to the large ionic currents carried by heteromeric P2X2/3 channels, whereas jugular neuron responses to ATP are modest as they lack P2X2/3, expressing only homomeric P2X3 channels which carry small desensitising currents when exposed to ATP.9,10 Consistent with this, ATP evoked cough in healthy animals is preserved when jugular ganglia pathways are disrupted through selective lesions of their recipient neurons in the brainstem.16
Clinical trial data supports an important role for ATP in chronic cough,2,13, 14, 15 although the nature of this role remains unclear. The preclinical data above would suggest that purinergic receptor antagonists reduce cough by targeting an action of ATP on nodose cough-evoking sensory neurons as jugular neurons are not readily activated by ATP. In this regard, ATP may sensitise or activate nodose neurons, leading to cough hypersensitivity and excessive coughing.2,11 However, these preclinical studies have been conducted in the absence of any pathology associated with chronic cough and it is conceivable that plasticity in purinergic receptor expression or function may alter the pattern of neural responsiveness to ATP in the disease settings.18 Indeed, individuals with chronic cough typically present with cough hypersensitivity, a syndrome characterised by excessive cough triggered by low levels of mechanical, chemical or thermal stimuli and consistent with plasticity in sensory neural function.2,3 However, we failed to see evidence for a change in medullary brainstem activation patterns that would be suggestive of a shift in the profile of sensory neuron responsivity to ATP in participants with chronic cough.
Despite the attractiveness of ATP as a key mediator causing cough hypersensitivity, the available evidence is inconsistent with this notion. For example, although the purinergic receptor antagonist Gefapixant inhibits ATP-evoked cough in individuals with RCC and UCC, it has no effect on heightened cough responses evoked by inhalation of either capsaicin or citric acid in these same people,13 arguing that ATP is not subserving the role of a generic sensitiser of cough sensory nerve fibres. This is consistent with the clinical trial data showing that P2X2/3 and P2X3 antagonists are not universally effective at reducing cough.2 Furthermore, the present data raises important questions about the role of peripheral nerve sensitisation in cough hypersensitivity and chronic cough. Our participants with chronic cough showed cough behavioural hypersensitivity to inhaled challenges for both capsaicin and ATP, resulting in significantly lower concentrations of stimuli needed to achieve comparable urge-to-cough ratings during fMRI scanning. However, BOLD signal responses in the medulla were also significantly lower in participants with chronic cough, seemingly representative of the lower stimulus concentration delivered and not the equivalent behavioural response experienced. One interpretation is that airway sensory nerve fibres activated by capsaicin and ATP are not hypersensitive to stimuli in the participants with chronic cough involved in our study, and instead stimuli like ATP may simply serve as activators of sensory neurons in some individuals. Heightened sensitivity or responsivity to these chemical stimuli may in fact manifest through processes elsewhere in the nervous system, such that even low levels of peripheral sensory inputs can be amplified or used more effectively to evoke coughing. This is consistent with existing data showing that individuals with chronic cough have a heightened Arnold nerve cough reflex, evoked by mechanical stimulation of the external auditory meatus, a site where putative airway-derived mediators of peripheral hypersensitivity are unlikely to reach and central amplification mechanisms seem more plausible.43,44 The findings are also consistent with clinical intervention studies demonstrating efficacy of cough behavioural therapy for treating RCC and UCC.45,46
The magnitude of BOLD signal responses in the primary sensory and cingulate cortical locations identified in the present study have previously been shown to correlate closely with urge-to-cough ratings during capsaicin inhalation,19,32 suggesting that they comprise part of a cortical network for encoding urge-to-cough intensity. As such, the comparable levels of neural activation in these regions of interest between control participants and participants with chronic cough, during comparable stimulus-evoked urge-to-cough experiences, are consistent with signal amplification occurring in the neural pathways at a level between the primary medullary brainstem integration sites and the higher brain. We have previously shown that regions of the midbrain encompassing the PAG and cuneiform nucleus show heightened responses in participants with chronic cough,20 and others have identified these midbrain regions as a putative site for pain sensitisation.47 In the present study we again noted that significantly more stimulus-evoked activation in the midbrain of participants with chronic cough compared to controls, in a region comparable to that in our previous study, perhaps providing further support for the midbrain acting as the cough sensory input amplifier.
Several considerations for the interpretation of our findings need to be explored. The neural processes involved in central amplification of sensory inputs in chronic cough are not known and we cannot rule out alternative processes including alterations in the sensitivity or activity of cough motor pathways or motor control systems. In this regard, a diminution of cough suppressive neural circuit activation in chronic cough has been reported.20,48, 49, 50 In the present study we noted that participants with chronic cough and controls displayed different relationships between their level of urge-to-cough experienced at their thresholds for eliciting cough responses. Thus, cough thresholds were reached in participants with chronic cough at significantly lower urge-to-cough ratings, indicative of a change in the efficacy of sensory-motor coupling in chronic cough. It is difficult to attribute this result solely to central amplification of incoming sensory signals, as one would expect both urge-to-cough and cough to shift in parallel with increasing sensitivity. Instead, a loss of central inhibition may reduce the sensory input needed to trigger coughing. It is also possible that complex changes occur in how individuals with chronic cough appraise their airway irritation. Although, it is interesting that we did not observe any significant differences in the qualitative description or location of the perceptible sensations associated with inhalation of capsaicin or ATP in control participants and participants with chronic cough, arguing that sensitisation in chronic cough is not accompanied by overt changes in these aspects of stimulus perception. Interrogation of the individual participant BOLD signal responses suggests a level of heterogeneity in regional responsivity, the cause of which is difficult to ascertain. One possibility is that anatomical organisation of functional responses in these small brainstem structures are not uniform between participants. Alternatively, different mechanisms (endotypes) leading to cough sensitisation may exist.2,51 Indeed, our findings may have been different if we investigated patients with cough and an existing lung disease (e.g., asthma) where pulmonary inflammation, and hence peripheral sensitisation, is expected. Lastly, it is important to recognise that brainstem BOLD signals may be adversely impacted by alternative sources of physiological noise which could be differentially expressed by control participants and participants with chronic cough. One of our incorporated measured estimates of baseline noise in the fMRI data was higher in cough participants, but importantly there was no systematic differential expression of increased noise associated with tussive stimulus blocks between the two groups (see Supplementary Material), suggesting that this is not a major confound in the BOLD activations reported.
In summary, the pattern of sensory neuron sensitivity to inhaled ATP does not appear to be a defining mechanism of cough hypersensitivity, at least in our participants with suspected RCC and UCC. Furthermore, our data challenges the notion that airway sensory neurons are hypersensitive in chronic cough. Instead, possible central amplifier mechanisms and losses in endogenous suppressive control systems may render the cough neural induction system in the brain more responsive to low levels of peripheral airway sensory neuron input. This finding adds to a growing body of evidence of both structural and functional plasticity in central neural networks as mechanisms underpinning cough hypersensitivity.20,52, 53, 54 Nevertheless, we cannot fully rule out the possibility that the chemically sensitive cough pathways activated by inhaled capsaicin and ATP in the present study are not the sensory pathways involved in chronic cough, even though capsaicin and ATP evoked cough and associated behaviours were hypersensitive. Furthermore, it is important to recognise that the data do not discount the therapeutic benefit of targeting peripheral sensory neuron activity in chronic cough,2 including the use of P2X3 receptor antagonists for individuals in which ATP plays a role in sensory neuron activation. However, the data do highlight the potential value of also considering centrally targeted approaches aimed at restoring normal cough circuit responsivity.
Contributors
AAKM and NS conducted the experiments and analysed the data; KC assisted with subject recruitment; MD conducted data analysis; LM, KFC, MM and ALR provided clinical input and assisted with subject recruitment and characterization; AEM, MF and SBM conceived and designed experiments. SBM, AAKM and MJF wrote the manuscript. All authors have access to the data and have contributed to its interpretation and editing of the manuscript. SBM and MJF have directly accessed and verified the underlying data reported in the manuscript. All authors have approved the final version of the manuscript and agree to be accountable for all aspects of the work. All persons designated as authors qualify for authorship and all those that qualify have been listed as authors.
Data sharing statement
All data supporting the results presented in the manuscript are included in the manuscript figures as mean ± SD of n = 28–29 per cohort. The de-identified data that support the findings of this study are available on request from the corresponding author.
Declaration of interests
SBM reports receiving grants from the National Health and Medical Research Council (NHMRC) of Australia and the Australian Research Council (ARC), Merck, Bellus Health and Reckitt Benkiser, and remuneration for consultancy from Merck, Trevi Therapeutics, Reckitt Benkiser and Nerre Therapeutics, has served on advisory committees for Reckitt Benkiser and has received payment from Reckitt Benkiser for assistance with manuscript writing. KFC reports research grants from Merck and GSK, remuneration for lectures from Novartis and AstraZeneca; has served on advisory boards for Roche, Merck, Reckitt Benckiser, and Shionogi & Co., Ltd., and a Data Safety Monitoring Board for Nocion. LMG reports research grants from Bayer AG, Bellus Health, Chiesi, Merck, and Shionogi, remuneration for lectures from Bayer AG, Bellus Health, Chiesi, GlaxoSmithKline, Merck, and Shionogi, remuneration for consultancy from Bayer AG, Bellus Health, Chiesi, Merck, NeRRe Therapeutics, Nocion Therapeutics, and Shionogi, and has served on advisory committees for Applied Clinical Intelligence, Bayer AG, Bellus Health, Chiesi, Merck, NeRRe Therapeutics, Nocion Therapeutics, and Shionogi and on a Data and Safety Monitoring Board for Bayer AG. All other authors declare no relevant conflict of interest.
Acknowledgements
Supported by a research grant from Investigator-Initiated Studies Program of Merck Sharp & Dohme (Australia) Pty Ltd, paid to institution.
Footnotes
Supplementary data related to this article can be found at https://doi.org/10.1016/j.ebiom.2024.104976.
Appendix A. Supplementary data
References
- 1.Song W.J., Chang Y.S., Faruqi S., et al. The global epidemiology of chronic cough in adults: a systematic review and meta-analysis. Eur Respir J. 2015;45(5):1479–1481. doi: 10.1183/09031936.00218714. [DOI] [PubMed] [Google Scholar]
- 2.Chung K.F., McGarvey L., Song W.J., et al. Cough hypersensitivity and chronic cough. Nat Rev Dis Primers. 2022;8(1):45. doi: 10.1038/s41572-022-00370-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Morice A.H., Faruqi S., Wright C.E., Thompson R., Bland J.M. Cough hypersensitivity syndrome: a distinct clinical entity. Lung. 2011;189(1):73–79. doi: 10.1007/s00408-010-9272-1. [DOI] [PubMed] [Google Scholar]
- 4.Kum E., Guyatt G.H., Devji T., et al. Cough symptom severity in patients with refractory or unexplained chronic cough: a systematic survey and conceptual framework. Eur Respir Rev. 2021;30(161) doi: 10.1183/16000617.0104-2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kum E., Guyatt G.H., Munoz C., et al. Assessing cough symptom severity in refractory or unexplained chronic cough: findings from patient focus groups and an international expert panel. ERJ Open Res. 2022;8(1) doi: 10.1183/23120541.00667-2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Morice A.H., Birring S.S., Smith J.A., et al. Characterization of patients with refractory or unexplained chronic cough participating in a phase 2 clinical trial of the P2X3-receptor antagonist gefapixant. Lung. 2021;199(2):121–129. doi: 10.1007/s00408-021-00437-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mazzone S.B., McGarvey L. Mechanisms and rationale for targeted therapies in refractory and unexplained chronic cough. Clin Pharmacol Ther. 2021;109(3):619–636. doi: 10.1002/cpt.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bonvini S.J., Birrell M.A., Grace M.S., et al. Transient receptor potential cation channel, subfamily V, member 4 and airway sensory afferent activation: role of adenosine triphosphate. J Allergy Clin Immunol. 2016;138(1):249–261.e12. doi: 10.1016/j.jaci.2015.10.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kwong K., Kollarik M., Nassenstein C., Ru F., Undem B.J. P2X2 receptors differentiate placodal vs. neural crest C-fiber phenotypes innervating guinea pig lungs and esophagus. Am J Physiol Lung Cell Mol Physiol. 2008;295(5):L858–L865. doi: 10.1152/ajplung.90360.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Nassenstein C., Taylor-Clark T.E., Myers A.C., et al. Phenotypic distinctions between neural crest and placodal derived vagal C-fibres in mouse lungs. J Physiol. 2010;588(Pt 23):4769–4783. doi: 10.1113/jphysiol.2010.195339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Garceau D., Chauret N. BLU-5937: a selective P2X3 antagonist with potent anti-tussive effect and no taste alteration. Pulm Pharmacol Ther. 2019;56:56–62. doi: 10.1016/j.pupt.2019.03.007. [DOI] [PubMed] [Google Scholar]
- 12.Farrell M.J., Bautista T.G., Liang E., Azzollini D., Egan G.F., Mazzone S.B. Evidence for multiple bulbar and higher brain circuits processing sensory inputs from the respiratory system in humans. J Physiol. 2020;598(24):5771–5787. doi: 10.1113/JP280220. [DOI] [PubMed] [Google Scholar]
- 13.Morice A.H., Kitt M.M., Ford A.P., et al. The effect of gefapixant, a P2X3 antagonist, on cough reflex sensitivity: a randomised placebo-controlled study. Eur Respir J. 2019;54(1) doi: 10.1183/13993003.00439-2019. [DOI] [PubMed] [Google Scholar]
- 14.Chuang M.H., Chen I.W., Chen J.Y., et al. Efficacy and safety of gefapixant for chronic cough: a meta-analysis of randomised controlled trials. Eur Respir Rev. 2023;32(168) doi: 10.1183/16000617.0219-2022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Dicpinigaitis P.V., Morice A.H., Smith J.A., et al. Efficacy and safety of eliapixant in refractory chronic cough: the randomized, placebo-controlled phase 2b PAGANINI study. Lung. 2023;201(3):255–266. doi: 10.1007/s00408-023-00621-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Driessen A.K., McGovern A.E., Behrens R., Moe A.A.K., Farrell M.J., Mazzone S.B. A role for neurokinin 1 receptor expressing neurons in the paratrigeminal nucleus in bradykinin-evoked cough in Guinea-pigs. J Physiol. 2020;598(11):2257–2275. doi: 10.1113/JP279644. [DOI] [PubMed] [Google Scholar]
- 17.Morice A.H., Jakes A.D., Faruqi S., et al. A worldwide survey of chronic cough: a manifestation of enhanced somatosensory response. Eur Respir J. 2014;44(5):1149–1155. doi: 10.1183/09031936.00217813. [DOI] [PubMed] [Google Scholar]
- 18.Fowles H.E., Rowland T., Wright C., Morice A. Tussive challenge with ATP and AMP: does it reveal cough hypersensitivity? Eur Respir J. 2017;49(2) doi: 10.1183/13993003.01452-2016. [DOI] [PubMed] [Google Scholar]
- 19.Mazzone S.B., Cole L.J., Ando A., Egan G.F., Farrell M.J. Investigation of the neural control of cough and cough suppression in humans using functional brain imaging. J Neurosci. 2011;31(8):2948–2958. doi: 10.1523/JNEUROSCI.4597-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ando A., Smallwood D., McMahon M., Irving L., Mazzone S.B., Farrell M.J. Neural correlates of cough hypersensitivity in humans: evidence for central sensitisation and dysfunctional inhibitory control. Thorax. 2016;71(4):323–329. doi: 10.1136/thoraxjnl-2015-207425. [DOI] [PubMed] [Google Scholar]
- 21.Birring S.S., Prudon B., Carr A.J., Singh S.J., Morgan M.D., Pavord I.D. Development of a symptom specific health status measure for patients with chronic cough: Leicester Cough Questionnaire (LCQ) Thorax. 2003;58(4):339–343. doi: 10.1136/thorax.58.4.339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Vertigan A.E., Bone S.L., Gibson P.G. Development and validation of the Newcastle laryngeal hypersensitivity questionnaire. Cough. 2014;10(1):1. doi: 10.1186/1745-9974-10-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Morice A.H., Fontana G.A., Belvisi M.G., et al. ERS guidelines on the assessment of cough. Eur Respir J. 2007;29(6):1256–1276. doi: 10.1183/09031936.00101006. [DOI] [PubMed] [Google Scholar]
- 24.Bautista T.G., Leech J., Mazzone S.B., Farrell M.J. Regional brain stem activations during capsaicin inhalation using functional magnetic resonance imaging in humans. J Neurophysiol. 2019;121(4):1171–1182. doi: 10.1152/jn.00547.2018. [DOI] [PubMed] [Google Scholar]
- 25.Smith S.M. Fast robust automated brain extraction. Hum Brain Mapp. 2002;17(3):143–155. doi: 10.1002/hbm.10062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Jenkinson M., Bannister P., Brady M., Smith S. Improved optimization for the robust and accurate linear registration and motion correction of brain images. Neuroimage. 2002;17(2):825–841. doi: 10.1016/s1053-8119(02)91132-8. [DOI] [PubMed] [Google Scholar]
- 27.Jenkinson M. Fast, automated, N-dimensional phase-unwrapping algorithm. Magn Reson Med. 2003;49(1):193–197. doi: 10.1002/mrm.10354. [DOI] [PubMed] [Google Scholar]
- 28.Woolrich M.W., Ripley B.D., Brady M., Smith S.M. Temporal autocorrelation in univariate linear modeling of FMRI data. Neuroimage. 2001;14(6):1370–1386. doi: 10.1006/nimg.2001.0931. [DOI] [PubMed] [Google Scholar]
- 29.Birn R.M., Diamond J.B., Smith M.A., Bandettini P.A. Separating respiratory-variation-related fluctuations from neuronal-activity-related fluctuations in fMRI. Neuroimage. 2006;31(4):1536–1548. doi: 10.1016/j.neuroimage.2006.02.048. [DOI] [PubMed] [Google Scholar]
- 30.Birn R.M., Smith M.A., Jones T.B., Bandettini P.A. The respiration response function: the temporal dynamics of fMRI signal fluctuations related to changes in respiration. Neuroimage. 2008;40(2):644–654. doi: 10.1016/j.neuroimage.2007.11.059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Birn R.M., Murphy K., Handwerker D.A., Bandettini P.A. fMRI in the presence of task-correlated breathing variations. Neuroimage. 2009;47(3):1092–1104. doi: 10.1016/j.neuroimage.2009.05.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Farrell M.J., Cole L.J., Chiapoco D., Egan G.F., Mazzone S.B. Neural correlates coding stimulus level and perception of capsaicin-evoked urge-to-cough in humans. Neuroimage. 2012;61(4):1324–1335. doi: 10.1016/j.neuroimage.2012.03.030. [DOI] [PubMed] [Google Scholar]
- 33.Andersson J.L. How to estimate global activity independent of changes in local activity. Neuroimage. 1997;6(4):237–244. doi: 10.1006/nimg.1997.0302. [DOI] [PubMed] [Google Scholar]
- 34.Worsley K.J., Evans A.C., Marrett S., Neelin P. A three-dimensional statistical analysis for CBF activation studies in human brain. J Cereb Blood Flow Metab. 1992;12(6):900–918. doi: 10.1038/jcbfm.1992.127. [DOI] [PubMed] [Google Scholar]
- 35.Paxinos G., Xu-Feng H., Sengul G., Watson C. In: The human nervous system. 3rd ed. Mai JKP G., editor. Academic Press; San Diego: 2012. Chapter 8 - Organization of brainstem nuclei. [Google Scholar]
- 36.Mumford J.A., Nichols T.E. Power calculation for group fMRI studies accounting for arbitrary design and temporal autocorrelation. Neuroimage. 2008;39(1):261–268. doi: 10.1016/j.neuroimage.2007.07.061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Wong C.H., Morice A.H. Cough threshold in patients with chronic obstructive pulmonary disease. Thorax. 1999;54(1):62–64. doi: 10.1136/thx.54.1.62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Fairhurst M., Fairhurst K., Berna C., Tracey I. An fMRI study exploring the overlap and differences between neural representations of physical and recalled pain. PLoS One. 2012;7(10) doi: 10.1371/journal.pone.0048711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Wager T.D., Rilling J.K., Smith E.E., et al. Placebo-induced changes in FMRI in the anticipation and experience of pain. Science. 2004;303(5661):1162–1167. doi: 10.1126/science.1093065. [DOI] [PubMed] [Google Scholar]
- 40.Mazzone S.B., Undem B.J. Vagal afferent innervation of the airways in health and disease. Physiol Rev. 2016;96(3):975–1024. doi: 10.1152/physrev.00039.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Driessen A.K., Farrell M.J., Dutschmann M., Stanic D., McGovern A.E., Mazzone S.B. Reflex regulation of breathing by the paratrigeminal nucleus via multiple bulbar circuits. Brain Struct Funct. 2018;223(9):4005–4022. doi: 10.1007/s00429-018-1732-z. [DOI] [PubMed] [Google Scholar]
- 42.Kollarik M., Ru F., Undem B.J. Phenotypic distinctions between the nodose and jugular TRPV1-positive vagal sensory neurons in the cynomolgus monkey. Neuroreport. 2019;30(8):533–537. doi: 10.1097/WNR.0000000000001231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Dicpinigaitis P.V., Kantar A., Enilari O., Paravati F. Prevalence of Arnold nerve reflex in adults and children with chronic cough. Chest. 2018;153(3):675–679. doi: 10.1016/j.chest.2017.11.019. [DOI] [PubMed] [Google Scholar]
- 44.Mai Y., Zhan C., Zhang S., et al. Arnold nerve reflex: vagal hypersensitivity in chronic cough with various causes. Chest. 2020;158(1):264–271. doi: 10.1016/j.chest.2019.11.041. [DOI] [PubMed] [Google Scholar]
- 45.Slovarp L.J., Jetté M.E., Gillespie A.I., Reynolds J.E., Barkmeier-Kraemer J.M. Evaluation and management outcomes and burdens in patients with refractory chronic cough referred for behavioral cough suppression therapy. Lung. 2021;199(3):263–271. doi: 10.1007/s00408-021-00442-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Vertigan A.E., Haines J., Slovarp L. An update on speech pathology management of chronic refractory cough. J Allergy Clin Immunol Pract. 2019;7(6):1756–1761. doi: 10.1016/j.jaip.2019.03.030. [DOI] [PubMed] [Google Scholar]
- 47.Zambreanu L., Wise R.G., Brooks J.C.W., Iannetti G.D., Tracey I. A role for the brainstem in central sensitisation in humans. Evidence from functional magnetic resonance imaging. Pain. 2005;114(3):397–407. doi: 10.1016/j.pain.2005.01.005. [DOI] [PubMed] [Google Scholar]
- 48.Cho P.S.P., Fletcher H.V., Patel I.S., Turner R.D., Jolley C.J., Birring S.S. Cough hypersensitivity and suppression in COPD. Eur Respir J. 2021;57(5) doi: 10.1183/13993003.03569-2020. [DOI] [PubMed] [Google Scholar]
- 49.Cho P.S.P., Fletcher H.V., Turner R.D., Jolley C.J., Birring S.S. Impaired cough suppression in chronic refractory cough. Eur Respir J. 2019;53(5) doi: 10.1183/13993003.02203-2018. [DOI] [PubMed] [Google Scholar]
- 50.Hilton E., Satia I., Holt K., Woodcock A.A., Belcher J., Smith J.A. The effect of pain conditioning on experimentally evoked cough: evidence of impaired endogenous inhibitory control mechanisms in refractory chronic cough. Eur Respir J. 2020 doi: 10.1183/13993003.01387-2020. [DOI] [PubMed] [Google Scholar]
- 51.Mazzone S.B., Chung K.F., McGarvey L. The heterogeneity of chronic cough: a case for endotypes of cough hypersensitivity. Lancet Respir Med. 2018;6(8):636–646. doi: 10.1016/S2213-2600(18)30150-4. [DOI] [PubMed] [Google Scholar]
- 52.Ando A., Mazzone S.B., Farrell M.J. Altered neural activity in brain cough suppression networks in cigarette smokers. Eur Respir J. 2019;54(3) doi: 10.1183/13993003.00362-2019. [DOI] [PubMed] [Google Scholar]
- 53.Arinze J.T., Vinke E.J., Verhamme K.M.C., et al. Chronic cough-related differences in brain morphometry in adults: a population-based study. Chest. 2023;164(1):169–178. doi: 10.1016/j.chest.2023.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Namgung E., Song W.J., Kim Y.H., An J., Cho Y.S., Kang D.W. Structural and functional correlates of higher cortical brain regions in chronic refractory cough. Chest. 2022;162(4):851–860. doi: 10.1016/j.chest.2022.04.141. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.