Dear Editor,
1.
Basal cell carcinoma (BCC) is the most common type of skin cancer, accounting for more than 80% of nonmelanoma skin cancer. 1 Although BCC has a low metastatic potential, it grows locally invasive and may cause significant destruction to crucial anatomical body parts, especially of the face. 2 Complete excision of BCC with adequate margins is essential for optimal treatment outcomes, minimizing recurrence rates and ensuring desired cosmetic outcomes. 3 However, traditional surgical techniques without imaging guidance may require additional excision steps, as clinically visible tumor margins might be significantly smaller than the “true” histopathological tumor expansion, resulting in an increased burden of healthcare costs and additional strain for the patient due to higher perioperative morbidity (Figure 1).
FIGURE 1.
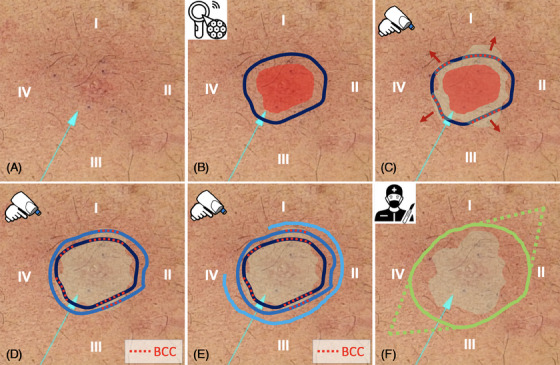
Step‐by‐step procedure showing the adjustment of tumor margins using LC‐OCT imaging prior to surgery. The basal cell carcinoma (BCC) lesion was located on the chest and divided into four quarters labeled I–IV.
The most recent development of line‐field confocal optical coherence tomography (LC‐OCT) allows noninvasive skin imaging at a high cellular resolution (1–2 μm) 4 not only for the in‐vivo diagnosis of skin cancer, but also for identifying tumor extension, subtype, and tissue penetration 5 , 6 , 7 , 8 .
Therefore, the aim of this proof‐of‐concept study was to evaluate the feasibility of presurgical BCC margin mapping using the deepLive LC‐OCT device (deepLive, DAMAE Medical, Paris, France) and establish a standard operating procedure for practical application (Figure 2).
FIGURE 2.
AI‐assistance while establishing tumor margins. Green lines indicate low probability (<30%), orange lines show equivocal lesions (30%–50%), and red lines depict high probability (>50%) for the presence of basal cell carcinoma (BCC) identified as hypo‐reflective nodules in corresponding vertical line‐field confocal optical coherence tomography (LC‐OCT) images in (C).
Patients with histologically diagnosed BCC were recruited at the Department of Dermatology and Allergy of the university hospital at Ludwig Maximilian University (LMU) in Munich and the university hospital in Augsburg and received presurgical margin mapping using LC‐OCT technology before undergoing surgery. Each patient gave written informed consent before inclusion, which was approved by the local ethics committee of the LMU university hospital (Ref.‐Nr. 17–699 and 18–0532).
Exemplary BCC cases demonstrate a step‐by‐step tutorial of the suggested presurgical application pathway, providing technical tricks and helpful hints for implementing this technique.
After clinical and dermoscopic inspection (Figure 1A), the macroscopic tumor margin was marked on the skin (Figure 1B, red area). According to the German guideline 9 , 10 an additional 3 mm safety margin, which normally serves as the excision line, was added and marked (Figure 1B, blue line) using surgical pens.
Presurgical imaging and margin mapping were performed using the LC‐OCT device in vertical “en‐coupe” mode (comparable to histology). The device allows real‐time processing and displays the LC‐OCT image together with the corresponding dermoscopic live surface image on a computer screen, providing high‐resolution images of the skin.
Imaging was performed in a standardized manner by placing the handheld probe on the skin surface on the BCC lesion and its surrounding areas. To visualize the tumor margins, the presurgical markings were found according to the integrated dermoscopic live surface camera and followed around the lesion in a circular manner using the vertical video mode and imaging one‐quarter of the circle at a time. A drop of paraffin oil was used before scanning to adjust the refractive indices of the probe optics, air, and skin (Figure 1C).
Beyond standard visual interpretation of the generated images, an integrated AI add‐on employs a color map to indicate the probability that the scanned area contains BCC (Figure 2A–C).
Each LC‐OCT scan was manually reviewed quarter‐wise by an experienced LC‐OCT assessor to check and validate the AI‐data and color map.
Subsequently, the margins were broadened and adjusted stepwise until they comprised the entire lesion and all margins were visually tumor‐free. Certain quarters needed more extension than others, due to residual BCC identified with LC‐OCT. Interestingly, these areas looked clinically unaffected (Figure 1D,E).
The final marked tumor expansion was then used to guide the excision, allowing complete tumor removal with narrow margins (Figure 1F). The standardized excision procedure was performed under local anesthesia.
Subsequent gold‐standard histology confirmed that the BCC lesions were removed entirely in all imaged cases by a single step of minimal tissue removal and avoidance of re‐excision.
Clinical and LC‐OCT imaging follow‐up data was assessed at 3 and 6 months after the procedure to monitor tumor recurrence and to evaluate cosmetic outcomes.
Using the newest dermoscopic application, a dermoscopic mosaic comprised of multiple overlapping, merged dermoscopic images helps navigate the probe to the right localization (Figure 3C,D).
FIGURE 3.
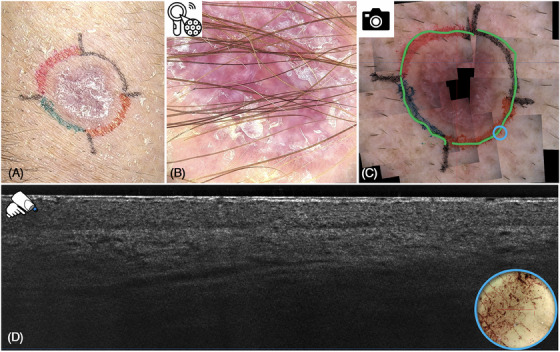
Sclerosing basal cell carcinoma (BCC) in clinical (A) and 30× optical magnification dermoscopic view (B), as well as supplementary dermoscopic mosaic (C). The blue circle indicates the localization of the line‐field confocal optical coherence tomography (LC‐OCT) probe visualizing the corresponding vertical LC‐OCT tumor‐free image (D).
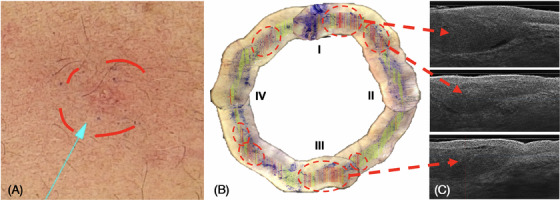
Limitations: Depending on the tumor localization (e.g., nostrils and triangular fossa of the outer ear, see Figure 4A–D, LC‐OCT imaging was difficult to assess. Because of artifacts due to crusting and inhomogeneous contact of the handheld probe with skin surface the entire presurgical margin mapping was not feasible. In these cases, we performed partial margin mapping as far as practicable and applied conventional dermoscopic margins in unreachable sites.
FIGURE 4.
Anatomical areas where it can be challenging to place the handheld probe in full contact with the skin surface, for example, nose/nostrils (A), outer ear triangular fossa (C), and dermoscopic view (B,D).
In addition, the depth of reliable tumor detection is technically limited to about 500–800 μm bearing the risk of missing deeper tumor parts in the lower dermis.
This proof‐of‐concept study is a first introduction for the use of LC‐OCT for presurgical BCC margin assessment and proposes an optimized user‐friendly imaging manual, striving for complete and accurate excision of BCC with narrow margins in a single setting. Further studies are needed to quantitatively validate the efficacy and safety of this technique in larger patient cohorts, including patients with different subtypes of BCC as well as different body areas that may impact the accuracy of LC‐OCT imaging. A multi‐center prospective, histologically controlled study on sensitivity, specificity, and positive/negative predicted value of LC‐OCT margin mapping is currently in progress.
ACKNOWLEDGMENTS
Open access funding enabled and organized by Projekt DEAL.
Maximilian Deußing, Quirine Louise Eijkenboom, Sandra Schuh, and Elke Christina Sattler contributed equally to this study.
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available on request from the corresponding author. The data are not publicly available due to privacy or ethical restrictions.
REFERENCES
- 1. Lomas, A , Leonardi‐Bee J, Bath‐Hextall F, A systematic review of worldwide incidence of nonmelanoma skin cancer. Br J Dermatol. 2012;166(5):1069‐1080. [DOI] [PubMed] [Google Scholar]
- 2. Cumberland, L , Dana A, Liegeois N, Mohs micrographic surgery for the management of nonmelanoma skin cancers. Facial Plast Surg Clin North Am. 2009;17(3):325‐335. [DOI] [PubMed] [Google Scholar]
- 3. Macfarlane, L , Waters A, Evans A, Affleck A, Seven years' experience of Mohs micrographic surgery in a UK centre, and development of a UK minimum dataset and audit standards. Clin Exp Dermatol. 2013;38(3):262‐269. [DOI] [PubMed] [Google Scholar]
- 4. Ruini, C , Schuh S, Sattler E, Welzel J. Line‐field confocal optical coherence tomography‐practical applications in dermatology and comparison with established imaging methods. Skin Res Technol. 2021;27(3):340‐352. [DOI] [PubMed] [Google Scholar]
- 5. Gust, C , Schuh S, Welzel J, Line‐field confocal optical coherence tomography increases the diagnostic accuracy and confidence for basal cell carcinoma in equivocal lesions: a prospective study. Cancers. 2022;14(4):1082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Ruini, C , Hartmann D, Bastian M, Non‐invasive monitoring of subclinical and clinical actinic keratosis of face and scalp under topical treatment with ingenol mebutate gel 150 mcg/g by means of reflectance confocal microscopy and optical coherence tomography: New perspectives and comparison of diagnostic techniques. J Biophotonics. 2019;12(7):e201800391. [DOI] [PubMed] [Google Scholar]
- 7. Verzì, AE , Russo A, Castellino N. Line‐field confocal optical coherence tomography of eyelid margin growths: a case series. Skin Res Technol. 2024;30(1):e13559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Cinotti, E , Bertello M, Cartocci A, Comparison of reflectance confocal microscopy and line‐field optical coherence tomography for the identification of keratinocyte skin tumours. Skin Res Technol. 2023;29(1):e13215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Lang, BM , Balermpas P, Bauer A, et al. S2k‐leitlinie basalzellkarzinom der haut—teil 2: therapie, prävention und nachsorge. J Dtsch Dermatol Ges. 2019;17(2):214‐231. [DOI] [PubMed] [Google Scholar]
- 10. Gulleth Y, Goldberg N, Silverman RP, Gastman BR, What is the best surgical margin for a Basal cell carcinoma: a meta‐analysis of the literature. Plast Reconstr Surg. 2010;126(4):1222‐1231. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data that support the findings of this study are available on request from the corresponding author. The data are not publicly available due to privacy or ethical restrictions.


