Abstract
Streptococcus pyogenes (group A Streptococcus) cell extracts (CE) have a remarkably powerful and dose-dependent inhibitory effect on antigen, superantigen, or mitogen-stimulated human peripheral blood mononuclear cell (PBMC) proliferation in vitro. Purification of the inhibitory component present in S. pyogenes type M5 (Manfredo strain) CE by anion-exchange chromatography followed by gel filtration chromatography showed that the inhibitor had an approximate native molecular mass of 100 kDa. Sodium dodecyl sulfate-polyacrylamide gel electrophoresis of purified inhibitory fractions followed by silver staining gave a single band with an approximate molecular mass of 47 kDa, indicating that the inhibitor is composed of two identical subunits. NH2-terminal sequencing of the protein revealed that it was identical to the previously characterized streptococcal acid glycoprotein (SAGP); this protein possesses between 31.5 and 39.0% amino acid identity with arginine deiminase (AD) from Mycoplasma hominis, Mycoplasma arginini, Pseudomonas putida, and Pseudomonas aeruginosa. AD enzyme activity was present in unfractionated CE prepared from a range of streptococcal strains, and partially purified inhibitory fractions of Manfredo CE also had high levels of activity. The inhibitory effect of Manfredo CE was overcome by the addition of l-arginine to proliferation assays in which human PBMC were stimulated with phytohemagglutinin. We conclude that SAGP, or its homolog, possesses AD activity and that the potent inhibition of proliferation of human T cells by streptococcal CE is due to activity of this enzyme.
Infection with group A streptococci leads commonly to acute or chronic pathogenic sequelae in humans, including pharyngitis, skin infections, toxic shock-like syndrome (29), and autoimmune diseases such as rheumatic fever (4, 5) and glomerulonephritis (37, 44). Although several group A streptococcal products have been proposed to have a role in pathogenesis, including enzymes (hyaluronidase, streptokinase, and DNase) and membrane-damaging toxins streptolysin O and streptolysin S (3, 16, 18, 27, 45), relatively little is known about human immune responses toward this extracellular bacterium. Studies have concentrated either on antibody and T-cell responses to serotypically diverse M proteins found extending from group A streptococcal cell surfaces (1, 13, 15, 39, 42) or on activation of T cells by superantigens of which group A streptococci produce a wide range, including streptococcal pyrogenic exotoxin A (SPEA) (7), SPEC (36), SPED (28), SPEF (22, 33), and SPEX (7), cytoplasmic membrane-associated protein (21, 52), and streptococcal superantigen (32, 38). However, the extent to which other streptococcal proteins may elicit human immune responses is not known.
In a recent study we screened a whole array of cellular and secreted proteins prepared from Streptococcus pyogenes for the ability to stimulate human T lymphocytes. S. pyogenes Manfredo (type M5) was used, and its effect on human peripheral blood mononuclear cell (PBMC) proliferation was determined in vitro (12). Proteins from bacterial cell extracts (CE) and spent culture supernatants were resolved into 22 fractions according to their molecular weights by electroelution from sodium dodecyl sulfate (SDS)-polyacrylamide gels. Then samples were added directly to proliferation assays using PBMC obtained from healthy donors. Using this technique, we showed that cell-derived proteins covering a wide range of sizes were capable of eliciting T-cell responses. Interestingly, however, proliferative responses toward unfractionated total CE were never detected.
In this report, we show that lack of PBMC proliferation to total Manfredo CE is due to the presence of a potent inhibitor of human T-cell proliferation in bacterial cell sonicates. We have screened several other S. pyogenes strains covering a variety of M types and demonstrated their ability to inhibit human PBMC proliferation in response to several different stimuli. In addition, we have purified the inhibitory component present in Manfredo CE by using a combination of anion-exchange and gel filtration column chromatography and have investigated the mechanism of S. pyogenes-mediated inhibition, using Manfredo as a representative streptococcal strain.
MATERIALS AND METHODS
Bacteria.
The group A streptococcal strains used in this study are listed in Table 4. Strains designated NCTC were purchased from the National Collection of Type Cultures, Colindale, United Kingdom. S. pyogenes M80, M5 R91/1974, PT2841, PT3875, and PT2110 were generously provided by A. Tanna, Streptococcal Reference Laboratory, Public Health Laboratory Service, Colindale, United Kingdom, while the M5 strains Manfredo and Smith were provided by the late Ed Beachey and are from the Memphis VA Hospital culture collection, Memphis, Tenn. Bacteria were maintained routinely on Todd-Hewitt agar (Difco, Detroit, Mich.) plates containing 5% (vol/vol) horse blood.
TABLE 4.
Comparison of AD levels present in different streptococcal CEa
| Strain or prepn | AD activity (μM citrulline produced/h/μg of protein) | ID50 (μg/ml) |
|---|---|---|
| Strains | ||
| M5 Smith | 40.3 | 1.0 |
| M5 NCTC 8193 | 30.7 | 1.2 |
| M80 | 39.2 | 1.5 |
| M14 NCTC | 11.4 | 1.2 |
| M5 Manfredo | 16.1 | 1.2 |
| M5 R91/1974 | 36.7 | 1.6 |
| PT2841 | 17.3 | 1.9 |
| PT3875 | 15.6 | 2.4 |
| M27 NCTC 8328 | 14.1 | 2.5 |
| M2 NCTC 8322 44/R64 | 8.6 | 3.7 |
| PT2110 | 4.1 | 7.0 |
| M15 NCTC | 2.7 | >10 |
| Manfredo FPLC-purified fraction | 84.7 | ND |
Results are representative of those obtained from two separate measurements. AD activity was assayed as described in Materials and Methods. ID50, CE concentration that causes 50% inhibition of proliferation of PBMC stimulated with PHA in the presence of a noninhibitory concentration of CE (0.625 μg/ml). ND, not determined.
Preparation of S. pyogenes CE.
Bacteria were grown in Todd-Hewitt broth (6,000 ml) containing filter-sterilized hyaluronidase (30 μg/ml) for 16.5 h at 37°C under stationary conditions, then harvested by centrifugation at 11,000 × g for 20 min at 10°C, and washed once with phosphate-buffered saline (PBS). Pelleted cells were suspended in 15 ml of double-distilled H2O and frozen at −80°C. After thawing, bacterial cells were disrupted by sonication (six bursts of 1 min each) at 12μ on ice, using a Sanyo MSE Soniprep 150. Cell wall debris and unbroken cells were removed from the CE by centrifugation at 17,000 × g for 20 min at 10°C. The supernatant was removed, dialyzed overnight against double-distilled H2O at 4°C, and filter sterilized through 0.2-μm-pore-size filter units (Gelman Sciences, Northampton, United Kingdom). Aliquots were stored at −80°C until required. Protein concentrations were determined by the bicinchoninic acid assay (Pierce, Chester, United Kingdom), using bovine serum albumin (Sigma Chemical Company, Poole, Dorset, United Kingdom) as a standard.
T-cell proliferation assays.
PBMC were separated from heparinized blood from healthy donors by centrifugation on Ficoll-Hypaque density gradients (Lymphoprep; Nycomed, Birmingham, United Kingdom). After being washed with RPMI 1640 (Sigma) supplemented with 10 mM HEPES buffer (Gibco, Paisley, Scotland), PBMC were suspended in medium (RPMI 1640 supplemented with glutamine [3 mM], penicillin [50 U], streptomycin [50 μg/ml], and 2.5% pooled human serum) and added at 2 × 105 per well to 96-well round-bottom plates (Corning Costar, Corning, N.Y.) containing test samples at different concentrations. Six hours before the end of the culture period, each well was pulsed with 15 kBq of tritiated thymidine (specific activity, 74 GBq/mmol; Amersham Life Science, Buckinghamshire, United Kingdom). After harvesting onto glass fiber filters, thymidine incorporation was determined with a Canberra Packard Matrix 96 gas-phase counter. Results are expressed as mean tritiated thymidine incorporation in triplicate microcultures ± standard error of the mean (SEM).
Treatment of unfractionated S. pyogenes CE.
CE (60 μg) was treated for 1 h at 37°C with 100 μl of one of the following: 2 N HCl, 2 N NaOH, trypsin (2.5 mg/ml), carboxypeptidase (1 mg/ml), leucine aminopeptidase (0.45 mg/ml), pronase (1 mg/ml), or proteinase K (0.5 mg/ml). Alternatively, CE was placed in a water bath at 44, 55, 64, 75, or 85°C for 10 min to test temperature sensitivity of the inhibitor. Subsequently, CE subjected to one of these treatments was added to PBMC stimulated with phytohemagglutinin (PHA; 1.0 μg/ml) to give a final CE concentration of 5.0 μg/ml. CE treated with acid or alkali was neutralized before addition to PBMC cultures, and the proteolytic activity of trypsin was stopped by adding bovine trypsin inhibitor (1.25 mg/ml). To determine the effect of each treatment itself on T-cell proliferation, controls were set up in which PBS, in place of CE, was incubated with 100 μl each of protease, acid, or alkali and then added to PBMC stimulated with PHA as described above. PBMC were cultured for 3 days as described above.
Column chromatography.
CE in PBS (2.2 mg) was fractionated by anion-exchange chromatography on Mono Q (HR5/5) (Pharmacia, Uppsala, Sweden), previously equilibrated with 20 mM HEPES (pH 7.4) buffer. At a flow rate of 1 ml/min, proteins were eluted in a HEPES-NaCl gradient (60 ml of 0 to 0.5 M followed by 20 ml of 0.5 to 1.0 M). The column eluate was monitored by absorbance at 280 nm. Fractions (1 ml) were collected and filter sterilized through 0.2-μm-pore-size filters (Gelman Sciences) before being assayed for T-cell inhibitory activity.
The fraction which exhibited maximal inhibition was concentrated and then resolved by gel filtration on Superose 12 (HR10/30) (Pharmacia). The column was equilibrated with PBS, and proteins were resolved at a flow rate of 0.25 ml/min. Fractions (0.5 ml) were collected and treated as described above. The column was calibrated by using aldolase (158 kDa), bovine serum albumin (66 kDa), hen egg lysozyme (45 kDa), chymotrypsinogen A (25 kDa), and cytochrome c (12.5 kDa), obtained from Pierce.
SDS-polyacrylamide gel electrophoresis (PAGE).
Samples were run on a polyacrylamide gel in the presence of 10% (wt/vol) SDS as described by Laemmli (26). A 4.5% stacking gel and 12.5% separating gel were used, and proteins were visualized by silver staining according to the method of Hochstrasser et al. (19).
NH2-terminal protein sequencing.
Manfredo CE was run on a polyacrylamide gel as described above and then blotted on to a polyvinylidene difluoride Fluortrans 0.2-μm-pore-size transfer membrane (Pall, Portsmouth, United Kingdom), using a wet tank electroblotter (Hoefer Scientific Instruments, San Francisco, Calif.). Blotting was done with 10 mM 3-(cyclohexylamino)-1-propanesulfonic acid (CAPS)–10% methanol (pH 11) transfer buffer at 5.5 mA/cm2 for 60 min. Transferred protein was stained with Coomassie blue. NH2-terminal protein sequencing was carried out in the Molecular Biology Unit of Newcastle University, using a Beckman LF3000 gas-phase sequencer.
Assay of AD activity.
l-Arginine deiminase (AD) activity was assayed by measuring the rate of conversion of l-arginine to citrulline. Briefly, streptococcal CE (0.1 ml) were incubated with 10 mM l-arginine contained in 0.1 M potassium phosphate (pH 6.5) buffer (0.4 ml) for 2 h at 37°C before the reaction was terminated by adding 0.25 ml of a mixture of H2SO4 and H3PO4 (1:3, vol/vol). The citrulline formed was then determined by using diacetyl monooxime according to the method of Oginsky (34).
Statistical analysis. Results are expressed as means ± standard errors of the means. Statistical differences were determined by Student’s t test.
Other reagents.
Unless otherwise stated, all chemicals were purchased from Sigma. Recombinant M5 (rM5) and recombinant SPEA (rSPEA) were prepared as previously described (9, 13).
RESULTS
Effect of S. pyogenes Manfredo CE on proliferation of human PBMC.
To determine whether the lack of response to unfractionated Manfredo CE was due to an inhibitory effect, CE was titrated into PBMC cultures stimulated with either the S. pyogenes superantigen rSPEA (1 nM, 3-day cultures), PHA (1.0 μg/ml, 3-day cultures), or the streptococcal recall antigen rM5 protein (5 μg/ml, 7-day cultures). Irrespective of the type of stimulus used, CE at concentrations above 0.5 μg/ml consistently caused potent inhibition of T-cell proliferation (Table 1). PBMC proliferative responses to stimulation with anti-CD3 monoclonal antibody, tetanus toxoid, or tuberculin purified protein derivative were also strongly inhibited by CE (data not shown).
TABLE 1.
Inhibition of human PBMC proliferation in response to rSPEA, rM5 protein, and PHA by S. pyogenes Manfredo unfractionated CEa
| CE (μg/ml) | Uptake of [3H]thymidine
(cpm)
|
||||
|---|---|---|---|---|---|
| 3-day assay
|
7-day assay
|
||||
| Cells only | PHA (1.0 μg/ml) | rSPEA (1 nM) | Cells only | rM5 (5.0 μg/ml) | |
| 0 | 54 ± 2 | 4,575 ± 413 | 1,543 ± 6 | 160 ± 13 | 606 ± 63 |
| 0.04 | 102 ± 10 | 4,356 ± 605 | 1,397 ± 56 | 283 ± 113 | 799 ± 189 |
| 0.2 | 155 ± 22 | 4,251 ± 309 | 996 ± 178 | 393 ± 62 | 758 ± 75 |
| 0.5b | 66 ± 30 | 1,811 ± 140 | 195 ± 25 | 36 ± 4 | 46 ± 10 |
| 1.0b | 31 ± 3 | 933 ± 80 | 56 ± 4 | 32 ± 6 | 42 ± 5 |
| 5.0b | 12 ± 2 | 26 ± 5 | 21 ± 1 | 20 ± 3 | 29 ± 2 |
Human PBMC (2 × 106/ml) were incubated in 96-well plates with CE at a range of concentrations and with either rSPEA (3 days), PHA (3 days), or rM5 (7 days) prior to pulsing with tritiated thymidine for the final 6 h of culture. Results show mean values ± SEM for triplicate cultures and are representative of data obtained from two separate experiments.
Proliferation of human PBMC was significantly inhibited (P < 0.004) by CE at final concentrations of 0.5 μg/ml or higher.
To investigate whether the inhibitory effect of CE on human PBMC proliferation was reversible, identical 96-well plates containing PBMC and Manfredo CE at 0, 0.008, 0.04, 0.2, 0.5, 1.0, and 5.0 μg/ml were set up concurrently. After 24 h of culture, PBMC in one plate were washed thoroughly by centrifuging the plate at 200 × g, exchanging 100 μl of supernatant with fresh medium, resuspending the cells, and repeating this procedure four times. PHA (final concentration, 1 μg/ml) was then added to both plates, which were cultured for a further 3 days. In contrast to unwashed cultures, the washed PBMC responded well to PHA (Table 2), showing that CE-pulsed PBMC were viable and capable of proliferative responses. The same result was obtained in assays using PBMC from three different donors. Responses to PHA were also inhibited when CE was added either 24 h before or 24 h after PHA, but not when CE was added concurrently with tritiated thymidine for the final 6 h of culture (data not shown).
TABLE 2.
Inhibition of PHA-stimulated PBMC proliferation by S. pyogenes Manfredo CE is reversiblea
| CE (μg/ml) | Uptake of [3H]thymidine (cpm)
|
|
|---|---|---|
| Washed PBMC | Unwashed PBMC | |
| 0 | 7,842 ± 77 | 6,194 ± 125 |
| 0.008 | 7,124 ± 144 | 6,627 ± 267 |
| 0.04 | 6,942 ± 792 | 7,781 ± 46 |
| 0.2 | 6,885 ± 122 | 7,691 ± 209 |
| 0.5b | 8,161 ± 372 | 375 ± 115 |
| 1.0b | 8,155 ± 224 | 70 ± 4 |
| 5.0b | 6,913 ± 281 | 35 ± 4 |
| Background | 94 ± 5 | 69 ± 3 |
Human PBMC (2 × 106/ml) were cultured with CE at a range of concentrations in 96-well round-bottom plates. After 24 h of incubation at 37°C, PBMC from one plate were washed with RPMI 1640 plus 10 mM HEPES before addition of PHA to each well to give a final concentration of 1.0 μg/ml. PBMC in the second plate were not washed before addition of PHA. Incorporation of [3H]thymidine into the cells was determined after a further 3 days of incubation. Results show mean incorporation of radioactivity for triplicate wells ± SEM for one donor studied and are representative of values obtained from four different individuals.
Proliferation of unwashed PBMC was significantly inhibited (P < 0.00005) by CE at final concentrations of 0.5 μg/ml or higher. No significant inhibition was observed with washed PBMC.
Screening of S. pyogenes strains for T-cell inhibitory activity.
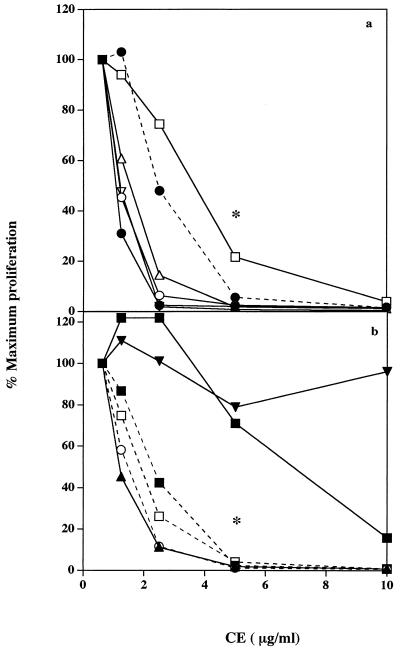
CE prepared from a further 11 streptococcal strains were assayed for the ability to inhibit human PBMC proliferation in response to rSPEA (1 nM), PHA (1.0 μg/ml), or tetanus toxoid (1.0 μg/ml) to determine whether this activity was a property unique to Manfredo or was widely distributed among S. pyogenes strains. CE from all strains examined, except that from S. pyogenes M15 NCTC, strongly inhibited PBMC proliferation. The same pattern of inhibition was observed for each strain regardless of the nature of the stimulus used; therefore, results are only shown for mitogen-stimulated cultures (Fig. 1). The most potent inhibitory activity was seen with CE from S. pyogenes M5 Smith, M5 R91/1974, M14, M5 NCTC, and M80 as can be seen from estimations of ID50 values for each CE (Table 4). The ID50 is the concentration of CE that caused 50% inhibition of the proliferative responses of human PBMC incubated with PHA (1.0 μg/ml) in the presence of a noninhibitory concentration of CE (0.625 μg/ml). Relatively weaker inhibition was detected with S. pyogenes M2, PT3875, PT2841, and M27 CE, while CE from S. pyogenes PT2110 was significantly inhibitory only at a concentration of 10 μg/ml. Subsequent experiments showed that S. pyogenes M15 NCTC CE was capable of suppressing PBMC proliferation but only at CE concentrations of 50 μg/ml or above.
FIG. 1.
Inhibition of human PBMC proliferative responses to PHA by S. pyogenes CE prepared from the following strains: (a) M5 Smith (-•-), M5 R91/1974 (▵), M5 NCTC 8193 (○), M2 NCTC 8322 44/R64 (□), M14 NCTC (▿), and M27 NCTC 8328 (----•----) and (b) PT2110 (-▪-), M15 NCTC (▾), PT2841 (----□----), M80 (----○----), PT3875 (----▪----), and Manfredo (▴). Human PBMC (2 × 106/ml were incubated in 96-well round-bottom plates with PHA (1.0 μg/ml) in the presence of a range of concentrations of streptococcal CE from various strains. After 3 days, the cells were pulsed with tritiated thymidine for the final 6 h of culture and the incorporated radioactivity was measured. Results show level of proliferation as a percentage of the proliferative response observed in the presence of 0.625 μg of each CE per ml. Maximum levels of PBMC proliferation in response to PHA (1.0 μg/ml) in the presence of 0.625 μg of each CE per ml were as follows (cpm ± SEM, n = 3): M5 Smith (6,213 ± 802), M5 R91/1974 (6,856 ± 792), M5 NCTC 8193 (5,828 ± 343), M2 NCTC 8322 44/R64 (4,866 ± 222), M14 NCTC (10,522 ± 1,665), M27 NCTC 8328 (7,644 ± 1,141), PT2110 (4,444 ± 918), M15 NCTC (9,625 ± 800), PT2841 (8,048 ± 1,096), M80 (7,654 ± 662), PT3875 (7,290 ± 988), and Manfredo (6,032 ± 331). Levels of background proliferation were not greater than 150 cpm. ∗, CE from all strains, except PT2110 and M15, significantly inhibited (P < 0.005) PBMC proliferation at concentrations of 5.0 μg/ml or higher.
Characterization of inhibitory component present in S. pyogenes Manfredo CE.
CE heated at 55, 64, 75, and 85°C for 10 min did not inhibit PHA responses, but temperatures of 22 and 44°C had no effect on its suppressive activity (Table 3). CE that had been heated to 80°C for 10 min and then added as a single stimulus to PBMC generated proliferative responses after 3 days of incubation (mean incorporation of [3H]thymidine, 613 ± 10 cpm; background level, 69 ± 14 cpm; n = 3), thus indicating that inactivation of the inhibitor by heat reveals immunostimulatory properties of the CE. Pretreatment of CE with either acid or alkali prior to its addition to PBMC stimulated with PHA also removed its inhibitory activity (Table 3).
TABLE 3.
Characterization of inhibitory component of S. pyogenes Manfredo CE
| Treatment of CE | [3H]thymidine incorporation (cpm) | |
|---|---|---|
| Untreated CE aloneb | 60 ± 10 | |
| Untreated CE + PHAb | 46 ± 3 | |
| PHA alone | 4,138 ± 370 | |
| CE heated at: | ||
| 44°Cb | 64 ± 6 | |
| 55°C | 3,833 ± 206 | |
| 64°C | 3,271 ± 80 | |
| 75°C | 4,103 ± 869 | |
| 85°C | 3,226 ± 461 | |
| 2 N HCl | 6,378 ± 328 | (5,672 ± 68) |
| 2 N NaOH | 6,847 ± 683 | (5,824 ± 259) |
| Pronase (1 mg/ml)b | 956 ± 140 | (3,520 ± 192) |
| Proteinase K (0.5 mg/ml)b | 2,342 ± 115 | (6,727 ± 428) |
| Trypsin (2.5 mg/ml)b | 73 ± 2 | (5,831 ± 519) |
| Carboxypeptidase (1 mg/ml)b | 115 ± 29 | (5,458 ± 206) |
| Leucine aminopeptidase (0.45 mg/ml)b | 44 ± 2 | (5,918 ± 535) |
CE (60 μg) was pretreated with 100 μl of acid, alkali, or enzymes for 1 h before addition to PBMC stimulated with PHA (1.0 μg/ml) to give a CE concentration of 5.0 μg/ml. PBMC were then incubated for 3 days before proliferation was determined by measuring incorporation of [3H]thymidine. Results are mean values obtained from triplicate wells ± SEM. Values in parentheses show levels of PBMC proliferation in response to PHA in the presence of each individual protease, acid, or alkali treatment alone. Results are representative of those obtained from three separate experiments.
Proliferation of human PBMC was significantly inhibited (P < 0.010) by CE.
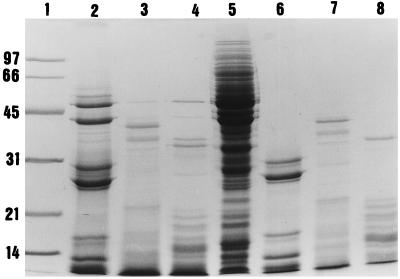
The effects on CE of several proteases were tested. Proliferation of human PBMC in response to PHA was obtained in the presence of CE that had been preincubated with either pronase or proteinase K (Table 3). In contrast, trypsin, carboxypeptidase, and leucine aminopeptidase had no effect on the inhibitory component of S. pyogenes despite the fact that these treatments resulted in extensive digestion of the CE constitutive proteins, as visualized by SDS-PAGE (Fig. 2).
FIG. 2.
SDS-PAGE of protease-digested S. pyogenes Manfredo CE. Lanes: 1, molecular weight markers (sizes are indicated in kilodaltons); 2, trypsinized CE; 3, pronase-treated CE; 4, proteinase K-digested CE; 5, total CE (15 μg); 6, trypsin alone; 7, pronase alone; 8, proteinase K alone.
Experiments were done to eliminate the possibility that the CE-mediated inhibition of PBMC proliferation was due to contaminating lipoteichoic acid. Lipoteichoic acid (0.04 to 5.0 μg/ml) was titrated into proliferation assays in which human PBMC were stimulated with PHA but was not found to be inhibitory at these concentrations, and addition of polymyxin B (a known inhibitor of lipoteichoic acid activity) to proliferation assays was unable to overcome the inhibitory effect of the CE (data not shown).
Column chromatography of Manfredo CE.
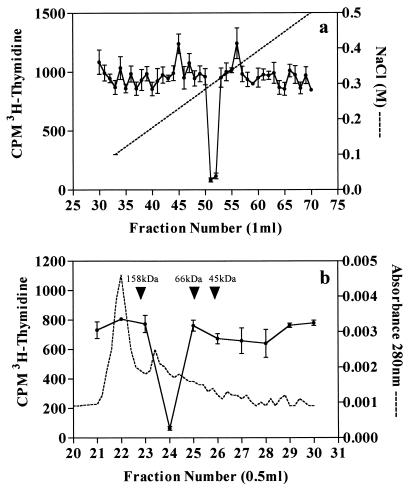
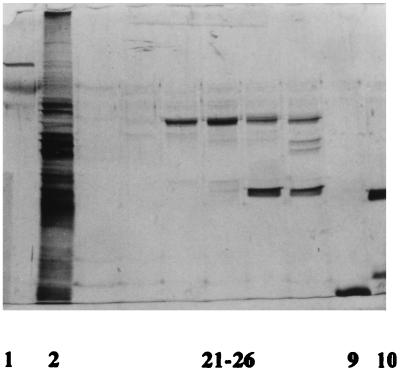
After it was shown that the inhibitory component present in Manfredo CE was resistant to the proteolytic action of trypsin and that this protease degraded a high proportion of other CE proteins, CE samples were routinely treated with trypsin for 2 h at 37°C as an initial purification step. Trypsinization of the CE did not alter the physicochemical properties of the inhibitor, the activity of which was consistently separated by anion-exchange chromatography to give a sharp peak that was eluted from the column with approximately 0.3 M NaCl during at least eight separate purifications (Fig. 3a). Pooling of the inhibitory fractions obtained by anion-exchange chromatography followed by their passage down a calibrated Superose 12 gel filtration column resulted in further resolution of inhibitory activity to give one sharp peak that corresponded to a native molecular mass of approximately 100 kDa (Fig. 3b). SDS-PAGE of inhibitory fractions obtained by serial column chromatography revealed the presence of a single band after silver staining (fraction 24) with an approximate molecular mass of 47 kDa, thus indicating that the inhibitor is composed of two identical subunits (Fig. 4). Although fractions 23, 25, and 26 also contain this 47-kDa band, there was not enough protein present to cause inhibition of proliferation by these samples. The NH2-terminal sequence of this band was determined after its transfer to a polyvinylidene difluoride Fluortrans transfer membrane (Pall) and was found to be TAQTPIHVYSEIGKL. A search of the Swissprot database (2) showed that this protein sequence is identical to that of the previously characterized streptococcal acid glycoprotein (SAGP). A further search also revealed that SAGP has between 31.5 and 39.0% amino acid identity with AD from Mycoplasma hominis, Mycoplasma arginini, Pseudomonas putida, and Pseudomonas aeruginosa. Figure 5 shows alignment of the amino acid sequences of SAGP and AD from M. hominis which have the highest homology.
FIG. 3.
(a) Separation of the inhibitory activity of Manfredo CE by anion-exchange chromatography. For details, see Materials and Methods. Broken line, NaCl gradient; •, incorporation of [3H]thymidine by human PBMC stimulated with PHA (1.0 μg/ml) in the presence of anion-exchange fraction. (b) Resolution of inhibitory activity present in pooled fractions obtained by anion-exchange chromatography by gel filtration chromatography. Broken line, A280; •, incorporation of [3H]thymidine by human PBMC stimulated with PHA (1.0 μg/ml) in the presence of gel filtration fraction. Results are representative of those obtained from at least eight separate experiments.
FIG. 4.
SDS-PAGE of Manfredo CE inhibitory fractions following anion-exchange chromatography and gel filtration chromatography. Lanes: 1, phosphorylase b (97 kDa); 2, total CE (0.5 μg); 21–26, fractions 21 to 26 after resolution of CE by anion-exchange chromatography and gel filtration chromatography (see Fig. 3); 9, cytochrome c (12 kDa); 10, chymotrypsinogen A (25 kDa). The gel was silver stained by the method of Hochstrasser et al. (19).
FIG. 5.
Alignment of the amino acid sequences of SAGP (accession no. P16962) and AD from M. hominis (accession no. P41141). Amino acid identity is indicated by vertical dashes, and similar amino acids are marked with colons.
Assay of streptococcal CE for AD activity.
Having shown that the inhibitor purified from Manfredo CE possesses very high sequence homology with AD, we assayed CE prepared from Manfredo, and also all other strains screened previously for inhibitory properties, for activity of this enzyme. Results in Table 4 show that AD activity was detected in all streptococcal strains tested, with highest amounts present in S. pyogenes M5 Smith, M5 R91/1974, M5 NCTC 8193, and M80. Moreover, fast protein liquid chromatography (FPLC)-purified inhibitory fraction from Manfredo CE (prepared subsequently to the material shown in Fig. 3 but a fraction corresponding to fraction 24) had even greater AD activity. Levels of AD measured in CE directly correlated (P < 0.02) the inhibitory potential of the different streptococcal strains, with high enzyme activities corresponding to strong inhibitory properties (low ID50 values) and vice versa. For example, S. pyogenes PT2110 and M15 NCTC CE had relatively weak inhibitory effects on human PBMC proliferation (Fig. 1) and were found to have low AD activity.
No AD activity was detected in any streptococcal CE assayed after it had been heated at 55°C for 10 min, or in Manfredo CE after it had been acid or alkali digested, treatments that all abrogate the inhibitory property of group A streptococcal CE. In contrast, Manfredo CE incubated with either carboxypeptidase or trypsin retained full AD activity whereas Manfredo CE treated with either proteinase K or pronase had lowered AD activity, which again directly correlates with these treatments either having no or partial effect on the inhibitory activity of Manfredo CE.
Effect of l-arginine on the inhibition of human PBMC proliferation by S. pyogenes Manfredo CE.
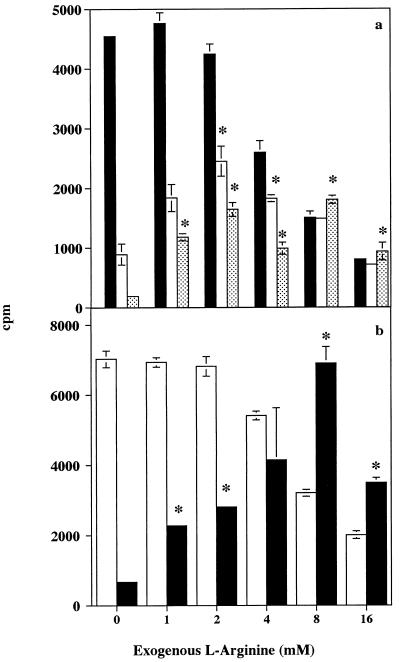
Addition of exogenous l-arginine to proliferation assays in which human PBMC were stimulated with PHA (1.0 μg/ml) overcame the suppressive activity of unfractionated Manfredo CE. No inhibition attributable to CE was seen in the presence of high concentrations of l-arginine (8 and 16 mM); however, at these levels the amino acid itself reduced PBMC proliferation (Fig. 6a). Although lower concentrations of l-arginine did not fully reverse the inhibitory effect of CE, up to 58 and 39% of the full proliferative response was observed in PBMC incubated with 2.5 and 5.0 μg, respectively, of CE per ml when an optimum concentration of l-arginine (2 mM) was included in the assay. In contrast, in the absence of exogenous l-arginine, CE at 5.0 μg/ml completely abolished the proliferative response to PHA and incubation with 2.5 μg of CE per ml resulted in only 20% of the full response.
FIG. 6.
Effect of l-arginine on the inhibition of human PBMC proliferation in response to PHA by (a) S. pyogenes Manfredo unfractionated CE at concentrations of 0 (▪), 2.5 (□), and 5.0 ( ) μg/ml and (b) FPLC partially purified inhibitory fractions from Manfredo CE at concentrations of 0 (□) and 4.0 (▪) μg/ml. See the legend to Fig. 1 for assay details. Results show mean [3H]thymidine uptake ± SEM for triplicate cultures. ∗, addition of l-arginine significantly overcame the inhibitory effect of CE (P < 0.01).
Addition of increasing concentrations of l-arginine to proliferation assays in which human PBMC were stimulated with PHA in the presence of partially purified Manfredo inhibitor (4.0 μg/ml) resulted in a gradual restoration of proliferation, with a full PHA response observed in the presence of 8 mM l-arginine (Fig. 6b). As described above, 16 mM l-arginine itself suppressed PBMC proliferation, but at this concentration no inhibition due to the partially purified inhibitor was seen.
DISCUSSION
In this report, we have shown that CE prepared from a variety of group A streptococcal strains strongly inhibited human PBMC proliferation in a dose-dependent manner. CE not only inhibited proliferative responses of PBMC to common recall antigens including streptococcal M5 protein but also markedly reduced stimulation of T cells by powerful polyclonal activators such as PHA and rSPEA (Fig. 1; Table 1). Purification of the inhibitory component from Manfredo CE by FPLC yielded a protein that had an NH2-terminal sequence identical to that of SAGP. A search of the Swissprot database (2) revealed that SAGP had a previously unreported high sequence homology with AD. The inhibitory protein isolated from Manfredo CE not only had biochemical and size characteristics similar to those of purified AD but also possessed AD enzyme activity. We therefore conclude that SAGP, or its homolog, has AD activity and that it is the activity of this enzyme that is responsible for inhibition of human PBMC proliferation.
Although SAGP has been well documented as having antiproliferative activity against a range of tumor cell lines, including murine fibrosarcoma Meth A cells, human HL60 cells, murine embryonic cells (BALB/3T3), HeLa cells, and murine leukemic L1210 cells (23, 50, 51), this is the first reported evidence that it also potently inhibits proliferation of human PBMC. SAGP was originally isolated from S. pyogenes Su following the observation that a lyophilized preparation, termed OK-432, of heat- and penicillin-inactivated streptococci of the Su strain had antitumor properties (17, 49). Indeed, OK-432 has been used clinically as an antitumor agent (35, 47), but it appears to mediate its tumoricidal effect by modulating the host immune response through pathways not involving the activity of SAGP. OK-432 is known to activate natural killer cells, T cells, and macrophages in vitro, and animals treated with OK-432 intraperitoneally develop antitumor cytotoxic macrophages (6). OK-432 induces proliferative responses of human PBMC, and they are thought to acquire antitumor activity which includes both cell- and cytokine-mediated mechanisms (20, 40). Since OK-432 is a lyophilized preparation of the Su strain of S. pyogenes, it would be expected to contain SAGP and inhibit rather than induce proliferative responses of human PBMC. Therefore, the activity of SAGP is presumably destroyed during the preparation of OK-432, perhaps during lyophilization, and it is the remaining mixture of bacterial proteins that elicits its antitumor effects.
While the inhibitory protein purified from Manfredo CE has an amino-terminal sequence identical to that of SAGP, it may differ from the latter in some aspects of its mode of action and also its size. SAGP is reported to have a direct cytotoxic effect on tumor cell lines (23, 24); however, results presented in Table 2 show that Manfredo CE does not have a direct cytotoxic effect on human PBMC. The native molecular mass of SAGP has been reported as 140 to 150 kDa (51) and also 220 kDa (24), although both sources agree that it is a tetramer composed of four 47- to 48-kDa monomers. In contrast, the protein that we have isolated from Manfredo CE has a native molecular mass of approximately 100 kDa (Fig. 3a) and appears to be composed of two identical subunits with approximate molecular masses of 47 kDa (Fig. 4). The difference in the molecular masses of SAGP and the Manfredo inhibitor may be explained by the fact that the SAGP tetramer disassociates very readily to yield the dimer under certain changes of pH and freeze-thawing cycles (24). Alternatively, this apparent discrepancy may represent a genuine difference in the protein derived from the two S. pyogenes strains.
As mentioned earlier, our search of the Swissprot protein database showed that SAGP and thus the inhibitor purified from Manfredo CE has between 31.5 and 39.0% amino acid sequence identity with AD from M. hominis, M. arginini, P. putida, and P. aeruginosa, the highest homology being with M. hominis AD (Fig. 5). AD is one of three enzymes that comprises the AD system, the other two being ornithine carbamoyltransferase and carbamate kinase (8, 10). AD catalyzes the conversion of l-arginine to citrulline, with the concomitant production of ammonia. The citrulline thus formed is then converted to ornithine and carbamoyl phosphate via the action of ornithine carbamoyltransferase. Carbamate kinase then catalyzes the breakdown of carbamoyl phosphate to yield carbon dioxide and ammonia, with the generation of 1 mol of ATP. The AD system functions to protect bacteria against acid damage by producing ammonia which combines with protons to give NH4+, provide a source of ATP for growth, and generate citrulline for biosynthetic pathways (8, 10, 11). The AD system is widely distributed among prokaryotes, including Enterococcus faecalis, Lactococcus lactis, Enterococcus faecium, and Clostridium perfringens, and in Mycoplasma the catabolism of l-arginine by this enzyme complex acts as a major nonglycolytic metabolic energy source (10, 41).
Data presented in this report strongly suggest that SAGP is the S. pyogenes equivalent of AD and that it is the breakdown of l-arginine by the activity of this enzyme that is responsible for inhibition of human PBMC proliferation mediated by CE from this organism. First, AD activity was found in all group A streptococcal CE used in this study and also in inhibitory Manfredo CE fractions purified by FPLC (Table 4). Second, heating CE at 55°C for 10 min destroyed activity of this enzyme and also abrogated the inhibitory property of streptococcal CE (Table 3). Third, levels of AD activity measured in each CE directly correlated with its inhibitory capability. Fourth, addition of l-arginine to cultures in which human PBMC were stimulated with PHA in the presence of Manfredo CE or FPLC partially purified protein restored proliferation (Fig. 6). Biochemical evidence further supports the view that the Manfredo inhibitor is group A streptococcal AD. AD purified from Mycoplasma arthritidis (48), P. putida (43), and M. arginini (30) have all been shown to be dimers composed of two identical subunits with molecular masses in the range of 46 to 54 kDa, which is in agreement with our estimations of the size characteristics of the Manfredo inhibitor. Moreover, M. arginini-derived AD was eluted from a DEAE-Sepharose column with 0.35 M NaCl (25), and the activity of P. putida AD was dramatically decreased at temperatures above 50°C (43), again both properties shared by the Manfredo inhibitor. We have carried out experiments to eliminate lipoteichoic acid as the inhibitory component present in Manfredo CE (results not shown). The inhibition is also unlikely to be due to contaminating streptolysin S or streptolysin O since both of these are cytotoxic and the data presented in Table 2 demonstrate that streptococcal CE did not have a cytotoxic effect on human PBMC. In addition, if the inhibition was due to either lipoteichoic acid, streptolysin S, or streptolysin O, it would be difficult to account for restoration of proliferation in the presence of l-arginine.
There is additional very strong published evidence to support our conclusion that it is the action of AD that potently inhibits PBMC proliferation. Recombinant M. arginini-derived AD expressed in Escherichia coli has antitumor properties and inhibits the growth of the two mouse cell lines, hepatoma MH134 and fibrosarcoma Meth A (30). In addition, Mycoplasma-derived AD isolated from a culture of a Rous sarcoma virus-transformed rat liver cell line powerfully inhibits the growth of several human tumor cell lines, including lung adenocarcinoma (A549) cells, hepatoma (HLE) cells, melanoma (VMRC) cells, and cervix squamous carcinoma (CaSki) cells (31). More recently, AD derived from M. arginini was purified from the serum-free culture medium of a B-precursor leukemia cell line, NALM-20, and found to strongly inhibit, in a dose-dependent manner, the growth of human T cells and T lymphoblastoid cell lines, but not B-precursor and myeloid cell lines (25). The morphologic features of dying cells and DNA fragmentation indicated that AD induced apoptotic cell death in T lymphoblasts. As we observed with inhibition of human PBMC proliferation (Fig. 6), the growth of T lymphoblastoid cells that had been inhibited by AD was completely restored by the addition of l-arginine to the cultures.
In vitro, l-arginine is essential for the optimal growth and replication of cells, but lack of extracellular l-arginine in the growth medium is thought not to lead to cell death (46). The action of AD will lead to a depletion of l-arginine in growth media, and it may be that in the absence of l-arginine cells are simply unable to synthesize new proteins, thus inhibiting growth and proliferation. However, l-arginine is not classified as an essential amino acid for mammalian cells, and homoarginine can replace l-arginine to give optimal proliferation even though the latter is not incorporated into proteins (14). An alternative explanation of how a lack of l-arginine inhibits proliferation may be that human PBMC are unable to synthesize nitric oxide in its absence. Nitric oxide is an essential requirement for optimal human peripheral blood lymphocyte DNA synthesis (14), and l-arginine is the sole substrate for its production. Nitric oxide is generated by the oxidation of one of the guanido nitrogens of l-arginine, and homoarginine is also able to donate a guanido nitrogen group for nitric oxide synthesis.
If its inhibitory effect on human PBMC proliferation is manifested in vivo, AD may have important consequences in modulating host immune response during infections. Normal colonization sites for group A streptococci are the pharynx and oral cavity, where AD activity is thought to play an important role in protecting bacteria found in dental plaque from highly acidic environments (8, 11). A consequence of AD activity at sites of inflammation may be the localized depletion of l-arginine and thus inhibition of T-cell proliferation which would enable S. pyogenes to downregulate the host immune response, thereby compromising the ability of the host to develop effective protective immunity to these common bacterial pathogens. Such effects may differ between strains depending on possible variation in levels of AD activity. The streptococcal inhibitor may also play an important role in pathogenesis of poststreptococcal autoimmune diseases. For example, the action of the inhibitor may play a role in determining the extent to which self cross-reactive T lymphocytes involved in autoimmune pathogenesis are activated during group A streptococcal infections. Alternatively, downregulation of protective T-cell immunity may allow bacterial persistence, thus potentiating other proinflammatory sequelae and increasing the potential for autoimmunity.
ACKNOWLEDGMENTS
This work was funded by project grant G0076 from the Arthritis and Rheumatism Council.
We thank J. Lakey for helpful discussion and advice and also J. Gray for help with protein sequencing.
REFERENCES
- 1.Baird R W, Bronze M S, Kraus W, Hill H R, Veasey L G, Dale J B. Epitopes of group A streptococcal M protein shared with antigens of articular cartilage and synovium. J Immunol. 1991;146:3132–3137. [PubMed] [Google Scholar]
- 2.Bairoch A, Apweiler R. The SWISS-PROT protein sequence data bank and its new supplement TrEMBL. Nucleic Acids Res. 1996;24:21–25. doi: 10.1093/nar/24.1.21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bernheimer A W. Hemolysins of streptococci: characterization and effects on biological membranes. In: Wannamaker L W, Matsen J M, editors. Streptococci and streptococcal diseases. New York, N.Y: Academic Press Inc.; 1972. pp. 19–31. [Google Scholar]
- 4.Bisno A L. Group A streptococcal infections and rheumatic fever. N Engl J Med. 1991;325:783–793. doi: 10.1056/NEJM199109123251106. [DOI] [PubMed] [Google Scholar]
- 5.Bisno A L. Acute rheumatic fever: a present-day perspective. Medicine. 1993;72:278–283. [Google Scholar]
- 6.Bonavida B, Jewett A. Activation of human peripheral blood-derived monocytes by OK-432 (Streptococcus pyogenes): augmented cytotoxicity and secretion of TNF and synergy with rIFN-γ. Cell Immunol. 1989;123:373–383. doi: 10.1016/0008-8749(89)90297-9. [DOI] [PubMed] [Google Scholar]
- 7.Braun M A, Gerlach D, Hartwig U F, Ozegowski J-H, Romanagne F, Carrel S, Kohler W, Fleischer B. Stimulation of human T cells by streptococcal “superantigen” erythrogenic toxins (scarlet fever toxins) J Immunol. 1993;150:2457–2466. [PubMed] [Google Scholar]
- 8.Casiano-Colon A, Marquis R E. Role of arginine deiminase system in protecting oral bacteria and an enzymatic basis for acid tolerance. Appl Environ Microbiol. 1988;54:1318–1324. doi: 10.1128/aem.54.6.1318-1324.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Charlton F G, Smith R J, Pyle G, Kehoe M A, Robinson J H. Hierarchy of SPEA presentation to T cells by murine MHC haplotypes. Eur J Immunogenet. 1997;24:423–430. doi: 10.1046/j.1365-2370.1997.d01-118.x. [DOI] [PubMed] [Google Scholar]
- 10.Cunin R, Glansdorff N, Pierard A, Stalon V. Biosynthesis and metabolism of arginine in bacteria. Microbiol Rev. 1986;50:314–352. doi: 10.1128/mr.50.3.314-352.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Curran T M, Lieou J, Marquis R E. Arginine deiminase system and acid adaptation of oral streptococci. Appl Environ Microbiol. 1995;61:4494–4496. doi: 10.1128/aem.61.12.4494-4496.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Degnan B A, Kehoe M A, Goodacre J A. Analysis of human T cell responses to group A streptococci using fractionated Streptococcus pyogenesproteins. FEMS Immunol Med Microbiol. 1997;17:161–170. doi: 10.1111/j.1574-695X.1997.tb01009.x. [DOI] [PubMed] [Google Scholar]
- 13.Degnan B A, Taylor J, Hawkes C, O’Shea U, Smith J, Robinson J H, Kehoe M A, Boylston A, Goodacre J A. Streptococcus pyogenestype 5 M protein is an antigen, not a superantigen, for human T cells. Hum Immunol. 1997;53:206–215. doi: 10.1016/S0198-8859(97)00028-1. [DOI] [PubMed] [Google Scholar]
- 14.Efron D T, Kirk S J, Regan M C, Wasserkrug H L, Barbul A. Nitric oxide generation from l-arginine is required for optimal human peripheral blood lymphocyte DNA synthesis. Surgery. 1991;110:327–334. [PubMed] [Google Scholar]
- 15.Fleischer B, Schmidt K-H, Gerlach D, Kohler W. Separation of T-cell stimulating activity from streptococcal M protein. Infect Immun. 1992;60:1767–1770. doi: 10.1128/iai.60.5.1767-1770.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ginsburg I. Mechanisms of cell and tissue injury induced by group A streptococci: relation to poststreptococcal sequelae. J Infect Dis. 1972;126:294–340. doi: 10.1093/infdis/126.3.294. [DOI] [PubMed] [Google Scholar]
- 17.Gravekamp C, Vreugdenhil R, Bolhuis R L H. OK-432 and IL-2-augmented cytotoxicity of human natural killer cells and cytotoxic T lymphocytes at the clonal level. FEMS Microbiol Immunol. 1988;47:31–40. doi: 10.1111/j.1574-6968.1988.tb02488.x. [DOI] [PubMed] [Google Scholar]
- 18.Haase A, Melder A, Kemp D, Mathews J. Streptokinase alleles and disease association in group A streptococci. FEMS Immunol Med Microbiol. 1994;10:75–80. doi: 10.1111/j.1574-695X.1994.tb00013.x. [DOI] [PubMed] [Google Scholar]
- 19.Hochstrasser D F, Patchornick A, Merril C R. Development of polyacrylamide gels that improve the separation of proteins and their detection by silver staining. Anal Biochem. 1989;173:412–423. doi: 10.1016/0003-2697(88)90208-4. [DOI] [PubMed] [Google Scholar]
- 20.Hu Z-L, Kubota E, Imamura H, Katano M, Katsuki T. Effective induction of human NK cells with OK-432 and further augmentation of their cytolytic function by rIL2. Microbiol Immunol. 1994;38:183–190. doi: 10.1111/j.1348-0421.1994.tb01763.x. [DOI] [PubMed] [Google Scholar]
- 21.Itoh T, Satoh H, Isono N, Rikiishi H, Kumagai K. Mechanism of stimulation of T cells by Streptococcus pyogenes: isolation of a major mitogenic factor, cytoplasmic membrane-associated protein. Infect Immun. 1992;60:3128–3135. doi: 10.1128/iai.60.8.3128-3135.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Iwasaki M, Igarashi H, Hinuma Y, Yutsudo T. Cloning, characterization and overexpression of a Streptococcus pyogenesgene encoding a new type of mitogenic factor. FEBS Lett. 1993;331:187–192. doi: 10.1016/0014-5793(93)80323-m. [DOI] [PubMed] [Google Scholar]
- 23.Kanaoka M, Kawanaka C, Negoro T, Fukita Y, Taya K, Agui H. Cloning and expression of the antitumour glycoprotein gene of Streptococcus pyogenes Su in Escherichia coli. Agric Biol Chem. 1987;51:2641–2648. [Google Scholar]
- 24.Kanaoka M, Negoro T, Kawanaka C, Agui H, Nabeshima S. Streptococcal antitumour protein: expression in Escherichia colicells and properties of the recombinant protein. Agric Biol Chem. 1991;55:743–750. [PubMed] [Google Scholar]
- 25.Komada Y, Zhang X-L, Zhou Y-W, Ido M, Azuma E. Apoptotic cell death of human T lymphoblastoid cells induced by arginine deiminase. Int J Hematol. 1997;65:129–141. doi: 10.1016/s0925-5710(96)00538-5. [DOI] [PubMed] [Google Scholar]
- 26.Laemmli U K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970;227:680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- 27.Marciel A M, Kapur V, Musser J M. Molecular population genetic analysis of a Streptococcus pyogenesbacteriophage-encoded hyaluronidase gene: recombination contributes to allelic variation. Microb Pathog. 1997;22:209–217. doi: 10.1006/mpat.1996.9999. [DOI] [PubMed] [Google Scholar]
- 28.McMillian R A, Bloomster T A, Saeed A M, Henderson K L, Zinn N E, Abernathy R, Watson D W, Greenberg R N. Characterization of a fourth streptococcal pyrogenic exotoxin (SPED) FEMS Microbiol Lett. 1987;44:317–322. [Google Scholar]
- 29.Michie C, Scott A, Cheesbrough J, Beverley P, Pasvol G. Streptococcal toxic shock-like syndrome: evidence of superantigen activity and its effects on T lymphocyte subsets in vivo. Clin Exp Immunol. 1994;98:140–144. doi: 10.1111/j.1365-2249.1994.tb06620.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Misawa S, Aoshima M, Takaku H, Matsumoto M, Hayashi H. High-level expression of Mycoplasma arginine deiminase in Escherichia coliand its efficient renaturation as an anti-tumor enzyme. J Biotechnol. 1994;36:145–155. doi: 10.1016/0168-1656(94)90050-7. [DOI] [PubMed] [Google Scholar]
- 31.Miyazaki K, Takaku H, Umeda M, Fujita T, Huang W, Kimura T, Yamashita J, Horio T. Potent growth inhibition of human tumor cells in culture by arginine deiminase purified from a culture medium of a Mycoplasma-infected cell line. Cancer Res. 1990;50:4522–4527. [PubMed] [Google Scholar]
- 32.Mollick J A, Miller G G, Musser J M, Cook R G, Grossman D, Rich R R. A novel superantigen isolated from pathogenic strains of Streptococcus pyogeneswith aminoterminal homology to staphylococcal enterotoxins B and C. J Clin Invest. 1993;92:710–719. doi: 10.1172/JCI116641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Norrby-Teglund A, Newton D, Kotb M, Holm S E, Norgren M. Superantigenic properties of the group A streptococcal exotoxin SpeF (MF) Infect Immun. 1994;62:5227–5233. doi: 10.1128/iai.62.12.5227-5233.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Oginsky E L. Isolation and determination of arginine and citrulline. Methods Enzymol. 1957;3:639–643. [Google Scholar]
- 35.Ogita S, Tsuto T, Nakamura K, Deguchi E, Tokiwa K, Iwai N. OK-432 therapy for lymphangioma in children: why and how does it work? J Pediatr Surg. 1996;31:477–480. doi: 10.1016/s0022-3468(96)90478-9. [DOI] [PubMed] [Google Scholar]
- 36.Ozegowski J H, Knoll H, Gerlach D, Kohler W. Investigation of erythrogenic toxin C produced by Streptococcus pyogenes. Zentbl Bakteriol Mikrobiol Hyg Ser A. 1984;257:38–50. [PubMed] [Google Scholar]
- 37.Rammelkamp C H, Weaver R S. Acute glomerulonephritis. The significance of the variation in the incidence of the disease. J Clin Invest. 1953;320:345–358. doi: 10.1172/JCI102745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Reda K B, Kapur V, Mollick J A, Lamphear J G, Musser J M, Rich R R. Molecular characterization and phylogenetic distribution of the streptococcal superantigen gene (ssa) from Streptococcus pyogenes. Infect Immun. 1994;62:1867–1874. doi: 10.1128/iai.62.5.1867-1874.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Robinson J H, Kehoe M A. Group A streptococcal M proteins: virulence factors and protective antigens. Immunol Today. 1992;13:362–367. doi: 10.1016/0167-5699(92)90173-5. [DOI] [PubMed] [Google Scholar]
- 40.Sato T, Fujii M, Satoh H, Itoh T, Rikiishi H, Kumagai K. Polyclonal activation of human lymphocytes and induction of cytotoxic lymphocytes by streptococcal preparations. Biotherapy. 1992;4:53–63. doi: 10.1007/BF02171710. [DOI] [PubMed] [Google Scholar]
- 41.Schimke R T, Berlin C M, Sweeney E W, Carroll W R. The generation of energy by the arginine dihydrolase pathway in Mycoplasma hominisO7. J Biol Chem. 1966;241:2228–2236. [PubMed] [Google Scholar]
- 42.Schmidt K H, Gerlach D, Wollweber L, Reichardt W, Mann K, Ozegowski J H, Fleischer B. Mitogenicity of M5 protein extracted from Streptococcus pyogenescells is due to streptococcal pyrogenic exotoxin C and mitogenic factor MF. Infect Immun. 1995;63:4569–4575. doi: 10.1128/iai.63.12.4569-4575.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Shibatani T, Kakimoto T, Chibata I. Crystallization and properties of l-arginine deiminase of Pseudomonas putida. J Biol Chem. 1975;250:4580–4583. [PubMed] [Google Scholar]
- 44.Stollerman G H. Rheumatogenic streptococci and autoimmunity. Clin Immunol Immunopathol. 1991;61:131–142. doi: 10.1016/s0090-1229(05)80019-4. [DOI] [PubMed] [Google Scholar]
- 45.Tewodros W, Norgren M, Kronvall G. Streptokinase activity among group A streptococci in relation to streptokinase genotype, plasminogen binding, and diseases manifestations. Microb Pathog. 1995;18:53–65. doi: 10.1016/s0882-4010(05)80012-9. [DOI] [PubMed] [Google Scholar]
- 46.Tytell A A, Neuman R E. Growth response of stable cell cultures to l-ornithine, l-citrulline, and l-arginine. Exp Cell Res. 1960;20:84–91. doi: 10.1016/0014-4827(60)90225-1. [DOI] [PubMed] [Google Scholar]
- 47.Uchida A, Micksche M. Intrapleural administration of OK-432 in cancer patients: activation of NK cells and reduction of suppressor cells. Int J Cancer. 1983;31:1–5. doi: 10.1002/ijc.2910310102. [DOI] [PubMed] [Google Scholar]
- 48.Weickmann J L, Fahrney D E. Arginine deiminase from Mycoplasma arthritidis. Evidence for multiple forms. J Biol Chem. 1977;252:2615–2620. [PubMed] [Google Scholar]
- 49.Yamamoto A, Nagamuta M, Usami H, Sugawara Y, Watanabe N, Niitsu Y, Urushizaki I. Production of cytotoxic factor into mouse peritoneal fluid by OK-432, a streptococcal preparation. Immunol Lett. 1985;11:83–88. doi: 10.1016/0165-2478(85)90147-6. [DOI] [PubMed] [Google Scholar]
- 50.Yoshida J, Takamura S, Suzuki S. Evidence for the involvement of sulfhydryl groups in the expression of antitumor activity of streptococcal acid glycoprotein (SAGP) purified from crude extract of Streptococcus pyogenes. Anticancer Res. 1994;14:1833–1838. [PubMed] [Google Scholar]
- 51.Yoshida J, Takamura S, Suzuki S, Nishio M. Streptococcal glycoprotein-induced tumour cell growth inhibition involves the modulation of a pertussis toxin-sensitive G protein. Br J Cancer. 1996;73:917–923. doi: 10.1038/bjc.1996.182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Yutsudo T, Murai H, Gonzalez J, Takao T, Shimonishi Y, Takeda Y, Igarashi H, Hinuma Y. A new type of mitogenic factor produced by Streptococcus pyogenes. FEBS Lett. 1992;1:30–34. doi: 10.1016/0014-5793(92)81043-l. [DOI] [PubMed] [Google Scholar]