Abstract
Walled-off pancreatic necrosis (WOPN) is a local complication of acute necrotizing pancreatitis frequently requiring intervention. Treatment is typically through the coordinated efforts of a multidisciplinary team. Current management guidelines recommend a step-up approach beginning with minimally invasive techniques (percutaneous or transmural endoscopic drainage) followed by escalation to more invasive procedures if needed. Although the step-up approach is an evidence-based treatment paradigm for management of pancreatic fluid collections, it lacks guidance regarding optimal invasive technique selection based on the anatomic characteristics of pancreatic fluid collections. Similarly, existing cross-sectional imaging-based classification systems of pancreatic fluid collections have been used to predict disease severity and prognosis; however, none of these systems are designed to guide intervention. We propose a novel classification system which incorporates anatomic characteristics of pancreatic fluid collections (location and presence of disconnected pancreatic duct) to guide intervention selection and clinical decision making. We believe adoption of this simple classification system will help streamline treatment algorithms and facilitate cross-study comparisons for pancreatic fluid collections.
Keywords: Acute pancreatitis, Walled-off necrosis, Necrotizing pancreatitis, Acute pancreatic fluid collections, Pseudocyst
Introduction
Historically, open surgical necrosectomy was the gold standard therapy for walled off pancreatic necrosis (WOPN). However, surgical necrosectomy is associated with increased morbidity [1]. Fortunately, minimally invasive modalities have been developed for the management of acute pancreatic fluid collections (PFCs) including percutaneous drainage, endoscopic transmural drainage and debridement, laparoscopic and robotic necrosectomy, and video-assisted retroperitoneal debridement (VARD) [[2], [3], [4], [5], [6]]. Current practice guidelines recommend a step-up approach starting with percutaneous drainage or transmural endoscopic drainage (if available or technically feasible) [7,8]. In the absence of clinical improvement with first-line treatment, providers can escalate therapy to more invasive procedures including VARD and open pancreatic necrosectomy.
While the step-up approach is an evidence-based treatment paradigm for managing severe acute pancreatitis, it lacks guidance regarding selection of the optimal index invasive technique based on the anatomic characteristics of PFCs [[9], [10], [11], [12]]. Specifically, both the location of the PFC and the presence of disconnected pancreatic duct syndrome (DPDS) significantly impact efficacy and feasibility of minimally invasive procedures [13,14]. We propose a novel classification system incorporating both collection location and presence of DPDS in an attempt to provide clinicians with a more tailored yet standardized approach to management of PFCs.
Methods
Over the last 10 years, our institution (Wake Forest University School of Medicine) has developed and adopted the proposed location-based anatomic classification system for PFCs. This classification system emerged out of a multidisciplinary conference that includes gastroenterology, emergency general surgery, hepatopancreaticobiliary surgery, and interventional radiology. As of May 2023, 304 patients with severe acute pancreatitis have been evaluated and managed in this multidisciplinary conference. The classification system has been further refined based on a retrospective analysis of cross-sectional imaging of 68 patients with acute pancreatitis treated with invasive procedures (endoscopic, percutaneous, or surgical). This anatomic pancreatic fluid collection classification system is based on expert opinion and consensus among the multidisciplinary conference members and focused on selection of the index intervention and not used in prediction of disease severity or clinical outcomes as proposed by other image-based classification systems [15,16].
Anatomic pancreatic fluid collection classification
The proposed Wake Forest anatomic pancreatic fluid collection classification system is summarized in Table 1 and should: (1) be applied only to well-defined or “mature” collections, (2) be applied prior to the index step-up intervention, and (3) not be applied to reactive ascites, pleural effusions, or amylase-rich pancreatic ascites. It is important to remember that many factors (e.g., location of pancreatic duct disruption, nutrition, immune suppression, infection) impact the development of PFCs and rarely does cross-sectional imaging before seven (7) days of the onset of acute pancreatitis have sufficient definition to characterize the anatomic location of PFCs [17,18]. Additionally, it is important to note that collections evolve over time with some resolving spontaneously and others developing in a delayed manner. Annotated and unannotated video images of each type of anatomic pancreatic fluid collection can be found in the Appendix.
Table 1.
Proposed anatomic classification scheme for acute pancreatic fluid collections.
| Collection type | Definition |
|---|---|
| Type A | Retrogastric position only; located entirely within the retroperitoneum. |
| Type B | Pericolic position only; located entirely within the retroperitoneum. |
| Type C | Both retrogastric and pericolic collections; located entirely within the retroperitoneum. |
| Type D | Dominant portion or entire collection posterior to the superior mesenteric vein and artery; displaces mesenteric vessels; may include retrogastric and/or pericolic collections; located entirely within the retroperitoneum. |
| Type E | Portion or entire collection outside of the retroperitoneum; may include retrogastric or pericolic collections; may include collections posterior to the mesenteric vessels. |
| Disconnected Pancreatic Duct Syndrome (*) | Any of the above collection types with disconnected pancreatic duct syndrome demonstrated on cross-sectional imaging or ERCP receives the modifier indicated by an asterisk. |
Type A retrogastric
Definition: Type A PFCs are located posterior (dorsal) to the stomach in a retroperitoneal, retrogastric position (Fig. 1A). Extension to the right along the posterior and lateral aspect of proximal duodenum (D1 and D2) may be present but should not extend beyond the foramen of Winslow or into Morrison's pouch. Extension to the left along the tail of pancreas and spleen may be present but should not extend beyond the splenic flexure of the colon. Type A collections exclude large, complex retrogastric collections that penetrate the peritoneal space through the hepato-gastric, gastro-colic, and gastro-splenic omental attachments, as well as, through the transverse mesocolon. Patients with discontinuous collections outside of the retrogastric position should be excluded.
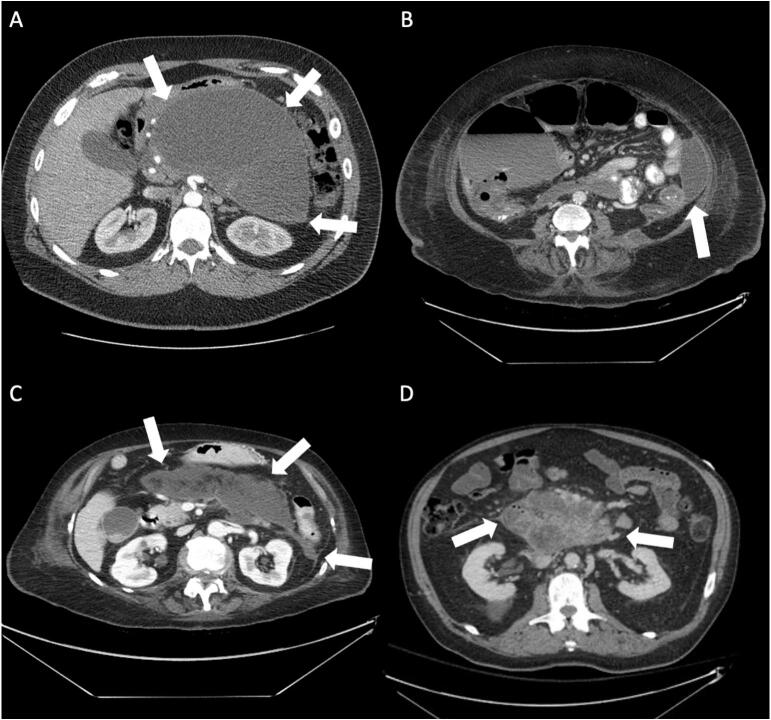
Fig. 1.
(A) A large retrogastric fluid collection in a 40-year-old male patient (Type A). (B) A pancreatic fluid collection in a 52-year-old male involving the left paracolic gutter (Type B). (C) A 74-year-old female with a Type C collection comprised of a retrogastric component which extends into the left paracolic gutter. (D) The majority of this collection is located posterior to the superior mesenteric artery and vein causing anterior displacement of the vessels (Type D).
Rationale: Type A retrogastric collections can be optimally treated with a transgastric approach. Endoscopic placement of a lumen apposing metal stent (LAMS) is generally preferred. Minimally invasive and open surgical techniques (laparoscopic or robotic-assisted) may be applied to Type A PFCs when cholecystectomy is indicated or if solid components in the collection are not favorable to an endoscopic approach.
Type B pericolic
Definition: Type B PFCs are located posterior (dorsal) and lateral to the colon (Fig. 1B). Pancreatic fluid dissects the natural anatomic plane between the colon and the kidney. On the right side, the collection most typically extends along Morrison's pouch and lateral to the ascending colon. On the left side, the collection is posterior to the tail of pancreas and extends posterior to the spleno-colic ligament. Collections can be bilateral along both pericolic gutters and can displace the colon anteriorly (ventrally) and medially. In rare cases, the fluid extends around to the medial aspect of the perinephric fat. Type B PFCs do not have a retrogastric component and do not have a peritoneal component.
Rationale: Type B collections are optimally treated with image-guided percutaneous drainage. In general, endoscopic intervention is not technically possible for Type B collections. Persistent collections may require video-assisted retroperitoneal debridement (VARD). Open or minimally invasive surgical intervention may be indicated when there are multiple large complex collections or if conditions indicate that more than two (2) percutaneous drains are required.
Type C combined retrogastric and pericolic
Definition: Type C PFCs are located adjacent to the stomach and/or duodenum and extend to the paracolic gutters (Fig. 1C). It is a combination of Type A and Type B fluid collections. The collection or collections do not have to be contiguous. Collections may extend to the right, left, or bilateral pericolic spaces. Type C PFCs do not have a peritoneal component.
Rationale: Type C collections are managed with endoscopic or image-guided percutaneous drainage. Combined endoscopic and percutaneous drainage (dual drainage) is also an option in carefully selected cases [[19], [20], [21]]. Persistent collections may require video-assisted retroperitoneal debridement (VARD). Open or minimally invasive surgical intervention may be indicated when there are multiple large complex collections or if conditions suggest multiple percutaneous drains will be inadequate.
Type D posterior to superior mesenteric artery and vein
Definition: Type D PFCs have a dominant component of the collection or the entire collection posterior (dorsal) to the mesenteric vessels (Fig. 1D). Type D collections typically displace the mesenteric root and small bowel mesentery anteriorly (ventrally). The collection or collections do not have to be contiguous and can extend into the retrogastric location (Type A) or pericolic spaces (Type B). Collections may extend to the right, left, or bilateral pericolic spaces. Type D PFCs do not have a peritoneal component.
Rationale: Type D collections present technical challenges for less invasive techniques. The mesenteric vessels may prevent endoscopic and image-guide percutaneous drainage. Minimally invasive or open surgery may be required as the index intervention. Alternatively, antibiotic therapy may be sufficient until the collection or collections become amenable to percutaneous or endoscopic management.
Type E external, outside of the retroperitoneum
Definition: Type E PFCs have any component of a collection or the entire collection outside of the retroperitoneum (Fig. 2). This includes retroperitoneal collections (Type A, B, C, or D) that extend through the hepato-gastric, gastro-colic, and gastro-splenic omental attachments, as well as, through the transverse mesocolon. Additional examples of PFCs are tracking collections into the mediastinum, inguinal canal, flank (external and/or internal oblique muscle), or subcutaneous tissues.
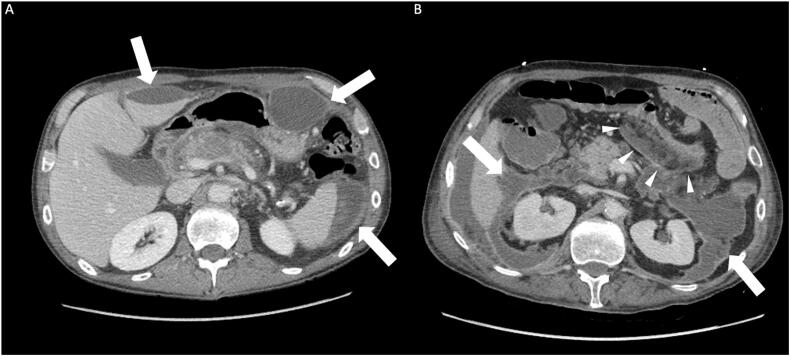
Fig. 2.
(A) A 54-year-old male with multiple fluid collections located outside the retroperitoneum (Type E). (B) A 64-year-old male with multiple complex fluid collections. A portion of the collection has extended through the transverse mesocolon (indicated by white arrowheads) causing compression of the adjacent transverse colon (Type E).
Rationale: Type E collections are usually managed initially with surgery given inability of endoscopic and image-guided percutaneous drainage to adequately access and treat these collections. Extension into the chest may require video-assisted thoracoscopic surgery (VATS). PFCs fistulizing into the inguinal region and subcutaneous tissues may require debridement, wide drainage, and use of a wound management system.
Disconnected duct syndrome modifier *
Definition: Disconnected duct syndrome (DDS) has been defined previously [22,23]. Briefly, DDS is a condition when cross-sectional imaging (CT or MR) or ERCP demonstrates disruption of the main pancreatic duct between segments of viable secreting pancreatic tissue. When imaging indicates DDS, the type of PFC has a modifier indicated by asterisk (*).
Rationale: Disconnected duct syndrome is associated with prolonged hospitalization, longer duration of percutaneous drainage, pancreatic fistula formation, and increased morbidity and mortality [13,14,23]. Intervention focuses on decreasing the risk of pancreatic fistula formation with preferential use of endoscopic drainage and/or internal surgical drainage (e.g. cystogastrostomy or cystoenterostomy). Efforts should be made to avoid percutaneous drainage. DDS can also impact the timing, duration, and prolonged use of stents in endoscopic necrosectomy [24].
Discussion
Severe acute pancreatitis is a potentially life-threatening condition that requires a multi-disciplinary team that can provide prompt and decisive treatment. Several classification systems have been proposed to stratify the severity and prognosis of acute pancreatitis based on clinical, biochemical, radiological, and histological criteria. The most widely used classification systems are the Atlanta classification, the revised Atlanta classification, the determinant-based classification, and the Japanese severity score [[25], [26], [27]]. These systems differ in their definitions and criteria, along with their own strengths and weaknesses. The choice of the most appropriate classification system depends on the clinical context, the availability of resources, and the purpose of the assessment. The characteristics of pancreatic fluid collections have primarily been used to predict the severity and clinic outcomes of acute pancreatitis [15,16,[28], [29], [30], [31], [32], [33]]. Despite this focus on pancreatic fluid collections, anatomic location of PFCs has not been formally applied to guiding invasive procedures.
We propose a novel anatomic classification system (Type A to E with disconnected duct modifier *) that provides a simple structure to characterize often complex pancreatic fluid collections in critically ill patients with severe acute necrotizing pancreatitis. While the simplicity of this classification system may facilitate widespread adoption, the optimal index intervention may vary based on the expertise and resources available locally. Application of this classification system across a variety of institutions will assist in the refinement of this classification system and crystalize the rationale for each PFC type. Evaluation of this classification system will also need to adjust for clinical condition (e.g. APACHE II) and other factors (e.g. active bleeding) that impact the ability to perform specific interventions. The proposed system does have the potential for inter-observer variation as seen in prior studies [34]. In our experience, inter-observer disagreement occurs during classification of PFCs early in the course of acute pancreatitis when collections are not well defined and when the collections are complex, posterior to the mesenteric vessels (Type D), or extend outside of the retroperitoneum (Type E). We envision that use of this classification system can help facilitate selection of the index intervention for severe acute pancreatitis. This classification system should also enable cross study and multi-institutional comparison of invasive procedures and assist with further refinement of the optimal treatment algorithm for severe acute pancreatitis.
Funding sources
Financial support for this manuscript and the associated study were provided by Department of Surgery, Atrium Health Wake Forest Baptist.
Ethics approval
Institutional review board approval was obtained for retrospective analysis portion of this study.
CRediT authorship contribution statement
Clancy J. Clark: Conceptualization, Data curation, Formal analysis, Investigation, Methodology, Project administration, Resources, Software, Supervision, Writing – review & editing, Funding acquisition, Validation, Visualization. Jonathan W. Ray: Data curation, Formal analysis, Investigation, Methodology, Writing – review & editing, Validation, Visualization. Swati Pawa: Methodology, Writing – review & editing. Darius Jahann: Methodology, Writing – review & editing. MaryAlyce McCullough: Conceptualization, Investigation, Methodology, Writing – review & editing. Preston Miller: Writing – review & editing, Conceptualization, Investigation, Methodology. Nathan Mowery: Conceptualization, Methodology, Writing – review & editing. Michael Miller: Conceptualization, Investigation, Methodology, Writing – review & editing. Ted Xiao: Data curation, Formal analysis, Investigation, Writing – original draft. Nicholas Koutlas: Data curation, Investigation, Methodology, Writing – original draft. Rishi Pawa: Conceptualization, Data curation, Formal analysis, Investigation, Methodology, Project administration, Resources, Supervision, Writing – review & editing.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Footnotes
Supported by: Department of Surgery, Atrium Health Wake Forest Baptist.
Disclaimer: The authors declare that they have no conflicts of interest.
Presentation Status: The manuscript has not been previously presented.
Supplementary data to this article can be found online at https://doi.org/10.1016/j.sopen.2024.01.008.
Appendix A. Supplementary data
The following are the supplementary data related to this article.
Type A
Type B
Type C
Type D
Type E
References
- 1.Bang J.Y., Arnoletti J.P., Holt B.A., et al. An endoscopic transluminal approach, compared with minimally invasive surgery, reduces complications and costs for patients with necrotizing pancreatitis. Gastroenterology. 2019;156(4):1027–1040.e3. doi: 10.1053/j.gastro.2018.11.031. [DOI] [PubMed] [Google Scholar]
- 2.Horvath K.D., Kao L.S., Wherry K.L., Pellegrini C., a, Sinanan MN. A technique for laparoscopic-assisted percutaneous drainage of infected pancreatic necrosis and pancreatic abscess. Surg Endosc. 2001;15(10):1221–1225. doi: 10.1007/s004640080166. [DOI] [PubMed] [Google Scholar]
- 3.van Santvoort H.C., Besselink M.G.H., Horvath K.D., et al. Videoscopic assisted retroperitoneal debridement in infected necrotizing pancreatitis. HPB (Oxford) 2007;9(2):156–159. doi: 10.1080/13651820701225688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bakker O.J., van Santvoort H.C., van Brunschot S., et al. Endoscopic transgastric vs surgical necrosectomy for infected necrotizing pancreatitis: a randomized trial. JAMA. 2012;307(10):1053–1061. doi: 10.1001/jama.2012.276. [DOI] [PubMed] [Google Scholar]
- 5.McGuire S.P., Maatman T.K., Ceppa E.P., et al. Contemporary intervention in necrotizing pancreatitis: improved understanding changing practice. J Gastrointest Surg. 2022;26(7):1445–1452. doi: 10.1007/s11605-022-05285-1. [DOI] [PubMed] [Google Scholar]
- 6.Dorrell R., Pawa S., Pawa R. Endoscopic Management of Pancreatic Fluid Collections. J Clin Med. 2021;10(2) doi: 10.3390/jcm10020284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Working Group IAP/APA IAP/APA evidence-based guidelines for the management of acute pancreatitis. Pancreatology. 2013;13(4 Suppl 2):e1–15. doi: 10.1016/j.pan.2013.07.063. [DOI] [PubMed] [Google Scholar]
- 8.Baron T.H., DiMaio C.J., Wang A.Y., Morgan K.A. American Gastroenterological Association clinical practice update: management of pancreatic necrosis. Gastroenterology. 2020;158(1):67–75.e1. doi: 10.1053/j.gastro.2019.07.064. [DOI] [PubMed] [Google Scholar]
- 9.van Santvoort H.C., Besselink M.G., Bakker O.J., et al. A step-up approach or open necrosectomy for necrotizing pancreatitis. N Engl J Med. 2010;362(16):1491–1502. doi: 10.1056/NEJMoa0908821. [DOI] [PubMed] [Google Scholar]
- 10.da Costa D.W., Boerma D., van Santvoort H.C., et al. Staged multidisciplinary step-up management for necrotizing pancreatitis. Br J Surg. 2014;101(1):e65–e79. doi: 10.1002/bjs.9346. [DOI] [PubMed] [Google Scholar]
- 11.Freeman M.L., Werner J., van Santvoort H.C., et al. Interventions for necrotizing pancreatitis: summary of a multidisciplinary consensus conference. Pancreas. 2012;41(8):1176–1194. doi: 10.1097/MPA.0b013e318269c660. [DOI] [PubMed] [Google Scholar]
- 12.Maatman T.K., Zyromski N.J. Management of Necrotizing Pancreatitis. Adv Surg. 2022;56(1):13–35. doi: 10.1016/j.yasu.2022.02.010. [DOI] [PubMed] [Google Scholar]
- 13.Maatman TK, Mahajan S, Roch AM, et al. Disconnected pancreatic duct syndrome predicts failure of percutaneous therapy in necrotizing pancreatitis: Percutaneous Drain in Necrotizing Pancreatitis. In: Pancreatology. Vol 20. Elsevier B.V.; 2020:362–368. doi: 10.1016/j.pan.2020.01.014. [DOI] [PubMed]
- 14.Jang J.W., Kim M.H., Oh D., et al. Factors and outcomes associated with pancreatic duct disruption in patients with acute necrotizing pancreatitis. Pancreatology. 2016;16(6):958–965. doi: 10.1016/j.pan.2016.09.009. [DOI] [PubMed] [Google Scholar]
- 15.Simchuk E.J., Traverso L.W., Nukui Y., Kozarek R.A. Computed tomography severity index is a predictor of outcomes for severe pancreatitis. Am J Surg. 2000;179(5):352–355. doi: 10.1016/s0002-9610(00)00375-5. [DOI] [PubMed] [Google Scholar]
- 16.Balthazar E.J., Robinson D.L., Megibow A.J., Ranson J.H. Acute pancreatitis: value of CT in establishing prognosis. Radiology. 1990;174(2):331–336. doi: 10.1148/radiology.174.2.2296641. [DOI] [PubMed] [Google Scholar]
- 17.van Grinsven J., van Brunschot S., van Baal M.C., et al. Natural history of gas configurations and encapsulation in necrotic collections during necrotizing pancreatitis. J Gastrointest Surg. 2018;22(9):1557–1564. doi: 10.1007/s11605-018-3792-z. [DOI] [PubMed] [Google Scholar]
- 18.van Grinsven J., van Brunschot S., Fockens P., et al. Diagnostic strategy and timing of intervention in infected necrotizing pancreatitis: an international expert survey and case vignette study. Hpb. 2016;18(1):49–56. doi: 10.1016/j.hpb.2015.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gluck M, Ross A, Irani S, et al. Dual modality drainage for symptomatic walled-off pancreatic necrosis reduces length of hospitalization, radiological procedures, and number of endoscopies compared to standard percutaneous drainage. J Gastrointest Surg 2012;16(2):248–256; discussion 256–7. doi: 10.1007/s11605-011-1759-4. [DOI] [PubMed]
- 20.Gluck M., Ross A., Irani S., et al. Endoscopic and percutaneous drainage of symptomatic walled-off pancreatic necrosis reduces hospital stay and radiographic resources. Clin Gastroenterol Hepatol. 2010;8(12):1083–1088. doi: 10.1016/j.cgh.2010.09.010. [DOI] [PubMed] [Google Scholar]
- 21.Ross A., Gluck M., Irani S., et al. Combined endoscopic and percutaneous drainage of organized pancreatic necrosis. Gastrointest Endosc. 2010;71(1):79–84. doi: 10.1016/j.gie.2009.06.037. [DOI] [PubMed] [Google Scholar]
- 22.Maatman T.K., Roch A.M., Lewellen K.A., et al. Disconnected pancreatic duct syndrome: Spectrum of operative management. Journal of Surgical Research. 2020;247:297–303. doi: 10.1016/j.jss.2019.09.068. [DOI] [PubMed] [Google Scholar]
- 23.Vanek P., Urban O., Trikudanathan G., Freeman M.L. Disconnected pancreatic duct syndrome in patients with necrotizing pancreatitis. Surg Open Sci. 2023;11:19–25. doi: 10.1016/j.sopen.2022.10.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Pawa R., Dorrell R., Russell G., Gilliam J., Mishra G., Pawa S. Long-term transmural drainage of pancreatic fluid collections with double pigtail stents following lumen-apposing metal stent placement improves recurrence-free survival in disconnected pancreatic duct syndrome. Dig Endosc. 2022;34(6):1234–1241. doi: 10.1111/den.14266. [DOI] [PubMed] [Google Scholar]
- 25.Thoeni R.F. The revised Atlanta classification of acute pancreatitis: its importance for the radiologist and its effect on treatment. Radiology. 2012;262(3):751–764. doi: 10.1148/radiol.11110947. [DOI] [PubMed] [Google Scholar]
- 26.Dellinger E.P., Forsmark C.E., Layer P., et al. Determinant-based classification of acute pancreatitis severity: an international multidisciplinary consultation. Ann Surg. 2012;256(6):875–880. doi: 10.1097/SLA.0b013e318256f778. [DOI] [PubMed] [Google Scholar]
- 27.Takada T., Isaji S., Mayumi T., et al. JPN clinical practice guidelines 2021 with easy-to-understand explanations for the management of acute pancreatitis. J Hepatobiliary Pancreat Sci. 2022;29(10):1057–1083. doi: 10.1002/jhbp.1146. [DOI] [PubMed] [Google Scholar]
- 28.Fabbri C., Baron T.H., Gibiino G., et al. The endoscopic ultrasound features of pancreatic fluid collections and their impact on therapeutic decisions: an interobserver agreement study. Endoscopy. 2022;54(6):555–562. doi: 10.1055/a-1640-4365. [DOI] [PubMed] [Google Scholar]
- 29.Easler J., Papachristou G.I. The morphologic evolution of necrotic pancreatic fluid collections and their management. Asymptomatic: Delay, Defer and Don’t Panic! 2014;27 www.annalsgastro.gr [PMC free article] [PubMed] [Google Scholar]
- 30.Van Santvoort H.C., Bollen T.L., Besselink M.G., et al. Describing peripancreatic collections in severe acute pancreatitis using morphologic terms: an international interobserver agreement study. Pancreatology. 2008;8(6):593–599. doi: 10.1159/000161010. [DOI] [PubMed] [Google Scholar]
- 31.Rana S.S., Bhasin D.K., Rami Reddy Y., et al. Morphological Features of Fluid Collections on Endoscopic Ultrasound in Acute Necrotizing Pancreatitis: Do They Change over Time? 2014;27 www.annalsgastro.gr [PMC free article] [PubMed] [Google Scholar]
- 32.Ding L., Li X.Y., Tan J.X., et al. Association between morphological features of necrotizing pancreatitis on endoscopic ultrasound and outcomes of the endoscopic transmural step-up approach. J Dig Dis. 2022;23(3):174–182. doi: 10.1111/1751-2980.13083. [DOI] [PubMed] [Google Scholar]
- 33.Ishikawa K., Idoguchi K., Tanaka H., et al. Classification of acute pancreatitis based on retroperitoneal extension: application of the concept of interfascial planes. Eur J Radiol. 2006;60(3):445–452. doi: 10.1016/j.ejrad.2006.06.014. [DOI] [PubMed] [Google Scholar]
- 34.Sternby H., Verdonk R.C., Aguilar G., et al. Significant inter-observer variation in the diagnosis of extrapancreatic necrosis and type of pancreatic collections in acute pancreatitis – an international multicenter evaluation of the revised Atlanta classification. Pancreatology. 2016;16(5):791–797. doi: 10.1016/j.pan.2016.08.007. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Type A
Type B
Type C
Type D
Type E