Abstract
Introduction
Percutaneous cholecystostomy (PCT) is an alternative to cholecystectomy (CCY) in high-risk surgical candidates with severe acute cholecystitis. A subset of these patients ultimately undergo delayed CCY. We therefore compared outcomes of delayed CCY in patients with grade III acute cholecystitis who received a PCT on index admission, to those who did not.
Methods
Non-elective adult hospitalizations for grade III acute cholecystitis that underwent delayed CCY were identified in the 2016–2020 Nationwide Readmission Database. Patients who received a PCT during their index admission comprised the PCT group (others: Non-PCT). Outcomes were assessed for the CCY hospitalization. Entropy balancing was used to generate sample weights to adjust for differences in baseline characteristics. Regression models were created to evaluate the association between PCT and the outcomes of interest.
Results
Of an estimated 13,782 patients, 13.3 % comprised PCT. Compared to Non-PCT, PCT were older (71.1 ± 13.1 vs 67.4 ± 15.3 years) and more commonly in the highest income quartile (22.5 vs 16.1 %, both p < 0.001). After risk adjustment, PCT was associated with reduced odds of respiratory (AOR 0.67, CI 0.54–0.83) and infectious (AOR 0.77, CI 0.62–0.96) complications after eventual CCY. Finally, PCT had comparable pLOS (β +0.31, CI [−0.14, 0.77]) and operative hospitalization costs (β $800, CI [−2300, +600]).
Conclusion
In the present study, PCT was associated with decreased odds of perioperative complications and comparable resource utilization upon readmission CCY. Our findings suggest that PCT may be helpful in bridging patients with grade III acute cholecystitis to eventual CCY.
Keywords: Percutaneous cholecystostomy, Cholecystectomy, Outcomes, Resource utilization
Introduction
Cholelithiasis afflicts >20 million people in the United States and accounts for ∼$5 billion in healthcare expenditures, annually. Importantly, an estimated 1 % of patients with cholelithiasis will develop acute cholecystitis (AC) requiring hospitalization [1,2]. Cholecystectomy (CCY) remains the definitive treatment for AC, as patients may otherwise experience recurrence and disease progression beyond their initial presentation [3]. However, contemporary management guidelines have endorsed the use of percutaneous cholecystostomy (PCT) or definitive antibiotic therapy in select patients who may be at increased perioperative risk for cholecystectomy [4]. While this approach may obviate the need for urgent surgery, PCT may, in fact, complicate future cholecystectomy due to increased risk of bile duct injury [5].
The use of PCT as a bridge to future CCY has shown mixed results in single center studies, partly due to the heterogeneity in study populations and small sample size [[6], [7], [8]]. The 2018 Tokyo Guidelines for the treatment of gallbladder and biliary pathology classifies cholecystitis into grade I-III, with grade III defined as acute pathology with additional organ dysfunction. In those with grade III disease who may be at increased risk for surgery, the guidelines have recommended initial percutaneous biliary drainage followed by delayed CCY [4,9]. Nevertheless, outcomes between nonoperatively managed patients who received PCT or definitive antibiotic therapy, rather than CCY, during their index admission for cholecystitis remain ill-defined. Given practice variability, additional research is required to examine the impact of prior PCT on outcomes of CCY deferred to a later date [10].
The present study sought to examine the association of initial PCT placement on perioperative outcomes and resource utilization of delayed CCY. We hypothesized that index PCT would be associated with shorter time to CCY, higher odds of perioperative complications, as well as increased postoperative length of stay (pLOS) and hospitalization costs.
Methods
We performed a retrospective cohort study using the 2016 to 2020 Nationwide Readmissions Database (NRD). Maintained by the Healthcare Cost and Utilization Project, the NRD is the largest readmissions database providing accurate estimates for 60 % of annual US hospitalizations [11]. The NRD uses unique hospital and patient identifiers, facilitating analyses across multiple hospitalizations within the calendar year.
All non-elective adult (≥18 years) hospitalizations with a primary diagnosis of acute cholecystitis were identified using relevant International Classification of Diseases, Tenth Revision (ICD-10) codes (Supplemental Table 1). Patients who underwent CCY at the index hospitalization were excluded from study. Grade III AC was defined as previously described by Dimou et al. [12]. Given the structure of NRD, only those who underwent CCY within the same calendar year of their initial admission were included for analysis. Patients with a diagnosis of choledocholithiasis, gallstone pancreatitis, or cholangitis were excluded. This was done to minimize the influence of outliers and complex cases that may be subject to more nuanced decision-making that may not be adequately represented within the NRD. Records with missing data for age, sex, costs, and length of stay, were also excluded (0.9 %; Fig. 1). Patients were then stratified into PCT and Non-PCT on the basis of whether they received a PCT during the index hospitalization.
Fig. 1.
Exclusion criteria.
Patient and hospital characteristics including age, sex, income quartile, primary payer, hospital setting, teaching status, and bed size were defined according to the NRD data dictionary [11]. Additionally, perioperative complications were determined using previously published ICD-10 codes [13]. The van Walraven modification of the Elixhauser Comorbidity Index was utilized to evaluate the burden of chronic comorbid conditions [14,15]. Hospitalization costs were computed using institution-specific cost-to-charge ratios, which was then adjusted for inflation to the 2020 Personal Health Index [11,16].
The primary outcomes of interest were perioperative complications. Secondary outcomes included pLOS, hospitalization costs, and rates of non-home discharge. All outcomes were assessed for the CCY hospitalization in order to ensure appropriate postoperative comparison between groups. Perioperative outcomes included neurologic (stroke or transient ischemic attack), cardiac (cardiac arrest or myocardial infarction), respiratory (acute respiratory failure, prolonged ventilation, pneumothorax, or acute respiratory distress syndrome), gastrointestinal or hepatobiliary (bile leak, upper gastrointestinal bleeding, iatrogenic bowel perforation, liver infarction, and hepatic vein thrombosis), thrombotic (deep vein thrombosis and pulmonary embolism), and infectious (urinary tract infection, sepsis, wound infection) complications.
Categorical data are reported as group proportions (%) and continuous data as means with standard deviation (SD) or medians with interquartile ranges (IQR). Adjusted Wald and Pearson's χ2 tests were used to determine the significance of intergroup differences for continuous and categorical variables, respectively. The significance of temporal trends were evaluated using Cuzick's non-parametric rank-based test (nptrend) [17]. A multivariable logistic regression model was constructed to examine factors associated with PCT. Prior to examining perioperative outcomes and resource utilization, entropy balancing was used to obtain optimal sample weights to balance covariates between groups (Supplemental Fig. 1). Unlike propensity score matching, this methodology maintains the entire cohort for analysis and reduces sampling bias [[18], [19], [20]]. Logistic and linear regression models using entropy balanced sample weights were then utilized to determine the association of PCT with the aforementioned outcomes. All models were optimized using Bayesian information criteria and receiver operating characteristics (C-statistic) [21]. Regression outputs are reported as adjusted odds ratios (AOR) or beta-coefficients (β) with 95 % confidence intervals (CI). An α <0.05 was set for significance.
Supplemental Fig. 1.

Pre- and post-covariate balancing after entropy balancing.
All statistical analyses were performed using Stata 16.1 software (StataCorp, College Station, TX). This study was deemed exempt from full review by the Institutional Review Board at the University of California, Los Angeles.
Results
Of an estimated 13,782 patients who met study criteria, 1827 (13.3 %) received a PCT at the index hospitalization for grade III acute cholecystitis, while others were initially managed medically. Throughout the study period, the proportion of patients having an index PCT and receiving a delayed CCY increased from 12.0 to 15.3 % (nptrend = 0.02; Fig. 2). Among the grade III AC group, 25.5 % of patients who received an index PCT underwent delayed CCY, while 33.2 % of those who did not required a subsequent readmission CCY. Compared to Non-PCT, PCT were older (71.1 ± 13.1 vs 67.4 ± 15.3 years, p < 0.001), less commonly female (34.1 vs 43.6 %, p < 0.001), and had a higher burden of comorbid disease as measured by the Elixhauser Index (5 [3–6] vs 4 [3–6], p < 0.001). Additionally, PCT were more likely to be in the highest income quartile (22.5 vs 16.1 %, p < 0.001), less commonly uninsured (1.6 vs 2.8 %, p = 0.002), and more frequently presented to metropolitan teaching hospitals (79.5 vs 64.6 %, p < 0.001; Table 1). Finally, prior PCT was associated with a shorter time to CCY readmission (51 ± 42 vs 72 ± 74 days, p < 0.001; Table 1).
Fig. 2.
National trends in overall volume of cholecystectomy and the proportion of cholecystectomies that are Non-Index, 2016–2020. nptrend < 0.001.
Table 1.
Demographic and hospital characteristics of patients who received a percutaneous cholecystostomy during index hospitalization (PCT) and those who did not (Non-PCT) from 2016 to 2020; SD, standard deviation; IQR, interquartile range.
| Non-PCT (n = 11,955) |
PCT (n = 1827) |
p-Value | |
|---|---|---|---|
| Age (years ± SD) | 67.4 ± 15.3 | 71.1 ± 13.1 | <0.001 |
| Female (%) | 43.6 | 34.1 | <0.001 |
| Elixhauser Comorbidity Index (median [IQR]) | 4 [3–6] | 5 [3–6] | <0.001 |
| Income quartile (%) | <0.001 | ||
| >75 % | 16.1 | 22.5 | |
| 51–75 % | 22.5 | 27.0 | |
| 26–50 % | 29.1 | 24.7 | |
| 0–25 % | 30.8 | 24.8 | |
| Insurance coverage (%) | 0.002 | ||
| Private | 14.9 | 15.1 | |
| Medicare | 68.1 | 73.1 | |
| Medicaid | 10.8 | 8.7 | |
| Uninsured | 2.8 | 1.6 | |
| Hospital teaching status (%) | <0.001 | ||
| Non-metropolitan | 9.6 | 2.5 | |
| Metropolitan non-teaching | 25.7 | 18.0 | |
| Metropolitan teaching | 64.6 | 79.5 | |
| Bed size (%) | <0.001 | ||
| Large | 53.5 | 55.6 | |
| Medium | 28.6 | 27.5 | |
| Small | 17.9 | 16.9 | |
| Comorbidities (%) | |||
| Cancer, non-metastatic | 3.9 | 4.9 | 0.15 |
| Cancer, metastatic | 1.7 | 1.3 | 0.32 |
| Cardiac arrhythmia | 34.0 | 39.1 | 0.003 |
| Chronic liver disease | 8.0 | 11.5 | 0.002 |
| Chronic lung disease | 28.1 | 25.4 | 0.09 |
| Coagulopathy | 11.1 | 16.5 | <0.001 |
| Congestive heart failure | 24.7 | 27.8 | 0.05 |
| Diabetes | 44.4 | 47.3 | 0.09 |
| End-stage renal disease | 34.2 | 34.9 | 0.68 |
| Hypertension | 78.9 | 82.6 | 0.01 |
| Neurologic disorder | 10.9 | 13.5 | 0.02 |
| Obesity | 23.8 | 28.4 | 0.004 |
| Peripheral vascular disease | 11.8 | 12.9 | 0.39 |
| Pulmonary circulatory disease | 7.0 | 7.8 | 0.41 |
| Rheumatologic disorder | 4.2 | 3.5 | 0.29 |
Following risk adjustment, increasing age (Adjusted Odds Ratio [AOR] 1.02/year, 95 % Confidence Interval [CI] 1.01–1.03, p < 0.001) and Elixhauser Index (AOR 1.14/unit, CI 1.08–1.21, p < 0.001) were associated with greater odds of receiving a PCT, while female sex (AOR 0.70, CI 0.60–0.82, p < 0.001) was linked with reduced odds of receiving index PCT. A full list of factors associated with receipt of PCT can be found in Table 2.
Table 2.
Factors associated with index PCT among patients with grade III cholecystitis who undergo delayed cholecystectomy. AOR, adjusted odds ratio; CI, confidence interval; IQR, interquartile range.
| AOR | 95 % CI | p-Value | ||
|---|---|---|---|---|
| Age | 1.02 | 1.01 | 1.03 | <0.001 |
| Female | 0.70 | 0.60 | 0.82 | <0.001 |
| Elixhauser Comorbidity Index (median [IQR]) | 1.14 | 1.08 | 1.21 | <0.001 |
| Heart failure | 0.94 | 0.78 | 1.14 | 0.66 |
| Chronic lung disease | 0.76 | 0.63 | 0.91 | 0.007 |
| Coagulopathy | 1.22 | 0.98 | 1.52 | 0.07 |
| Hospital teaching status | <0.001 | |||
| Non-metropolitan hospital | Reference | |||
| Metropolitan non-teaching | 2.36 | 1.46 | 3.80 | <0.001 |
| Metropolitan hospital | 4.21 | 2.65 | 6.68 | <0.001 |
| Income quartile | ||||
| 0–25th percentile | Reference | |||
| 26th to 50th percentile | 0.96 | 0.79 | 1.18 | 0.71 |
| 51st to 75th percentile | 1.26 | 1.01 | 1.56 | 0.04 |
| 76 to 100th percentile | 1.37 | 1.10 | 1.69 | 0.004 |
On unadjusted analysis, PCT had lower rates of respiratory (13.3 vs 18.2 %, p = 0.001), infectious (11.3 vs 14.3 %, p = 0.02), and gastrointestinal complications (2.5 vs 4.4 %, p = 0.01) compared to non-PCT. Furthermore, PCT had comparable pLOS (4 [2–7] vs 4 [2–6] days, p = 0.10), hospitalization costs ($17,300 [11,700 - 26,700] vs $17,700 [12,900 - 26,200], p = 0.99), and a similar incidence of nonhome discharge (19.5 vs 21.8 %, p = 0.12).
After entropy balancing, PCT was associated with reduced odds respiratory (AOR 0.67, CI 0.54–0.83, p < 0.001), thrombotic (AOR 0.40, CI [0.18–0.87], p = 0.02), and infectious complications (AOR 0.77, CI 0.62–0.96, p < 0.001; Fig. 3). Additionally, PCT was linked with similar odds of gastrointestinal complications (AOR 0.60, CI 0.35–1.01, p = 0.06; Fig. 3). Finally, PCT was associated with comparable pLOS (β +0.31, CI [−0.14, +0.77], p < 0.18) and operative hospitalization costs (β -$800, 95%CI [−2300, +600], p < 0.27), as well as lower odds of non-home discharge (AOR 0.73, CI 0.61–0.89, p = 0.001; Table 3) compared to non-PCT.
Fig. 3.
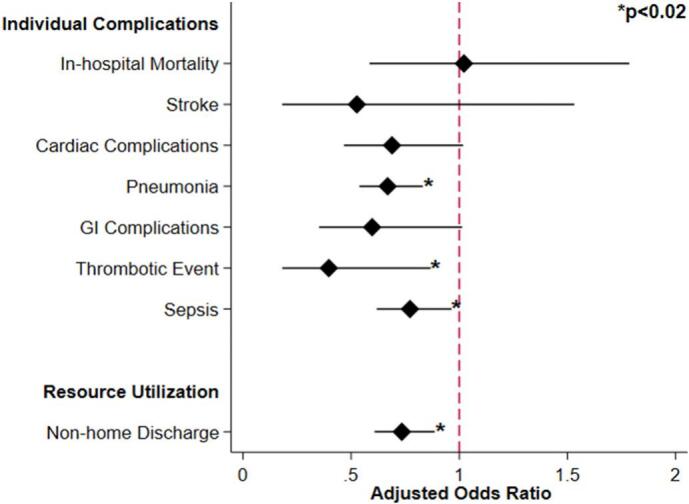
Association of percutaneous cholecystectomy tube with outcomes of those undergoing delayed cholecystectomy (Reference = Non-PC).
Table 3.
Risk-adjusted outcomes following delayed cholecystectomy for patients with a history of cholecystitis who underwent initial cholecystectomy tube placement relative to those who did not. Outcomes reported as adjusted odds ratio or beta coefficient (reference group: Non-PCT). AOR, adjusted odds ratio; β, beta coefficient; CI, confidence interval; CCY, Cholecystectomy; GI, gastrointestinal; pLOS, postoperative length of stay.
| AOR or β Coefficient | 95 % CI | p-Value | |
|---|---|---|---|
| Resource utilization | |||
| CCY hospitalization cost (USD $1000) [IQR] | +800 | [−2300 − +600] | 0.27 |
| pLOS (days) [IQR] | 0.31 | [−0.14 − +0.77] | 0.18 |
| Non-home discharge | 0.73 | [0.61–0.89] | <0.001 |
| Complications | |||
| Neurologicala | 0.53 | [0.18–1.53] | 0.24 |
| Cardiacb | 0.69 | [0.47–1.02] | 0.06 |
| Respiratoryc | 0.67 | [0.54–0.83] | <0.001 |
| GI/hepatobiliaryd | 0.60 | [0.35–1.01] | 0.06 |
| Thrombotic | 0.40 | [0.18–0.87] | 0.02 |
| Sepsis | 0.77 | [0.62–0.96] | 0.02 |
Stroke or transient ischemic attack.
Cardiac arrest or myocardial infarction.
Acute respiratory failure, prolonged mechanical ventilation, or acute respiratory distress syndrome.
Bile leak, upper gastrointestinal bleeding, iatrogenic bowel perforation, liver infarction, and hepatic vein thrombosis.
Discussion
Nonoperative management of AC has been increasingly employed in recent years, with the COVID-19 pandemic further altering surgical decision-making [22,23]. In this nationwide retrospective study, we observed a significant increase in the utilization of PCT as a bridge to CCY among those managed nonoperatively at the index hospitalization for grade III AC. Factors associated with receipt of PCT included increased age, sex, and burden of comorbid disease. Receiving an index PCT was associated with lower odds of experiencing perioperative complications at the time of delayed CCY. Furthermore, index PCT was associated with comparable pLOS and operative hospitalization costs, as well as decreased odds of experiencing non-home discharge. Several of these findings warrant further discussion.
Prior research has provided evidence for the use of PCT in high-risk surgical populations as either definitive treatment for cholecystitis or as a bridge to CCY [24,25]. Although PCT is one non-operative modality to treat AC, others have suggested that bowel rest and definitive antibiotics may be sufficient for patients with AC who are felt to be poor surgical candidates at initial presentation [26]. In the present analysis, 25.5 % of patients who received a PCT underwent delayed inpatient CCY, compared to the 33 % delayed inpatient CCY rate observed among those managed with antibiotics and supportive care. This is in congruence with a single-center retrospective study of 245 patients who received PCT for cholecystitis, where 29 % of patients went on to require interval CCY [27]. Additionally, Suzuki et al. observed that among patients receiving antibiotic treatment for initial presentation of AC, 43 % of patients readmitted for reoccurrence required urgent CCY [28]. While the need for delayed surgery is well established, current literature suggests that deferred CCY is associated with increased risk of perioperative complications and wound infections compared to those who received CCY on initial presentation [29,30]. However, patients who receive nonoperative management during the index admission often have more severe gallbladder inflammation and a higher burden of comorbid disease [31]. Given this, the two cohorts are difficult to compare, and it is imperative to delineate how different non-operative treatment options impact delayed perioperative outcomes within this distinct population. Therefore, the increasing utilization of index PCT as a bridge to deferred CCY warrants further discussion due to its potential impact on clinical outcomes and expenditures.
Given the significant recurrent rate of AC after cholecystostomy, prior literature has recommended its use only as a bridge to eventual elective CCY. In our analysis, which was limited to solely patients who were managed non-operatively at the initial hospitalization for AC, rates of PCT use for Tokyo grade III increased from 12.0 to 15.3 % from 2016 to 2020. Despite this rise, however, only a small fraction of the cohort received a PCT, despite guidelines suggesting it should be strongly considered if CCY will be delayed beyond the initial hospitalization. This may be related to lack of access to PCT or concerns that PCT tubes may delay eventual CCY. On the contrary, our data suggests that, in severe AC that is managed non-operatively, PCT appears to be associated with superior outcomes compared to antibiotics alone. The present study demonstrates that PCT followed by deferred CCY is increasingly more common in an acutely ill population and further establishes potential clinical benefit within this distinct cohort. Given the unique pathology and high rate of disease recurrence within this vulnerable population, our work emphasizes the importance of index care choices on outcomes of delayed CCY.
In our analysis, index PCT was associated with decreased odds of respiratory, thrombotic, and infectious complications at the surgical hospitalization for CCY. There are several plausible explanations for these findings. Given the benefit of continual drainage and potential symptomatic benefit from PCT, patients who received a PCT for cholecystitis may have lower rates of recurrent cholecystitis while the tube is still in place. Indeed, we noted non-elective admissions for definitive CCY to be greater among those who did not initially receive a PCT. The use of PCT has additionally been associated with reduced systemic inflammatory response and less chronic inflammation of the gallbladder, which may facilitate later CCY [32,33]. Alternatively, patients who received PCT may have been fit enough to tolerate PCT placement, though this can often be performed under local anesthesia [34]. Clinical endpoints aside, we found that those bridged with PCT had comparable pLOS and hospitalization costs, as well as reduced odds of nonhome discharge. While prior research has found PCT to be associated with increased cumulative care costs compared to index CCY, the present analysis demonstrates no increase when we look only among those initially managed nonoperatively [35,36]. The findings of the present study demonstrate that index PCT is associated with superior clinical outcomes and comparable resource utilization at deferred CCY. These findings should be considered when evaluating recommendations for value-based care practices.
Our study has several limitations related to its design and the structure of the NRD. The NRD does not contain data regarding specific imaging findings or laboratory reports, though these data points are abstracted into diagnoses at each hospital. Additionally, given the retrospective design, we are unable to make causal associations between index PCT and the observed outcomes during deferred CCY. Moreover, outpatient or emergency room visits for management of cholecystostomy tubes were unable to be studied, and likely influence costs. Furthermore, NRD tracks admissions within one calendar year, and is as such unable to track patients who ultimately received a CCY in a subsequent year. In addition, patient-reported outcomes were not able to be studied, as PCT may influence quality of life. Notwithstanding, this study utilized the largest all-payer database and comprehensive statistical analysis to decrease bias and enhance the generalizability of our findings.
Conclusion
Our findings suggest that percutaneous cholecystostomy tubes are increasingly utilized among patients undergoing non-operative management of severe acute cholecystitis. Compared to antibiotics alone, PCT was associated with lower adjusted odds of respiratory, thrombotic, and infectious complications in patients receiving deferred CCY. Finally, index PCT was associated with comparable postoperative length of stay and operative hospitalization costs, as well as decreased odds of non-home discharge. In conclusion, PCT as a bridge to cholecystectomy for grade III AC is safe, does not delay cholecystectomy, and may be associated with reduced complications and resource use compared to management without PCT.
The following are the supplementary data related to this article.
International Classification of Diseases, 10th Edition, Clinical-Modification Diagnosis Codes to Identify Grade III Cholecystitis, Cholecystectomy, and Cholecystostomy.
Funding/financial support
No external financial support was received from any source.
Ethics approval
This study was deemed exempt from full review by the Institutional Review Board at the University of California, Los Angeles.
CRediT authorship contribution statement
Joanna Curry: Conceptualization, Methodology, Formal analysis, Resources, Writing – original draft, Writing – review & editing. Nikhil Chervu: Conceptualization, Methodology, Formal analysis, Writing – review & editing. Nam Yong Cho: Conceptualization, Methodology, Writing – review & editing. Joseph Hadaya: Writing – review & editing. Amulya Vadlakonda: Methodology, Writing – review & editing. Shineui Kim: Methodology, Writing – review & editing. Jessica Keeley: Conceptualization. Peyman Benharash: Writing – review & editing, Supervision.
Declaration of competing interest
The authors of this manuscript have no related conflicts of interest to declare.
References
- 1.Gallaher J.R., Charles A. Acute cholecystitis: a review. JAMA. 2022;327(10):965–975. doi: 10.1001/jama.2022.2350. [DOI] [PubMed] [Google Scholar]
- 2.Strasberg S.M. Acute calculous cholecystitis. N Engl J Med. 2008;358(26):2804–2811. doi: 10.1056/NEJMcp0800929. [DOI] [PubMed] [Google Scholar]
- 3.Popowicz A., Lundell L., Gerber P., et al. Cholecystostomy as bridge to surgery and as definitive treatment or acute cholecystectomy in patients with acute cholecystitis. Gastroenterol Res Pract. 2015;2016 doi: 10.1155/2016/3672416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Okamoto K., Suzuki K., Takada T., et al. Tokyo guidelines 2018: flowchart for the management of acute cholecystitis. J Hepato-Biliary-Pancreat Sci. 2018;25(1):55–72. doi: 10.1002/jhbp.516. [DOI] [PubMed] [Google Scholar]
- 5.Altieri M.S., Yang J., Yin D., Brunt L.M., Talamini M.A., Pryor A.D. Early cholecystectomy (≤ 8 weeks) following percutaneous cholecystostomy tube placement is associated with higher morbidity. Surg Endosc. 2020;34(7):3057–3063. doi: 10.1007/s00464-019-07050-z. [DOI] [PubMed] [Google Scholar]
- 6.Zeren S. Bridge treatment for early cholecystectomy in geriatric patients with acute cholecystitis; percutaneous cholecystostomy. Turk J Trauma Emerg Surg Published online. 2017 doi: 10.5505/tjtes.2017.63668. [DOI] [PubMed] [Google Scholar]
- 7.Yeo C.S.W., Tay V.W.Y., Low J.K., Woon W.W.L., Punamiya S.J., Shelat V.G. Outcomes of percutaneous cholecystostomy and predictors of eventual cholecystectomy. J Hepato-Biliary-Pancreat Sci. 2016;23(1):65–73. doi: 10.1002/jhbp.304. [DOI] [PubMed] [Google Scholar]
- 8.Nikfarjam M., Shen L., Fink M.A., et al. Percutaneous Cholecystostomy for treatment of acute cholecystitis in the era of early laparoscopic cholecystectomy. Surg Laparosc Endosc Percutan Tech. 2013;23(5):474. doi: 10.1097/SLE.0b013e318290142d. [DOI] [PubMed] [Google Scholar]
- 9.Inoue K., Ueno T., Nishina O., et al. Optimal timing of cholecystectomy after percutaneous gallbladder drainage for severe cholecystitis. BMC Gastroenterol. 2017;17(1):71. doi: 10.1186/s12876-017-0631-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Riall T.S., Zhang D., Townsend C.M., Kuo Y.F., Goodwin J.S. Failure to perform cholecystectomy for acute cholecystitis in elderly patients is associated with increased morbidity, mortality, and cost. J Am Coll Surg. 2010;210(5):668–677. doi: 10.1016/j.jamcollsurg.2009.12.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.NRD Overview. Accessed April 25, 2023. https://hcup-us.ahrq.gov/nrdoverview.jsp.
- 12.Dimou F.M., Adhikari D., Mehta H.B., Riall T.S. Outcomes in older patients with grade III cholecystitis and Cholecystostomy tube placement: a propensity score analysis. J Am Coll Surg. 2017;224(4):502–511.e1. doi: 10.1016/j.jamcollsurg.2016.12.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cho N.Y., Chervu N.L., Sakowitz S., et al. Effect of surgical timing on outcomes after cholecystectomy for mild gallstone pancreatitis. Surgery. 2023;174(3):660–665. doi: 10.1016/j.surg.2023.05.009. [DOI] [PubMed] [Google Scholar]
- 14.Elixhauser A., Steiner C., Harris D.R., Coffey R.M. Comorbidity measures for use with administrative data. Med Care. 1998;36(1):8–27. doi: 10.1097/00005650-199801000-00004. [DOI] [PubMed] [Google Scholar]
- 15.van Walraven C., Austin P.C., Jennings A., Quan H., Forster A.J. A modification of the Elixhauser comorbidity measures into a point system for hospital death using administrative data. Med Care. 2009;47(6):626–633. doi: 10.1097/MLR.0b013e31819432e5. [DOI] [PubMed] [Google Scholar]
- 16.Using Appropriate Price Indices for Expenditure Comparisons. Accessed April 25, 2023. https://meps.ahrq.gov/about_meps/Price_Index.shtml.
- 17.Cuzick J. A Wilcoxon-type test for trend. Stat Med. 1985;4(1):87–90. doi: 10.1002/sim.4780040112. [DOI] [PubMed] [Google Scholar]
- 18.Vegetabile B.G., Ann Griffin B., Coffman D.L., Cefalu M., Robbins M.W., McCaffrey D.F. Nonparametric estimation of population average dose-response curves using entropy balancing weights for continuous exposures. Health Serv Outcomes Res Methodol. 2021;21(1):69–110. doi: 10.1007/s10742-020-00236-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Amusa L., Zewotir T., North D. Examination of entropy balancing technique for estimating some standard measures of treatment effects: a simulation study. Electron J Appl Stat Anal. 2019;12(2):491–507. doi: 10.1285/i20705948v12n2p491. [DOI] [Google Scholar]
- 20.Hainmueller J. Entropy balancing for causal effects: a multivariate reweighting method to produce balanced samples in observational studies. Polit Anal. 2012;20(1):25–46. doi: 10.1093/pan/mpr025. [DOI] [Google Scholar]
- 21.Tilford J.M., Roberson P.K., Fiser D.H. Using lfit and lroc to evaluate mortality prediction models. Stata Tech Bull. 1996;5(28) https://ideas.repec.org//a/tsj/stbull/y1996v5i28sbe12.html Accessed April 25, 2023. [Google Scholar]
- 22.McGillicuddy E.A., Schuster K.M., Barre K., et al. Non-operative management of acute cholecystitis in the elderly. Br J Surg. 2012;99(9):1254–1261. doi: 10.1002/bjs.8836. [DOI] [PubMed] [Google Scholar]
- 23.Purdy A.C., Smith B.R., Hohmann S.F., Nguyen N.T. The impact of the novel coronavirus pandemic on gastrointestinal operative volume in the United States. Surg Endosc. 2022;36(3):1943–1949. doi: 10.1007/s00464-021-08477-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Boules M., Haskins I.N., Farias-Kovac M., et al. What is the fate of the cholecystostomy tube following percutaneous cholecystostomy? Surg Endosc. 2017;31(4):1707–1712. doi: 10.1007/s00464-016-5161-x. [DOI] [PubMed] [Google Scholar]
- 25.Bergman S., Al-Bader M., Sourial N., et al. Recurrence of biliary disease following non-operative management in elderly patients. Surg Endosc. 2015;29(12):3485–3490. doi: 10.1007/s00464-015-4098-9. [DOI] [PubMed] [Google Scholar]
- 26.Rosa F., Covino M., Cozza V., et al. Management of acute cholecystitis in elderly patients: a propensity score-matched analysis of surgical vs. medical treatment. Dig Liver Dis. 2021;53(12):1620–1626. doi: 10.1016/j.dld.2021.01.011. [DOI] [PubMed] [Google Scholar]
- 27.Khasawneh M.A., Shamp A., Heller S., et al. Successful laparoscopic cholecystectomy after percutaneous cholecystostomy tube placement. J Trauma Acute Care Surg. 2015;78(1):100. doi: 10.1097/TA.0000000000000498. [DOI] [PubMed] [Google Scholar]
- 28.Suzuki K., Bower M., Cassaro S., Patel R.I., Karpeh M.S., Leitman I.M. Tube Cholecystostomy before cholecystectomy for the treatment of acute cholecystitis. JSLS. 2015;19(1):e2014.00200 doi: 10.4293/JSLS.2014.00200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Cao A.M., Eslick G.D., Cox M.R. Early cholecystectomy is superior to delayed cholecystectomy for acute cholecystitis: a Meta-analysis. J Gastrointest Surg. 2015;19(5):848–857. doi: 10.1007/s11605-015-2747-x. [DOI] [PubMed] [Google Scholar]
- 30.Cao A.M., Eslick G.D., Cox M.R. Early laparoscopic cholecystectomy is superior to delayed acute cholecystitis: a meta-analysis of case–control studies. Surg Endosc. 2016;30(3):1172–1182. doi: 10.1007/s00464-015-4325-4. [DOI] [PubMed] [Google Scholar]
- 31.Ridtitid W., Kulpatcharapong S., Piyachaturawat P., Angsuwatcharakon P., Kongkam P., Rerknimitr R. The impact of empiric endoscopic biliary sphincterotomy on future gallstone-related complications in patients with non-severe acute biliary pancreatitis whose cholecystectomy was deferred or not performed. Surg Endosc. 2019;33(10):3325–3333. doi: 10.1007/s00464-018-06622-9. [DOI] [PubMed] [Google Scholar]
- 32.Little M.W., Briggs J.H., Tapping C.R., et al. Percutaneous cholecystostomy: the radiologist’s role in treating acute cholecystitis. Clin Radiol. 2013;68(7):654–660. doi: 10.1016/j.crad.2013.01.017. [DOI] [PubMed] [Google Scholar]
- 33.Jang W.S., Lim J.U., Joo K.R., Cha J.M., Shin H.P., Joo S.H. Outcome of conservative percutaneous cholecystostomy in high-risk patients with acute cholecystitis and risk factors leading to surgery. Surg Endosc. 2015;29(8):2359–2364. doi: 10.1007/s00464-014-3961-4. [DOI] [PubMed] [Google Scholar]
- 34.Bakkaloglu H., Yanar H., Guloglu R., et al. Ultrasound guided percutaneous cholecystostomy in high-risk patients for surgical intervention. World J Gastroenterol WJG. 2006;12(44):7179–7182. doi: 10.3748/wjg.v12.i44.7179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Sanaiha Y., Hadaya J., Aguayo E., Chen F., Benharash P. Comparison of diversion strategies for Management of Acute Complicated Diverticulitis in a US Nationwide cohort. JAMA Netw Open. 2021;4(11) doi: 10.1001/jamanetworkopen.2021.30674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Knaapen L. Evidence-based medicine or cookbook medicine? Addressing concerns over the standardization of care. Sociol Compass. 2014;8(6):823–836. doi: 10.1111/soc4.12184. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
International Classification of Diseases, 10th Edition, Clinical-Modification Diagnosis Codes to Identify Grade III Cholecystitis, Cholecystectomy, and Cholecystostomy.




