Abstract
Urinary tract infection, most frequently caused by Escherichia coli, is one of the most common bacterial infections in humans. A vast amount of literature regarding the mechanisms through which E. coli induces pyelonephritis has accumulated. Although cystitis accounts for 95% of visits to physicians for symptoms of urinary tract infections, few in vivo studies have investigated possible differences between E. coli recovered from patients with clinical symptoms of cystitis and that from patients with symptoms of pyelonephritis. Epidemiological studies indicate that cystitis-associated strains appear to differ from pyelonephritis-associated strains in elaboration of some putative virulence factors. With transurethrally challenged mice we studied possible differences using three each of the most virulent pyelonephritis and cystitis E. coli strains in our collection. The results indicate that cystitis strains colonize the bladder more rapidly than do pyelonephritis strains, while the rates of kidney colonization are similar. Cystitis strains colonize the bladder in higher numbers, induce more pronounced histologic changes in the bladder, and are more rapidly eliminated from the mouse urinary tract than pyelonephritis strains. These results provide evidence that cystitis strains differ from pyelonephritis strains in this model, that this model is useful for the study of the uropathogenicity of cystitis strains, and that it would be unwise to use pyelonephritis strains to study putative virulence factors important in the development of cystitis.
Although the bladder, particularly in women, is often exposed to bacteria and although urine generally supports bacterial growth, the combined effects of bladder emptying by urination and an intrinsic defense mechanism associated with bladder epithelium assist in resisting bacterial infection of the bladder (4, 28, 29). However, a breach of natural bladder defense mechanisms resulting from anatomical abnormalities of the urinary tract or, more commonly, virulence factors expressed by the colonizing bacteria results in urinary tract infection (UTI). Symptomatic UTI is manifest in two syndromes. One is acute pyelonephritis clinically identified by flank pain and fever and generally perceived as being a kidney infection. The second is cystitis, characterized by dysuria and increased frequency and urgency of urination, which is generally perceived to be a bladder infection and accounts for 95% of all visits to physicians for UTIs (7).
In the normal urinary tract, most UTIs are caused by Escherichia coli. Studies on the uropathogenicity of E. coli have focused primarily on the development of pyelonephritis. Epidemiologic studies have implicated adhesins, particularly P fimbriae, and other factors, such as hemolysin, in the development of acute pyelonephritis; some of these have been confirmed to be virulence factors by in vitro and in vivo studies (9, 10, 18–20). Only recently have epidemiologic studies begun to focus on cystitis, the more frequent UTI syndrome. Although some putative virulence factors appear to be common, as a group cystitis-associated strains differ from pyelonephritis-associated strains. For instance, cystitis strains less frequently possess P fimbriae than acute pyelonephritis strains (2, 22, 28).
In this study, we tested the hypothesis that the widely used mouse model of pyelonephritis introduced by Hagberg et al. (9) distinguishes E. coli strains causing acute pyelonephritis from those causing acute cystitis.
MATERIALS AND METHODS
Bacterial strains.
Seven strains of E. coli recovered from cultures of the urinary tracts of patients with clinical symptoms of cystitis (36) and three strains from patients with pyelonephritis (39) were studied. The following characteristics of the bacterial strains were assessed: type 1 fimbriae, by mannose-sensitive agglutination of guinea pig erythrocytes (26); P fimbriae, by mannose-resistant agglutination of human type O erythrocytes (26); and cytotoxic necrotizing factor (CNF), by dot blots with the CNF-encoding gene (11a). All strains expressed type 1 fimbriae, hemolysin was expressed by one of the three pyelonephritis-inducing and six of the seven cystitis-inducing strains, all three pyelonephritis strains and six of the cystitis strains expressed P fimbriae, and six cystitis strains expressed CNF. In preliminary studies pyelonephritis strains CPZ 005, CFT 073 (26), and CFT 325 (unpublished observation) colonized mouse bladder, kidneys, and urine in higher numbers than other pyelonephritis strains tested.
For mouse challenge experiments E. coli strains were cultured overnight on Trypticase soy agar (BBL, Cockeysville, Md.). Cells were harvested into phosphate-buffered 0.9% sodium chloride, pH 7.2 (PBS;BBL), and adjusted to approximately 2 × 1010 CFU per ml by comparison to McFarland turbidity standards confirmed by enumeration by the spread plate technique (Spiral Systems, Bethesda, Md.).
Mouse model of urinary tract infection.
Female virus antibody-free CBA/J/Hsd mice, weighing 22 to 24 g (Harlan Sprague Dawley, Indianapolis, Ind.), were quarantined for 1 week after receipt and allowed ad libitum access to tap water and Purina Lab Chow during quarantine and throughout the experiment. For inoculation mice were anesthetized with methoxyflurane (Metofane; Pitman-Moore, Washington Crossing, N.J.). After cleansing of the periurethral area with povidone-iodine solution which was removed with sterile water, a sterile polyethylene catheter (0.28-mm inside diameter, 0.61-mm outer diameter, and 25-mm length) was inserted into the bladder through the urethra. An inoculum of 0.05 ml containing approximately 109 organisms was infused into the bladder through the urethral catheter over 30 s through a tuberculin syringe attached to an infusion pump (Harvard Apparatus, Millis, Mass.) controlled by a timer (Dimco-Gray Co., Dayton, Ohio). The catheter was removed immediately after challenge, and mice were returned to their cages and cared for by the normal routine.
As described previously (27), in each experiment one mouse was used to assess whether the inoculum refluxed into the kidney during the challenge procedure. The inoculum, suspended in sterile India ink (27), was infused into the bladder, as described above. The reflux assessment mouse was sacrificed immediately after challenge; the bladder, ureters, and kidneys were visually inspected for evidence of India ink, and the kidneys were aseptically removed and quantitatively cultured.
Experimental mice were sacrificed by CO2 overdose at 1, 3, 5, or 7 days after challenge. At sacrifice, the abdomen was opened aseptically by a midline incision and urine was aspirated from the bladder with a tuberculin syringe for quantitative bacteriologic culture. After tying of the proximal end of each ureter, the bladder was washed by injecting and aspirating sterile saline. The bladder and each kidney were removed aseptically and halved. One half of each organ was separately homogenized in PBS by using a sterile glass grinder (Kontes, Inc., Vineland, N.J.), and the other half was preserved in 10% neutral buffered formalin for histologic examination. Urine and the homogenized tissue were quantitatively cultured on Trypticase soy agar by the spread plate technique, and the mean number of CFU per milliliter of urine or per gram of bladder or kidney was determined after 24 h of incubation at 37°C.
Histological examination.
Each bladder and kidney was divided longitudinally, and half the organ (including areas with gross abnormality) was fixed overnight in 10% neutral buffered formalin. Paraffin-embedded sections were stained with hematoxylin and eosin and were examined by light microscopy by a pathologist blinded to the infecting organism. The histologic criteria used for evaluation of renal lesions included dilation of the pelvis, neutrophilic infiltration and fibrosis of the pelvis, necrosis of tubular epithelium, interstitial edema, interstitial infiltrates of neutrophils and mononuclear cells in the cortex or medulla, presence of neutrophils or mononuclear cells in collecting ducts and tubules, intraparenchymal abscesses, interstitial fibrosis, tubular atrophy, and periglomerular fibrosis. The location and distribution of the lesions were evaluated, and the severity of lesions were graded with a scoring system in which +, ++, and +++ indicated mild, moderate, and severe pyelitis, respectively. The severity of pyelitis was graded based on low (+) or moderate (++) numbers of inflammatory cells accumulated beneath the epithelium lining the pelvic cavity and within the pelvic cavity, with severe pyelitis (+++) indicated by moderate to large numbers of inflammatory cell infiltrates surrounding the pelvis and extending into the adjacent parenchyma. Focal areas of necrosis and desquamation of the pelvic epithelium were more prominent in severe pyelitis and were accompanied by local neutrophil infiltration and moderate to pronounced purulent exudate in the lumen.
The urinary bladder was evaluated for abnormalities of the luminal epithelium, inflammatory cell infiltrates, and the presence of interstitial edema. The severity of lesions in the urinary bladder was graded as follows: 1+, mild (infiltration of low numbers of neutrophils in the lamina propria, little or no interstitial edema, and absence or local evidence of regenerative hyperplasia in the luminal epithelium); 2+, moderate (infiltration of moderate numbers of neutrophils in the lamina propria, moderate interstitial edema, and moderate generalized hyperplasia of the luminal epithelium); and 3+, severe (diffuse infiltration of moderate to large numbers of neutrophils in the lamina propria, severe interstitial edema, and severe generalized hyperplasia of the luminal epithelium).
Urinary bladders from unchallenged CBA mice were used to examine adherence of E. coli strains to bladder mucosa by a previously described technique (12). Mice were sacrificed by CO2 overdose. Each urinary bladder was aseptically removed, cut to expose the mucosal surface, and placed in a plastic jig. The jig consisted of two polycarbonate squares (a 12-mm-thick base and a 12-mm-thick upper piece containing a 6-mm-diameter hole) held together with a nut and bolt at each corner. The cut mouse bladder was placed into the jig with the mucosal surface up (facing the 6-mm-diameter hole). When the upper piece of the jig was secured to the base, the bladder tissue created a watertight seal at the base of the hole. A bacterial suspension of a washed broth culture (200 μl; 1010 CFU/ml) was placed in the 6-mm-diameter well and allowed to remain in contact with the bladder mucosa for up to 2 h at 37°C. The bacterial suspension was aspirated from the well, and the bladder mucosa was washed three times with PBS (pH 7.2). Bladders were fixed in 2.5% gluteraldehyde in 0.1 M cacodylate buffer (pH 7.4), postfixed in 1% osmium tetroxide in 0.1 M cacodylate buffer (pH 7.4), dehydrated in ethanol and hexamethyldisilazone, gold and palladium coated (Hummer II sputter coater), and examined by scanning electron microscopy (JEOL model JSM T200).
Statistical analysis.
Mean numbers of CFU per milliliter or per gram from cultures of urine or tissue homogenates and mean histologic scores were compared by Student’s t test. Differences in the numbers of mice with bladder or kidney colonized by the challenge organism were compared by chi-square analysis. P values for the tests of the bladder/kidney ratios were calculated using Wilcoxon’s signed rank test. To evaluate the association between urine or tissue counts and tissue histology scores, product-moment correlation coefficients were determined for each day of animal examination and then pooled.
RESULTS
Inspection of the reflux assessment mouse from each experiment immediately after inoculation revealed that India ink was present in each bladder but could not be visualized in the ureters or kidneys of any mice, and the challenge organism was not recovered from culture of kidney homogenates of any reflux assessment mouse.
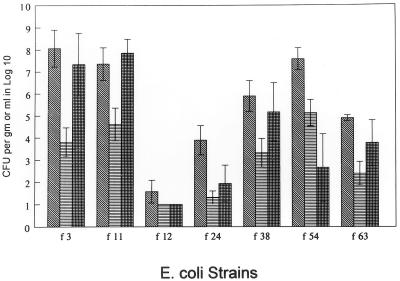
Seven cystitis strains randomly selected from a total of 20 strains in our collection were evaluated in mice to assess uropathogenicity (Fig. 1). At sacrifice 1 week after challenge, strains f3, f11, and f54 were found to colonize the bladder and kidney better than the other four strains. These three strains were used in additional experiments to compare their uropathogenicities with those of the three pyelonephritis strains in our collection that had been found in previous screening experiments to best colonize mouse urine, bladder, and kidneys (CFT 073, CFT 325, and CPZ 005).
FIG. 1.
Colonization of bladder (), kidney (▤), or urine ( ) in mice 7 days after experimental transurethral challenge with E. coli strains originally isolated from patients with clinical symptoms of cystitis. The challenge dose was 109 CFU per mouse. Data are means ± standard errors of the means for five mice per E. coli strain.
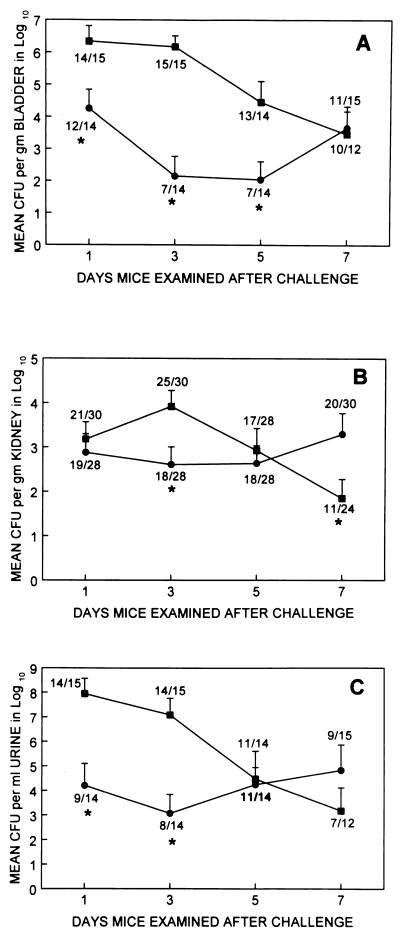
The patterns of mouse UTI produced by cystitis and pyelonephritis strains were different. These were compared by examining the mean bacterial density and prevalence of infection (proportion of animals with ≥103 CFU per ml of urine or per g of tissue) at each time point (Fig. 2).
FIG. 2.
Colonization of mouse bladder (A), kidney (B), and urine (C) following experimental transurethral challenge with E. coli strains originally isolated from patients with clinical symptoms of cystitis (▪) or pyelonephritis (•). The challenge dose was 109 CFU per mouse. Data points (*P ≤ 0.05) are means ± standard errors of the means for 15 mice examined per day (5 mice for each of the cystitis strains f3, f11, and f54 and 5 mice for each of the pyelonephritis strains CFT 073, CFT 325, and CPZ 005). Numbers at each data point are numbers of mice with ≥103 CFU per g or per ml of specimen per the total number of mice for which bacterial growth was detected in at least one specimen.
With cystitis strains, most animals were infected on days 1 and 3 with concentrations of 106 to 108 per g in the bladder and per ml of urine and about 103 per g in the kidney. After day 3, though most animals remained infected, the concentrations of organisms began to fall in each site, so that by day 7 the mean concentrations were <104 per g in the bladder and per ml of urine and <102 per g in the kidneys. In the same experiments, the pyelonephritis strains, though infecting the majority of animals, were initially found at concentrations of only 103 to 104 per g or tissue or per ml of urine. However, these organisms tended to persist at these concentrations in the kidneys and urine throughout the observation period.
A comparison of the cystitis and pyelonephritis strains is instructive. At 1 day after challenge, there was significantly greater colonization of the bladder (2 × 106 versus 2 × 104; P = 0.013) and urine (9 × 107 versus 2 × 104; P = 0.003) by cystitis strains than by pyelonephritis strains; the kidney colonization results were similar (2 × 103 versus 8 × 102; P = 0.62). At 3 days after challenge, the cystitis strains continued to colonize the bladder (2 × 106 versus 1 × 102; P < 0.001) and urine (107 versus 103; P = 0.001) better than the pyelonephritis strains; they also colonized the kidney (8 × 103 versus 4 × 102; P = 0.02) better. At 5 days after challenge, cystitis strains were still recovered from bladder homogenates in significantly higher numbers than were pyelonephritis strains (3 × 104 versus 1 × 102; P = 0.002). But by 7 days after challenge, due to declining colonization by cystitis strains combined with persistent or increasing colonization by pyelonephritis strains, recovery of cystitis strains was similar to that of pyelonephritis strains from the bladder (3 × 103 versus 5 × 103; P = 0.83) and from the urine (2 × 103 versus 6 × 104; P = 0.67). At this time pyelonephritis strains colonized the kidney better than did cystitis strains (2 × 103 versus 7 × 101; P = 0.03).
These findings were generally reflected in the prevalence of infection in tissue and urine. Cystitis strains were found at a significantly higher prevalence than pyelonephritis strains in the bladder on days 3 (15 of 15 versus 7 of 14; P < 0.002) and 5 (13 of 14 versus 7 of 14; P = 0.012) and in urine on days 1 (14 of 15 versus 9 of 14; P = 0.05) and 3 (14 of 15 versus 8 of 14; P = 0.02). Over the 7-day observation period, cystitis strains resulted in significantly higher proportions than pyelonephritis strains of animals with ≥103 CFU per g or per ml in bladder tissue (52 of 56 versus 37 of 57; P = 0.0006) and urine (46 of 56 versus 37 of 57; P = 0.036). However, in the kidney, at no time point were cystitis strains more prevalent than pyelonephritis strains (≥25 of 30 versus 18 of 28; P ≥ 0.09); over the observation period, the proportions of infected kidneys did not differ for cystitis and pyelonephritis strains (74 of 112 versus 75 of 114; P = 0.92).
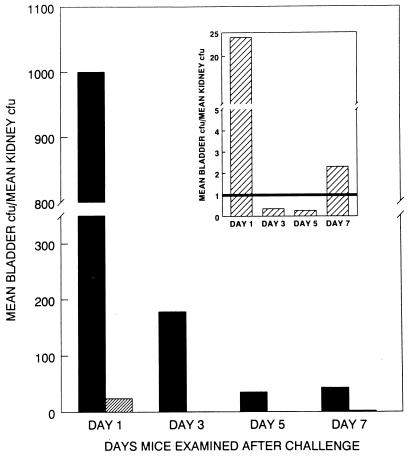
Figure 3 represents the ratio of bladder to kidney bacterial concentration at the different time points. At all time points for cystitis strains and on day 1 for pyelonephritis strains, the ratio was significantly greater than 1 (P ≤ 0.044). For days 3, 5, and 7, the ratio for pyelonephritis strains was not significantly different than 1 (P ≥ 0.35). This demonstrates that cystitis strains throughout the 7-day experiment infected bladder tissue at significantly greater concentration than kidney tissue. Indeed, even as cystitis strains declined in bacterial density throughout the urinary tract, they continued to infect the bladder in higher concentrations than the kidneys. Cystitis strains were found in the bladder on day 1 at 1,000 times the concentration in the kidney (Fig. 2 and 3), on day 3 at about 180 times, and by days 5 and 7 at about 40 times. On the other hand, pyelonephritis strains were found in the bladder at substantially higher concentrations than in the kidney (24 times) (Fig. 2 and inset in Fig. 3) only on day 1. On days 3 through 7, either pyelonephritis strains were found in greater concentration in the kidney (days 3 and 5) or the bladder concentration was only twice the kidney concentration (day 7).
FIG. 3.
Ratio of mean bladder CFU to mean kidney CFU for mice challenged transurethrally with E. coli strains isolated from patients with clinical symptoms of cystitis (▪) or pyelonephritis ( ). The inset shows the ratios for mice challenged with pyelonephritis strains only.
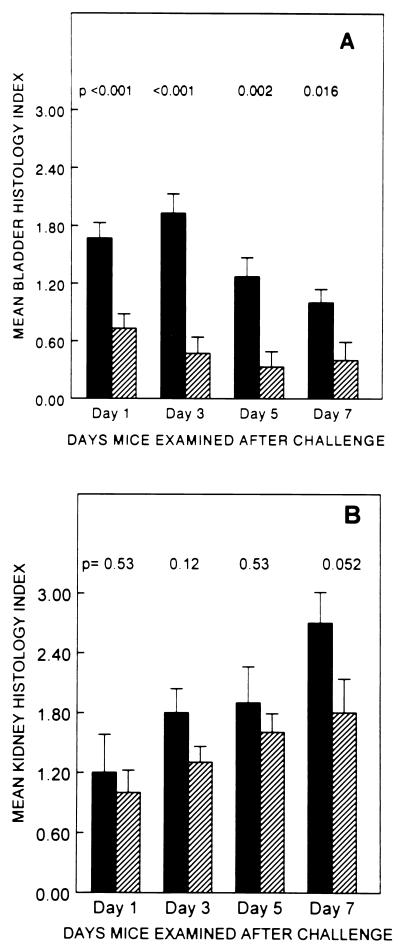
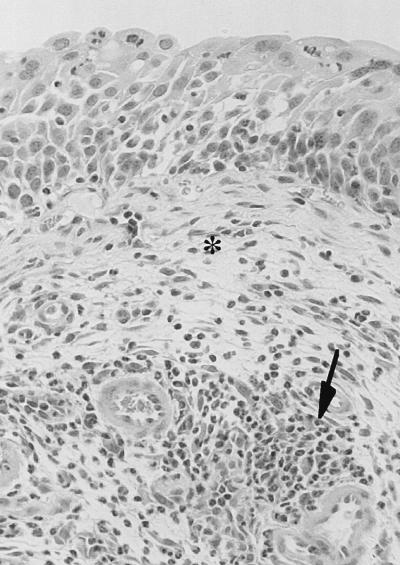
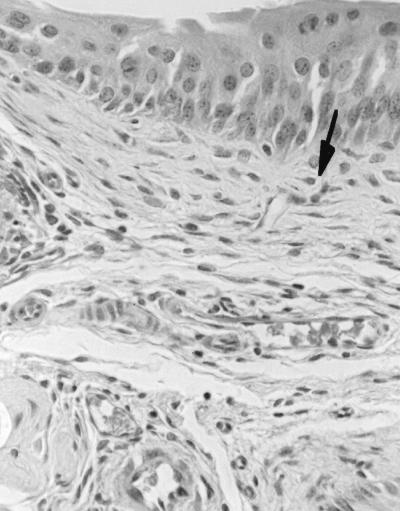
The cystitis strains caused mild to moderate cystitis which persisted through day 7 (Fig. 4). Bladders from mice challenged with cystitis strains had interstitial infiltrates of large numbers of inflammatory cells, mostly neutrophils, prominent interstitial edema, and generalized thickening of the bladder epithelium attributed to regenerative hyperplasia (Fig. 5). On the other hand, the pyelonephritis strains elicited very minimal changes in bladder histology (Fig. 6), significantly less marked than those elicited by cystitis strains on days 1, 3, and 7. Of the 60 mice challenged with cystitis strains and examined over the 7-day observation period, 56 (94%) had histologic evidence of cystitis, compared to only 24 of 60 (40%) mice challenged with pyelonephritis strains (P < 0.0001). Histologic changes in the kidneys increased over the 7-day period and were greater than changes seen in the bladder. However, unlike the changes seen in the bladder, the histologic changes in the kidneys induced by cystitis strains and by pyelonephritis strains on days 1, 3, and 5 were not significantly different (P ≥ 0.12). On day 7 greater changes in kidney histology were seen in mice challenged with cystitis strains than in mice challenged with pyelonephritis strains (P = 0.052).
FIG. 4.
Mean histology index scores (± standard errors of the means) for mice (15 mice per group) that received transurethral challenge with 109 CFU of E. coli strains isolated from patients with clinical symptoms of cystitis (▪) or pyelonephritis ( ).
FIG. 5.
Photomicrograph of mouse bladder showing moderate cystitis (2+) on day 3 after infection with E. coli f11 (a human cystitis strain). The histopathologic changes are characterized by prominent interstitial edema (asterisk) and diffuse infiltration of large numbers of neutrophils (arrow) in the lamina propria. The epithelium is thickened due to regenerative hyperplasia.
FIG. 6.
Photomicrograph of mouse bladder showing mild cystitis (1+) on day 3 after infection with E. coli CFT 073 (a human pyelonephritis strain). The lamina propria appears normal because there is no edema and only low numbers of neutrophils (arrow) are seen. The epithelium exhibits only localized hyperplasia (above arrow).
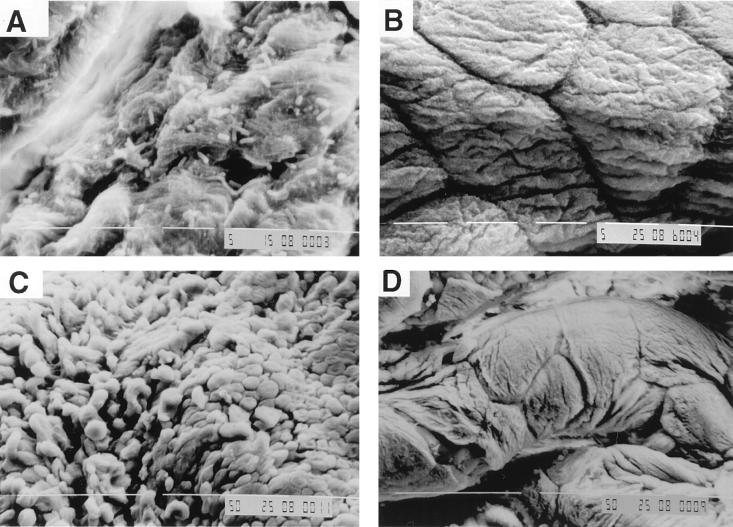
Differences between cystitis and pyelonephritis strains were also observed when mouse bladders exposed in vitro to bacterial suspensions were examined by scanning electron microscopy. As seen in Fig. 7, after 1 h of exposure of the bladder mucosa to E. coli suspension, cells of cystitis strain f11 (Fig. 7A), but not of pyelonephritis strain CFT 073 (Fig. 7B), were observed adhering to the bladder mucosa. After 2 h of exposure cells of f11 were still adhering (Fig. 7C) and no CFT 073 adherence was observed (Fig. 7D); the mucosal surface appeared to be disrupted after f11 exposure for 2 h (Fig. 7C) but not after CFT 073 exposure (Fig. 7D). These observations appear to be consistent with microbiologic results, which showed significantly greater colonization of the bladder by cystitis strains, and with light microscopic results, which showed early epithelial damage followed by generalized regenerative hyperplasia of bladder mucosa in mice challenged with cystitis strains but not in those challenged with pyelonephritis strains.
FIG. 7.
Scanning electron photomicrographs of mouse bladder mucosa after in vitro exposure to E. coli suspensions. After 1 h of exposure cells of cystitis strain f11 are observed adhering to the bladder mucosa (A) but no cells of pyelonephritis strain CFT 073 are observed (B). After 2 h of exposure to cystitis strain f11, the bladder mucosa appears to be disrupted (C), unlike mucosa exposed to pyelonephritis strain CFT 073 for 2 h (D), which appears similar to mucosa exposed to PBS (pH 7.2) for 2 h (data not shown). Magnifications, ×2,000 (A and B) and ×500 (C and D).
Figure 8 shows the correlation coefficients for bladder, kidney, and urine quantitative counts plotted against bladder and histology scores. The strongest correlations were between kidney quantitative counts and kidney histology for cystitis strains (r = 0.36; P = 0.005) and for pyelonephritis strains (r = 0.38; P = 0.003). For cystitis strains, but not for pyelonephritis strains, bladder histology scores were significantly correlated with bladder and urine colony counts. For pyelonephritis strains the only significant correlation was that between kidney histology scores and kidney colony counts.
FIG. 8.
Correlations for the relationship of bladder (b), kidney (k), or urine (u) log10 CFU to bladder histology (BH) or kidney histology (KH) scores for mice challenged with E. coli strains isolated from patients with clinical symptoms of cystitis or pyelonephritis. The correlation coefficients were derived from data for mice examined 1, 3, 5, and 7 days after transurethral challenge. The error bars show the 95% confidence intervals for the pooled correlation coefficients.
DISCUSSION
The majority of UTIs are caused by E. coli (34, 36). Strains that infect the urinary tract are a subset of strains which colonize the colon and possess characteristics, or virulence factors, which allow them to interact with host urinary cells and induce symptomatic urinary infections (38). E. coli strains isolated from patients with clinical symptoms of acute pyelonephritis have been studied extensively. These strains express a number of virulence factors associated epidemiologically with acute pyelonephritis. P fimbriae, the most extensively studied urovirulence factor, mediate the adherence of these organisms to the digalactoside receptor, α-d-Gal-(1→4)-β-d-Gal, the predominant glycolipid found in human kidney tissue (18, 24, 37). The association of P-fimbrial expression and renal infection in mice has been confirmed (9).
A reasonable question might be whether the only distinction between cystitis and pyelonephritis is the site where the organism happens to attach. There are reports suggesting that this sometimes may be the case, i.e., that apparently the same organism earlier causing acute pyelonephritis has caused cystitis upon recurrence in the same patient (2, 33). However, there are other data indicating that cystitis strains as a group are different from pyelonephritis strains. The two groups differ in distribution of O, K, and H serotypes (33). Cystitis strains adhere less well to uroepithelial cells, are less likely to mediate mannose-resistant hemagglutination, and are less often P fimbriated (2, 33, 36). PapG III but not PapG II adhesins appear to be prominent in P-fimbriated cystitis strains (2, 36). Dr adhesins may be associated with cystitis strains more than pyelonephritis strains, particularly in children (2, 36). A multivariate analysis demonstrated that cystitis strains are 40 times more likely than fecal strains to display mannose-resistant hemagglutination not mediated by P fimbriae, a feature not characteristic of pyelonephritogenic strains (35). Taken together, these data suggest that many cases of cystitis are caused by E. coli strains different from those causing acute pyelonephritis.
Although others have assessed the interaction of E. coli strains and mouse bladder (1, 5, 8, 21) or the development of cystitis and pyelonephritis following experimental challenge of mice or nonhuman primates with E. coli (16, 31, 32), this is the first study to assess differences between the uropathogenicities of pyelonephritis and cystitis strains of E. coli in an animal model of UTI. We chose to use the transurethrally challenged mouse model which was originally described by Hagberg et al. (9), who documented that a P-fimbria-expressing strain colonized the kidney better than P-fimbria-negative strains. Subsequent studies have shown that the model may be useful in assessing in vivo potential immunogens for prevention of pyelonephritis (30) and may be adapted to study other aspects of UTI, including Providencia stuartii (12) and Proteus mirabilis (15, 17, 25) uropathogenicity and the effects of an indwelling bladder catheter (13) and urethral obstruction (14).
We hypothesized that the model could be used to study whether patterns of uropathogenicity from E. coli strains isolated from patients with cystitis and from those with pyelonephritis differed. The constraints of cost and time prevented us from comparing the uropathogenicities of all of the 71 pyelonephritis strains and the 20 cystitis strains in our collection. We chose to compare the most virulent strains in each group as determined by separate screening studies in mice. We should note that the screening procedures for the two groups were different. The pyelonephritis strains were screened in mice challenged transurethrally with 1010 CFU/mouse and examined 2 days after challenge, whereas the cystitis strains were screened in mice challenged transurethrally with 109 CFU/mouse and examined 7 days after challenge. The three most uropathogenic strains (greatest colonizers of the bladder and kidney) in each group were selected for comparative studies of mice in which the challenge dose (109 CFU/mouse) and examination times (1, 3, 5, and 7 days after challenge) were standardized.
In the comparative in vivo and in vitro infection studies in mice, the cystitis strains colonized the bladder and urine significantly better than did the pyelonephritis strains. Moreover, significantly more mice challenged with cystitis strains had histologic evidence of cystitis, the mean bladder histology scores were significantly higher than they were in mice challenged with pyelonephritis strains, and, by scanning microscopy, the bladder mucosa was observed to be disrupted after 2 h of exposure to cystitis strains but not after exposure to pyelonephritis strains. These observations demonstrate that the rapid urinary tract colonization by cystitis strains has a propensity for bladder colonization. Over the 7-day observation period there was a significant reduction in bladder, kidney, and urine colonization by cystitis strains, while colonization of the urinary tract by pyelonephritis strains persisted or increased slightly.
This model does appear to distinguish cystitis from pyelonephritis strains. The cystitis strains infect the bladder and urine at high concentrations for up to 3 days and then begin to clear from the urinary tract. The pyelonephritis strains, though infecting at initially lower concentrations, tend to persist in the bladder, kidney, and urine, so that by the end of a 7-day observation period they are present in higher concentrations in the kidney than are the cystitis strains. Even as the cystitis strains are declining in concentration throughout the urinary tract, they tend to have greater bacterial densities in the bladder than in the kidney. On the other hand, the pyelonephritis strains are present at higher concentrations in the bladder first, and thereafter the kidney concentrations are either higher than or equivalent to the bladder concentrations.
For pyelonephritis strains the only significant correlation between bacterial count and tissue histology was that between kidney counts and kidney histology scores. This correlation may reflect the propensity of these strains for preferentially infecting and damaging the kidney. These results appear to be consistent with observations by other authors who have noted a lack of correlation between urine counts and bladder or kidney counts when studying pyelonephritis strains (9, 11). In contrast, for cystitis strains there were weak but significant correlations between urine or bladder counts and bladder histology scores and urine and between bladder or kidney counts and kidney histology scores. This is another example of differences between cystitis and pyelonephritis strains and suggests that for cystitis strains quantitative urine culture results may be useful in monitoring bladder and kidney infection in mice.
We believe that this model mimics in many ways the important features of cystitis in humans. The model is of females, the inoculum is introduced into the bladder, and bacteriuria is a concomitant part of the infection. Cystitis strains infect the bladder in greater concentrations than the kidney. It is interesting that while most clinicians ascribe cystitis to an infection of the bladder, 15 to 25% of “cystitis” cases may actually have bacteria above the level of the bladder as well (3, 6). In our animal model cystitis strains spontaneously began to clear from the urine and urinary tract. While the natural history of cystitis in humans is difficult to ascertain in the era of antibiotics, in a randomized trial Mabeck assigned 53 nonpregnant women with symptomatic bacterial cystitis to placebo therapy (23). Of these, eight required antibiotic therapy because of persistent symptoms. However, of the remaining 45, 43 cleared the bacteriuria without antibiotics, 32 within 1 month (23).
The mechanisms responsible for the observed differences in urinary tract colonization by cystitis and pyelonephritis strains are not known. Presumably, pyelonephritis strains persist in the urinary tract as a result of expression of adhesins that promote colonization of the uroepithelium. Studies are ongoing to determine bacterial and host factors which are important in the induction of cystitis by E. coli strains isolated from patients with clinical symptoms of cystitis.
Differences between patterns of urinary tract colonization by cystitis and pyelonephritis strains were clearly demonstrated by using the transurethrally challenged mouse model. This suggests two important consequences. First, the model should be useful in defining factors that promote infection of the lower urinary tract by cystitis strains. Secondly, since the patterns of urinary tract colonization by cystitis and pyelonephritis strains are different, it would be unwise to attempt to study cystitis by using bacterial strains isolated from patients with clinical symptoms of pyelonephritis.
ACKNOWLEDGMENTS
This work was supported by Public Health Service grant 1 PO1 DK49720-01 from the National Institute of Diabetes and Digestive and Kidney Diseases and by the Research Service, Department of Veterans Affairs.
REFERENCES
- 1.Aronson M, Medalia O, Schori L, Mirelman D, Sharon N, Ofek I. Prevention of colonization of the urinary tract of mice with Escherichia coli by blocking of bacterial adherence with methyl α-d-mannopyranoside. J Infect Dis. 1979;139:329–332. doi: 10.1093/infdis/139.3.329. [DOI] [PubMed] [Google Scholar]
- 2.Arthur M, Johnson C E, Rubin R H, Arbeit R D, Campanelli C, Kim C, Steinbach S, Agarwal M, Wilkinson R, Goldstein R. Molecular epidemiology of adhesin and hemolysin virulence factors among uropathogenic Escherichia coli. Infect Immun. 1989;57:303–313. doi: 10.1128/iai.57.2.303-313.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Busch R, Huland J. Correlation of symptoms and results of direct bacterial localization in patients with urinary tract infections. J Urol. 1984;132:282–285. doi: 10.1016/s0022-5347(17)49595-4. [DOI] [PubMed] [Google Scholar]
- 4.Cox C E, Hinman F. Experiments with induced bacteriuria, vesical emptying and bacterial growth on the mechanism of bladder defense to infection. J Urol. 1961;86:739–748. doi: 10.1016/S0022-5347(17)65257-1. [DOI] [PubMed] [Google Scholar]
- 5.Dalal E, Medalia O, Harari O, Aronson M. Moderate stress protects female mice against bacterial infection of the bladder by eliciting uroepithelial shedding. Infect Immun. 1994;62:5505–5510. doi: 10.1128/iai.62.12.5505-5510.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Eykyn S, Lloyd-Davies R W, Shuttleworth K E, Vinnicombe J. The localization of urinary tract infection by ureteric catheterization. Investig Urol. 1972;9:271–275. [PubMed] [Google Scholar]
- 7.Ferry S, Burman L G, Holm S E. Clinical and bacteriological effects of therapy of urinary tract infection in primary health care: relation to in vitrosensitivity testing. Scand J Infect Dis. 1988;20:535–544. doi: 10.3109/00365548809032503. [DOI] [PubMed] [Google Scholar]
- 8.Gillon G, Small M, Medalia O, Aronson M. Sequential study of bacterial clearance in experimental cystitis. J Med Microbiol. 1984;18:319–326. doi: 10.1099/00222615-18-3-319. [DOI] [PubMed] [Google Scholar]
- 9.Hagberg L, Engberg I, Freter R, Lam J, Olling S, Svanborg Edén C. Ascending, unobstructed urinary tract infection in mice caused by pyelonephritogenic Escherichia coliof human origin. Infect Immun. 1983;40:273–283. doi: 10.1128/iai.40.1.273-283.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hull R A, Gill R E, Hsu P, Minshew B H, Falkow S. Construction and expression of recombinant plasmids encoding type 1 or d-mannose-resistant pili from a urinary tract infection Escherichia coliisolate. Infect Immun. 1981;33:933–938. doi: 10.1128/iai.33.3.933-938.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hultgren S J, Porter T N, Schaeffer A J, Duncan J L. Role of type 1 pili and effects of phase variation on lower urinary tract infections produced by Escherichia coli. Infect Immun. 1985;50:370–377. doi: 10.1128/iai.50.2.370-377.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11a.Island, M. D. Personal communication.
- 12.Johnson D E, Lockatell C V, Hall-Craggs M, Mobley H L T, Warren J W. Uropathogenicity in rats and mice of Providencia stuartiifrom long-term catheterized patients. J Urol. 1987;138:632–635. doi: 10.1016/s0022-5347(17)43287-3. [DOI] [PubMed] [Google Scholar]
- 13.Johnson D E, Lockatell C V, Hall-Craggs M, Warren J W. Mouse models of short- and long-term foreign body in the urinary bladder: analogies to the bladder segment of urinary catheters. Lab Anim Sci. 1991;41:451–455. [PubMed] [Google Scholar]
- 14.Johnson D E, Russell R G, Lockatell C V, Zulty J C, Warren J W. Urethral obstruction of 6 hours or less causes bacteriuria, bacteremia, and pyelonephritis in mice challenged with “nonuropathogenic” Escherichia coli. Infect Immun. 1993;61:3422–3428. doi: 10.1128/iai.61.8.3422-3428.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Johnson D E, Russell R G, Lockatell C V, Zulty J C, Warren J W, Mobley H L T. Contribution of Proteus mirabilisurease to persistence, urolithiasis, and acute pyelonephritis in a mouse model of ascending urinary tract infection. Infect Immun. 1993;61:2748–2754. doi: 10.1128/iai.61.7.2748-2754.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Johnson J R, Berggren T, Newburg D S, McCluer R H, Manivel J C. Detailed histopathological examination contributes to the assessment of Escherichia coliurovirulence. J Urol. 1992;147:1160–1166. doi: 10.1016/s0022-5347(17)37507-9. [DOI] [PubMed] [Google Scholar]
- 17.Jones B D, Lockatell C V, Johnson D E, Warren J W, Mobley H L T. Construction of a urease-negative mutant of Proteus mirabilis: analysis of virulence in a mouse model of ascending urinary tract infection. Infect Immun. 1990;58:1120–1123. doi: 10.1128/iai.58.4.1120-1123.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kallenius G, Molby R. Adhesion of Escherichia colito human periurethral cells correlated to mannose-resistant agglutination of human erythrocytes. FEMS Microbiol Lett. 1979;5:295–299. [Google Scholar]
- 19.Kallenius G, Svenson S B, Molby R, Cedergren B, Hultberg H, Winberg J. Structure of carbohydrate part of receptor on human uroepithelial cells for pyelonephritogenic Escherichia coli. Lancet. 1981;ii:604–606. doi: 10.1016/s0140-6736(81)92743-4. [DOI] [PubMed] [Google Scholar]
- 20.Korhonen T K, Eden S, Svanborg-Eden C. Binding of purified Escherichia colipili to human urinary tract epithelial cells. FEMS Microbiol Lett. 1980;7:237–240. [Google Scholar]
- 21.Liao J, Tomochika K, Watanabe S, Kanemasa Y. Establishment of a mouse model of cystitis and roles of type 1 fimbriated Escherichia coliin its pathogenesis. Microbiol Immunol. 1992;36:243–256. doi: 10.1111/j.1348-0421.1992.tb01662.x. [DOI] [PubMed] [Google Scholar]
- 22.Lindstedt R, Larson G, Falk P, Jodal U, Leffler H, Svanborg C. The receptor repertoire defines the host range for attaching Escherichia colistrains that recognize globo-A. Infect Immun. 1991;59:1086–1092. doi: 10.1128/iai.59.3.1086-1092.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mabeck C E. Treatment of uncomplicated urinary tract infection in non-pregnant women. Postgrad Med J. 1972;48:69–75. [PMC free article] [PubMed] [Google Scholar]
- 24.Marcus D M, Janis R. Localization of glycosphingolipids in human tissues by immunofluorescence. J Immunol. 1970;104:1530–1539. [PubMed] [Google Scholar]
- 25.Massad G, Lockatell C V, Johnson D E, Mobley H L T. Proteus mirabilis fimbriae: construction of an isogenic pmfAmutant and analysis of virulence in a CBA mouse model of ascending urinary tract infection. Infect Immun. 1994;62:536–542. doi: 10.1128/iai.62.2.536-542.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Mobley H L T, Green D M, Trifillis A L, Johnson D E, Chippendale G R, Lockatell C V, Jones B D, Warren J W. Pyelonephritogenic Escherichia coliand killing of cultured human renal proximal tubular epithelial cells: role of hemolysin in some strains. Infect Immun. 1990;58:1281–1289. doi: 10.1128/iai.58.5.1281-1289.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mobley H L T, Jarvis K G, Elwood J P, Whittle D I, Lockatell C V, Russell R G, Johnson D E, Donnenberg M S, Warren J W. Isogenic P-fimbrial deletion mutants of pyelonephritogenic Escherichia coli: the role of αGal(1-4)βGal binding in virulence of a wild-type strain. Mol Microbiol. 1993;10:143–155. doi: 10.1111/j.1365-2958.1993.tb00911.x. [DOI] [PubMed] [Google Scholar]
- 28.Norden C W, Green G M, Kass E H. Antibacterial mechanisms of the urinary bladder. J Clin Invest. 1968;47:2689–2700. doi: 10.1172/JCI105952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.O’Grady F, Cattell W R. Kinetics of urinary tract infection. II. The bladder. Br J Urol. 1966;38:156–162. doi: 10.1111/j.1464-410x.1966.tb09694.x. [DOI] [PubMed] [Google Scholar]
- 30.O’Hanley P, Lark D, Falkow S, Schoolnik G. Molecular basis of Escherichia colicolonization of the upper urinary tract in BALB/c mice. J Clin Invest. 1985;75:347–359. doi: 10.1172/JCI111707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Roberts J A, Kaak M B, Fussell E N. Bacterial adherence in urinary tract infections: preliminary studies in a primate model. Infection. 1989;17:401–404. doi: 10.1007/BF01645559. [DOI] [PubMed] [Google Scholar]
- 32.Roberts R A, Surarez G M, Kaack B, Kallenius G, Svenson S B. Experimental pyelonephritis in the monkey. VII. Ascending pyelonephritis in the absence of vesicoureteral reflux. J Urol. 1985;133:1068–1075. doi: 10.1016/s0022-5347(17)49382-7. [DOI] [PubMed] [Google Scholar]
- 33.Sandberg T, Kaijser B, Lidin-Janson G, Lincoln K, Ørskov F, Ørskov I, Stokland E, Svanborg-Edén C. Virulence of Escherichia coliin relation to host factors in women with symptomatic urinary tract infection. J Clin Microbiol. 1988;26:1471–1476. doi: 10.1128/jcm.26.8.1471-1476.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Savage D C L, Howie G, Adler K, Wilson M I. Controlled trial of therapy in covert bacteriuria of childhood. Lancet. 1975;i:358–361. doi: 10.1016/s0140-6736(75)91277-5. [DOI] [PubMed] [Google Scholar]
- 35.Siitonen A, Martikainen R, Ikaheimo R, Palmgren J, Makela P H. Virulence-associated characteristics of Escherichia coliin urinary tract infection: a statistical analysis with special attention to type 1C fimbriation. Microb Pathog. 1993;15:65–75. doi: 10.1006/mpat.1993.1057. [DOI] [PubMed] [Google Scholar]
- 36.Stapleton A, Moseley S, Stamm W E. Urovirulence determinants in Escherichia coliisolates causing first-episode and recurrent cystitis in women. J Infect Dis. 1991;163:773–779. doi: 10.1093/infdis/163.4.773. [DOI] [PubMed] [Google Scholar]
- 37.Svanborg-Eden C, Hanson L A, Jodal U, Lindberg U, Akerlund A S. Variable adherence to normal human urinary-tract epithelial cells of Escherichia colistrains associated with various forms of urinary-tract infection. Lancet. 1976;ii:490–492. [PubMed] [Google Scholar]
- 38.Warren J W. Host-parasite interactions and host defense mechanisms. In: Schrier R W, Gottschalk C W, editors. Diseases of the kidney. 6th ed. Vol. 1. Boston, Mass: Little, Brown and Company; 1997. pp. 873–893. [Google Scholar]
- 39.Warren J W, Miller E H, Jr, Fitzpatrick B, DiFranco D E, Caplan E S, Tenney J H, Anthony W C. A randomized, controlled trial of cefoperazone vs. cefamandole-tobramycin in the treatment of putative, severe infections with gram-negative bacilli. Rev Infect Dis. 1983;5:S173–S180. doi: 10.1093/clinids/5.supplement_1.s173. [DOI] [PubMed] [Google Scholar]