Abstract
Abnormalities on breast ultrasound (US) images which do not meet the criteria for masses are referred to as nonmass lesions. These features and outcomes have been investigated in several studies conducted by Asian researchers. However, the term “nonmass” is not included in the American College of Radiology (ACR) Breast Imaging Reporting and Data System (BI-RADS) 5th edition for US. According to the Japan Association of Breast and Thyroid Sonology guidelines, breast lesions are divided into mass and nonmass. US findings of nonmass abnormalities are classified into five subtypes: abnormalities of the ducts, hypoechoic areas in the mammary glands, architectural distortion, multiple small cysts, and echogenic foci without a hypoechoic area. These findings can be benign or malignant; however, focal or segmental distributions and presence of calcifications suggest malignancy. Intraductal, invasive ductal, and lobular carcinomas can present as nonmass abnormalities. For the nonmass concept to be included in the next BI-RADS and be widely accepted in clinical practice, standardized terminologies, an interpretation algorithm, and outcome-based evidence are required for both screening and diagnostic US.
Keywords: BI-RADS, Breast cancer, Breast ultrasound, Ductal carcinoma in situ, JABTS, Nonmass lesions
INTRODUCTION
Most breast abnormalities identified on ultrasound (US) are three-dimensional (3D) masses and are distinguished by their shape, margin, orientation, echo pattern, and posterior features [1]. However, some discrete findings observed in practice do not meet these criteria for masses, thus leading to the US concept of “nonmass lesions” [2,3,4]. The features and outcomes of screening and diagnostic US have been investigated in multiple studies by Asian researchers [5,6,7,8,9,10,11]. Currently, nonmass lesions are not part of the American College of Radiology (ACR) Breast Imaging Reporting and Data System (BI-RADS) (5th edition). However, in Japan, US findings of breast lesions have been divided into mass and nonmass since the 1980s; guidelines including the definition, classifications, and differential diagnoses of masses and nonmass abnormalities have been published and updated by the Japan Society of Ultrasonics in Medicine and the Japan Association of Breast and Thyroid Sonology (JABTS) [12,13,14,15]. Although nonmass abnormalities in the JABTS guidelines are different and more comprehensive than those mentioned in recent studies [2,4], it is necessary to understand these features to further discuss the nonmass concept.
In this review, we explain the normal structure of the mammary glands based on US images and summarize the nonmass components in the BI-RADS lexicon. We also introduce the JABTS classifications of nonmass abnormalities with corresponding histological findings and diseases, and the key points for differentiating between benign and malignant diseases. Finally, we discuss the issues and research directions related to the inclusion of nonmass lesions in the next BI-RADS lexicon.
Normal Structure of the Mammary Gland
The smallest unit of the mammary gland is the terminal duct lobular unit (TDLU), comprising a lobule and a terminal duct. The TDLUs come together and largely open into the major subareolar ducts, which then converge to form and open into the nipple. TDLUs are the primary structures from which breast cancers and their precursors arise; age-related lobular involution is associated with the risk of breast cancer [16,17]. The anatomy of the ducts and TDLUs has been shown to be extremely detailed in US, particularly with recent improvements in US technology.
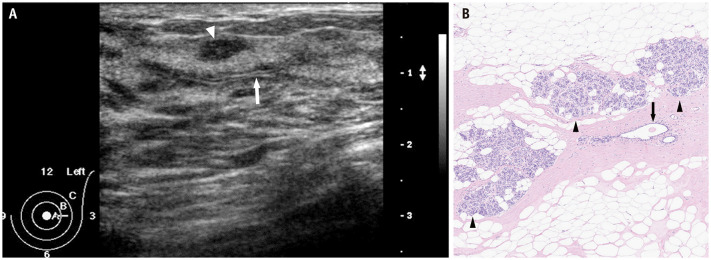
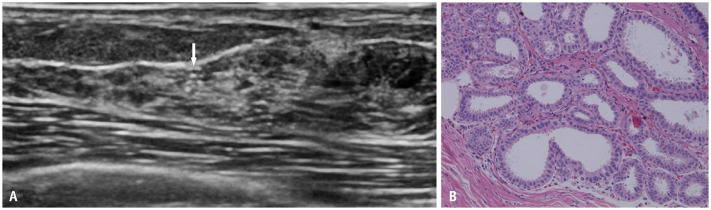
By radially scanning the probe around the nipple, the normal mammary ducts leading to the periphery can be recognized as thick linear to tubular hypoechoic structures in the relatively hyperechoic surrounding tissue. When the US probe is applied orthogonally to the radial direction, neatly aligned patchy or mottled hypoechoic structures are observed. Occasionally, two parallel hyperechoic lines are detected within the structure owing to the US reflections from the mammary duct wall (Fig. 1A). The hypoechoic area surrounding the ducts histologically corresponds to a stroma of densely packed fibrous connective tissue (Fig. 1B). In contrast, the surrounding uniform hyperechoic area corresponds to the stroma of loose fibrous connective tissues [18]. The dense stroma of collagen fibers is thought to remain relatively intact with advancing age, whereas the loose stroma changes with age and body mass index, which is characterized by adipose tissues replacing fibrous connective tissues. When the loose stroma and entire breast are replaced by fat, the US echogenicity of the entire breast is equal to that of the subcutaneous fat.
Fig. 1. Normal structure of the mammary gland. A: Radial US scan shows normal lobules (arrowhead) and ducts extending from the nipple to the periphery as hypoechoic tubular structures. Two parallel hyperechoic lines (arrow) within the structure are due to US reflections from the mammary duct wall. B: Histopathology (hematoxylin and eosin stain) obtained from a different patient shows normal mammary ducts (arrow), lobules (arrowheads), and fibrous and adipose stroma. US = ultrasound.
By scanning the probe with an understanding of normal structures, the presence of nonmass lesions can be identified [19]. However, the proportion of iso- or hypoechoic areas in the fibroglandular tissue, termed “glandular tissue content”, varies considerably among individuals [20,21,22]. Thus, heterogeneous background breast tissue should not be interpreted as a nonmass lesion.
Nonmass Components in ACR BI-RADS US
Although the term, “nonmass”, is not used in the current BI-RADS for the US lexicon, the components of nonmass abnormalities are included. Ductal changes and architectural distortions are listed among the associated features. Moreover, calcifications are a separate major category after masses; the descriptors for calcifications are as follows: 1) in a mass, 2) outside of a mass, or 3) intraductal. Cysts and clustered microcysts are included in special case categories. Notably, the definition of a mass in the BI-RADS US lexicon omitted the convexity criterion to allow for the inclusion of lesions with diffuse growth patterns, such as invasive lobular carcinoma or ductal carcinoma in situ (DCIS).
The 6th edition of BI-RADS is currently under review and may include the term, “nonmass lesions”, as a new category in the revised lexicon. Nonmass lesions are defined as discrete areas that can be differentiated from normal tissues, lack the margins of a mass, and cannot be assigned a specific shape [23]. Several features and descriptors of masses and the proposed nonmass lesions are summarized in Table 1, and discussed in another review article and in an accompanying editorial [24,25].
Table 1. Definitions and features of masses and proposed nonmass lesions in ACR BI-RADS ultrasound.
| Classifications | Definition* | Major features | Associated features |
|---|---|---|---|
| Masses | Three-dimensional and occupying space; should be seen in 2 different planes | Shape, margin, orientation, echo pattern, and posterior features | Calcifications, architectural distortion, duct changes, skin changes, edema, vascularity, and elasticity |
| Nonmass lesions | Discrete finding distinctly different from normal tissue; lacks the margination of a mass and cannot be assigned a specific shape; often subtle and may be visualized primarily in 1 plane only | Distribution and echo pattern | Calcifications, architectural distortion, duct changes, posterior features, vascularity, and elasticity |
*Definitions and features of masses are based on the 5th edition of BI-RADS [1], and those of nonmass lesions are based on presentations at the Society of Breast Imaging symposium in May 2023 [23]. In the BI-RADS lexicon, calcifications are not in “associated features” category and are a separate major category after mass. Modified from Choi et al. J Breast Imaging 2023 [24].
ACR = American College of Radiology, BI-RADS = Breast Imaging Reporting and Data System
Nonmass Abnormalities in the JABTS Guidelines
In the JABTS guidelines, a mass is defined as a space-occupying lesion or lump formed by components that differ from the surrounding tissue, whereas nonmass abnormalities refer to lesions that are difficult to discern as a mass on US images [14]. The term, “abnormality,” was chosen to emphasize a finding with pathological significance, particularly with a texture that differed from the normal surrounding tissue. The US findings of nonmass abnormalities are classified into five subtypes: 1) abnormalities of the ducts, 2) hypoechoic areas in the mammary glands, 3) architectural distortion, 4) multiple small cysts, and 5) echogenic foci without a hypoechoic area (Table 2). While some cases presented findings of only one subtype, many exhibited a mixture of five subtypes. Although nonmass abnormalities may co-exist with masses, the abovementioned findings were often depicted as isolated findings without mass formation. Therefore, it remains clinically essential to understand these nonmass abnormalities.
Table 2. Definition and differential diagnosis of five subtypes of nonmass abnormalities in the JABTS guidelines.
| Classifications | Definition | Differential diagnosis | |
|---|---|---|---|
| Benign | Malignant | ||
| Abnormalities of the ducts | The properties of the ducts such as caliber, wall thickness, or regularity are different from those of normal ducts | Intraductal papillomas, epithelial hyperplasia, duct ectasia | DCIS, invasive ductal carcinoma with a predominant intraductal component, invasive ductal carcinoma |
| Hypoechoic area in the mammary gland* | A hypoechoic area with properties that differ from those of the surrounding mammary gland or contralateral mammary gland that is difficult to discern as a mass | Epithelial hyperplasia, fibrosis, radial scar, complex sclerosing lesions, sclerosing adenosis, mastitis | DCIS, invasive ductal carcinoma with a predominant ductal component, invasive ductal carcinoma, invasive lobular carcinoma, inflammatory carcinoma |
| Architectural distortion | Findings of mammary gland structure-concentrated tightening/distortion at one spot or in a localized area in the mammary gland | Sclerosing adenosis, complex sclerosing lesions, postsurgical scar, fat necrosis, granulomatous mastitis, fibrosis | DCIS, invasive ductal carcinoma, invasive lobular carcinoma |
| Multiple small cysts | A finding in which multiple lesions recognized as small cysts several millimeters in size are observed in the mammary gland | Fibrocystic change, mucocele-like lesions | DCIS† |
| Echogenic foci without a hypoechoic area | Solitary finding of spotty echogenic foci without ductal abnormalities, hypoechoic areas, or distortion | Fibrocystic change, epithelial hyperplasia | DCIS, invasive ductal carcinoma with a predominant intraductal component |
Definitions in English are according to Ito et al. J Med Ultrason 2023;50:331-339 [15].
*In the JABTS guidelines, the echogenicity of the hypoechoic area is defined relative to the surrounding mammary tissue. However, both Breast Imaging Reporting and Data System and JABTS define the echogenicity of mass findings relative to the subcutaneous fat, †This is only possible when small cysts are present in a localized area.
JABTS = Japanese Association of Breast and Thyroid Sonology, DCIS = ductal carcinoma in situ
Various benign and malignant diseases can present as nonmass abnormalities. These findings are most useful in detecting DCIS; they are reflected in the anatomic structures of the ducts and lobules. In a multicenter study of 705 DCIS lesions, 277 (39%) were masses whereas 428 (61%) were nonmass abnormalities [26]. Some invasive carcinomas can also be appropriately recognized as nonmass abnormalities rather than masses. The following sections defines each nonmass abnormality subtype, describes the differential diagnoses, and discusses important considerations in clinical and screening situations. The goal of each section is to guide the detection of clinically significant cancers without increasing false-positive results.
Abnormalities of the Ducts
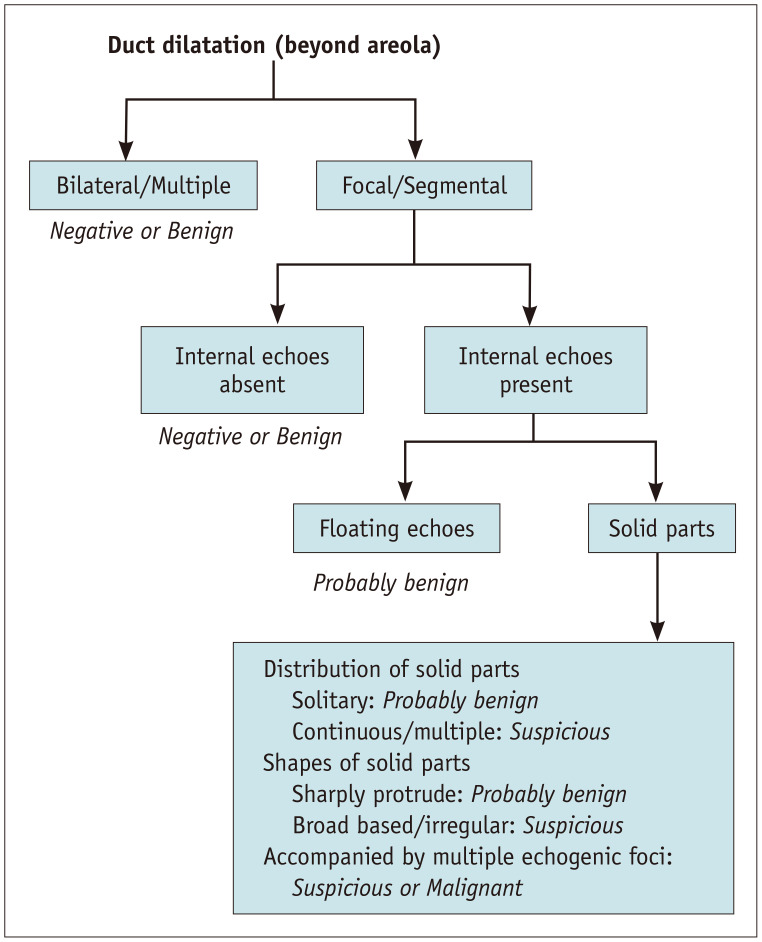
In lactating breasts, multiple dilated mammary ducts with milk are visible on US. However, bilateral or unilateral ductal dilatations with smooth walls and absent internal echogenicity can be observed below the nipple or areola in normal non-lactating breasts. Fluid secretions in the ducts sometimes appear as internal echoes; however, checking for floating can help differentiate whether they are fluid or solid. Therefore, dilated ducts observed only in certain areas beyond the nipple, ducts with irregular widening and narrowing, ducts with solid echoes, and ducts with thickened walls should be recognized as ductal abnormalities and distinguished from ducts without any pathological significance.
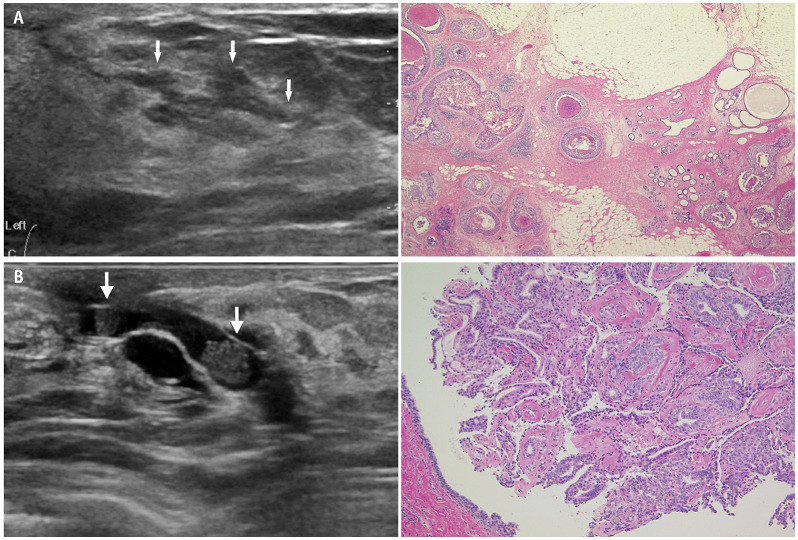
Diseases recognized as ductal abnormalities include, intraductal papillomas, epithelial hyperplasia, ductal ectasia, DCIS, and invasive ductal carcinoma with predominant intraductal component [27,28]. Suspicious findings for cancer include the presence of a unilateral segmental distribution, irregularity of ductal caliber, continuous or multiple lesions, a gradual angle between intraductal proliferative lesions and the duct wall, and calcifications in the solid component (Fig. 2A) [29]. In benign diseases such as intraductal papillomas, the solid component in the dilated duct forms a steep angle with the duct wall and has high homogeneous echoes (Fig. 2B). The interpretation guidelines for ductal abnormalities are shown in Figure 3.
Fig. 2. Abnormalities of the ducts. A: High-grade ductal carcinoma in situ. US (left) shows a dilated duct with irregular calipers (arrows). Mammary ducts in other directions are normal. Histopathology (right, hematoxylin and eosin stain) shows cancer cells proliferating with solid and comedo patterns. B: Intraductal papilloma. US (left) shows two solid components (arrows) in the dilated ducts with steep rise and relatively high homogeneous echoes. Histopathology (right, hematoxylin and eosin stain) from core needle biopsy shows a papilloma with typical frond-like epithelium surrounding a fibrovascular core. US = ultrasound.
Fig. 3. Assessment of abnormalities of the ducts suggested by the Japan Association of Breast and Thyroid Sonology guidelines. Modified from Ban et al. J Med Ultrason 2020;47:107-115 [29].
Hypoechoic Areas in the Mammary Glands
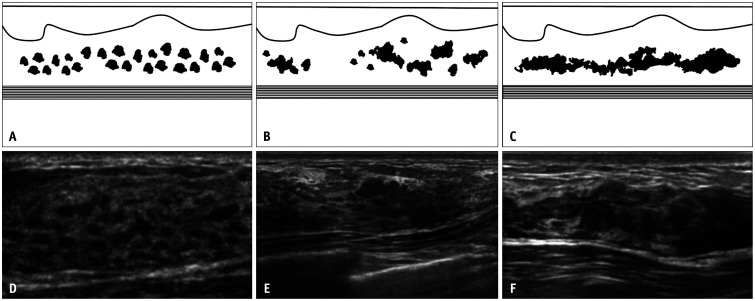
A hypoechoic area has different properties as compared with the surrounding or contralateral mammary gland but does not meet the criteria for a mass. In the case of a mass, the internal echoes are defined relative to the fatty tissue, whereas the term, “hypoechoic,” here refers to the echogenicity relative to the surrounding mammary or fibro-glandular tissue. The terms, “patchy” or “mottled,” are used to describe areas consisting of relatively small, speckled, low echoes, whereas the term, “geographic” is used when such areas have fused together; the term, “indistinct,” is used to describe areas that have indistinguishable boundaries (Fig. 4). Because this type of abnormality is determined by a deviation from the normal mammary structure, the JABTS terminology and diagnostic criteria committee has concluded that it is clinically more appropriate to compare echogenicity with the easily comparable internal echoes of mammary tissue rather than with distant fatty tissue, where comparison of echo levels is often ambiguous.
Fig. 4. Three patterns of hypoechoic areas in the mammary gland. A-C: Schematics depict typical patchy or mottled (A), geographic (B), and indistinct (C) patterns of hypoechoic areas. D-F: Ultrasound images from three different women show patchy or mottled (D), geographic (E), and indistinct (F) patterns, respectively.
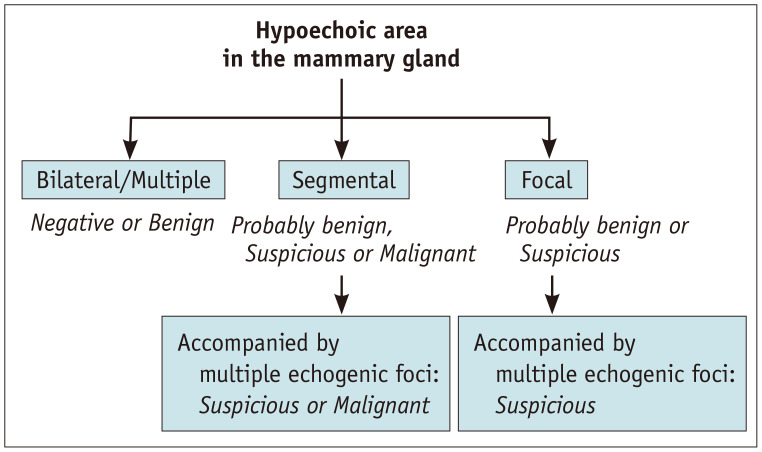
The presence of hypoechoic areas in the mammary glands is the most important finding for understanding breast-related diseases. However, distinguishing pathological findings that deviate from normal physiologic patterns or fibrocystic changes is difficult. The key point of differentiation is the distribution of the hypoechoic areas. Multiple similar findings in the bilateral breasts suggest normal or benign lesions, whereas focal or segmental distribution suggests suspicious or malignant lesions (Fig. 5). Therefore, if a hypoechoic area is found, it should be compared with the normal ipsilateral or contralateral side for evaluation.
Fig. 5. Assessment of hypoechoic areas in the mammary gland suggested by the Japan Association of Breast and Thyroid Sonology guidelines.
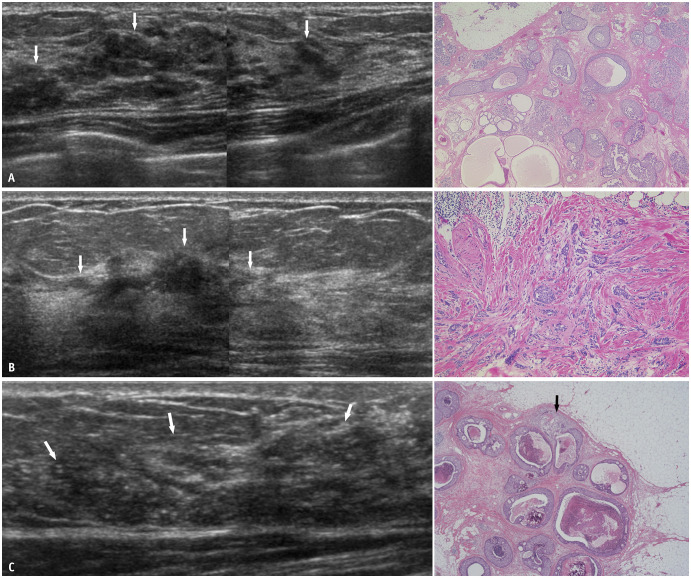
Diseases that can be depicted as hypoechoic areas in the mammary glands include benign pathologies such as epithelial hyperplasia, fibrosis, radial scar, sclerosing adenosis, and mastitis. Malignant diseases include DCIS (Fig. 6A), invasive ductal carcinoma with predominant intraductal component, invasive ductal carcinoma, invasive lobular carcinoma (Fig. 6B), and inflammatory carcinoma [30,31,32]. The presence of calcifications raises concerns regarding the possibility of malignancy [33,34,35]. It is difficult to distinguish whether a lesion is localized within the ducts (in situ) or has an invasive component (Fig. 6C). In some cases, a preoperative diagnosis of DCIS may change to invasive ductal carcinoma after surgery. Invasion should be considered particularly when lesions are extensive.
Fig. 6. Hypoechoic areas in the mammary gland. A: Intermediate-grade DCIS. US (left) shows partly patchy and partly geographic hypoechoic areas (arrows) with segmental distribution. Histopathology (right, hematoxylin and eosin stain) shows papillary-cribriform-solid growth patterns of DCIS. B: Invasive lobular carcinoma. US (left) shows a geographic hypoechoic area (arrows) with segmental distribution. The interface between adipose and glandular tissues appears to be partially interrupted. Histopathology (right, hematoxylin and eosin stain) shows small non-cohesive cells invading the stroma. C: Microinvasive carcinoma. US (left) shows an indistinct hypoechoic area (arrows) with segmental distribution. Numerous echogenic foci suggesting calcifications are present within the hypoechoic area. On histopathology (right, hematoxylin and eosin stain), most of the areas are non-invasive, comedo, solid, cribriform, papillary proliferation; however, focal areas of tumor invasion (arrow) are present. DCIS = ductal carcinoma in situ, US = ultrasound.
Architectural Distortion
Architectural distortion pertains to a lesion that distorts breast tissue without mass formation. This reflects convergent changes in the tissue, which can be attributed to both benign and malignant findings. Surgery and trauma are common causes of architectural distortion; history taking is important for diagnosis [36]. Fibrosis and scarring caused by preoperative chemotherapy can also cause distortion [37].
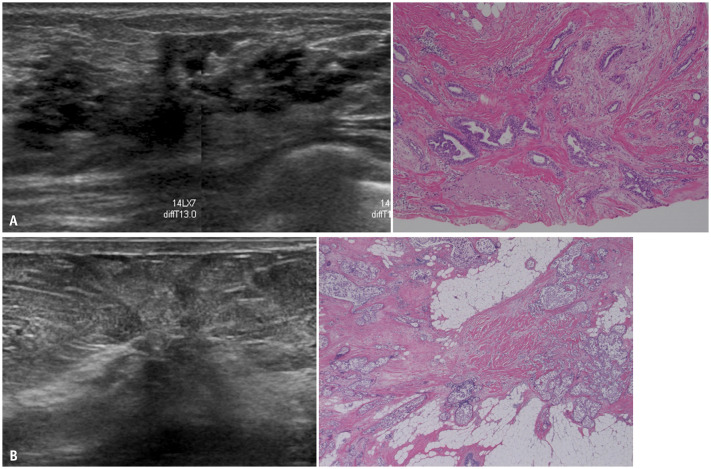
In benign cases, distortion occurs in sclerosing adenosis, radial scar/complex sclerosing lesions, fat necrosis, granulomatous mastitis, and fibrosis (Fig. 7A) [38,39,40]. In malignant cases, DCIS, invasive ductal carcinoma, and invasive lobular carcinoma can present as distortion [30,41,42]. When DCIS develops from a background of sclerosing adenosis, it could be mistaken for invasive cancer on imaging or pathological examination (Fig. 7B) [43]. It is difficult to establish the extent of cancer when the pathology of DCIS involves sclerosing adenosis. DCIS associated with sclerosing adenosis is often bilateral [44,45]. Moreover, sclerosing adenosis is known to be a risk factor for breast cancer. Thus, careful follow-up may be required [46].
Fig. 7. Architectural distortion. A: Complex sclerosing lesions. US (left) shows a hypoechoic area with intense retraction. Histopathology (right, hematoxylin and eosin stain) of vacuum-assisted biopsy shows complex sclerosing lesions, including sclerosing adenosis, ductal hyperplasia, and cysts. After more than 10 years of follow-up, the lesions are found to be gradually decreasing in size. B: Intermediate-grade DCIS with sclerosing adenosis. On US (left), strong convergence is detected toward one point. The anterior interface between adipose and glandular tissues appears to be interrupted by distortion. On histopathology (right, hematoxylin and eosin stain), solid-type DCIS cells are seen from a background of sclerosing adenosis. US = ultrasound, DCIS = ductal carcinoma in situ.
Multiple Small Cysts
Breast cysts are circumscribed anechoic masses with accentuated posterior echoes and imperceptible walls. However, multiple small cysts, which are several millimeters in size, may easily be understood when considering them as a single entity rather than when recognizing each cyst as a mass. If no cysts are found in other parts of the breast while a cluster of small cysts is observed in a localized area, the term, “clustered microcysts,” can be used.
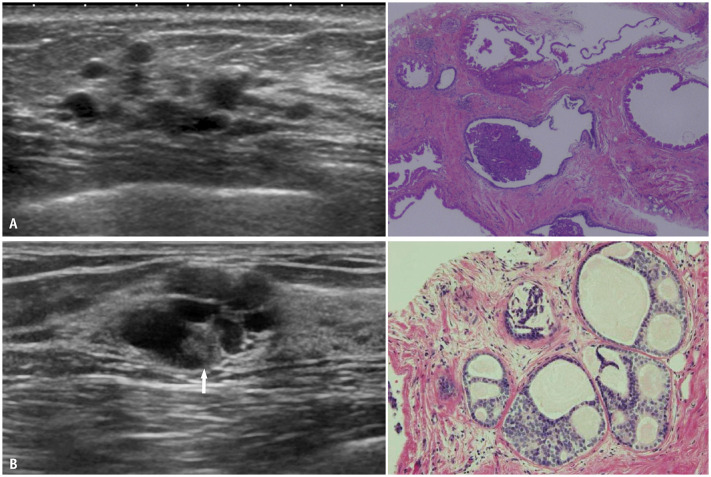
Multiple small cysts are classified into two groups based on their distribution: 1) the presence of multiple scattered cysts in the bilateral breasts, which are typically benign, due to fibrocystic changes, 2) the presence of multiple small cysts with focal or segmental distributions and clustered microcysts. Most of these lesions are benign (Fig. 8A), including apocrine metaplasia, epithelial hyperplasia, or mucocele-like lesions; however, low-grade DCIS (Fig. 8B) is also considerable if the lesions are accompanied by surrounding hypoechoic areas or calcifications [47,48,49]. Thus, a core needle biopsy may be required if clustered microcysts are associated with solid components, thick walls, or other suspicious features. However, on screening US, multiple small cysts alone do not require further examination because the frequency of malignancy is extremely low when multiple small cysts are the only findings, which are often indolent and seldom malignant [50,51].
Fig. 8. Multiple small cysts. A: Ductal epithelial hyperplasia. US (left) shows several small cysts in a localized area. Histopathology (right, hematoxylin and eosin stain) from core needle biopsy shows multiple cysts and epithelial hyperplasia. B: Low-grade DCIS. US (left) shows clustered small cysts; a small solid portion (arrow) is suspected. Histopathology (right, hematoxylin and eosin stain) from core needle biopsy shows a flat, low papillary, and cribriform type of DCIS. US = ultrasound, DCIS = ductal carcinoma in situ.
Echogenic Foci without a Hypoechoic Area
Microcalcifications on mammography can sometimes be recognized on US as echogenic foci without definite ductal abnormalities or hypoechoic areas. However, advancements in US equipment have made these findings more common for both benign and malignant diseases.
When suspicious calcifications are detected on mammographic screening, careful scanning of the corresponding areas can distinguish echogenic foci which cannot be identified on routine examinations (Fig. 9) [52,53]. These are helpful in determining the target for needle biopsy. However, echogenic foci can also be caused by normal breast tissue or artifacts. The detection of calcifications without any other associated findings can lead to increased false-positive results and over-diagnosis. Thus, echogenic foci alone are not included in the recall criteria for US screening [14,50].
Fig. 9. Echogenic foci without a hypoechoic area. A: Targeted ultrasound for the evaluation of clustered calcifications detected on screening mammography shows echogenic foci (arrow) without an associated hypoechoic area or mass in the corresponding location. B: Histopathology (hematoxylin and eosin stain) shows a low-grade, flat and low papillary-type of ductal carcinoma in situ.
Additional Findings: Vascularity and Elasticity
The evaluation of nonmass abnormalities is based on B-mode images; however, color Doppler and elastography may also aid in evaluation. Information on vascularity is important because early-stage breast cancers, including DCIS, are often hypervascular. In the hypoechoic areas of mammary glands, increased blood flow is more likely to indicate malignancy [6]. It has also been reported that micro-invasive carcinoma is more hypervascular than DCIS, thus rendering it useful for differentiating the presence of micro-invasion [54]. Furthermore, it has been reported that high vascularity is useful for improving specificity during screening [55].
Information on elasticity by either strain or shear-wave elastography can be used to characterize benign and malignant lesions in nonmass hypoechoic or distorted areas [56]. In strain elastography, elasticity is scored from 1 (soft) to 5 (hard) by light compression, where scores of 4 and 5 indicate malignancy [57]. However, some DCIS cases are soft and similar to the surrounding tissue. Thus, as compared to masses, the criteria for use with nonmass abnormalities are not established [58,59].
Issues and Research Directions Related to Inclusion in BI-RADS
For the nonmass concept of breast US to be included in the next BI-RADS (6th edition) and be widely accepted in clinical practice, the following issues need to be addressed. First, terminology needs to be standardized. Clear definitions for nonmass lesions should be established such that the same lesions are less likely to be classified as either mass or nonmass. In addition, the terms, “nonmass abnormalities” and “nonmass lesions”, must also be distinguished; “nonmass abnormalities” should be used when referring to all five subtypes included in the JABTS guidelines, whereas “nonmass lesions” should be used when referring to nonmass hypoechoic areas in the mammary glands. Therefore, the definitions and usage of these terms require further discussion. Second, there is a need for standardizing the feature descriptors and assessments of nonmass lesions. Multiple groups have reported on nonmass lesions over the past 20 years; however, they have included different lesions and analyzed varying characteristics [5,6,7,8,9,10,11]. Therefore, a standardized interpretation algorithm based on several key features, such as lesion distribution and the presence of associated calcifications or vascularity, can aid in the management of nonmass lesions [24]. Finally, the outcomes of nonmass lesions on US, including cancer detection rates and positive predictive values, should be reported in both the screening and diagnostic settings. However, increased attention to nonmass lesions might promote more false-positive results on screening US, thus making it a current concern. In screening US, nonmass lesions requiring needle biopsy are uncommon [5,6] and should be distinguished from heterogeneous breast tissues or normal variants. The likelihood of malignancy increases when the US lesions are correlated with findings on other imaging modalities or clinical presentations.
CONCLUSION
In the JABTS guidelines, US findings of breast lesions are divided into mass and nonmass. Nonmass abnormalities have five subtypes, including abnormalities of the ducts, hypoechoic areas in the mammary glands, and architectural distortion. Nonmass abnormalities are an important concept in breast US imaging and must be correctly understood based on the knowledge of normal mammary structures. This leads to a more accurate understanding of breast cancer development and progression as well as its corresponding US findings, which is important when performing US examinations and establishing a diagnosis. The BI-RADS 5th edition are not sufficient for nonmass findings; therefore, a new lexicon corresponding to the type of lesion described as “hypoechoic areas in the mammary glands”, in the JABTS guidelines may be added under the name of “nonmass lesions” in the next revision to address this issue. For the nonmass concept of breast US to be widely accepted in clinical practice, standardized terminologies, an interpretation algorithm, and outcome-based evidence are required for both screening and diagnostic US.
Acknowledgments
The authors thank Dr. Naoki Kanomata, Department of pathology of St. Luke’s International Hospital and Dr. Han Suk Ryu, Department of pathology of Seoul National University Hospital for histological images related to this work.
Footnotes
Conflicts of Interest: The authors have no potential conflicts of interest to disclose.
- Conceptualization: Hiroko Tsunoda.
- Project administration: Woo Kyung Moon.
- Resources: Hiroko Tsunoda.
- Supervision: Woo Kyung Moon.
- Writing—original draft: Hiroko Tsunoda.
- Writing—review & editing: all authors.
Funding Statement: WKM was supported by the National Research Foundation of Korea and funded by the Ministry of Science, ICT, and Future Planning (2022R1A2C1091282).
References
- 1.Mendelson EB, Böhm-Vélez M, Berg WA, Whitman GJ, Feldman MI, Madjar H, et al. American College of Radiology, editors. ACR BI-RADS® atlas, breast imaging reporting and data system. 5th ed. Reston, VA: American College of Radiology; 2013. ACR BI-RADS® ultrasound; pp. 41–102. [Google Scholar]
- 2.Uematsu T. Non-mass-like lesions on breast ultrasonography: a systematic review. Breast Cancer. 2012;19:295–301. doi: 10.1007/s12282-012-0364-z. [DOI] [PubMed] [Google Scholar]
- 3.Giess CS, Chesebro AL, Chikarmane SA. Ultrasound features of mammographic developing asymmetries and correlation with histopathologic findings. AJR Am J Roentgenol. 2018;210:W29–W38. doi: 10.2214/AJR.17.18223. [DOI] [PubMed] [Google Scholar]
- 4.Choe J, Chikarmane SA, Giess CS. Nonmass findings at breast US: definition, classifications, and differential diagnosis. Radiographics. 2020;40:326–335. doi: 10.1148/rg.2020190125. [DOI] [PubMed] [Google Scholar]
- 5.Lee J, Lee JH, Baik S, Cho E, Kim DW, Kwon HJ, et al. Non-mass lesions on screening breast ultrasound. Med Ultrason. 2016;18:446–451. doi: 10.11152/mu-871. [DOI] [PubMed] [Google Scholar]
- 6.Kim SJ, Park YM, Jung HK. Nonmasslike lesions on breast sonography: comparison between benign and malignant lesions. J Ultrasound Med. 2014;33:421–430. doi: 10.7863/ultra.33.3.421. [DOI] [PubMed] [Google Scholar]
- 7.Park JW, Ko KH, Kim EK, Kuzmiak CM, Jung HK. Non-mass breast lesions on ultrasound: final outcomes and predictors of malignancy. Acta Radiol. 2017;58:1054–1060. doi: 10.1177/0284185116683574. [DOI] [PubMed] [Google Scholar]
- 8.Park KW, Park S, Shon I, Kim MJ, Han BK, Ko EY, et al. Non-mass lesions detected by breast US: stratification of cancer risk for clinical management. Eur Radiol. 2021;31:1693–1706. doi: 10.1007/s00330-020-07168-y. [DOI] [PubMed] [Google Scholar]
- 9.Ko KH, Jung HK, Kim SJ, Kim H, Yoon JH. Potential role of shear-wave ultrasound elastography for the differential diagnosis of breast non-mass lesions: preliminary report. Eur Radiol. 2014;24:305–311. doi: 10.1007/s00330-013-3034-4. [DOI] [PubMed] [Google Scholar]
- 10.Ko KH, Hsu HH, Yu JC, Peng YJ, Tung HJ, Chu CM, et al. Non-mass-like breast lesions at ultrasonography: feature analysis and BI-RADS assessment. Eur J Radiol. 2015;84:77–85. doi: 10.1016/j.ejrad.2014.10.010. [DOI] [PubMed] [Google Scholar]
- 11.Wang ZL, Li N, Li M, Wan WB. Non-mass-like lesions on breast ultrasound: classification and correlation with histology. Radiol Med. 2015;120:905–910. doi: 10.1007/s11547-014-0493-x. [DOI] [PubMed] [Google Scholar]
- 12.Ueno E, Tohno E, Itoh K. [Classification and diagnostic criteria in breast echography] Jpn J Med Ultrasonics. 1986;13:19–31. Japanese. [Google Scholar]
- 13.Japan Association of Breast and Thyroid Sonology. [Guideline for breast ultrasound: management and diagnosis] Tokyo: Nankodo; 2004. Japanese. [Google Scholar]
- 14.Japan Association of Breast and Thyroid Sonology. [Guideline for breast ultrasound: management and diagnosis] 4th ed. Tokyo: Nankodo; 2020. Japanese. [Google Scholar]
- 15.Ito T, Ueno E, Endo T, Omoto K, Kuwajima A, Taniguchi N, et al. The Japan Society of Ultrasonics in Medicine guidelines on non-mass abnormalities of the breast. J Med Ultrason. 2023;50:331–339. doi: 10.1007/s10396-023-01308-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wellings SR, Jensen HM, Marcum RG. An atlas of subgross pathology of the human breast with special reference to possible precancerous lesions. J Natl Cancer Inst. 1975;55:231–273. [PubMed] [Google Scholar]
- 17.Milanese TR, Hartmann LC, Sellers TA, Frost MH, Vierkant RA, Maloney SD, et al. Age-related lobular involution and risk of breast cancer. J Natl Cancer Inst. 2006;98:1600–1607. doi: 10.1093/jnci/djj439. [DOI] [PubMed] [Google Scholar]
- 18.Izumori A, Horii R, Akiyama F, Iwase T. Proposal of a novel method for observing the breast by high-resolution ultrasound imaging: understanding the normal breast structure and its application in an observational method for detecting deviations. Breast Cancer. 2013;20:83–91. doi: 10.1007/s12282-011-0313-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Uematsu T, Izumori A, Moon WK. Overcoming the limitations of screening mammography in Japan and Korea: a paradigm shift to personalized breast cancer screening based on ultrasonography. Ultrasonography. 2023;42:508–517. doi: 10.14366/usg.23047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lee SH, Ryu HS, Jang MJ, Yi A, Ha SM, Kim SY, et al. Glandular tissue component and breast cancer risk in mammographically dense breasts at screening breast US. Radiology. 2021;301:57–65. doi: 10.1148/radiol.2021210367. [DOI] [PubMed] [Google Scholar]
- 21.Lee SH, Moon WK. Glandular tissue component on breast ultrasound in dense breasts: a new imaging biomarker for breast cancer risk. Korean J Radiol. 2022;23:574–580. doi: 10.3348/kjr.2022.0099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Acciavatti RJ, Lee SH, Reig B, Moy L, Conant EF, Kontos D, et al. Beyond breast density: risk measures for breast cancer in multiple imaging modalities. Radiology. 2023;306:e222575. doi: 10.1148/radiol.222575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.DeMartini WB, Destounis SV, Eby PR, Leung JWT. BI-RADS update: the edition formerly known as fifth; Proceedings of the 2023 SBI Breast Imaging Symposium; 2023 May 4-7; National Harbor, MD, USA. Society of Breast Imaging; 2023. p. 9. [Google Scholar]
- 24.Choi JS, Tsunoda H, Moon WK. Nonmass lesions on breast ultrasound: an international perspective on clinical use and outcomes. J Breast Imaging. 2023 Oct 27; doi: 10.1093/jbi/wbad077. [Epub] [DOI] [PubMed] [Google Scholar]
- 25.Leung JWT. Nonmass descriptor at breast US to expand clinical utility. J Breast Imaging. 2023 Dec 27; doi: 10.1093/jbi/wbad095. [Epub] [DOI] [PubMed] [Google Scholar]
- 26.Watanabe T, Yamaguchi T, Tsunoda H, Kaoku S, Tohno E, Yasuda H, et al. Ultrasound image classification of ductal carcinoma in situ (DCIS) of the breast: analysis of 705 DCIS lesions. Ultrasound Med Biol. 2017;43:918–925. doi: 10.1016/j.ultrasmedbio.2017.01.008. [DOI] [PubMed] [Google Scholar]
- 27.Hsu HH, Yu JC, Hsu GC, Chang WC, Yu CP, Tung HJ, et al. Ultrasonographic alterations associated with the dilatation of mammary ducts: feature analysis and BI-RADS assessment. Eur Radiol. 2010;20:293–302. doi: 10.1007/s00330-009-1546-8. [DOI] [PubMed] [Google Scholar]
- 28.Sabatier R, Sabiani L, Zemmour C, Taix S, Chereau E, Gonçalves A, et al. Invasive ductal breast carcinoma with predominant intraductal component: clinicopathological features and prognosis. Breast. 2016;27:8–14. doi: 10.1016/j.breast.2015.12.003. [DOI] [PubMed] [Google Scholar]
- 29.Ban K, Tsunoda H, Watanabe T, Kaoku S, Yamaguchi T, Ueno E, et al. Characteristics of ultrasonographic images of ductal carcinoma in situ with abnormalities of the ducts. J Med Ultrason. 2020;47:107–115. doi: 10.1007/s10396-019-00981-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Selinko VL, Middleton LP, Dempsey PJ. Role of sonography in diagnosing and staging invasive lobular carcinoma. J Clin Ultrasound. 2004;32:323–332. doi: 10.1002/jcu.20052. [DOI] [PubMed] [Google Scholar]
- 31.Sotome K, Yamamoto Y, Hirano A, Takahara T, Hasegawa S, Nakamaru M, et al. The role of contrast enhanced MRI in the diagnosis of non-mass image-forming lesions on breast ultrasonography. Breast Cancer. 2007;14:371–380. doi: 10.2325/jbcs.14.371. [DOI] [PubMed] [Google Scholar]
- 32.Jones KN, Magut M, Henrichsen TL, Boughey JC, Reynolds C, Glazebrook KN. Pure lobular carcinoma of the breast presenting as a hyperechoic mass: incidence and imaging characteristics. AJR Am J Roentgenol. 2013;201:W765–W769. doi: 10.2214/AJR.12.9742. [DOI] [PubMed] [Google Scholar]
- 33.Moon WK, Myung JS, Lee YJ, Park IA, Noh DY, Im JG. US of ductal carcinoma in situ. Radiographics. 2002;22:269–280. doi: 10.1148/radiographics.22.2.g02mr16269. [DOI] [PubMed] [Google Scholar]
- 34.Scoggins ME, Fox PS, Kuerer HM, Rauch GM, Benveniste AP, Park YM, et al. Correlation between sonographic findings and clinicopathologic and biologic features of pure ductal carcinoma in situ in 691 patients. AJR Am J Roentgenol. 2015;204:878–888. doi: 10.2214/AJR.13.12221. [DOI] [PubMed] [Google Scholar]
- 35.Guo W, Wang T, Li F, Jia C, Zheng S, Zhang X, et al. Non-mass breast lesions: could multimodal ultrasound imaging be helpful for their diagnosis? Diagnostics (Basel) 2022;12:2923. doi: 10.3390/diagnostics12122923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ko ES, Han H, Lee BH, Choe DH. Sonographic changes after removing all benign breast masses with sonographically guided vacuum-assisted biopsy. Acta Radiol. 2009;50:968–974. doi: 10.3109/02841850903130836. [DOI] [PubMed] [Google Scholar]
- 37.Seymour MT, Moskovic EC, Walsh G, Trott P, Smith IE. Ultrasound assessment of residual abnormalities following primary chemotherapy for breast cancer. Br J Cancer. 1997;76:371–376. doi: 10.1038/bjc.1997.392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lee E, Wylie E, Metcalf C. Ultrasound imaging features of radial scars of the breast. Australas Radiol. 2007;51:240–245. doi: 10.1111/j.1440-1673.2007.01719.x. [DOI] [PubMed] [Google Scholar]
- 39.Soo MS, Kornguth PJ, Hertzberg BS. Fat necrosis in the breast: sonographic features. Radiology. 1998;206:261–269. doi: 10.1148/radiology.206.1.9423681. [DOI] [PubMed] [Google Scholar]
- 40.Lee JH, Oh KK, Kim EK, Kwack KS, Jung WH, Lee HK. Radiologic and clinical features of idiopathic granulomatous lobular mastitis mimicking advanced breast cancer. Yonsei Med J. 2006;47:78–84. doi: 10.3349/ymj.2006.47.1.78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Takei J, Tsunoda-Shimizu H, Kikuchi M, Kawasaki T, Yagata H, Tsugawa K, et al. Clinical implications of architectural distortion visualized by breast ultrasonography. Breast Cancer. 2009;16:132–135. doi: 10.1007/s12282-008-0085-5. [DOI] [PubMed] [Google Scholar]
- 42.Yang WT, Tse GM. Sonographic, mammographic, and histopathologic correlation of symptomatic ductal carcinoma in situ. AJR Am J Roentgenol. 2004;182:101–110. doi: 10.2214/ajr.182.1.1820101. [DOI] [PubMed] [Google Scholar]
- 43.Sekine K, Tsunoda-Shimizu H, Kikuchi M, Saida Y, Kawasaki T, Suzuki K. DCIS showing architectural distortion on the screening mammogram - comparison of mammographic and pathological findings. Breast Cancer. 2007;14:281–284. doi: 10.2325/jbcs.14.281. [DOI] [PubMed] [Google Scholar]
- 44.Yoshida A, Hayashi N, Akiyama F, Yamauchi H, Uruno T, Kikuchi M, et al. Ductal carcinoma in situ that involves sclerosing adenosis: high frequency of bilateral breast cancer occurrence. Clin Breast Cancer. 2012;12:398–403. doi: 10.1016/j.clbc.2012.08.002. [DOI] [PubMed] [Google Scholar]
- 45.Oiwa M, Endo T, Ichihara S, Moritani S, Hasegawa M, Iwakoshi A, et al. Sclerosing adenosis as a predictor of breast cancer bilaterality and multicentricity. Virchows Arch. 2015;467:71–78. doi: 10.1007/s00428-015-1769-9. [DOI] [PubMed] [Google Scholar]
- 46.Wong HN, Tsunoda H, Matsuda N, Suzuki K, Li CP, Fok EWS, et al. Sclerosing adenosis: should we still regard it as a simple benign disease? Report of two patients with subsequent development of invasive or in-situ breast cancer. Hong Kong J Radiol. 2014;17:49–56. [Google Scholar]
- 47.Usami Y, Tsunoda H, Kajiura Y, Kawauchi N, Kikuchi M, Honda S, et al. [Ultrasonographic evaluation of multiple cystic lesions in breast sonography] Jpn J Med Ultrasonics. 2011;38:455–460. Japanese. [Google Scholar]
- 48.Kim SM, Kim HH, Kang DK, Shin HJ, Cho N, Park JM, et al. Mucocele-like tumors of the breast as cystic lesions: sonographic-pathologic correlation. AJR Am J Roentgenol. 2011;196:1424–1430. doi: 10.2214/AJR.10.5028. [DOI] [PubMed] [Google Scholar]
- 49.Tanaka A, Imai A, Goto M, Konishi E, Shinkura N. Which patients require or can skip biopsy for breast clustered microcysts? Predictive findings of breast cancer and mucocele-like tumor. Breast Cancer. 2016;23:590–596. doi: 10.1007/s12282-015-0607-x. [DOI] [PubMed] [Google Scholar]
- 50.Terminology and Diagnostic Criteria Committee, Japan Society of Ultrasonics in Medicine. Recall criteria for ultrasound breast cancer screening. J Med Ultrason. 2016;43:301–313. doi: 10.1007/s10396-015-0694-5. [DOI] [PubMed] [Google Scholar]
- 51.Berg WA, Campassi CI, Ioffe OB. Cystic lesions of the breast: sonographic-pathologic correlation. Radiology. 2003;227:183–191. doi: 10.1148/radiol.2272020660. [DOI] [PubMed] [Google Scholar]
- 52.Moon WK, Im JG, Koh YH, Noh DY, Park IA. US of mammographically detected clustered microcalcifications. Radiology. 2000;217:849–854. doi: 10.1148/radiology.217.3.r00nv27849. [DOI] [PubMed] [Google Scholar]
- 53.Cheung YC, Wan YL, Chen SC, Lui KW, Ng SH, Yeow KM, et al. Sonographic evaluation of mammographically detected microcalcifications without a mass prior to stereotactic core needle biopsy. J Clin Ultrasound. 2002;30:323–331. doi: 10.1002/jcu.10074. [DOI] [PubMed] [Google Scholar]
- 54.Yao JJ, Zhan WW, Chen M, Zhang XX, Zhu Y, Fei XC, et al. Sonographic features of ductal carcinoma in situ of the breast with microinvasion: correlation with clinicopathologic findings and biomarkers. J Ultrasound Med. 2015;34:1761–1768. doi: 10.7863/ultra.15.14.07059. [DOI] [PubMed] [Google Scholar]
- 55.Yoshida Y, Tsunoda H, Tsurugi S, Uzawa I, Yagishita K, Kanomata N. [Do all intraductal lesions identified during breast ultrasound screening require further examination?] J Jpn Assoc Breast Cancer Screen. 2022;31:203–210. Japanese. [Google Scholar]
- 56.Hong S, Li W, Gao W, Liu M, Song D, Dong Y, et al. Diagnostic performance of elastography for breast non-mass lesions: a systematic review and meta-analysis. Eur J Radiol. 2021;144:109991. doi: 10.1016/j.ejrad.2021.109991. [DOI] [PubMed] [Google Scholar]
- 57.Itoh A, Ueno E, Tohno E, Kamma H, Takahashi H, Shiina T, et al. Breast disease: clinical application of US elastography for diagnosis. Radiology. 2006;239:341–350. doi: 10.1148/radiol.2391041676. [DOI] [PubMed] [Google Scholar]
- 58.Cho N, Moon WK, Park JS. Real-time US elastography in the differentiation of suspicious microcalcifications on mammography. Eur Radiol. 2009;19:1621–1628. doi: 10.1007/s00330-009-1335-4. [DOI] [PubMed] [Google Scholar]
- 59.Lee SH, Chang JM, Cho N, Koo HR, Yi A, Kim SJ, et al. Practice guideline for the performance of breast ultrasound elastography. Ultrasonography. 2014;33:3–10. doi: 10.14366/usg.13012. [DOI] [PMC free article] [PubMed] [Google Scholar]